Abstract
The architecture of a Sphingomonas biofilm was studied during early phases of its formation, using strain L138, a gfp-tagged derivative of Sphingomonas sp. strain LB126, as a model organism and flow cells and confocal laser scanning microscopy as experimental tools. Spatial and temporal distribution of cells and exopolymer secretions (EPS) within the biofilm, development of microcolonies under flow conditions representing varied Reynolds numbers, and changes in diffusion length with reference to EPS production were studied by sequential sacrificing of biofilms grown in multichannel flow cells and by time-lapse confocal imaging. The area of biofilm in terms of microscopic images required to ensure representative sampling varied by an order of magnitude when area of cell coverage (2 × 105 μm2) or microcolony size (1 × 106 μm2) was the biofilm parameter under investigation. Hence, it is necessary to establish the inherent variability of any biofilm metric one is attempting to quantify. Sphingomonas sp. strain L138 biofilm architecture consisted of microcolonies and extensive water channels. Biomass and EPS distribution were maximal at 8 to 9 μm above the substratum, with a high void fraction near the substratum. Time-lapse confocal imaging and digital image analysis showed that growth of the microcolonies was not uniform: adjacently located colonies registered significant growth or no growth at all. Microcolonies in the biofilm had the ability to move across the attachment surface as a unit, irrespective of fluid flow direction, indicating that movement of microcolonies is an inherent property of the biofilm. Width of water channels decreased as EPS production increased, resulting in increased diffusion distances in the biofilm. Changing hydrodynamic conditions (Reynolds numbers of 0.07, 52, and 87) had no discernible influence on the characteristics of microcolonies (size, shape, or orientation with respect to flow) during the first 24 h of biofilm development. Inherent factors appear to have overriding influence, vis-à-vis environmental factors, on early stages of microcolony development under these laminar flow conditions.
Biofilms are ubiquitous in all natural and many industrial environments. Researchers study different aspects of biofilm development and processes in fields such as biofouling (3), biocorrosion (23, 35), bioremediation (13), wastewater treatment (62, 68), human health (59), and ecology (37). Earlier work on biofilms focused on elucidation of the process of adhesion of bacteria to surfaces (6). However, more recent research has dealt with complexities of the structural and functional aspects of biofilms (42, 48, 65), associations between different physiological and metabolic groups of microorganisms (60), material traffic into and out of biofilm matrices (34, 41), involvement of genetic factors on biofilm formation (46), response of biofilms to changing environmental conditions (40), and cell-cell communication systems (9, 25, 63).
Biofilms are characterized by a complex architecture. Biofilm architecture in medical scenarios (e.g., on implants) or industrial scenarios (e.g., on heat exchanger surfaces or in biofilm reactors) assumes importance in the context of mass/heat transport and biofilm control using antibiotics or biocides. The distribution of cells and exopolymer secretions (EPS) is a manifestation of the complex physical, chemical, and biological organization of the biofilm. The earlier concept of homogeneous biofilms had assumed that transport of materials (dissolved oxygen, nutrients, and waste products) into and out of biofilms took place mainly through diffusional processes. However, recent work indicates that biofilms exhibit more complex architecture (53).
The objective of the present study was to understand the development of the architecture of a monoculture biofilm during the initial phases of its formation. Early stages of biofilm genesis represent a very dynamic phase of biofilm growth (7, 42, 57). Monoculture biofilms are relatively rare in nature, but they do exist and are important in many medical scenarios. From an experimental point of view there are advantages to studying them, because variations in architecture introduced by interactions with other species are avoided and only interactions of the cells themselves are taken into account. For this purpose we used strain L138, a gfp-labeled derivative of Sphingomonas sp. strain LB126 (1), as a model organism. Sphingomonas spp. have been shown to possess unique abilities to degrade refractory contaminants and secrete highly useful gellan exopolysaccharides (15, 24). They are biotechnologically important bacteria and have been used in the removal and degradation of several pollutants and xenobiotics under natural and bioreactor conditions (17-19, 55). Our experimental strategy involved development of a biofilm in a flow cell mounted on a microscope stage and time series observations using a confocal laser scanning microscope (CLSM). CLSM was preferred as it allows nondestructive optical sectioning of fully hydrated biofilms, rendering images that are amenable to digital image processing (33).
MATERIALS AND METHODS
Bacterial strains, antibiotic selection, and growth conditions.
Sphingomonas sp. strain LB126 was isolated from a polycyclic aromatic hydrocarbon-contaminated soil by liquid enrichment with fluorene as sole source of carbon and energy (1) and is naturally resistant to streptomycin. The strain was grown routinely in phosphate minimal medium (PMM), which is identical to Tris medium (39) but with 50 mM phosphate buffer (pH 7.4) instead of Tris and supplemented with either 0.2% (wt/vol) glucose or with a few fluorene crystals (approximately 0.4% [wt/vol]) as sole carbon source. Flow cell experiments were carried out using a gfp-labeled derivative strain (L138) of Sphingomonas sp. strain LB126. The cells were chromosomally tagged with a mini-Tn5-tet-gfp transposon, resulting in strong constitutive expression of the green fluorescent protein (GFP) as previously described (64). Cells for experiments were harvested from a mid-exponential-phase culture grown in PMM containing 0.2% glucose. The medium contained antibiotics at the following concentrations: tetracycline, 5 μg ml−1; streptomycin, 100 μg ml−1.
gfp marker stability.
The stability of the gfp marker in strain L138 was tested as described by Taghavi et al. (56) over a period of 100 generations under nonselective conditions. Briefly, the recombinant strain was pregrown in PMM containing fluorene, supplied in the form of crystals, as sole carbon source and 5 μg ml−1 tetracycline to ensure the presence of the introduced mini-Tn5-tet-gfp. In the late-logarithmic growth phase, cells were washed and diluted to an optical density at 600 nm (OD600) of 0.0005 in PMM containing glucose and incubated at 30°C. The cultures were diluted 103-fold in PMM containing glucose when the OD600 reached 0.5, corresponding to 10 generations, and grown for the next 10 generations. This treatment continued for 100 generations. Every 20 generations, samples were plated on PMM agar containing glucose for estimating the actual number of generations that the cells had grown under nonselective conditions. Subsequently, 100 colonies were replica plated and checked for growth under nonselective and selective conditions inherent to the introduced marker. Tetracycline resistance was tested on PMM plates containing either fluorene or glucose and 5 μg ml−1 tetracycline. In addition, the resulting replicated colonies were scored for the expression of the gfp marker by examining green fluorescence appearance when the plates were placed under UV light.
Flow cell experiments.
The experimental biofilms were generated in a stainless steel flow cell with four single channels (channel size, 4 cm by 0.4 cm by 0.4 cm) (30). Glass microscope coverslips, glued (with silicone glue) to the top and bottom of the flow cell, served as substratum for biofilm growth and allowed direct microscopic observation. The experimental setup consisted of a medium (same as used for cell culture) reservoir, a peristaltic pump (Ismatec PC-04; Ismatec SA, Zurich, Switzerland), flow cell, and a waste reservoir, all connected by silicone tubing. The entire setup was autoclaved prior to each experiment.
For inoculating each channel, cells harvested by centrifugation (6,000 rpm, 5 minutes) were washed in sterile phosphate-buffered saline (PBS) and resuspended in sterile PBS. The cells were vortexed for 4 to 5 min to disrupt any cell clumps present. Trial experiments had shown that any clumps present in the flow cell inoculum tended to grow as microcolonies, thereby biasing experimental results. Disruption ensured that the biofilm developed from single cells. Two milliliters of the bacterial suspension (OD600 ≈ 0.17) was injected into each channel of the flow cell, replacing the nutrient medium present inside. Inoculation was carried out under sterile conditions, carefully avoiding formation of air bubbles in the flow cell. After 2 h, pumping through the flow cell was resumed at a rate of 8 ml h−1 using the peristaltic pump (configuration mode 1). The biofilm was allowed to grow for 24 h, unless otherwise stated, before CLSM images were taken.
Hydrodynamic conditions.
Considering the physical principle of the fluid mass balance governing the multichannel flow cell with n single channels, the continuity equation shows
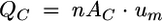 |
(1) |
where QC is the flow rate in the multichannel flow cell, n is number of used single channels within the multichannel flow cell, AC is the cross-sectional area of one single channel in the flow cell, and um is the mean bulk fluid velocity in the flow cell.
We note with equation 1 that
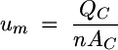 |
(2) |
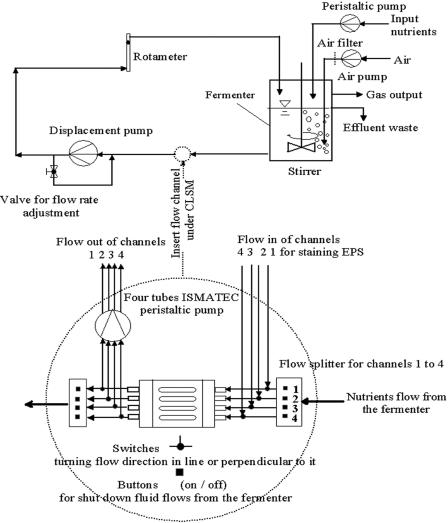
If during experiments, which are carried out in the “recirculating” configuration mode 2, some single channels have to be shut down, then the input flow rates of the other still-“active” channels have to be adjusted in order to keep constant the same mean bulk fluid velocity in the remaining channels as when all channels are in use. This can be done by inserting the corresponding value n in equation 1 and by keeping constant the previous flow velocity value for um in equation 2.
The Reynolds number, Re, is a dimensionless parameter characterized by the ratio of inert forces to frictional forces in a flow. It is used here to describe the type of flow conditions in the channel. For internal flows in rectangular systems, the Reynolds number is usually defined as follows:
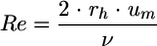 |
(3) |
with
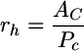 |
(4) |
where rh is the hydraulic radius, PC is the wetted perimeter of the flow channel, and ν is the coefficient of kinematic viscosity of the fluid (νwater = 0.01 cm2 s−1 at 20°C).
The Reynolds number is a useful parameter in fluid dynamics and a dominant factor in transition to turbulent flow. In a clean rectangular system, the transition from laminar to turbulent flow is theoretically defined as Recrit of ∼2,100. Under the experimental conditions used for configuration mode 1, um was 3.47 × 10−3 cm s−1 and was associated with Reynolds number Re = 0.07; therefore, the adhesion and growth of bacteria took place under laminar flow conditions.
Experiments were also carried out to compare the microcolony formation at Re = 0.07 with that at higher flow rates of 6 liters h−1 (Re = 52) and 10 liters h−1 (Re = 87). These higher fluid flow velocities (um = 2.60 cm s−1 and um = 4.34 cm s−1, respectively) in each single channel were obtained by inserting the multichannel flow cell (Fig. 1) in a recirculation loop (configuration mode 2). In contrast to the open flowthrough configuration mode 1, configuration mode 2 was a closed recirculating configuration, as described previously (31). The nutrient medium was pumped by a pressure-independent displacement pump (Netsch, Waldkraiburg, Germany) into an aerated and stirred fermentor (2 liters) and then recirculated through the flow cell. Unlike configuration mode 1, in configuration 2 the flow velocities in the channels are independent of the nutrient supply and high volume flows can be achieved by incorporating the flow cell into the suction branch of the recirculation loop. A nutrient reservoir continuously supplied nutrients to the fermentor at a rate of 8 liters day−1. The flow rate was monitored using a rotameter (type 47 R; Krohne, Duisburg, Germany). The overall dilution rate, D, in the medium mixing vessel was 4.88 h−1 and 7.96 h−1 for Re = 52.1 and Re = 86.8, respectively. The growth rate on glucose in planktonic culture was determined independently as 0.14 h−1. It follows that no cell division was possible in the mixing vessel. The hydraulic retention time, θ, in the flow channel for the three hydrodynamic conditions was 19.2 min for Re = 0.07, 1.54 s for Re = 52.1, and 0.92 s for Re = 86.8.
FIG. 1.
Simplified schematic of a multichannel flow cell used for experiments on biofilm formation by Sphingomonas sp. strain L138 under different hydrodynamic conditions. For details of the experimental setup, see reference 31.
The individual components of the complete system were either autoclaved or sterilized with 0.1× peracetic acid and rinsed with autoclaved distilled water before use (16). Before inoculation, sterile nutrient medium was circulated through the experimental system to prime the tubes and purge air from the system.
Automated image acquisition.
We used a Zeiss LSM 410 confocal system with a Zeiss Axiovert 135 TV inverted microscope (Carl Zeiss, Germany). The images were obtained (unless specified otherwise) using a 63×, 1.2 numerical aperture (NA) (C-Apochromat) water immersion objective lens. A series of horizontal x-y optical sections (512 by 512 pixels) of the biofilms were automatically acquired at a Δz of 1 μm, so as to cover the entire thickness of the biofilm, from 9 to 12 fields (each with a substratum area of 202.8 μm by 202.8 μm). This was done with the help of a user-specified macro procedure that operated the motorized focusing and stage movement controls and enabled automated acquisition of images (30, 31). In order to accommodate the flow velocity profile, three transects were scanned to generate confocal stacks: one close to the flow cell wall (transect 1), one in the middle of the channel (transect 3), and one in between the two (transect 2). Cells and EPS (see below) were imaged after 24, 48, 72, and 96 h of growth (unless otherwise mentioned) by sequentially sacrificing each of the four channels in the multichannel flow cell.
EPS staining and imaging.
Cells, as mentioned, were imaged using their inherent GFP fluorescence. In addition, EPS distribution in the biofilm was visualized after staining with fluorescent lectin concanavalin A (ConA) (24). Two milliliters of tetramethyl rhodamine isothiocyanate (TRITC)-labeled ConA (Molecular Probes) solution (1 mg/ml in PBS containing the following, in grams/liter: Na2HPO4, 0.2; NaH2PO4, 1.44; NaCl, 8.0, KCl, 0.2, CaCl2, 0.011; MnCl2, 0.013; pH 6.8) was injected into the flow cell, replacing its liquid content. After 15 min of incubation, the flow cell was flushed with excess PBS for 10 min and confocal image stacks were collected by the procedure mentioned above. The microscope stage was not moved during the staining procedure and, hence, the cell and EPS images were obtained from the same microscope fields. GFP signal was collected by exciting the fluorochrome using an Ar laser (488 nm) and using the emission filter BP 515-540, while TRITC was excited using a He/Ne 543 nm laser and the fluorescence signals were collected using the filter BP 590-610.
Experiment to study comparability of flow cell channels.
The experimental procedure followed in this study assumed biofilms growing in different channels of the flow cell to be comparable, as they were generated under identical conditions. This assumption was verified by growing triplicate biofilms in three channels of a flow cell under identical conditions and then comparing them. Microcolony size was used as the variable for the purpose of comparison. After 24 h of growth, the biofilms in the channels were imaged. The height (i.e., z position) of the confocal slice above the substratum, at which plane the cell coverage was maximum, was 8 μm in all channels. Subsequently, eight optical thin x-y sections were randomly collected at this height from each channel using a 40×, 1.3 NA objective, totaling an area of 8.2 × 106 μm2 per channel. The images were subsequently analyzed and compared by digital image analysis (see below).
Determination of representative area.
To estimate the minimum biofilm area required to be sampled, an experiment was carried out as described by Korber et al. (26). This was done by plotting variations in a suitable parameter, which ideally represented the heterogeneity of biofilm, against increasing biofilm sampling area and determining from the graph the sampling area where distribution of the parameter became independent of the analysis area. Percent cell area coverage and microcolony size were used as variables to evaluate the representative area. Biofilms grown in a flow cell were imaged after 24 h of growth. Images of optical thin sections were collected using a 63×, 1.2 NA objective from a total biofilm area of 1.11 × 106 μm2. A macro routine was written to cut each digital image into four equal quadrants; subsequently, each quadrant was analyzed as if it were a separate image. This quadratization was done to provide greater sensitivity to regional variability within the biofilm, by mimicking the use of a higher-power objective (26, 27 32). The sizes of microcolonies as well as percentage cell coverage in each quadrant were determined. Microcolonies less than 5 μm2 as well as those touching the edges were excluded from the former analysis. Using a computer program, 2, 3, 4, 8, 16, etc. quadrants were randomly selected and mean colony size and mean cell coverage were computed. Thus, by combining an increasing number of quadrants we could generate data for the above two parameters from a consistently larger virtually “scanned” biofilm area. Areas much larger than the original analysis area could be randomly synthesized by this method. The process required iterative computation, and a Visual Basic program was written for this purpose.
Time-lapse study of microcolony development.
This experiment was carried out by time-lapse imaging of a biofilm growing at Re = 0.07 for 24 h. A flow cell was inoculated with a suspension of cells as described earlier. After 2 h of incubation (designated zero hours), nutrient flow was resumed. Starting at time zero hours, confocal image stacks (spanning the entire thickness of biofilm, Δz = 1 μm) were collected at 1-h intervals. The microscope stage (x and y positions) was held stationary during the entire experiment, and the same field area (202.8 μm by 202.8 μm) was repeatedly scanned to follow the dynamics of microcolony formation.
Digital image analysis.
The confocal image stacks were processed using digital image analysis software programs such as NIH Image (Windows version freely available from Scion Corporation [http://www.scioncorp.com]) and Quantimet 570 (Leica, Cambridge, United Kingdom) to derive quantitative information. The area covered by cells in sequential slices was measured as the percentage of pixels detected relative to the area of the measurement frame. Biovolumes (cell as well as EPS volumes) were calculated using the Leica software by numerical integration of area of cell (or EPS) coverage, following the trapezoidal rule (31).
Diffusion length and void width.
Images of the cells and EPS were processed to get information about the internal architecture of the biofilms, especially related to the clusters and water channels. Changes in the distance a solute molecule had to travel from a water channel into the interior of the microcolony were studied by calculating the diffusion length (DL), defined here as a dimensionless ratio of the perpendicular distance from the middle of the microcolony to its edge and the width of the water channel adjoining it (equation 5).
 |
(5) |
This method was used to take into account both colony size and water channel width. Colony width decides the distance the solute molecule has to travel to reach the interior of the colony, while width of the water channel determines the size of the solute reservoir. The spatially calibrated confocal images, after interactive thresholding and binarization, were “outlined,” a process that sequentially removed pixels from the interior of the images, leaving behind a single-pixel-wide outline. A copy of the original image was similarly thresholded and binarized, after which it was “skeletonized.” This process, available in Scion Image, sequentially removed pixels from the exterior of the images until a single-pixel-wide line, representing the middle of the microcolonies, remained. The “outline” image and “skeleton” image were then digitally combined. The resultant image showed an outline of the original microcolonies, with the skeleton representing the median line that ran along the middle of the colony. Distance from the middle of the colony to the edge of the colony was measured by counting the intervening pixels. The width of the adjoining void was also measured in a similar way. The colony size and void width measurements were done first using the images of the cells alone (GFP signal) and later on using digitally combined images of cells and EPS (ConA signal), so as to demonstrate the influence of EPS production on diffusion length and void width.
Microcolony characteristics.
Image analysis was used to determine morphological characteristics of the microcolonies in the images. It was assumed that ambient flow rate would influence the shape and orientation of the microcolonies in the developing biofilm. It was hypothesized that at low flow rates the biofilm might produce colonies with serrated edges, so as to increase surface area. This would make the outline of the colonies more tortuous, and the degree of such tortuousness can be studied by comparing the ratio of their perimeter length to colony area. Similarly, the ratio of the major axis of a microcolony to its minor axis was used as an indicator of the roundness of the colony (the major and minor axes were determined by best-fitting an ellipse over the microcolony, a feature available in Scion Image). The angle of orientation of all microcolonies with respect to nutrient flow in the flow cell was also measured. Microcolonies, which were oriented vertically and horizontally vis-à-vis the direction of nutrient flow, were counted. Microcolonies with their major axis lying at 90 ± 10° with respect to the flow were considered vertically oriented, while those whose major axes were in the range 0 to 10° or 170 to 180° were considered horizontally oriented.
Growth and migration of microcolonies.
Scion Image was used to compare the growth of microcolonies as well as their movement on the substratum. The time-lapse images (taken from the same microscope field at defined time intervals) were spatially calibrated, segmented, and binarized. The images of the microcolonies were outlined as described earlier. Digital superimposition of two outlined sequential images (one of which was pseudo-colored to aid easy recognition) was used to determine the change in the sizes and positions of microcolonies over time.
RESULTS
Fluorescent marker stability.
After 80 generations, the gfp and Tc resistance markers were still present in 100% of the population in the derivative strain L138. Moreover, the strain exhibited growth rates on fluorene and glucose similar to the wild-type strain LB126. The growth rate on glucose in planktonic culture was 0.14 h−1.
Comparability of biofilms.
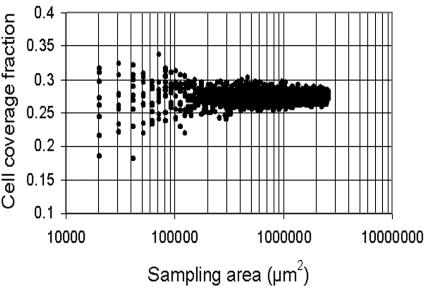
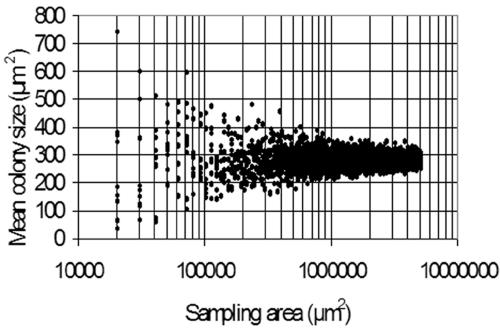
As the experiments were performed using multichannel flow cells, it was necessary to confirm that biofilm growth in identically treated channels of the same flow cell was comparable. For this, Sphingomonas sp. strain L138 biofilms growing in three identically treated channels of the flow cell were imaged after 24 h of growth. A comparison of the mean size of the microcolonies in the three channels showed that there were no significant differences (analysis of variance F = 1.326; P = 0.1426). Hence, the three biofilm channels and the three transects of each channel had morphological features that were statistically comparable. Results of the experiments to determine the representative biofilm area are given in Fig. 2 and 3. If percent cell coverage is used as a parameter to study Sphingomonas sp. strain L138 biofilms, a minimum area of 2 × 105 μm2 needs to be scanned to obtain representative data (Fig. 2). On the other hand, if one uses a more variable parameter such as microcolony size as a variable to compare biofilms, then the sampling area needs to be increased substantially, by almost an order of magnitude (Fig. 3).
FIG. 2.
Analysis of minimum representative area of Sphingomonas sp. strain L138 biofilm to be sampled when cell coverage is used as the quantifying parameter.
FIG. 3.
Analysis of minimum representative area of Sphingomonas sp. strain L138 biofilm to be sampled when mean size of microcolonies is used as the biofilm parameter.
Biofilm architecture.
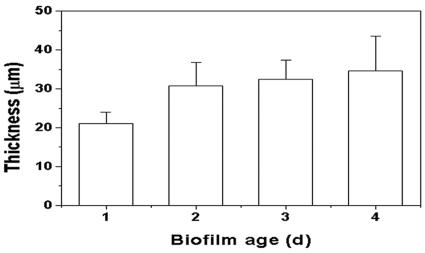
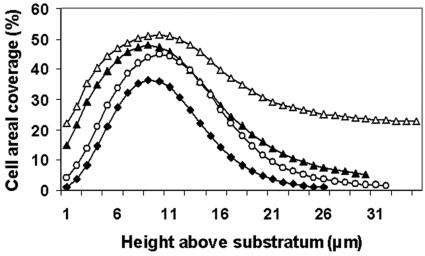
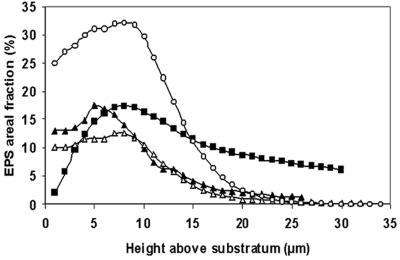
Sphingomonas sp. strain L138 produced microcolonies that were typically mushroom shaped, with small bases and expanding columns (Fig. 4). The thickness of the biofilm changed as a function of biofilm age (Fig. 5). It can be seen that the average thickness did not vary significantly during the period from 48 to 96 h. The mean cell biovolume in the case of creeping flow experiments (Re = 0.07) increased from 5,973 μm3 after 24 h to 48,528 μm3 after 96 h of growth (calculated per confocal stack) (data not shown). Examination of the distribution of cell material in the confocal slices revealed that biomass was more concentrated at about 7 to 9 μm above the substratum (Fig. 6). The pattern remained the same even after 96 h of biofilm growth. EPS development was poor initially, but there was a sudden increase in EPS production after 96 h. Moreover, distribution of EPS in the confocal slices showed a pattern similar to the cell distribution (see Fig. S1 in the supplemental material), with maximum EPS being present at about 7 to 9 μm above the substratum (Fig. 7).
FIG. 4.
Sagittal (x-z) section of a Sphingomonas sp. strain L138 biofilm showing a mushroom-shaped microcolony. Bar, 8 μm.
FIG. 5.
Changes in Sphingomonas sp. strain L138 biofilm thickness as a function of biofilm age at low Reynolds number (Re = 0.07). Error bars indicate standard deviations.
FIG. 6.
Distribution of cell area coverage in the confocal image slices of a Sphingomonas sp. strain L138 biofilm (grown at Re = 0.07) plotted as a function of biofilm depth for different biofilm ages. Symbols: ♦, 1 day; ▴, 2 days; ○, 3 days; ▵, 4 days.
FIG. 7.
Changes in EPS area coverage in Sphingomonas sp. L138 biofilm (grown at Re = 0.07) plotted as a function of biofilm age (in days). Symbols: ▴, 1 day; ▪, 2 days; ○, 3 days; ▵, 4 days.
Microcolony characteristics.
Table 1 lists the characteristics of microcolonies that developed in the flow cells during the first 24 h under three flow rates: 8 ml h−1, 6 liters h−1, and 10 liters h−1 (Re = 0.07, 52, and 87, respectively). The data did not show any noticeable trend in microcolony characteristics when the biofilms were grown at low (8 ml h−1) or higher (6 and 10 liters h−1) flow rates. Changes in hydrodynamic flow had little influence on these parameters during the very early stages (24 h) of biofilm growth. Similarly, microcolony orientation (with respect to fluid flow) did not seem to be influenced by flow direction.
TABLE 1.
Morphological features of microcolonies formed at high and low flow rates in a 24-h-old biofilme
| Condition and transect no. | L/Aa ± SEM | Mj/Mnb ± SEM | % Horizc ± SEM | % Vertd ± SEM |
|---|---|---|---|---|
| Re = 0.07 | ||||
| 1 | 1.17 ± 0.06 | 1.80 ± 0.04 | 22 ± 2 | 27 ± 2 |
| 2 | 1.17 ± .06 | 1.82 ± 0.03 | 19 ± 2 | 18 ± 2 |
| 3 | 1.12 ± 0.03 | 1.68 ± 0.06 | 29 ± 2 | 23 ± 1 |
| Re = 52 | ||||
| 1 | 1.12 ± 0.04 | 1.69 ± 0.05 | 27 ± 4 | 21 ± 1 |
| 2 | 1.22 ± 0.04 | 1.55 ± 0.04 | 28 ± 5 | 15 ± 7 |
| 3 | 1.35 ± 0.04 | 1.62 ± 0.01 | 24 ± 5 | 21 ± 7 |
| Re = 87 | ||||
| 1 | 1.39 ± 0.06 | 1.90 ± 0.02 | 24 ± 2 | 20 ± 2 |
| 2 | 1.27 ± 0.03 | 1.85 ± 0.03 | 24 ± 1 | 19 ± 2 |
| 3 | 1.10 ± 0.01 | 1.65 ± 0.16 | 29 ± 5 | 18 ± 8 |
L/A = ratio of the perimeter length of the colony to the area of the colony.
Mj/Mn = ratio of major axis of the colony to the minor axis.
% Horiz = percentage of colonies oriented horizontally to the direction of flow.
% Vert = percentage of colonies oriented vertically to the flow direction.
Data shown are from three different scan transects (see the text for details). SEM, standard error of the mean.
Diffusion length changes in biofilm.
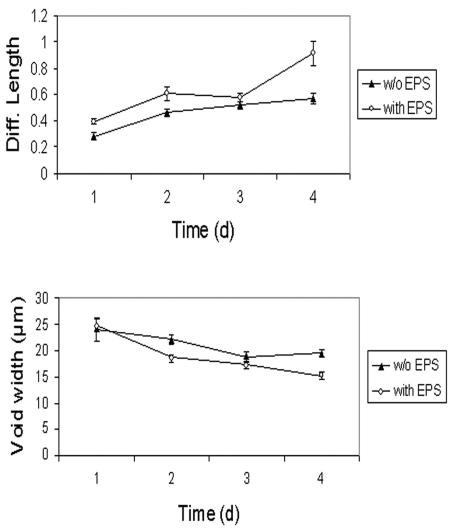
Figure 8 shows the changes in diffusion length (DL) and void (water channel) width in a Sphingomonas sp. strain L138 biofilm grown at Re = 0.07. The parameters have been calculated first using the cell (i.e., GFP) signal alone and then after digitally combining the cell image with the corresponding EPS (ConA signal) image, so that the effect of EPS production on DL and void width could be clearly understood. There was an increase in the DL as a function of time. The increase was quite marked on the fourth day, obviously due to a sudden increase in EPS development.
FIG. 8.
Changes in diffusion length and void (water channel) width as a function of biofilm age. Data are presented based on analysis of images of cells alone (without EPS [▴]) and images of cells and EPS digitally combined (with EPS [○]) to delineate the effect of EPS production. Error bars are standard errors of the means, with n = 160; that is, the values plotted are the means of 160 measurements per image. For details of the image analysis protocol, see the text.
Microcolony development.
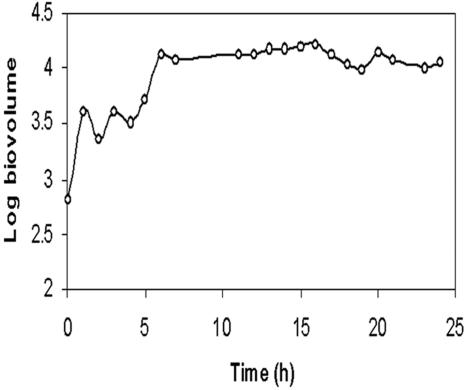
Images from the time-lapse experiment carried out at Re = 0.07 were processed to study the development of microcolonies. Analysis of biofilm volume showed that the early stages of microcolony development (0 to 8 h) were characterized by rapid changes in biovolume, indicating growth or accretion and/or removal of cell material (Fig. 9). Comparison of images taken at 1-h intervals (Fig. 10) showed that growth of microcolonies was not uniform throughout the image field. Some of the colonies increased their size substantially, while the size of adjacent colonies in the same microscope field remained unchanged. Comparison of processed images also showed that microcolonies had the ability to move across the substratum as a unit (Fig. 10). The images, taken within a gap of 2 h, clearly showed migration of microcolonies in a direction opposite to that of the ambient flow. Figure 11 also demonstrates the coalescence of two microcolonies to form a larger colony.
FIG. 9.
Changes in biovolume (calculated per confocal stack) during early stages of biofilm development in Sphingomonas sp. strain L138 (grown at Re = 0.07). Confocal image stacks were collected from the same biofilm location, with a stationary (x-y) microscope stage.
FIG. 10.
Digitally superimposed images of Sphingomonas sp. strain L138 biofilm taken 18 and 19 h after flow cell initiation (grown at Re = 0.07). Microcolonies are outlined in red (18 h) and black (19 h). An increase in the size of the microcolony at the upper right-hand corner is clearly seen, while some other colonies did not exhibit any observable growth during the same period. Arrow indicates flow. Bar, 12 μm.
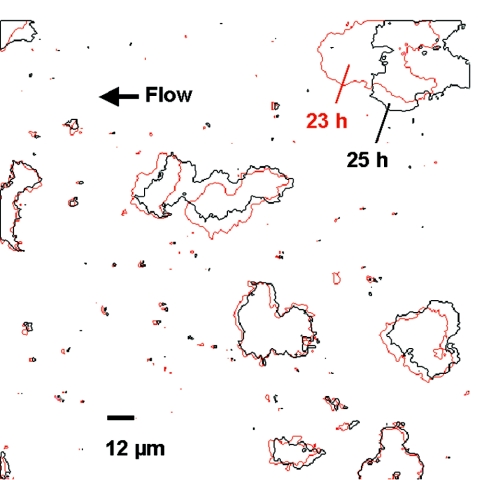
FIG. 11.
Digitally superimposed images of Sphingomonas sp. strain L138 biofilm taken 23 and 25 h after flow cell initiation (grown at Re = 0.07). Microcolonies are outlined in red (23 h) and black (25 h). The microcolony on the upper right-hand side has migrated in a direction opposite to that of the flow (arrow). Coalescence of two microcolonies in the center can also be seen. Bar, 12 μm.
DISCUSSION
Flow cell biofilms.
Understanding biofilm architecture is important for evaluating environmental influences on biofilm development and for interpreting biofilm processes that are influenced by biofilm structure (49). Moreover, quantification of biofilm heterogeneity is required in the context of its importance in mass transport dynamics (68). Flow cells are ideal tools for the generation of such data using single-species or multispecies biofilms, under varied experimental conditions. Such studies, which permit in situ observation under flow conditions, have yielded insights into the structural complexity of biofilms (49). Multichannel flow cells, as used in the present case, make possible comparison of biofilm development between two species or by the same species under different conditions, such as different flow or nutrient regimens.
Though flow cells are simple, inexpensive, and amenable to experimental manipulations, questions have been raised about the reproducibility of biofilms generated using flow cells. Heydorn et al. (20) presented a method for assessment of experimental reproducibility by quantitative comparison of certain biofilm structures. They also showed that such analysis could be undertaken by looking at different variables describing biofilm structures, such as mean biofilm thickness, roughness, substratum coverage, and surface-to-volume ratio (21). In the present study, we approached the issue of comparability of biofilms grown in different channels of a flow cell by looking at the size of microcolonies. In a developing biofilm, the size of microcolonies represents a highly variable parameter. In a 24-h-old Sphingomonas sp. biofilm, the size varied from essentially single cells to colonies as large as 1,317 μm2 (with average diameter of 179 μm2). Results of our study showed that the differences in the mean colony size between different channels were not significant, indicating that biofilms were comparable. This finding is important in the context of the present study (as well as similar studies involving flow cells), where channels were simultaneously initiated but sequentially sacrificed, to follow the temporal progression of biofilm development.
Biofilms are extremely heterogeneous systems (65) and, therefore, have an inherent spatial variability with respect to their structure. Microscopic analysis of biofilms requires sampling from a minimum area that would be representative. Determination of a representative area is very important, not only to ensure the minimum sampling area necessary to reliably quantify fundamental features of biofilms, but also to avoid unnecessary oversampling. Korber et al. (26, 27) presented a method to estimate representative biofilm areas based on the representative elementary volume analysis and extrapolated the principle to two-dimensional biofilm systems. They concluded that representative areas could be obtained when a set of parameter values ceased to fluctuate with increasing sampling size. In practice, this can be achieved by sampling the system (here, the biofilm) in such a way as to incorporate the entire spectrum of variability and then determining the scale of analysis at which the parameter remains constant with increasing sampling area. Using a Pseudomonas fluorescens biofilm, Korber et al. (26) have shown that a minimum area of 1 × 105 μm2 would have to be sampled to adequately describe the biofilm architecture. Those authors used cell coverage as the parameter to determine the representative area. In the present study, we showed that a minimum sampling area of 2 × 105 μm2 would suffice to accommodate the inherent variability of a Sphingomonas sp. strain L138 biofilm, based on cell coverage (Fig. 2). However, if a more variable parameter such as microcolony size were used, the above representative area would no longer be valid and one would have to sample close to 1 × 106 μm2, an increase of an order of magnitude (Fig. 3). It is concluded that a representative sampling area for a biofilm will have to be established and depends on the inherent variability of the biofilm parameter one is attempting to quantify.
Biofilm architecture.
The structures of biofilm colonies have variously been described as “mushroom” shaped or as “inverted pyramids” (7, 43). Generally, such typical structures have been described for multispecies “real world” biofilms occurring in natural, medical, and industrial environments (50, 58). De Beer et al. (11) and Massol-Deya et al. (38) reported that multispecies biofilms form highly complex structures containing cells arranged in clusters interspersed with voids. Complex architecture has been found in a variety of biofilms, such as aerobic wastewater biofilms and anaerobic fixed-bed reactors. The present study showed that even single-species biofilms, such as the ones produced by Sphingomonas sp. strain L138, exhibit complex morphological features. The distribution of cell material and void spaces in the biofilm was such that environmental fluids could have access to the base of the biofilm. Zhang and Fang (71) have also reported a similar distribution of cell biomass in confocal images of seawater biofilms grown on stainless steel coupons. P. fluorescens showed a top-heavy kind of architecture with maximal cell distribution near the top of the biofilm (44). In our Sphingomonas sp. strain L138 biofilms, the maximum cell distribution was at about 7 to 9 μm from the substratum, with a higher void fraction at the top and base of the biofilm. These results disagree with a previous study that analyzed an 8-day-old biofilm of the parent strain, LB126, where the greatest development of cells and EPS occurred at the substratum (5). The flow velocity in that experiment was also low (2.4 mm s−1; Re ≈ 5), and the experimental setup was similar. It is not known whether the insertion of the miniTn5-tet-gfp transposon in L138 could have interrupted gene expression responsible for the spatial organization of the developing biofilm. The two different architectures may be explained by the fact that the LB126 biofilm in the previous study was significantly older (8 days) compared to the 24-h-old biofilm used in the present study. It is also possible that the base of the biofilm was defined differently in the two studies.
The forces determining the three-dimensional structure of a biofilm consisting of microcolonies, EPS, and voids are still not completely resolved (65). Hydrodynamic and substrate loading effects can account for much of the biofilm architecture seen in controlled experiments, as exemplified by the increasingly comprehensive nature of physically based computer simulations describing biofilm structures that model biological factors only in terms of growth yield and substrate conversion rates (29, 61). Genetic analysis has largely been focused on initial adhesion steps, and experiments are usually performed in hydrodynamically undefined batch systems (69). Genotypic factors are considered important for defined-species biofilms, apart from other factors such as physico-chemical properties of the substratum, stochastic processes, species interactions, nutrient concentration, and type and shear forces. Hunt et al. (22) showed using a three-dimensional computer program that chemically mediated cell detachment from bottom layers may cause formation of mushroom-shaped microcolonies in biofilms. Cluster size and shape are important structural features of biofilms, as they relate to mass transport due to their importance in hydrodynamics (70). Results of the present study showed that microcolony characteristics (such as shape and orientation with respect to flow) were not influenced by flow, at least during the initial stages (24 h) of biofilm formation. The flow rate was varied from 8 ml h−1 to 10 liters h−1, but morphological characteristics of the microcolonies did not show any recognizable pattern that could be correlated to flow rate. Earlier, Bowden and Li (4) reported that nutritional conditions had little effect on the initial stages of development of oral biofilms. Stoodley et al. (51, 52) used the length-width ratio of microcolonies as an indicator of the shape differences caused by flow. They observed an elongation of cell clusters at relatively higher flow rates. However, their experiments were performed on mature biofilms (21 to 29 days old), while the present study was performed on nascent 24-h-old biofilms. Other recent studies, such as that of Beyenal and Lewandowski (2), which showed that biofilms grown at different flow velocities had different structures, were also focused on older biofilms. It appears that environmental influences on biofilm architecture take effect during the later stages of biofilm development (see below).
EPS production and its implications.
Davy and O'Toole (10) gave a graphical representation of biofilm formation in a typical gram-negative bacterium. They identified three distinct phases in the development process: (i) an initial attachment phase, (ii) a second phase characterized by the formation of microcolonies, and (iii) a final maturation phase involving formation of an EPS-ensconced mature biofilm. The importance of EPS in the development of characteristic biofilm structure is now well acknowledged (8). However, its exact role in varied functions, such as cell adhesion, aggregation, and other features of biofilms, is still not clear (71). Sutherland (54) reported that EPS production was not required during the initial attachment phase of biofilm development but was required for the development of architecture. Palmer and Sternberg (43) argued that conclusions regarding architecture of the extracellular matrix of biofilms would be speculative, unless explored using appropriate techniques, such as fluorescent lectins. EPS have been quantified by various methods, but different methods give different results (36, 47, 67, 71).
In the present study, we employed ConA to visualize and quantify the EPS produced by Sphingomonas sp. The results showed low levels of EPS production during the initial 3 days and a rapid increase on the fourth day. The experiments were repeated thrice and, on all three occasions, a sudden increase on the fourth day was observed (data not shown). It appears that this sudden increase in EPS production marks the advent of the maturation phase suggested by Davy and O'Toole (10), when EPS and environmental factors begin shaping the biofilm structure. Kreft and Wimpenny (28) simulated nitrifying biofilms to study the effect of EPS production on biofilm structure and function. Their data showed that the architecture of a biofilm was dramatically influenced by EPS production. As in the present case, they also reported maximum EPS production in the middle of the vertical profile.
Diffusion length and void width.
This sudden increase in EPS production had significant impact on the architecture of the biofilm. Our data show that the average width of water channels showed a marked decrease on the fourth day (Fig. 8) which was caused mainly by the increase in EPS. The diffusion lengths in the present study were calculated as the ratio of the distance from the center to the edge of the microcolony to the width of the adjoining water channel. We took into account the sizes of the clusters and width of the water channel, both of which are important variables that influence mass transfer. Yang et al. (70) have written an algorithm for the calculation of diffusion distance in biofilm clusters, taking the maximum distance from a cluster pixel to its nearest void pixel. However, this does not take into account the size of the void (water channel) at that point. Obviously, larger voids provide a larger reservoir of solute molecules for diffusion into the colony (or, conversely, a larger sink for waste molecules diffusing out), compared to smaller voids. The present data show a decrease in DL with an increase in EPS production. Obliteration of the water channels by EPS would make the cells in the interior of the colonies susceptible to nutrient depletion. Sternberg et al. (48) observed reduced growth activity of Pseudomonas putida biofilms in the centers of microcolonies due to decreased penetration of nutrients as the biofilm grew older and thicker. De Beer et al. (12) observed anaerobic zones in the centers of cell clusters away from substratum. Others have also reported that EPS hinders diffusion of nutrients into a biofilm (34, 66).
The existence of water channels in the biofilm would enhance mass transfer kinetics through convectional transport. Water channels also help penetration of biocides into the biofilm interior, speeding up kill kinetics. Moreover, the significance of water channels in maintaining chemical (pH, redox) heterogeneity in the biofilm cannot be overemphasized. Such heterogeneities play a major role in biofilm-induced corrosion. Fang et al. (14) reported pitting corrosion occurring close to voids in between cell clusters of sulfate-reducing bacteria biofilms.
Microcolony development.
Microcolonies are the basic structural units of biofilms (59). Movement of microcolonies in a biofilm was reported earlier by Stoodley et al. (49, 50). They observed that microcolony migration along the substratum occurred in the direction of the flow. However, in the present study, we observed movement in a direction opposite to that of the ambient flow, suggesting that the movement of microcolonies in a biofilm is not necessarily induced by the flow but is rather an inherent property of the biofilm. We used low flow rates during the time-lapse experiment (8 ml/h); therefore, it needs to be investigated if such migration could occur at relatively higher flow rates. Migration of microcolonies has profound implications for the colonization of industrial pipelines and implanted medical devices (49). Another significant fact brought out by the time-lapse experiment was that microcolonies exhibited differential growth rates. Mathematical models that simulate biofilm development assume that biofilm clusters have uniform growth rates (28). For example, in biomass-based models, the biofilm grows by spreading of biomass distributed in discrete grids (cellular automata principle) (45). It is assumed that biofilm structural complexity arises due to differential biomass spreading caused by the growth of different species. From the present work it appears that the differential growth rate of a given species present in different microcolonies of the same biofilm needs to be factored into such models. Further studies are required to examine why colonies growing under similar conditions exhibit variable growth rates and what impact this has on biofilm architecture. Diffusion limitation does not seem to be the reason, as some of the colonies that remained static with respect to size were smaller than those colonies that increased in size during the same period.
In conclusion, the present study shows that monospecies biofilms also present complexities similar to those of multispecies biofilms. The very early stages of microcolony growth in the biofilm seem to be more influenced by inherent (probably genotypic) factors than by the external ambient factors, based on the fact that there was no significant effect of varying shear forces on microcolony morphology. A sudden increase in EPS production appears to mark the advent of the maturation phase in a Sphingomonas sp. biofilm. It is hypothesized that the environmental influence, which results in complex biofilm architecture, takes effect during the maturation phase, which is further contributed by increased EPS production. Microcolonies in biofilms exhibit properties (movement not influenced by flow and differential growth rate) hitherto undescribed in the biofilm literature. Additional studies using a combination of flow cells, confocal microscopy, and digital image analysis with different flow regimens are required to throw more light on the development of structural complexity in biofilms.
Supplementary Material
Acknowledgments
V.P.V. gratefully acknowledges financial support provided by the Department of Biotechnology, Government of India, New Delhi. This study was partially funded by a grant from the European Union, under the project BIOSTIMUL (QLK3-199-00326), and by the German Research Foundation through its Research Center for Fundamental Studies of Aerobic Biological Wastewater Treatment, Munich, Germany (SFB411).
Joseph Winston, IGCAR, Kalpakkam, kindly wrote the Visual Basic program used for analysis of representative biofilm area. We thank A. Ryngaert for assistance with GFP stability tests.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bastiaens, L. D., D. Springael, P. Wattiau, H. Harms, R. de Wachter, H. Verachtert, and L. Diels. 2000. Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl. Environ. Microbiol. 66:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyenal, H., and Z. Lewandowski. 2000. Combined effect of substrate concentration and flow velocity on effective diffusivity in biofilms. Water Res. 34:528-538. [Google Scholar]
- 3.Bott, T. R. 1995. Fouling of heat exchangers. Elsevier, Amsterdam.
- 4.Bowden, G. H. W., and Y. H. Li. 1997. Nutritional influence on biofilm development. Adv. Dental Res. 11:81-99. [DOI] [PubMed] [Google Scholar]
- 5.Bungartz, H.-J., M. Kuehn, M. Mehl, M. Hausner, and S. Wuertz. 2000. Fluid flow, transport, and biomass growth in defined biofilms: experiments and numerical simulations on a microscale. Water Sci. Technol. 41(4-5):331-338. [Google Scholar]
- 6.Busscher, H. J., and H. C. van der Mei. 1997. Physico-chemical interactions in initial microbial adhesion and relevance for biofilm formation. Adv. Dental Res. 11:24-32. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 8.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, G., M. R. Marsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 210:295-298. [DOI] [PubMed] [Google Scholar]
- 10.Davy, M. E., and O. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBeer, D., P. Stoodley, and Z. Lewandowski. 1996. Liquid flow and mass transport in heterogeneous biofilms. Water Res. 30:2761-2765. [Google Scholar]
- 12.DeBeer, D., P. Stoodley, F. Roe, and Z. Lewandowski. 1994. Effect of biofilm structures on oxygen distribution and mass transfer. Biotechnol. Bioeng. 43:1131-1138. [DOI] [PubMed] [Google Scholar]
- 13.Ebihara, T., and P. L. Bishop. 1999. Biofilm structural forms utilized in bioremediation of organic compounds. Water Sci. Technol. 39(7):203-210. [Google Scholar]
- 14.Fang, H. H. P., L.-C. Xu, and K.-W. Chan. 2000. Influence of Cr3+ on microbial cluster formation in biofilm and on steel corrosion. Biotechnol. Lett. 22:801-805. [Google Scholar]
- 15.Fialho, A. M., L. O. Martins, M.-L. Donval, J. H. Leitão, M. J. Ridout, A. J. Jay, V. J. Morris, and I. Sá-Correia. 1999. Structures and properties of gellan polymers produced by Sphingomonas paucimobilis ATCC 31461 from lactose compared with those produced from glucose and from cheese whey. Appl. Environ. Microbiol. 65:2485-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemming, H.-C. 1984. Bakterienwachstum auf Ionenaustauscher-Harz Untersuchungen an einem stark sauren Kationen-Austauscher. Teil III: Desinfektion mit Peressigsäure. Z. Wasser-Abwasser-Forsch. 17:229-234. [Google Scholar]
- 17.Fredrickson, J. K., D. L. Balkwill, G. R. Drake, M. F. Romine, D. B. Ringelberg, and D. C. White. 1995. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl. Environ. Microbiol. 61:1917-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halden, R. U., B. G. Halden, and D. F. Dwyer. 1999. Removal of dibenzofuran, dibenzo-p-dioxin, and 2-chlorodibenzo-p-dioxin from soils inoculated with Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 65:2246-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernáez, M. J., W. Reineke, and E. Santero. 1999. Genetic analysis of biodegradation of tetralin by a Sphingomonas strain. Appl. Environ. Microbiol. 65:1806-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heydorn, A., B. K. Ersbøll, M. Hentzer, M. R. Parsek, M. Givskov, and S. Molin. 2000. Experimental reproducibility in flow-chamber biofilms. Microbiology 146:2409-2415. [DOI] [PubMed] [Google Scholar]
- 21.Heydorn, A., A. T. Nielsen, M. Hentze, C. Sternberg, M. Givskov, B. K. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program Comstat. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 22.Hunt, S. M., M. A. Hamilton, J. T. Sears, G. Harkin, and J. Reno. 2003. A computer investigation of chemically mediated detachment in bacterial biofilms. Microbiology 149:1155-1163. [DOI] [PubMed] [Google Scholar]
- 23.Jayaraman, A., A. K. Sun, and T. K. Wood. 1998. Characterization of axenic Pseudomonas fragi and Escherichia coli biofilms that inhibit corrosion of SAE 1018 steel. J. Appl. Microbiol. 84:485-492. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen, A. R., M. Hausner, A. Schnell, and S. Wuertz. 2000. Evaluation of fluorescently labeled lectins for noninvasive localization of extracellular polymeric substances in Sphingomonas biofilms. Appl. Environ. Microbiol. 66:3487-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjelleberg, S., and S. Molin. 2002. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 5:254-258. [DOI] [PubMed] [Google Scholar]
- 26.Korber, D. R., J. R. Lawrence, M. J. Hendry, and D. E. Caldwell. 1992. Programs for determining statistically representative areas of microbial biofilms. Binary 4:204-210. [Google Scholar]
- 27.Korber, D. R., J. R. Lawrence, M. J. Hendry, and D. E. Caldwell. 1993. Analysis of spatial variability within Mot+ and Mot− Pseudomonas fluorescens biofilms using representative elements. Biofouling 7:339-358. [Google Scholar]
- 28.Kreft, J. U., and J. W. Wimpenny. 2001. Effect of EPS on biofilm structure and function as revealed by an individual-based model biofilm growth. Water Sci. Technol. 43(6):135-141. [PubMed] [Google Scholar]
- 29.Kreft, J. U., C. Picioreanu, J. W. Wimpenny, and M. C. M. van Loosdrecht. 2001. Individual based modeling of biofilms. Microbiology 147:2897-2912. [DOI] [PubMed] [Google Scholar]
- 30.Kuehn, M., M. Mehl, M. Hausner, H. J. Bungartz, and S. Wuertz. 2001. Time-resolved study of biofilm architecture and transport processes using experimental and simulation techniques: the role of EPS. Water Sci. Technol. 43(6):143-151. [PubMed] [Google Scholar]
- 31.Kuehn, M., M. Hausner, H. J. Bungartz, M. Wagner, P. A. Wilderer, and S. Wuertz. 1998. Automated confocal laser scanning microscopy and semiautomated image processing for analysis of biofilms. Appl. Environ. Microbiol. 64:4115-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1989. Computer-enhanced dark field microscopy for the quantitative analysis of bacterial growth and behaviour on surface. J. Microbiol. Methods 10:123-138. [Google Scholar]
- 33.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence, J. R., G. M. Wolfaardt, and D. R. Korber. 1994. Determination of diffusion coefficients in biofilms by confocal laser microscopy. Appl. Environ. Microbiol. 60:1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, W., and W. G. Characklis. 1993. Corrosion of mild steel under anaerobic biofilm. Corrosion 49:186-197. [Google Scholar]
- 36.Liu, H., and H. H. Fang. 2002. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95:249-256. [DOI] [PubMed] [Google Scholar]
- 37.Manz, W., K. Wendt-Potthoff, T. R. New, U. Szewzyk, and J. R. Lawrence. 1999. Phylogenetic composition, spatial structure and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb. Ecol. 37:225-237. [DOI] [PubMed] [Google Scholar]
- 38.Massol-Deya, A. A., J. Whallon, R. F. Hickey, and J. M. Tiedje. 1995. Channel structures in aerobic biofilms of fixed film reactors treating contaminated groundwater. Appl. Environ. Microbiol. 61:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. Van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norwood, D. E., and A. Gilmour. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520. [DOI] [PubMed] [Google Scholar]
- 41.Okabe, S., H. Kuroda, and Y. Watanabe. 1998. Significance of biofilm structure on transport of inert particulates into biofilms. Water Sci. Technol. 38(8-9):163-170. [Google Scholar]
- 42.O'Toole, G. A., H. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, R. J., Jr., and C. Sternberg. 1999. Modern microscopy in biofilm research: confocal microscopy and other approaches. Curr. Opin. Biotechnol. 10:263-268. [DOI] [PubMed] [Google Scholar]
- 44.Pereira, M. O., M. Kuehn, S. Wuertz, T. Neu, and L. F. Melo. 2002. Effect of flow regime on the architecture of a Pseudomonas fluorescens biofilm. Biotechnol. Bioeng. 78:164-171. [DOI] [PubMed] [Google Scholar]
- 45.Picioreanu, C., M. C. van Loosedrecht, and J. J. Heijnen. 1998. A new combined differential-discrete cellular automaton approach to biofilm modeling application for growth in gel beads. Biotechnol. Bioeng. 57:718-731. [PubMed] [Google Scholar]
- 46.Pratt, L. A., and R. Kolter. 1999. Genetic analysis of bacterial biofilm formation. Curr. Opin. Microbiol. 2:598-603. [DOI] [PubMed] [Google Scholar]
- 47.Spaeth, R., and S. Wuertz. 2000. Extraction and quantification of extracellular polymeric substances from wastewaters, p. 51-68. In H.-C. Flemming, U. Szewzyk, and T. Griebe (ed.), Biofilms: investigative methods and applications. Technomic Publishers, Lancaster, Pa.
- 48.Sternberg, C., B. B. Christensen, T. Johansen, A. T. Nielsen, J. B. Anderson, M. Givskov, and S. Molin. 1999. Distribution of bacterial growth activity in flow chamber biofilms. Appl. Environ. Microbiol. 65:4108-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoodley, P., I. Dodds, J. D. Boyle, and H. M. Lappin-Scott. 1999. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. Symp. Suppl. 85:19S-28S. [DOI] [PubMed] [Google Scholar]
- 50.Stoodley, P., Z. Lewandowski, J. D. Boyle, and H. M. Lappin-Scott. 1999. The formation of migratory ripples in a mixed species bacterial biofilm growing in turbulent flow. Environ. Microbiol. 1:447-455. [DOI] [PubMed] [Google Scholar]
- 51.Stoodley, P., J. D. Boyle, D. DeBeer, and H. M. Lappin-Scott. 1999. Evolving perspectives in biofilm structure. Biofouling 14:75-90. [Google Scholar]
- 52.Stoodley, P., S. Wilson, L. H. Stoodley, J. D. Boyle, H. M. Lappin-Scott, and J. W. Costerton. 2001. Growth and detachment of cell clusters from mature mixed species biofilms. Appl. Environ. Microbiol. 67:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutherland, I. W. 2001. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 9:222-227. [DOI] [PubMed] [Google Scholar]
- 54.Sutherland, I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 55.Tabata, K., K.-I. Kasuya, H. Abe, K. Masuda, and Y. Doi. 1999. Poly(aspartic acid) degradation by a Sphingomonas sp. isolated from freshwater. Appl. Environ. Microbiol. 65:4268-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taghavi, S., A. Provoost, M. Mergeay, and D. van derLelie. 1996. Identification of a partition and replication region in the Alcaligenes eutrophus megaplasmid pMOL28. Mol. Gen. Genet. 250:169-179. [DOI] [PubMed] [Google Scholar]
- 57.Takenaka, S., M. Iwaku, and E. Hoshino. 2001. Artificial Pseudomonas aeruginosa biofilms and confocal laser scanning microscopic analysis. J. Infect. Chemother. 7:87-93. [DOI] [PubMed] [Google Scholar]
- 58.Tolker-Nielson, A., T. Tolker-Nielson, K. B. Barken, and S. Molin. 2000. Role of commensal relationships on the spatial structure of a surface-attached microbial consortium. Environ. Microbiol. 2:59-68. [DOI] [PubMed] [Google Scholar]
- 59.Tolker-Nielson, A., U. C. Brinch, P. C. Ragas, J. B. Anderson, C. S. Jacobsen, and S. Molin. 2000. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Loosdrecht, M. C. M., D. Eikelboom, A. Gjaltema, A. Mulder, A. Tijhuis, and J. J. Heijnen. 1995. Biofilm structures. Water Sci. Technol. 32(8):35-43. [Google Scholar]
- 61.van Loosdrecht, M. C. M., J. J. Heijnen, H. Eberl, J. Kreft, and C. Picioreanu. 2002. Mathematical modelling of biofilm structures. Antonie Leeuwenhoek 81:245-256. [DOI] [PubMed] [Google Scholar]
- 62.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watnick, P. I., and R. Kolter. 2000. Steps in the development of a Vibrio cholerae El biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wick, L. J., P. A. Mattle, P. Wattiau, and H. Harms. 2004. Electrokinetic transport of PAH-degrading bacteria in model aquifers and soil. Environ. Sci. Technol. 38:4596-4602. [DOI] [PubMed] [Google Scholar]
- 65.Wimpenny, J., W. Manz, and U. Szewzyk. 2000. Heterogeneity in biofilms. FEMS Microbiol. Rev. 24:661-671. [DOI] [PubMed] [Google Scholar]
- 66.Wolfaardt, G. M., J. R. Lawrence, D. Robarts, S. J. Caldwell, and D. E. Caldwell. 1994. Multicellular organization in a degradative biofilm community. Appl. Environ. Microbiol. 60:434-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wuertz, S., R. Spaeth, A. Hinderberger, T. Griebe, H.-C. Flemming, and P. A. Wilderer. 2001. A new method for extraction of extracellular polymeric substances from biofilms and activated sludge suitable for direct quantification of sorbed metals. Water Sci. Technol. 43(6):25-31. [PubMed] [Google Scholar]
- 68.Wuertz, S., P. L. Bishop, and P. A. Wilderer (ed.). 2003. Biofilms in wastewater treatment: an interdisciplinary approach. IWA Publishing, London, Great Britain.
- 69.Wuertz, S., S. Okabe, and M. Hausner. 2004. Microbial communities and their interactions in biofilm systems: an overview. Water Sci. Technol. 49(11-12):327-336. [PubMed] [Google Scholar]
- 70.Yang, X., H. Beynal, G. Harkin, and Z. Lewandowski. 2000. Quantifying biofilm structure using image analysis. J. Microbiol. Methods 39:109-119. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, T., and H. H. P. Fang. 2001. Quantification of extracellular polymeric substances in biofilms by confocal laser scanning microscopy. Biotechnol. Lett. 23:405-409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.