Abstract
The ubiquitous arbuscular mycorrhizal fungi consume significant amounts of plant assimilated C, but this C flow has been difficult to quantify. The neutral lipid fatty acid 16:1ω5 is a quantitative signature for most arbuscular mycorrhizal fungi in roots and soil. We measured carbon transfer from four plant species to the arbuscular mycorrhizal fungus Glomus intraradices by estimating 13C enrichment of 16:1ω5 and compared it with 13C enrichment of total root and mycelial C. Carbon allocation to mycelia was detected within 1 day in monoxenic arbuscular mycorrhizal root cultures labeled with [13C]glucose. The 13C enrichment of neutral lipid fatty acid 16:1ω5 extracted from roots increased from 0.14% 1 day after labeling to 2.2% 7 days after labeling. The colonized roots usually were more enriched for 13C in the arbuscular mycorrhizal fungal neutral lipid fatty acid 16:1ω5 than for the root specific neutral lipid fatty acid 18:2ω6,9. We labeled plant assimilates by using 13CO2 in whole-plant experiments. The extraradical mycelium often was more enriched for 13C than was the intraradical mycelium, suggesting rapid translocation of carbon to and more active growth by the extraradical mycelium. Since there was a good correlation between 13C enrichment in neutral lipid fatty acid 16:1ω5 and total 13C in extraradical mycelia in different systems (r2 = 0.94), we propose that the total amount of labeled C in intraradical and extraradical mycelium can be calculated from the 13C enrichment of 16:1ω5. The method described enables evaluation of C flow from plants to arbuscular mycorrhizal fungi to be made without extraction, purification and identification of fungal mycelia.
Arbuscular mycorrhizal (AM) fungal mycelia acquire hexoses released by the roots of their host (3, 39, 42, 43) and metabolize them to lipids, mainly neutral lipids, such as triacylglycerols (39). Neutral lipids are transported throughout the fungal mycelium (4), are metabolized through the glyoxalate cycle (3, 24), and probably provide the major fungal energy source. The mechanisms that regulate C transfer from plant to fungus are not well understood (21). However, AM fungal colonization affects plant C metabolism (13, 38, 51, 52) and the genes that regulate this metabolism (20, 40).
AM fungal neutral lipids usually are stored in intraradical vesicles or in spores and make up a large proportion of the AM fungal biomass (6, 22, 36). The fatty acids of these lipids have a characteristic and specific composition (7). In Glomus intraradices, 50 to 70% of the neutral lipids are the fatty acid 16:1ω5 (19, 35), which is uncommon in other groups of fungi (28, 32, 47) and can be used as an AM fungal signature (31, 37). 13C nuclear magnetic resonance has been used to determine C-metabolic pathways after 13C labeling of monoxenic root cultures (3). The incorporation and turnover of C in AM fungal mycelium can be difficult to measure because the hyphae are not easily extracted and separated from roots or soil.
13C enrichment or 13C dilution of signature compounds, such as the neutral lipid fatty acid (NLFA) 16:1ω5 can be quantified by gas chromatography-mass spectrometry, which enables the measurement of the C turnover in AM fungi in complex materials, roots and soil in particular (2). Extraction and purification of mycelia (see reference 44) is therefore not needed. However, all AM fungi do not contain the same amount of NLFA 16:1ω5 (19). The root lipid NLFA 18:1ω6,9 does not increase in roots after AM colonization and is rare in AM fungi, so it can be used to estimate turnover of plant lipids in AM-colonized plants (35). Similarly, some phospholipid fatty acids (PLFAs) are good indicators of soil bacteria (16). NLFAs are not as good for estimating bacterial biomass since bacteria usually do not store neutral lipids (32).
The objective of the present study was to determine whether 13C labeling of plant assimilates, followed by compound specific isotope ratio mass spectrometry (IRMS), can be used to quantify the C transfer from plant to fungus in the AM symbiosis. We hypothesized (i) that 13C incorporation into the AM fungal signature compound NLFA 16:1ω5 is correlated to the total 13C incorporation in the fungal mycelium and (ii) that the transfer of C to the fungus would result in 13C enrichment in the AM fungal signature fatty acid 16:1ω5 at a similar speed and quantity as in plant lipids. The advantages of the proposed method include that it is a relatively fast and easy way to estimate C transfer from plant to intraradical mycelium and that the estimates of C translocation to the extraradical mycelium are sensitive enough for the technique to be used in complicated substrates, such as soil. 13C nuclear magnetic resonance and 14C autoradiography also can be used to study C allocation, but neither of these techniques can be used for quantitative measurements.
MATERIALS AND METHODS
Monoxenic arbuscular mycorrhiza cultures.
The AM fungus Glomus intraradices Schenck & Smith (DAOM 197198; Biosystematics Research Center, Ottawa, Canada) was grown monoxenically in mycorrhizal association with root-organ cultures of carrot (Daucus carota L.). The cultures were clones of carrot roots (line DC1) that had been transformed with the T-DNA of the root-inducing (Ri) plasmid from the bacterium Agrobacterium rhizogenes (5). The cultures were originally established as described by St-Arnaud et al. (48). AM-colonized cultures were maintained at 24°C in petri dishes on a minimal nutrient medium (MM) (5) containing sucrose at 10 g liter−1 as the C source and 35 μM P (KH2PO4 [4.8 mg liter−1]) gelled with 0.3% Phytagel (Sigma Chemical Co., St. Louis, MO).
Monoxenic time course study.
Monoxenic experimental plates were initiated by transferring a 14-mm diameter plug of solid MM from 4-month-old cultures, containing colonized roots, extraradical mycelium and spores, into the center of an 85-mm plate containing sterile solid MM. After 9 weeks of growth, the plates were supplied with sterile filtered d-[13C]glucose solution (7 mg of glucose per dish, U-13C6 at 99% 13C; Cambridge Isotope Laboratories, Andover, MD) in a ring, 5 mm outside the initial inoculum plug. The agar surface at this stage was completely covered by roots and mycelia. Plates were analyzed 1, 2, and 7 days after labeling (two replicate plates at each harvest). MM (ca. 15 ml) with only hyphae was cut out from the plates and dissolved in 250 ml of 10 mM sodium citrate (12) by being mixed on a magnetic stirrer for 1 h at low speed at 20°C to leave extraradical mycelia and spores. The mycelia (0.56 ± 0.11 mg of dry mycelium per plate) were collected by filtration on a 25-μm-pore-size nylon mesh and washed in deionized water in a 500-ml glass beaker. Roots were collected with forceps from the plates and washed in 10 mM sodium citrate to remove any remaining solid medium. Mycelia and roots were stored at −20°C until analyzed for 13C enrichment and fatty acid content and composition. Two plates with axenic root cultures (without AM fungus) were included to measure the fatty acid content in noncolonized roots. These cultures were not 13C labeled.
Monoxenic P experiment.
For a detailed description of this experiment, see experiment 2 in Olsson et al. (36). The data from this experiment are already published (36), but here we use the data from three different inorganic P treatments to calculate a conversion factor for the estimation of total C flow based on C flow to the signature compound NLFA 16:1ω5c. Briefly, plugs of solid MM containing carrot roots, mycelia, and spores of G. intraradices were transferred at the start of the experiment from a 3- to 4-month-old culture to fresh medium in one compartment of a two-compartment petri dish (27). Liquid media were added to the second compartment 54 days after the inoculation of the first (root) compartment. The liquid medium contained one of three P levels: (i) no P (P-free), (ii) 25 μM P as KH2PO4 (low-P), and (iii) 2.5 mM P as KH2PO4 (high-P). Root compartments were labeled with d-[13C]glucose (10 mg of glucose per dish) 30 days after the addition of the liquid medium. The aqueous [13C]glucose solution (100 μl, 100 mg ml−1, filter sterilized) was pipetted onto the solid medium 0.5 cm inside the barrier to the liquid compartment. Cultures were harvested 7 days after labeling. Mycelia were collected by using forceps, and the few roots (roots had been trimmed once during the growth period) growing together with the mycelia in the liquid compartment were removed under a dissecting microscope by using forceps. Mycelia were stored at −20°C until freeze-dried for the measurement of 13C and lipids. The solid medium of the root compartment was dissolved in 250 ml of 10 mM sodium citrate by being mixed on a magnetic stirrer for 1 h at low speed at 20°C. These roots also were collected, freeze-dried, weighed, and analyzed, as were the roots from the liquid media.
Whole-plant study with three different host species.
Allium porrum L. (12 per pot), Plantago lanceolata L. (9 per pot), or Trifolium subterraneum L. (5 per pot) were planted in 0.5-liter pots filled with 500 g of a mixture containing 80% acid-washed sand, 10% sterilized sandy loam soil (see reference 18 for soil details), and a 10% pot culture inoculum of G. intraradices (BEG 87) or Gigaspora margarita (MAFF520054; MAFF GenBank, National Institute of Agribiological Sciences, Tsukuba, Japan). Seeds were surface sterilized (5% Ca-hypochlorite, 15 min), pregerminated in petri dishes, and transplanted into the pots. Two replicate pots for each combination of plant species and fungus were placed in a greenhouse with average 22°C day and 18°C night temperatures, a minimum of 270 μmol m2 s−1 photosynthetic photon flux density supplemented with 400 W OSRAM light bulbs as required, and an 18-h photoperiod. Plants were watered weekly with a low-P nutrient solution (50) per pot (60 ml in all were added during the growth period to each pot). Additional N, as NH4NO3, was provided with 1.2 mg of N per pot for Plantago and Trifolium and 0.5 mg of N per pot for Allium during the growth period. Two pots of two-month-old plants were labeled in a closed chamber (volume, 4.2 dm3) with 25 ml of 13C-CO2 (99% 13CO2; Larodan Fine Chemicals, Malmö, Sweden) injected with a gas-tight syringe through a septum. Removing the lid of the chamber interrupted the 3-h pulse period. Approximately 6.6 mg of 13C were assimilated per pot, assuming that all of the 25 ml of added 13C-CO2 were assimilated. At 7 days after labeling, plants were harvested, and root samples were collected and rinsed carefully with water on a sieve before further processing. Soil samples free of roots were collected for fatty acid analysis. Roots were stained with trypan blue and AM fungal colonization was determined microscopically (50). Fatty acid composition and 13C enrichment of lipids extracted from roots and from soil were determined on samples that had been stored frozen (−20°C).
Extraction of intraradical mycelium.
Intraradical mycelium was collected after enzymatic digestion of the remaining roots from the whole-plant study. Fresh root samples (cleaned from debris, extraradical mycelium, and spores) were kept on ice, cut into pieces 5 mm in length, and placed in a freshly prepared, filtered enzyme digestion solution as described by Saito (41). The digestion solution contained 2% cellulase (from Trichoderma harzianum, 11.6 U mg−1; Sigma) and 1% pectinase (from Rhizopus sp. 0.60 U mg−1; Sigma). The samples were placed under vacuum (created by water suction) for 10 to 15 min to remove air from the roots and then covered with Parafilm and incubated at 30°C for 2 h on a shaker at 120 rpm. Digested root samples were collected on a 25-μm-pore-size nylon mesh, washed clean with washing buffer (41), and kept on ice. The intraradical mycelium was separated by hand from the stele and cortical cells under a dissecting microscope by using needles and forceps and then transferred to fresh washing buffer. Almost pure intraradical mycelia could be extracted from Allium roots but not from the other two plant species. Samples from Plantago and Trifolium are therefore best regarded as enriched for intraradical mycelia. For Allium, we collected root pieces from which the intraradical mycelium had been removed to use as fungus-free samples of cortex and stele. For Plantago and Trifolium, we collected root pieces that lacked AM fungal colonization on the basis of dissecting microscope observations. Total 13C enrichment was determined in the collected intraradical mycelia, in samples enriched for intraradical mycelia, and in the mycelium-free root samples.
Whole-plant study with mycelium in root-free sand compartments.
Gavito and Olsson (18) provide a detailed description of this experiment. The results for NLFA 16:1ω5 from this experiment were used previously to calculate C flow to AM fungal lipids (17) in response to amendment treatments ([i] organic matter [dry yeast], [ii] a full nutrient solution without P, or [iii] a solution containing only P). Here we present the 13C-enrichment data (%) of NLFA 16:1ω5, 18:2ω6,9, and solid tissue. Rectangular polyvinyl chloride boxes (22-cm length by 11-cm width by 9.5-cm height) were divided into three compartments with two screens of 25-μm-pore-size nylon mesh. AM fungal hyphae can pass through this nylon mesh, but the roots cannot (37). The central compartment (5.5-cm width) was filled with nonsterilized soil (18) serving both as the growth substrate and as AM fungal inoculum for the host plants. One of the two side hyphal compartments received the amendment treatment, and the other side was left unamended as a control. The two side compartments were equal in size and were filled with 750 g of autoclaved quartz sand (Ahlsell-Mineral, Malmö, Sweden) that had been washed five times in deionized water. There were five complete randomized blocks.
Seven pregerminated seeds of P. lanceolata were planted in a row across the root compartment to create a line of plants equidistant from the two side compartments. Plantago lanceolata shoots in three polyvinyl chloride boxes at a time were labeled with 13CO2 (99% 13CO2; Larodan Fine Chemicals) in a closed chamber (volume, 33 dm3). The transparent lid of a greenhouse box was altered, with a fan and gas sampling tubing connected to an infrared gas analyzer. The initial CO2 concentration was recorded. 13CO2 (125 ml) was injected with a gas tight syringe through a septum. The CO2 concentration inside the box increased from 240 to 270 ppm to 500 to 650 ppm after injection. The labeling period lasted until the CO2 concentration inside the box decreased to its initial level (between 60 and 90 min). Approximately 20 mg of 13C was assimilated per pot. Plants were harvested 1 week after labeling, and soil and root samples were collected. 13C-enrichment was determined in fatty acids extracted from mycelium collected from the sand compartment by wet sieving (18).
Lipid analysis.
Mycelial samples were milled with stainless steel balls (7-mm diameter) by shaking by hand for 15 s in 50-ml Teflon tubes, and roots (100 mg) were ball milled (15 s, 300 strokes min−1) in stainless steel beakers. The lipids from the mycelia (0.5 to 1 mg [dry mass]), roots (15 to 30 mg), and soil (10 g) were extracted by vortex mixing (1 min) in a one-phase mixture of citrate buffer, methanol, and chloroform (0.8:2:1 [vol/vol/vol]; pH 4.0) (8). The lipids were fractionated into neutral lipids, glycolipids, and phospholipids on prepacked silica columns (100 mg of sorbent mass; Varian Medical Systems, Palo Alto, CA) by elution with 1.5 ml of chloroform, 6 ml of acetone, and 1.5 ml of methanol, respectively. The fatty acid residues in the neutral lipids and the phospholipids were transformed into free fatty acid methyl esters that were analyzed by gas chromatography using a 50-m HP5 capillary fused silica column (Hewlett Packard, Palo Alto, CA) with H2 as the carrier gas (for further details, see reference 17). The fatty acids were identified from their retention times relative to that of the internal standard (fatty acid methyl ester 19:0). These were compared to those earlier determined by gas chromatography-mass spectrometry.
Signature fatty acids.
NLFA 16:1ω5 is a sensitive signature of AM fungi in both roots and soil (19, 34). PLFA 16:1ω5 is a constituent of AM fungal membranes, with rather low specificity as a signature due to relatively low content in AM fungi and a high background in soil originating from bacteria (34). NLFA 16:0 is a general NLFA that often increases in concentration in plant roots after AM colonization due to the high content of storage lipids in AM fungi (34). NLFA 18:2ω6,9 is present in plant storage lipids. It also is the dominant fatty acid in most fungi but occurs at very low levels in AM fungi (19, 35). PLFAs i15:0, a15:0, and cy19:0 are three bacterium-specific PLFAs that can be used as indicators of bacterial biomass (16).
Determination of 13C enrichment in crude tissue samples and fatty acids.
Freeze-dried mycelia (ca. 20 μg) or ball-milled root material (ca. 100 μg) were placed in tin capsules (crude tissue samples) and analyzed by continuous-flow IRMS with an ANCA-NT 20-20 stable isotope analyzer interfaced to a solid-liquid preparation module (PDZ Europa Scientific Instruments, Crewe, United Kingdom). The 13C/12C ratios of CO2 of the combusted samples (total C) were determined with a 0.01% precision. The data were expressed as 13C atom% with reference to a sucrose (BDH Laboratory Supplies, Poole, United Kingdom) standard, calibrated against the Pee Dee Belemite standard (the limestone fossil Belemnitella americana from the Cretaceous Pee Dee formation in South Carolina; International Atomic Energy Agency [11]).
The 13C enrichment in fatty acid methyl esters was determined in the 20-20 IRMS interfaced with a Hewlett-Packard 6890 gas chromatograph equipped with a split/splitless injector, a flame ionization detector, a high-temperature reaction furnace mounted, and a CTC Combi Prep and Load System (Crelab Instruments, Stockholm, Sweden). The chromatographic conditions were as described for the lipid analysis except that He was used as the carrier gas at a constant pressure of 36 lb/in2. The effluent from the capillary column passed through an Al tube with CuO wires at 860°C, where the fatty acids were converted to CO2. Cogenerated water vapor was removed via a Nafion membrane (PDZ Europa Scientific Instruments), and the purified CO2 was released into the 20-20 IRMS.
The 13C atom% values were calculated based on atom 13C of the reference CO2 gas, injected three times at the beginning and end of a chromatographic run. The reference CO2 was standardized with the Pee Dee Belemite standard by using a solid-liquid preparation module. Integration for each peak was checked and corrected manually. The 13C enrichment of fatty acids was calculated after correction for the C added in the methanolysis step of the fatty acid analysis procedure.
RESULTS
Monoxenic time course study.
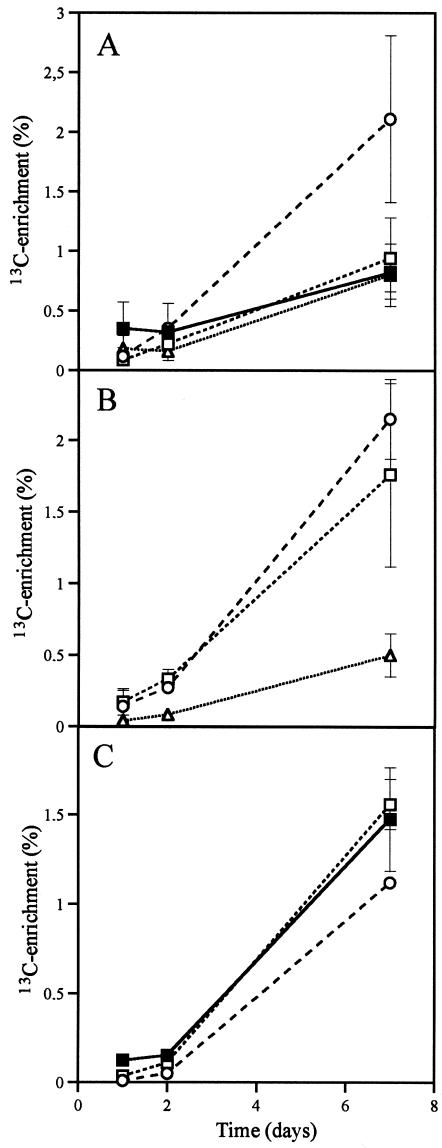
13C enrichment was observed in roots and hyphae 1 day after application of [13C]glucose to monoxenic carrot roots cultures with G. intraradices (Fig. 1). Rapid 13C enrichment in the AM fungal fatty acid 16:1ω5 showed that new lipids were being synthesized. The background levels of fatty acid 16:1ω5 in noncolonized carrot roots were low. The content of PLFA 16:1ω5 increased from 5 nmol g−1 in noncolonized roots to 140 nmol g−1 in AM-colonized roots, whereas NLFA 16:1ω5 increased from 80 nmol g−1 to 7,600 nmol g−1 and NLFA 16:0 from 1.0 to 6.6 nmol g−1. Thus, the majority of this PLFA and these NLFAs were mainly of AM fungal origin in the colonized roots (as expected from previous studies [33-36]), while the content of the PLFAs 16:0 and 18:1ω6,9 and NLFA 18:2ω6,9 did not increase due to AM colonization and can be considered as primarily plant root signatures. At 7 days after labeling, the root PLFAs, 16:0 and 18:2ω6,9 were enriched for 13C in a manner similar to that for total root C, but the PLFA 16:1ω5 had a higher 13C enrichment than did the root material (Fig. 1A). The 13C-enrichment of the NLFAs 16:0 and 16:1ω5 (Fig. 1B) in AM colonized roots was higher than in the root lipids (represented by NLFA 18:2ω6,9). 13C enrichment was slightly higher in intraradical mycelium (Fig. 1B) than in extraradical mycelium (Fig. 1C), as indicated by the signature NLFAs 16:1ω5 and 16:0.
FIG. 1.
Time course study of monoxenic AM carrot root system. (A) 13C enrichment of total root C and PLFAs within roots. (B) 13C-enrichment of NLFAs in roots. (C) 13C enrichment of total C and NLFAs within extraradical mycelium. Symbols: ▪, total C; ○, fatty acid 16:1ω5; □, fatty acid 16:0; ▵, fatty acid 18:1ω6,9. 13C enrichments of roots and AM mycelium were estimated at different times after labeling (n = 2, ± the standard error [SE]). Where no error bars can be seen, the SE is smaller than the symbol.
Monoxenic P experiment.
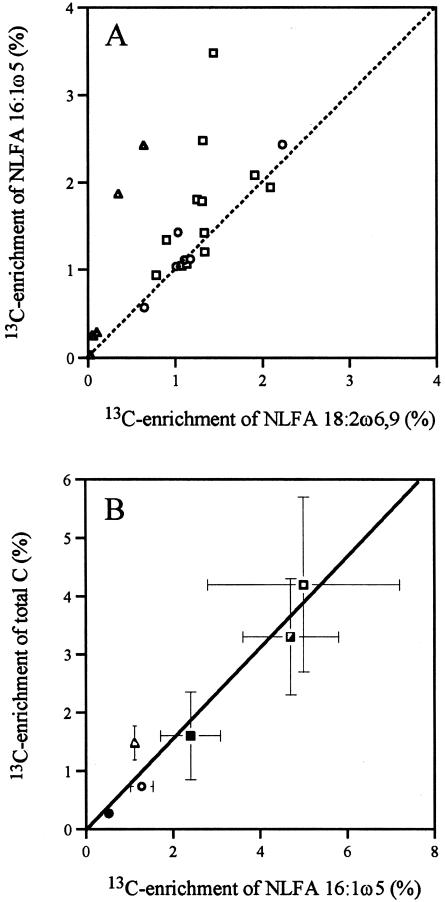
Available phosphorus reduced 13C enrichment in the extraradical mycelia, and the 13C enrichment of NLFA 16:1ω5 was significantly higher in extraradical mycelia than in intraradical mycelia (4.0% ± 0.84% compared to 1.7% ± 0.21%, P < 0.05 [paired t test, n = 12]). The PLFA with the greatest enrichment in the extraradical mycelia was 16:1ω5 (3.9% ± 0.40%, mean over all P treatments), whereas PLFAs 16:0 and 18:2ω6,9 had similar enrichment (3.2% ± 0.24% and 3.0% ± 0.28%, respectively). The 13C enrichment was slightly higher in NLFA 16:1ω5 than in NLFA 18:2ω6,9 (Fig. 2A). The lowest 13C enrichment in extraradical mycelia was observed in the high-P treatment, but the relation between 13C enrichment in total C and in NLFA 16:1ω5 was similar for all three P treatments (Fig. 2B). This experiment demonstrated that 13C was translocated since the labeled substrate was applied to one compartment, and the 13C enrichment was measured in extraradical mycelia collected from a second compartment.
FIG. 2.
Comparisons of 13C enrichment of NLFA 16:1ω5 to enrichment of NLFA 18:2ω6,9 and total C. (A) Plant roots studied in three experiments where the dotted line represents k = 1. (B) AM fungal mycelium across four experiments (y = 0.77x, r2 = 0.94, P < 0.01). Triangles indicate monoxenic time course study, squares indicate monoxenic P experiment (open squares denotes no P, half-filled squares indicate low-P, and filled squares indicate high-P), open circles represent experiments with multiple plant hosts (extracted intraradical mycelium was used for determination of total hyphal 13C in panel B), and closed circles represent the sand compartment study. Means (n = 2 for the monoxenic time course study, n = 12 for the monoxenic P experiment, 6 for experiment with multiple plant hosts and 30 for sand compartment study) ± the SE; where no error bars can be seen, the SE is smaller than the symbol.
Whole-plant study with three different host species.
Glomus intraradices colonized all three host plants at a rate ranging from 41% (Plantago) to 56% (Trifolium) of the root length (Table 1). Glomus intraradices colonization increased the content of both PLFA and NLFA 16:1ω5 in the host roots and in the soil due to growth of extraradical mycorrhizal mycelia. The neutral lipid fraction of G. intraradices colonized roots was dominated in particular by NLFAs 16:1ω5 and 16:0 (Fig. 3A). In the roots, PLFA 16:1ω5, NLFA 16:1ω5, and NLFA 16:0 were mainly of AM fungal origin (Table 1). NLFA 18:2ω6,9 was mainly of root origin because it was present at similar levels in control and AM-colonized roots. The content of NLFA 18:2ω6,9 varied between the different plant species but did not increase due to AM colonization.
TABLE 1.
Root colonization of three plant species inoculated with Glomus intraradices, the content of fatty acid 16:1ω5 in roots and soil, and 13C enrichment in the experiment with multiple plant hosts
| Background FAa (mean ± SE) | Mean result for Glomus-colonized roots ± SEb
|
|||
|---|---|---|---|---|
| Allium | Plantago | Trifolium | ||
| Colonization (% root length) | ||||
| Total | 55 ± 3.2 | 41 ± 0.2 | 56 ± 1.4 | |
| Arbuscular | 32 ± 9.0 | 20 ± 0.3 | 32 ± 9.8 | |
| Vesicles | 20 ± 2.5 | 11 ± 1.0 | 15 ± 0.3 | |
| Root FA (nmol g−1) | ||||
| PLFA 16:1ω5 | 12 ± 2.3 | 350 ± 180 | 120 ± 33 | 170 ± 10 |
| NLFA 16:1ω5 | 71 ± 21 | 17000 ± 3500 | 24000 ± 910 | 41000 ± 3500 |
| NLFA 16:0 | 2200 ± 820 | 13000 ± 3800 | 11000 ± 1300 | 21000 ± 90 |
| NLFA 18:2ω6,9 | 3400 ± 570 | 8100 ± 2100 | 2300 ± 120 | 1800 ± 270 |
| Soil FA (nmol g−1) | ||||
| PLFA 16:1ω5 | 0.29 ± 0.02 | 0.57 ± 0.10 | 0.43 ± 0.09 | 0.51 ± 0.21 |
| NLFA 16:1ω5 | 0.23 ± 0.04 | 50 ± 4.2 | 39 ± 8.0 | 33 ± 18 |
| 13C enrichmentc (%) | ||||
| Roots (total C) | 0.61 ± 0.13 | 0.46 ± 0.18 | 0.40 ± 0.06 | |
| Intraradical mycelium (total C) | 0.96 ± 0.48 | 0.56 ± 0.02 | 0.68 ± 0.04 | |
| Root NLFA 16:1ω5 | 1.9 ± 0.51 | 0.80 ± 0.23 | 1.1 ± 0.004 | |
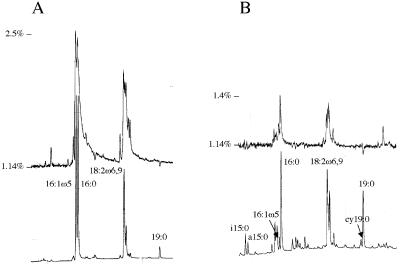
FIG. 3.
Parts of fatty acid chromatograms (fatty acid methyl esters) from the experiment with multiple plant hosts shows the total amount of each fatty acid (lower chromatograms) and the 13C enrichment as the 13C/12C ratio (upper chromatograms; the baseline represents natural abundance 13C/12C ratio). (A) Neutral lipid fatty acids from Allium porrum roots colonized by Glomus intraradices; (B) phospholipid fatty acids in a soil sample from a pot culture with Allium colonized by G. intraradices. The identities of the fatty acids are indicated. Fatty acid methyl ester 19:0 was added as an internal standard. Relative retention times compared to 19:0 were 0.699 (i15:0), 0.706 (a15:0), 0.788 (16:1ω5), 0.794 (16:0), 0.913 (18:2ω6,9), and 0.995 (cy19:0).
13C enrichment of total C was higher in intraradical mycelium than in root pieces from the same root system but devoid of fungal structures (Table 1). 13C enrichment also was higher in NLFA 16:1ω5 than in total C of the extracted intraradical mycelium (Table 1). The 13C enrichment was slightly higher in NLFA 16:1ω5 than in NLFA 18:2ω6,9, and their 13C enrichment was correlated (r2 = 0.92, P < 0.001; Fig. 3A).
The background level of NLFA 16:1ω5 in the soil was very low (Table 1), and >99% of this fatty acid was estimated to be from extraradical fungal mycelia of G. intraradices. The same fatty acid had higher 13C enrichment in the soil than did three bacterial PLFAs (Table 2). Of the analyzed PLFAs, 16:1ω5 was most enriched with 13C. The extraradical mycelia of G. intraradices contributed 30 to 50% of the PLFA 16:1ω5 in the soil (Table 1), assuming that the background amounts came from other microorganisms. 13C enrichment also was found in the bacterial PLFAs i15:0, a15:0, and cy19:0 (Table 2, Fig. 3B). The 13C enrichment in PLFAs a15:0 and cy19:0 was significantly higher in soil with G. intraradices than without (Table 2). In the soil without G. intraradices the enrichment of PLFA 16:1ω5 and the bacterial PLFAs was similar.
TABLE 2.
The 13C enrichment in NLFAs and PLFAs in soil from pots of Allium, Plantago, or Trifolium grown with or without (control) the AM fungus Glomus intraradices in the multiple host experiment
| Fatty acid | % 13C enrichmenta (mean ± SE)
|
|
|---|---|---|
| Control | Glomus | |
| Glomus signatures | ||
| NLFA 16:1ω5 | 0.43 ± 0.14 | 0.81 ± 0.15 |
| PLFA 16:1ω5 | 0.083 ± 0.027 | 0.26 ± 0.06* |
| Bacterial signatures | ||
| PLFA i15:0 | 0.042 ± 0.009 | 0.047 ± 0.006 |
| PLFA a15:0 | 0.035 ± 0.009 | 0.077 ± 0.014* |
| PLFA cy19:0 | 0.064 ± 0.019 | 0.12 ± 0.010* |
Natural abundance of 13C was subtracted (n = 6). *, Significantly different values between control and AM (P < 0.05, t test).
Whole-plant study with mycelium in root free sand compartments.
13C enrichment of the NLFA 16:1ω5 in extraradical mycelia varied between 0.16 and 0.84% and was similar to that measured in the experiment with multiple plant hosts. The addition of nutrient medium to the sand compartments did not alter the 13C enrichment in the NLFA 16:1ω5 of the extraradical mycelium (data not shown). In the present experiment we used a field inoculum of AM fungi in which the fungi present had not been identified. The relation between 13C enrichment in NLFA 16:1ω5-C and total C was similar to that found in defined systems.
DISCUSSION
We developed a new methodology to quantify the flow of plant assimilated C to microorganisms. We use a signature lipid, such as NLFA 16:1ω5, to quantify the amount of C transferred in complex media with higher sensitivity and specificity than is possible through measurement of total 13C in roots and soil. AM fungal 13C can also be measured in purified extraradical mycorrhizal mycelia, but only after laborious picking and subjective identification (44). Staddon et al. (45) obtained a good signal in shoots after 4 h of pulse-labeling with 13CO2 in field vegetation but had a very low signal in roots (maximum 0.1% enrichment) and almost no signal in soil, illustrating the low sensitivity that results unless a specific compound with a high turnover rate is targeted. The same methodology can be used to study C from plants to other rhizosphere microorganisms such as pathogenic fungi and bacteria, which have signature fatty acids that differ from those in roots and AM fungi (16, 25). Carbon that is sequestered by the fungus can be separated from that metabolized by the plant to yield lipids and thus allow a test of the hypothesis that C transferred from one host plant to the fungal mycelium is retained there and not returned or moved to new plants (14). It is now easy to acquire pure extraradical mycelium from monoxenic mycorrhizal cultures. Such monoxenic cultures provide a simple, easy-to-handle system to study carbon transfer in pure cultures (4, 39). Thus far, at least 27 AM fungal species have been cultured monoxenically (15), most commonly with transformed carrot roots (5) but also with Medicago truncatula (9).
Quantification of transferred carbon.
The correspondence between 13C enrichment in NLFA 16:1ω5 and 13C enrichment in total mycelium (Fig. 2B) suggests that the flow of C from host plants to fungal mycelia can be calculated from 13C enrichment in NLFA 16:1ω5. We used the data from the monoxenic P experiment to calculate a conversion factor to be used in the experiment with multiple plant hosts.
The total C flow to NLFA 16:1ω5 was calculated according to the following equation: NLFA 16:1ω5-C (μg) × 13C enrichment of NLFA 16:1ω5 (%/100) = C flow to NLFA 16:1ω5 (equation 1).
The molecular weight of NLFA 16:1ω5 is 253 g mol−1 and contains 192 g of C mol−1. There was a mean of 318 nmol NLFA 16:1ω5 of the mycelium collected from one culture, corresponding to 61 μg of C and the mean 13C enrichment of NLFA 16:1ω5 was 4.0%, which gives a C flow to NLFA 16:1ω5 of 2.5 μg.
The total amount of the AM mycelium was determined from the weight of freeze-dried mycelium from the monoxenic P experiment (all three P treatments). The mean amount of mycelium was 0.44 mg. The biomass-C of the mycelium was assumed to represent 50% of its dry weight corresponding to 220 μg of C. The mean 13C enrichment of total mycelium C was 3.0%, which gives a total C flow of 6.6 μg calculated according to the following equation: mycelium biomass C (μg) × total 13C enrichment (%/100) = C flow to mycelium (equation 2).
By using the results from equations 1 and 2 we can calculate the ratio of C flow to NLFA 16:1ω5 to total C flow to AM fungal mycelium as follows: C flow to mycelium/C flow to NLFA 16:1ω5 = conversion factor (equation 3).
This ratio (6.6/2.5 = 2.6) can be used as a conversion factor to estimate the flow of plant C to the AM fungal mycelia based on measurements of the signature NLFA 16:1ω5. Thus, for every C atom incorporated in NLFA 16:1ω5, 2.6 C atoms were incorporated into the fungal mycelia.
In the experiment with multiple plant hosts, the largest C flow to fungal mycelium per g of root (and the largest NLFA 16:1ω5 concentration) was found in Trifolium, and the lowest was found in Plantago, which also had the lowest 13C enrichment in NLFA 16:1ω5 (Table 3). Around 6,600 μg of 13C was assimilated per pot, which can be compared to the 100 to 230 μg of enriched 13C in intraradical AM fungal mycelium per g of root.
TABLE 3.
Calculation of C flow to the intraradical mycelium in roots of three plant species
| Plant type | NLFA 16:1ω5a (μmol g−1) | NLFA 16:1ω5 (mg of C g−1) | 13C enrichment in NLFA 16:1ω5 (%) | C flow to NLFA 16:1ω5 (μg g−1) | Total C flow to AM fungal myceliumb (μg g−1) |
|---|---|---|---|---|---|
| Allium | 17 | 3.3 | 1.9 | 63 | 163 |
| Plantago | 24 | 4.6 | 0.80 | 37 | 96 |
| Trifolium | 41 | 7.9 | 1.1 | 87 | 226 |
Data for the conversion of NLFA 16:1ω5 and its 13C enrichment are taken from Table 1.
The C flow to NLFA 16:1ω5 is calculated from equation 1, and the total C flow to AM fungal mycelium was calculated by multiplying that value with the conversion factor 2.6.
Sensitivity and specificity of the method.
The natural abundance of 13C (δ13C) is generally around δ −30 (corresponds to 1.15% 13C) in C3 plants, and the 13C abundance in AM fungi is similar to that of their host plant (30, 31, 46). Abraham et al. (1) provided various substrates to different microorganisms and observed that the 13C abundance in the fatty acid 16:0 extracted from the microorganisms resembled that of the substrate. With a 13C-labeled substrate, we reach a much higher sensitivity when the C metabolism was traced than can be obtained by using just the naturally occurring differences in 13C abundance between various substrates.
The dominance of the fatty acid 16:1ω5 in AM fungi and its rareness in other fungi (28, 47) makes it a useful biomarker probably for all Glomus species (19, 34) and also for Scutellospora species (19, 49) but not for Gigaspora species (19). Neutral lipids are important for energy storage, and 16:1ω5 is particularly common in this fraction of AM fungal lipids (35). Triacylglycerols are the main type of neutral lipids found in AM fungal spores and vesicles (6, 10, 29), although other fractions, e.g., diacylglycerols and free fatty acids, also are important in Glomus. All of them are, however, dominated by 16:1ω5.
16:1ω5 is suitable for labeling because the amount of this fatty acid is correlated with total 13C enrichment in hyphae and because this fatty acid represents a significant portion of the AM fungal biomass, which is consistent with the hypothesis of Bago et al. (4) that up to 50% of the hyphal volume may be lipid bodies. That a high proportion of AM fungal biomass is lipids is probably the main reason for the strong correlation between 13C enrichment in hyphae and in NLFA 16:1ω5 extracted from the same hyphae (Fig. 2B). Higher 13C enrichment of NLFA 16:1ω5 than of total C in mycelium indicates that lipids are the main C compounds translocated in AM fungal mycelia (39). Although the total 13C enrichment varied between experiments and was reduced by the high-P treatment in the monoxenic P experiment, the relative allocation to NLFA 16:1ω5 was the same (Fig. 2B). High P availability reduces the allocation to AM in plants (33), but our results indicate that the relative allocation to neutral lipids within the mycelium is the same.
Timing of measurements.
Timing is extremely important in all labeling experiments, particularly for studies of C allocation, because C is continually respired. However, a large proportion of AM fungal C is contained in lipids in vesicles inside plant roots and in spores on the external mycelium where they accumulate and may be stored for long periods of time. As long as the mycelium is expanding and spores are formed, there should be a continuous allocation of carbon to long-term storage. In field-labeled AM fungal mycelium, labeled respiration ceased within 7 days after pulse-labeling (23), indicating that only labeled stored material remained at that time. We observed a similar level of enrichment in the extraradical and intraradical mycelia after 7 days (Fig. 1), indicating that there was enough time for C translocation between different parts of the mycelium. Higher 13C enrichment in lipids of the intraradical mycelia than in plant root lipids shows that lipid metabolism is more active in the intraradical mycelia than in the plant. This difference was particularly evident in the monoxenic experiments with [13C]glucose (Fig. 2A), which suggests that this substrate can be directly transferred to AM fungi without being metabolized first.
The method described here provides an objective method to compare C allocation to intraradical and extraradical AM hyphae and to track C allocation in plant roots to fungal symbionts. Plant regulation of C allocation can be followed by this method after the symbiosis has been established. The regulation of colonization establishment in AM has been well studied, whereas the regulation of the C transfer in established symbiosis has been little studied due to methodological difficulties. Our method may be used to test hypotheses regarding the relative C allocation to AM fungi under different environmental conditions and to estimate how much C different plant species allocate to the AM symbiosis. This method also might be used to calculate carbon fixation in AM fungi in field ecosystems, but this application needs further evaluation since the turnover rates in the field probably are lower due to lower mean temperatures and since the fungal community composition is much more complex than in the laboratory experimental systems.
Acknowledgments
The Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas) and the Carl Trygger Foundation supported this study.
We thank Nancy Johnson for valuable comments on the manuscript.
REFERENCES
- 1.Abraham, W.-R., C. Hesse, and O. Pelz. 1998. Ratios of carbon isotopes in microbial lipids as an indicator of substrate usage. Appl. Environ. Microbiol. 64:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arao, T. 1999. In situ detection of changes in soil bacterial and fungal activities by measuring 13C incorporation into soil phospholipid fatty acids from 13C acetate. Soil Biol. Biochem. 31:1015-1020. [Google Scholar]
- 3.Bago, B., P. E. Pfeffer, and Y. Shachar-Hill. 2000. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 124:949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bago, B., W. Zipfel, R. M. Williams, J. Jun, R. Arreola, P. J. Lammers, P. E. Pfeffer, and Y. Shachar-Hill. 2002. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol. 128:108-124. [PMC free article] [PubMed] [Google Scholar]
- 5.Bécard, G., and J. A. Fortin. 1988. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 108:211-218. [DOI] [PubMed] [Google Scholar]
- 6.Beilby, J. P., and D. K. Kidby. 1980. Biochemistry of ungerminated and germinated spores of the vesicular-arbuscular mycorrhizal fungus, Glomus caledonius, changes in neutral and polar lipids. J. Lipid Res. 21:739-750. [PubMed] [Google Scholar]
- 7.Bentivenga, S. P., and J. B. Morton. 1996. Congruence of fatty acid methyl ester profiles and morphological characters of arbuscular mycorrhizal fungi in Gigasporaceae. Proc. Natl. Acad. Sci. USA 93:5659-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 9.Boisson-Dernier, A., M. Chabaud, F. Garcia, G. Bécard, C. Rosenberg, and D. G. Barker. 2001. Agrobacterium rhizogenes-transformed roots of Medicago truncatula for studying nitrogen-fixing and endomycorrhizal symbiotic associations. Mol. Plant-Microbe Interact. 14:693-700. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, K. M., and D. M. Lösel. 1978. Lipid physiology of vesicular-arbuscular mycorrhiza. I. Composition of lipids in roots of onion, clover, and ryegrass infected with Glomus mosseae. New Phytol. 80:143-151. [Google Scholar]
- 11.Dawson, T. E., and P. D. Brooks. 2001. Fundamentals of stable isotope chemistry, p. 1-18. In M. Unkovich, J. Pate, A. McNeill, and D. J. Gibbs (ed.), Stable isotope techniques in the study of biological processes and functioning of ecosystems. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 12.Doner, L. W., and G. Becard. 1991. Solubilization of gellan gels by chelation of cations. Biotech. Tech. 5:25-28. [Google Scholar]
- 13.Douds, D. D., Jr., C. R. Johnson, and K. E. Koch. 1988. Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol. 86:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitter, A. H., J. D. Graves, N. K. Watkins, D. Robinson, and C. Scrimgeour. 1998. Carbon transfer between plants and its control in networks of arbuscular mycorrhizas. Functional Ecol. 12:406-412. [Google Scholar]
- 15.Fortin, J. A., G. Bécard, S. Declerck, Y. Dalpé, M. St-Arnaud, A. P. Coughlan, and Y. Piché. 2002. Arbuscular mycorrhiza on root-organ cultures. Can. J. Bot. 80:1-20. [Google Scholar]
- 16.Frostegård, Å., and E. Bååth. 1996. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 22:59-65. [Google Scholar]
- 17.Frostegård, Å., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavito, M. E., and P. A. Olsson. 2003. Allocation of plant carbon to foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 45:181-187. [DOI] [PubMed] [Google Scholar]
- 19.Graham, J. H., N. C. Hodge, and J. B. Morton. 1995. Fatty acid methyl ester profiles for characterization of Glomalean fungi and their endomycorrhizae. Appl. Environ. Microbiol. 61:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison, M. J. 1996. A sugar transporter from Medicago truncaula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J. 9:491-503. [DOI] [PubMed] [Google Scholar]
- 21.Harrison, M. J. 1997. The arbuscular mycorrhizal symbiosis: an underground association. Tr. Plant Sci. 2:54-60. [Google Scholar]
- 22.Jabaji-Hare, S., A. Deschene, and B. Kendrick. 1984. Lipid content and composition of vesicles of a vesicular-arbuscular mycorrhizal fungus. Mycologia 76:1024-1030. [Google Scholar]
- 23.Johnson, D., J. R. Leake, N. Ostle, P. Ineson, and D. J. Read. 2002. In situ 13CO2 pulse-labeling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol. 153:327-334. [Google Scholar]
- 24.Lammers, P. J., J. Jun, J. Abubaker, R. Arreola, A. Gopalan, B. Bago, C. Hernandez-Sebastia, J. W. Allen, D. D. Douds, P. E. Pfeffer, and Y. Schachar-Hill. 2001. The glyoxylate cycle in an arbuscular mycorrhizal fungus: carbon flux and gene expression. Plant Physiol. 127:1287-1298. [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen, J., and L. Bødker. 2001. Interactions between pea root-inhabiting fungi examined using signature fatty acids. New Phytol. 149:487-493. [DOI] [PubMed] [Google Scholar]
- 26.Madan, R., C. Pankhurst, B. Hawke, and S. Smith. 2002. Use of fatty acids for identification of AM fungi and estimation of the biomass of AM spores in soil. Soil Biol. Biochem. 34:125-128. [Google Scholar]
- 27.Maldonado-Mendoza, I. E., G. R. Dewbre, and M. J. Harrison. 2001. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol. Plant-Microbe Interact. 14:1140-1148. [DOI] [PubMed] [Google Scholar]
- 28.Müller, M. M., R. Kantola, and V. Kitunen. 1994. Combining sterol and fatty acid profiles for the characterization of fungi. Mycol. Res. 98:593-603. [Google Scholar]
- 29.Nagy, S., H. E. Nordby, and S. Nemec. 1980. Composition of lipids in roots of six citrus cultivars infected with the vesicular arbuscular mycorrhizal fungus Glomus mosseae. New Phytol. 85:377-384. [Google Scholar]
- 30.Nakano, A., K. Takahashi, and M. Kimura. 1999. The carbon origin of arbuscular mycorrhizal fungi estimated from δ13C values of individual spores. Mycorrhiza 9:41-47. [Google Scholar]
- 31.Nakano, A., K. Takahashi, and M. Kimura. 2001. Effect of host shoot clipping on carbon and nitrogen sources for arbuscular mycorrhizal fungi. Mycorrhiza 10:287-293. [Google Scholar]
- 32.Olsson, P. A. 1999. Minireview: signature fatty acids provide tools for determination of distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 29:303-310. [Google Scholar]
- 33.Olsson, P. A., E. Bååth, and I. Jakobsen. 1997. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl. Environ. Microbiol. 63:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsson, P. A., E. Bååth, I. Jakobsen, and B. Söderström. 1995. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 99:623-629. [Google Scholar]
- 35.Olsson, P. A., and A. Johansen. 2000. Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol. Res. 104:429-434. [Google Scholar]
- 36.Olsson, P. A., I. M. van Aarle, W. G. Allaway, A. E. Ashford, and H. Rouhier. 2002. Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures. Plant Physiol. 130:1162-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson, J. N., and I. Jakobsen. 1993. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 124:481-488. [Google Scholar]
- 38.Peng, S., D. M. Eissenstat, J. H. Graham, K. Williams, and N. C. Hodge. 1993. Growth depression in mycorrhizal citrus at high-phosphorus supply. Analysis of carbon cost. Plant Physiol. 101:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeffer, P. E., D. D. Douds, Jr., G. Bécard, and Y. Shachar-Hill. 1999. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 120:587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravnskov, S., Y. Wu, and J. H. Graham. 2003. Arbuscular mycorrhizal fungi differentially affect expression of genes coding for sucrose synthases in maize roots. New Phytol. 157:539-545. [DOI] [PubMed] [Google Scholar]
- 41.Saito, M. 1995. Enzyme activities of the internal hyphae and germinated spores of an arbuscular mycorrhizal fungus, Gigaspora margarita Becker & Hall. New Phytol. 129:425-431. [Google Scholar]
- 42.Shachar-Hill, Y., P. E. Pfeffer, D. D. Douds, Jr., S. A. Osman, L. W. Doner, and R. G. Ratcliffe. 1995. Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhizal leek. Plant Physiol. 108:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solaiman, M. D. Z., and M. Saito. 1997. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol. 136:533-538. [DOI] [PubMed] [Google Scholar]
- 44.Staddon, P. L., C. Bronik Ramsey, N. Ostle, P. Ineson, and A. H. Fitter. 2003. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 300:1138-1140. [DOI] [PubMed] [Google Scholar]
- 45.Staddon, P. L., N. Ostle, L. A. Dawson, and A. H. Fitter. 2003. The speed of soil carbon throughput in an upland grassland is increased by liming. J. Exp. Bot. 54:1461-1469. [DOI] [PubMed] [Google Scholar]
- 46.Staddon, P. L., D. Robinson, J. D. Graves, and A. H. Fitter. 1999. The d13C signature of the external phase of Glomus mycorrhizal fungus: determination and implications. Soil Biol. Biochem. 31:1067-1070. [Google Scholar]
- 47.Stahl, P. D., and M. J. Klug. 1996. Characterization and differentiation of filamentous fungi based on fatty acid composition. Appl. Environ. Microbiol. 62:4136-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St-Arnaud, M., C. Hamel, Vimard, B., Caron, M., and J. A. Fortin. 1996. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol Res. 100:328-332. [Google Scholar]
- 49.Van Aarle, I. M., and P. A. Olsson. 2003. The relation of fungal lipid accumulation to development of mycelial structures in two arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 69:6762-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Aarle, I. M., H. Rouhier, and M. Saito. 2002. Phosphatase activities of arbuscular mycorrhizal intraradical and extraradical mycelium, and their relation to phosphorus availability. Mycol. Res. 106:1224-1229. [Google Scholar]
- 51.Wright, D. P., D. J. Read, and J. D. Scholes. 1998. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 21:881-891. [Google Scholar]
- 52.Wright, D. P., J. D. Scholes, and D. J. Read. 1998. Effect of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ. 21:209-216. [Google Scholar]