Abstract
Three small antimicrobial anionic peptides (AP) were originally isolated from an ovine pulmonary surfactant. However, their presence in bronchoalveolar lavage (BAL) fluid and tissues of the respiratory tract is unknown. In this study, we made affinity-purified rabbit polyclonal and mouse monoclonal antibodies to synthetic H-DDDDDDD-OH. Antibody specificity was assessed by a competitive enzyme-linked immunosorbent assay (ELISA), and the exact epitope binding sites were determined with analog peptides synthesized on derivatized cellulose. These antibodies were used to detect AP in BAL fluid by ELISA and in respiratory tissues by Western blot analysis and immunocytochemistry. BAL fluid from 25 sheep contained 0.83 ± 0.33 mM AP (mean ± standard deviation; range, 0.10 to 1.59 mM) and was antimicrobial. The presence of AP in BAL fluid was confirmed by reverse-phase high-pressure liquid chromatography fractionation followed by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry on those fractions which were positive by competitive ELISA and demonstrated antimicrobial activity. In Western blots, polyclonal antibody PAB96-1 and monoclonal antibody 1G9-1C2 (5.0 μg/ml) detected four bands in solubilized turbinate and tracheal epithelial cells (53.7, 31.2, 28.0, and 25.7 kDa) and five bands in lung homogenates (53.5, 37.1, 31.2, 28.0, and 25.7 kDa). Only a single band was seen in solubilized liver and small-intestine homogenates, and no bands were seen in blots containing BAL fluid, albumin, or kidney or spleen homogenates. In pulmonary-tissue sections, both antibodies PAB96-1 and 1G9-1C2 identified accumulated protein in the apical cytoplasm of the bronchial and bronchiolar epithelia, in the cytoplasm of pulmonary endothelial cells, and in an occasional alveolar macrophage. As a first step in identifying a candidate AP precursor gene(s), degenerate oligonucleotides representing all possible coding combinations for H-GADDDDD-OH and H-DDDDDDD-OH were synthesized and used to probe Southern blots of sheep genomic DNA. Following low-stringency washes and a 2-day exposure, strongly hybridizing bands could be identified. One degenerate oligonucleotide, SH87, was used as a hybridization probe to screen a sheep phage genomic library. Two independent phage contained the H-GADDDDD-OH coding sequence as part of a larger predicted protein. AP may originate as part of an intracellular precursor protein, with multistep processing leading to the release of the heptapeptide into mucosal secretions. There it may interact with other innate pulmonary defenses to prevent microbial infection.
Inhaled air and aspirated aerosols of upper-respiratory secretions contain large quantities of microorganisms, often approaching 108 to 109 bacteria per ml (1). Despite the continuous exposure to both environmental and commensal organisms, the respiratory tract remains remarkably free from infections. Innate pulmonary immunity is generally thought to be provided by overlapping mechanical, chemical, and cell-mediated clearance mechanisms (16). Recent evidence suggests that antimicrobial peptides play an integral role in protection of the respiratory tree (17). Examples include both cationic defensins (10, 13) and anionic peptides (AP) (8, 12), isolated from epithelial cells and from pulmonary secretions, respectively. Overall, these peptides have broad-spectrum activity against both gram-positive and gram-negative bacteria (3, 7, 9).
Ovine AP were isolated originally from a pulmonary surfactant (and are called surfactant-associated AP) (6). The biochemical features and requirements for antimicrobial activity were similar to those of the partially purified peptides found in mouse, rabbit, and human bronchoalveolar lavage (BAL) fluid (8, 12). Ovine AP are small (721.6 to 823.7 Da) and hydrophilic and contain homopolymeric regions (e.g., 5 to 7 residues) of aspartic acid (6). MICs of AP and similar analogs (4) are comparable to those of other vertebrate antimicrobial peptides (3, 7, 9).
The biochemistry and localization of AP synthesis remain to be characterized. In this study, we used both polyclonal and monoclonal antibodies generated against the synthetic peptide H-DDDDDDD-OH to detect and quantify the level of AP in BAL fluid, to localize sites of AP expression within the lung, and to identify putative AP precursor proteins.
MATERIALS AND METHODS
Animals.
Seven Columbia sheep, weighing 60 to 80 kg each, were bled, euthanized (with sodium pentobarbital at 60 mg/kg of body weight), and exsanguinated in accordance with procedures outlined by the American Association for Accreditation of Laboratory Animal Care and the Institutional Animal Care and Use Committee of the National Animal Disease Center. In addition, excised lungs were obtained from 20 lambs at a local slaughter plant.
BAL.
The lungs of the seven sheep were excised and lavaged to total lung capacity with 5 liters of 0.14 M NaCl. The lungs of the 20 lambs from the slaughter plant were lavaged to total lung capacity with 0.14 M NaCl, and an effluent lavage volume of 1 liter was collected. The BAL fluids from all sheep were centrifuged at 200 × g for 30 min at 4°C to remove alveolar cells and residual debris. Blood urea nitrogen concentrations (1.3 ± 0.5 mg/dl) (mean ± standard deviation [SD]) were determined (Clinical Pathology Laboratory, College of Veterinary Medicine, Iowa State University, Ames) and used to calculate the epithelial lining fluid (ELF) volume in each BAL fluid sample (15). BAL fluid was then adjusted to contain 0.5 ml of ELF/liter of BAL fluid for a comparative assessment of AP concentration among animals.
Peptide synthesis.
Peptides H-DDDDDDD-OH, H-GADDDDD-OH, H-GDDDDDD-OH, and H-VDDDDK-OH were synthesized by Multiple Peptide Systems (San Diego, Calif.) by using Merrifield resins and standard tert-butoxycarbonyl chemistry in combination with simultaneous multiple-peptide synthesis (SMPS or “tea-bag” methodology). Cyclohexyl was used as a side chain-protecting group for aspartic acid. H-TQDDGGK-OH, H-GGEEK-OH, and H-SGSGSGS-OH were synthesized by Chiron Mimotopes Pty. Ltd. (Australia) on a grafted polymer surface in a Multipin peptide synthesis format with N-α-9-fluorenylmethoxycarbonyl-protected amino acids. The peptides were side chain deprotected and cleaved from the solid support by acidolysis. Peptides were purified by high-pressure liquid chromatography (HPLC), characterized by analytical HPLC and by plasma desorption mass spectral analysis on a Biolon 20 Mass Analyzer, and lyophilized. Peptides were 95 to 99% pure and were verified by amino acid analysis.
Antibody.
The α-amino group of H-DDDDDDD-OH (5.0 mg) was coupled to 5.0 mg of keyhole limpet hemocyanin with glutaraldehyde and used as an antigen to immunize both rabbits and mice. To immunize rabbits (9-month-old New Zealand rabbits), the conjugate was suspended in 10 mM sodium phosphate buffer (pH 7.2) with 140 mM NaCl (PBS) (3.1 mg/ml), emulsified in Freund’s adjuvant (50% emulsion; total volume of 0.6 ml), and injected into four subcutaneous dorsal sites. Subsequent immunizations (days 14, 42, and 56 post-initial immunization) utilized the same conjugate, substituting incomplete Freund’s adjuvant. Antiserum was collected on day 70. Antibody (PAB96-1) was affinity purified on a gel prepared by coupling H-DDDDDDD-OH (3 mg) through the N-terminal amino group to aldehyde-activated agarose (3 ml). Bound antibody was eluted with 0.1 M glycine-HCl buffer (pH 2.7), neutralized with Tris buffer, assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and adjusted to contain 0.1 mg of protein/ml.
To immunize CF1 mice, 0.25 ml of conjugate (1.0 mg/ml), 0.125 ml of PBS, and 0.125 ml of muramyl dipeptide (1.0 mg/ml) were emulsified in 0.5 ml of incomplete Freund’s adjuvant (50% emulsion; total volume of 1.0 ml) and injected subcutaneously (0.2 ml). After 20 days, the mice were euthanized for removal of their spleens. Spleen cells were fused with myeloma cells (Sp2/0 Ag 14 myeloma cell line CRL1581; American Type Culture Collection, Manassas, Va.), and hybrid cells were propagated and screened for antibody production by an enzyme-linked immunosorbent assay (ELISA) using H-DDDDDDD-OH conjugated to bovine serum albumin (BSA-DDDDDDD conjugate) as the antigen. Two positive primary hybrids, 1G9-1C2 and 1G9-2B10, were then selected for single-cell cloning. Monoclonal antibody in supernatants was then isolated on a Protein A-Sepharose column (HiTrap; Pharmacia Biotech, Piscataway, N.J.). Bound antibody was eluted with 0.1 M glycine-HCl buffer (pH 2.7), neutralized with Tris buffer, and assessed by SDS-PAGE. 1G9-1C2 was adjusted to a stock solution of 1.0 mg of protein/ml, and 1G9-2B10 contained 0.586 mg of protein/ml.
Assessment of antibody specificity.
Antibody specificity was assessed by a competitive ELISA. BSA-DDDDDDD-OH conjugate (50 ng of conjugate/well) was used as the adsorbed antigen, and varying graded concentrations (1.0 to 0.002 mM) of H-DDDDDDD-OH, H-GADDDDD-OH, H-VDDDDK-OH, H-TQDDGGK-OH, H-GGEEK-OH, and H-SGSGSGS-OH were used as the competitive antigens in the presence of antibody PAB96-1, 1G9-1C2, or 1G9-2B10 (1.0 μg/ml).
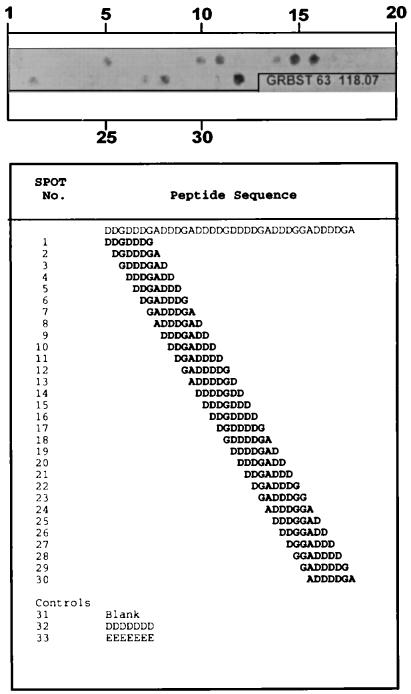
The epitope binding sites of the three antibodies were also determined. Thirty peptides (7 residues each) corresponding to a single-residue frameshift of the sequence in Fig. 3 were synthesized simultaneously on a derivatized cellulose sheet (SPOTs; Genosys, The Woodlands, Tex.). For example, peptide 1, starting at residue 30 of AP 87-8, was H-DDGDDDG-OH, peptide 2 was H-DGDDDGA-OH, peptide 3 was H-GDDDGAD-OH, etc. SPOT 32 contained H-DDDDDDD-OH and SPOT 33 contained H-EEEEEEE-OH as peptide controls. The strip was incubated overnight in casein-based blocking buffer (Genosys), washed in 0.05 M Tris buffer (pH 8.0) with 0.14 M NaCl, 2.7 mM KCl, and 0.05% Tween 20 (T-TBS), and incubated in casein-based blocking buffer containing antibody for 3 h at room temperature. Preimmunized rabbit serum (1:40), preimmunized mouse serum (1:40), rabbit antibody PAB96-1 (5 and 25 μg/ml), mouse antibodies 1G9-2B10 and 1G9-1C2 (5 and 25 μg/ml), secondary β-galactosidase-conjugated sheep polyclonal antibody to mouse immunoglobulin G (IgG) (5 μg/ml), and secondary β-galactosidase-conjugated sheep polyclonal antibody to rabbit IgG (5 μg/ml) were all tested. After incubation, the membrane was washed in T-TBS and incubated in secondary β-galactosidase-conjugated sheep polyclonal antibody to rabbit or mouse IgG (5 μg/ml) for 2 h at room temperature. Bound antibody was detected with Signal Development solution (Genosys).
FIG. 3.
Nucleotide and predicted peptide sequences for candidate AP precursor genes, comprising exons from sheep genomic library clones. The predicted ORF for AP 87-8 extends in the 5′ direction. ORF sequences are capitalized, and potential AP coding sequences are in boldface.
ELISA.
The concentration of AP in BAL fluids was assessed by a direct ELISA. Briefly, samples (100 μl) were incubated overnight at 26°C in styrene plates (Immulon 1; Dynatech Laboratories, Inc., Chantilly, Va.). The negative control was a surfactant from sheep 3149 (100 μl), previously shown not to be bactericidal (6). The positive control was synthetic peptide H-DDDDDDD-OH added to a surfactant (1.0 to 0.063 mM). Wells were blocked with gelatin blocking buffer. Antibody PAB96-1 or 1G9-1C2 (1.0 μg/ml of blocking buffer) was added and incubated for 1 h. The wells were washed, and antibody was detected with peroxidase-labeled goat anti-rabbit IgG or goat anti-mouse IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), respectively. Antibody binding reactions were detected with tetramethylbenzidine (TMB substrate and stop system; Kirkegaard & Perry Laboratories, Inc.).
The competitive ELISA was also used to find AP in reverse-phase (RP) HPLC fractions of BAL fluid and epithelial-cell extracts. BSA-DDDDDDD-OH conjugate (50 ng of conjugate/well) was used as the adsorbed antigen, varying graded concentrations (1.0 to 0.002 mM) of H-DDDDDDD-OH were used as the competitive control antigen, and column fractions were used as the competitive antigens in the presence of antibody PAB96-1, 1G9-1C2, or 1G9-2B10 (1.0 μg/ml).
Identification of AP genes.
Degenerate oligonucleotides, representing H-GADDDDD-OH (SH87, 5′ GGTGCTGAYGAYGAYGAYGAY 3′, where Y stands for T or C) and H-DDDDDDD-OH (SH88, 5′ GAYGAYGAYGAYGAYGAYGAY 3′, where Y stands for T or C) were synthesized, end labeled with 32P, and used to probe a Southern blot containing sheep genomic DNA to identify candidate AP precursor genes. Oligonucleotide SH87 was also used as a hybridization probe to screen a sheep phage genomic library (Clonetech) by standard techniques (11).
RP-HPLC.
BAL fluid from each sheep (1.6 ml) and lysed tracheal epithelial-cell extracts from two sheep (1.6 ml) were mixed with chloroform (2 ml) and methanol (4 ml) to extract lipids as described by Bligh and Dyer (2). The methanol-water phase was then removed and dried by rotary evaporation. The residue was resuspended in distilled water and filtered, first in a sterilizing centrifugal filter (Ultrafree-CL; 0.22 μm; Millipore Corp., Bedford, Mass.) and then through a centrifugal ultrafilter (Biomax-10; Millipore Corp.). The 10-kDa ultrafiltrate was collected and separated (50-μl aliquots) on a C18 column (Jupiter 300; 5-μm particle size; 4.6 by 250 mm; Phenomenex, Torrance, Calif.) by using a 12114M pump and a 406 Analog Interface Module (Beckman Instruments, Inc., Palo Alto, Calif.). Timed fractions over 30 min were eluted with a gradient of acetonitrile (0 to 100%) in 0.1% trifluoroacetic acid (TFA) with a flow rate of 1.0 ml/min. Fractions were collected (SC100 fraction collector; Beckman Instruments, Inc.). Fractions were pooled with similar fractions of previous runs and evaporated to dryness under a vacuum. Residues were dissolved in distilled water and tested for antimicrobial activity. Antimicrobial fractions were separated again by HPLC and eluted in a 0 to 30% acetonitrile gradient over 30 min with a flow rate of 0.5 ml/min. Fractions were collected, evaporated to dryness, dissolved in distilled water, and again tested for antimicrobial activity, analyzed for amino acid content, or checked for peptide content by ELISA. Synthetic peptides H-DDDDDDD-OH, H-GADDDDD-OH, and H-GDDDDDD-OH were added to BAL fluid before sample preparation and used as internal standard column controls.
Mass spectrometry.
HPLC fractions with antimicrobial activity and evidence of AP by competitive ELISA were examined for H-DDDDDDD-OH, H-GADDDDD-OH, H-GDDDDDD-OH, (M + H)+, (M + Na)+, or (M + K)+ masses by matrix-assisted laser desorption/ionization (MALDI) (Finnigan MAT, Lasermat 2000; Protein Facility, Iowa State University). The matrix was α-cyano-4-hydroxycinnamic acid (50%, vol/vol).
Antimicrobial activity.
Pasteurella haemolytica serotype A1 strain 82-25, isolated from a sheep with enzootic pneumonia, was grown in tryptose broth at 37°C for 3 h, pelleted by centrifugation at 5,900 × g for 15 min at 4°C, and resuspended in 140 mM NaCl. The suspensions were adjusted in the spectrophotometer (78% transmittance at 600 nm; Coleman model 35; Bacharach Instrument Co.) to contain 1.0 × 108 CFU/ml and were diluted 10−5-fold to 103 CFU/ml of 140 mM NaCl (6). A dilution susceptibility test was used to obtain the percent killed bacteria in BAL fluids compared to that in the control solution (4). Percent killing was calculated as [1 − (CFU in BAL fluids/CFU in saline control mixtures)] × 100.
SDS-PAGE and Western blotting.
BAL fluid, respiratory epithelial cells (scraped from either turbinate or tracheal surfaces), and small pieces of tissue (e.g., lung, liver, spleen, kidney, small intestine, and bladder wall) were ground in Tenbrock tissue grinders, diluted 4:5 with sample buffer (ImmunoPure; Pierce, Rockford, Ill.), and heated for 5 min at 100°C. Proteins in solubilized BAL fluid and cell and tissue homogenates were then separated by SDS-PAGE (14) with a 6.0% stacking gel over a 12.5% resolving gel. Prestained and unstained protein ladders (10 to 184 kDa; Benchmark; Life Technologies, Gaithersburg, Md.) were used as standards. Proteins were blotted onto Immobilon-P transfer membranes (Millipore Corp.) and cut into strips. One strip of each tissue blot was stained with Coomassie blue, and the other strip was blocked overnight with gelatin blocking buffer containing 10 mM Tris buffer, 145 mM NaCl, 1.0% fish gelatin, and 0.05% Tween 20. Strips were incubated for 1 h with antibody PAB96-1 or 1G9-1C2 (5.0 μg/ml) and washed. Bound antibody was detected with peroxidase-labeled goat anti-rabbit IgG or goat anti-mouse IgG (0.5 μg/ml; Kirkegaard & Perry Laboratories, Inc.), respectively, and a 4-chloro-1-naphthol substrate system (Kirkegaard & Perry Laboratories, Inc.). Blots were photographed (digital camera RD-175; Minolta, Ramsey, N.J.), and the molecular masses of the reactive bands were determined (GelCompar, v. 4.0; Applied Maths, Kortrijk, Belgium) by extrapolation from a standard curve of the molecular masses of the prestained bands on the protein ladder.
As controls, strips of membrane containing separated tracheal and lung proteins were incubated with preimmunized rabbit serum (1:200), preimmunized mouse serum (1:200), or peroxidase-labeled goat anti-rabbit IgG (0.5 μg/ml) or goat anti-mouse IgG (0.5 μg/ml). No reactions were seen. In addition, preincubation of antibody PAB96-1, 1G9-2B10, or 1G9-1C2 (5.0 μg/ml) with 1.0 mM H-DDDDDDD-OH eliminated specific staining of all bands.
Histopathology and immunocytochemistry.
Pulmonary tissue, taken from each lung, was fixed in 10% neutral buffered formalin solution, dehydrated and cleared, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Pulmonary tissues for immunocytochemical analysis for AP were sectioned onto ProbeOn Plus microscope slides (Fisher Scientific, Pittsburgh, Pa.) as previously described (5). Paraffin was removed with xylene, and the ovine tissue sections were rehydrated sequentially in 100% ethanol, 90% ethanol, and 70% ethanol and then were rinsed in water. The sections were first incubated in a gelatin blocking buffer and then digested with trypsin for 15 min at 37°C. After an additional wash first in gelatin blocking buffer and then in blocking serum, the sections were incubated with antibody PAB96-1 (0.5 μg/ml) or 1G9-1C2 (2.0 μg/ml) for 1.5 h at 37°C. Sections were then washed in gelatin blocking buffer, incubated with biotinylated goat anti-mouse or goat anti-rabbit antibody (Kirkegaard & Perry Laboratories, Inc.), respectively, washed in buffer, and incubated in streptavidin-alkaline phosphatase for 30 min at room temperature. The color was developed with HistoMark Red (Kirkegaard & Perry Laboratories, Inc.). Sections were then counterstained with hematoxylin for 1 min (Shannon Lipshaw), dehydrated, infiltrated, and mounted.
The immunohistochemical procedure was optimized after a series of trials. Control tissue sections were processed as described above without antibody 1G9-1C2 or with antibody 1G9-1C2 preincubated with 0.5 mM H-DDDDDDD-OH. In both of these controls, staining specific for AP could not be detected. Tissue sections were also incubated with trypsin for 15 or 30 min or heated in a microwave for 10 min in either citrate buffer (pH 6.0; Biogenex) or Tris buffer (pH 10.0) to further expose AP in cells. None of these treatments dramatically increased staining specific for AP. Tissue sections were also incubated in a variety of blocking buffer solutions, including solutions containing fish gelatin, 10, 50, or 100% normal goat serum, or fish gelatin with either 10, 50, or 100% normal goat serum, or in a commercial blocker (Powerblock; Biogenex). Gelatin blocking buffer containing 50% normal goat serum was chosen; it enhanced specificity as well as reducing nonspecific background and nuclear staining.
RESULTS
Assessment of antibody specificity.
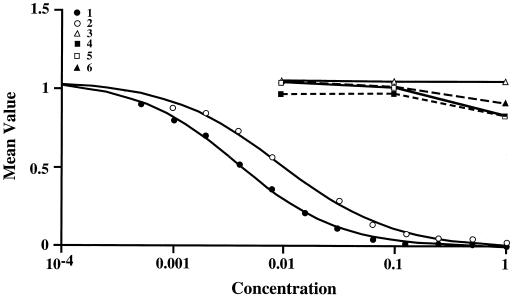
Polyclonal antibody PAB96-1 and monoclonal antibodies 1G9-1C2 and 1G9-2B10 were specific for peptides with C-terminal Asp residues (Fig. 1 and 2). In the competitive ELISA, all three antibodies reacted similarly (results with antibody 1G9-1C2 are shown in Fig. 1, and the results with antibodies PAB96-1 and 1G9-2B10 are not shown). All antibodies recognized H-GADDDDD-OH nearly as well as H-DDDDDDD-OH. However, H-VDDDDK-OH, H-TQDDGGK-OH, H-GGEEK-OH, and H-SGSGSGS-OH were not recognized and did not cross-react.
FIG. 1.
The competitive ELISA was used to assess the specificity of monoclonal antibody 1G9-1C2. BSA-DDDDDDD-OH conjugate (50 ng of conjugate/well) was used as the adsorbed antigen, and varying graded concentrations (1.0 to 0.002 mM) of H-DDDDDDD-OH (peptide 1), H-GADDDDD-OH (peptide 2), H-TQDDGGK-OH (peptide 3), H-GGEEK-OH (peptide 4), H-VDDDDK-OH (peptide 5), and H-SGSGSGS-OH (peptide 6) were used as the competitive antigens in the presence of antibody 1G9-1C2 (1.0 μg/ml). Antibody 1G9-1C2 was specific and recognized H-GADDDDD-OH nearly as well as H-DDDDDDD-OH. However, the sequences with internal Asp or Glu residues (e.g., H-VDDDDK-OH, H-TQDDGGK-OH, and H-GGEEK-OH) were not recognized and did not cross-react. H-SGSGSGS-OH was a negative-control peptide.
FIG. 2.
The epitope binding sites of antibody 1G9-1C2 (25 μg/ml) were determined with 30 peptides (7 residues each) corresponding to a single-residue frameshift of the 7-residue sequence starting at residue 30 of the sequence identified in AP 87-8 of Fig. 3. For example, peptide 1 was H-DDGDDDG-OH, peptide 2 was H-DGDDDGA-OH, peptide 3 was H-GDDDGAD-OH, etc. Peptides were synthesized simultaneously on a derivatized cellulose sheet (SPOTs; Genosys). SPOT 32 contained H-DDDDDDD-OH and SPOT 33 contained H-EEEEEEE-OH as peptide controls. SPOTs were detected after the strip was incubated in casein-based blocking buffer, incubated in antibody, washed, incubated in secondary β-galactosidase-conjugated antibody, and incubated in Signal Development solution. Epitopes that contained more than 2 terminal Asp residues were recognized.
With the SPOTs Epitope Mapping Kit, the epitope binding sites of antibodies PAB96-1, 1G9-1C2, and 1G9-2B10 were determined with 30 peptides (7 residues each) corresponding to a single-residue frameshift of the 7-residue sequence starting at residue 30 of the sequence identified in AP 87-8 of Fig. 3. Antibody 1G9-1C2 (25 μg/ml only; Fig. 2) recognized epitopes that contained more than 2 C-terminal Asp residues. Antibody PAB96-1 (both 5 and 25 μg/ml), affinity purified on a gel coupled with H-DDDDDDD-OH, recognized only H-DDDDDDD-OH (data not shown). Antibody 1G9-2B10 (5 and 25 μg/ml) did not react with any epitope in this assay (data not shown). As in the reactions with the SPOTs Epitope Mapping Kit, antibodies PAB96-1 and 1G9-1C2 worked well to detect proteins by Western blot, but 1G9-2B10 did not. Preimmunized rabbit serum (1:40), preimmunized mouse serum (1:40), and secondary β-galactosidase-conjugated sheep polyclonal antibody to mouse or rabbit IgG (5 μg/ml) did not react with any peptide.
Identification of AP genes.
Both degenerate oligonucleotides SH87 and SH88, representing coding combinations for H-GADDDDD-OH and H-DDDDDDD-OH, respectively, detected candidate AP precursor genes in sheep DNA.
Based on this result, SH87 was used as a hybridization probe to screen a sheep phage genomic library. One million PFU were screened, and 12 duplicate positives were identified. Three rounds of purification led to the isolation of two independent phage whose genomic DNA inserts contained sequences encoding the H-GADDDDD-OH sequence. The peptides encoded are referred to as AP 87-7 and AP 87-8 (Fig. 3), and their predicted open reading frames (ORF) show distinct sequences surrounding the heptapeptide codons. AP 87-8 is unique in encoding variable-length repeats of combinations of glycine, alanine, and aspartic acid.
PCR-generated probes specific for the putative AP 87-7 and AP 87-8 coding sequences (see Fig. 3) hybridized strongly to genomic ovine DNA blots. Using these same probes against a Northern blot of parenchymal lung RNA obtained from adult and 4-day-old sheep, we were unable to detect a hybridization signal (data not shown).
Detection of AP in BAL fluid.
AP in BAL fluid or a pulmonary surfactant could be detected with a direct ELISA. As a positive control, H-DDDDDDD-OH was added to an ELISA-negative surfactant. The peptide could be detected with antibody PAB96-1 or antibody 1G9-1C2 in a linear relationship (correlation coefficient, 0.995; slope, 0.370). With antibody 1G9-1C2, BAL fluid contained 0.83 ± 0.33 mM AP (mean ± SD; range, 0.10 to 1.59 mM). Results were similar with antibody PAB96-1 (data not shown).
BAL fluids varied greatly in antimicrobial activity, ranging from 0.0 to 98.0% killing.
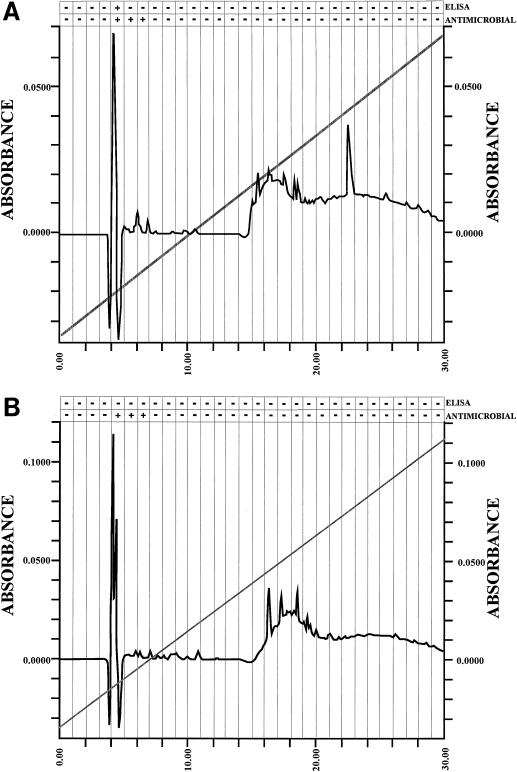
To confirm that AP was the molecule detected by ELISA and was responsible for the antimicrobial activity of the BAL fluids, BAL fluid was separated by RP-HPLC, and fractions were again tested for antimicrobial activity and were tested for the presence of AP by competitive ELISA and MALDI mass spectroscopy. RP-HPLC fraction 5, eluted with 13 to 14% acetonitrile (Fig. 4A), induced killing of P. haemolytica. This fraction was also positive for AP by competitive ELISA and contained H-DDDDDDD-OH [(M + H)+; 824.4 Da] and H-GDDDDDD-OH [(M + H)+; 766.1 Da].
FIG. 4.
RP-HPLC chromatograms of ovine BAL fluid (A) and ovine epithelial-cell extracts (B), showing peak fraction 5, eluted in 13 to 14% acetonitrile, with antimicrobial activity and evidence of AP by competitive ELISA with antibody 1G9-1C2. BSA-DDDDDDD-OH conjugate (50 ng of conjugate/well) was used as the adsorbed antigen, and RP-HPLC fractions were used as the competitive antigens in the presence of antibody 1G9-1C2 (1.0 μg/ml). To confirm the presence of AP, fractions were examined by MALDI mass spectrometry. H-DDDDDDD-OH [(M + H)+; 824.4 Da], H-GDDDDDD-OH [(M + H)+; 766.1 Da], and H-GADDDDD-OH [(M + H)+; 718.24 Da] were detected.
Detection of AP in epithelial tissue.
AP was also present in epithelial-cell extracts (Fig. 4B). RP-HPLC fraction 5 induced killing of P. haemolytica. Although AP could not be detected by competitive ELISA, this fraction contained H-DDDDDDD-OH [(M + H)+; 824.4 Da] and H-GDDDDDD-OH [(M + H)+; 766.1 Da].
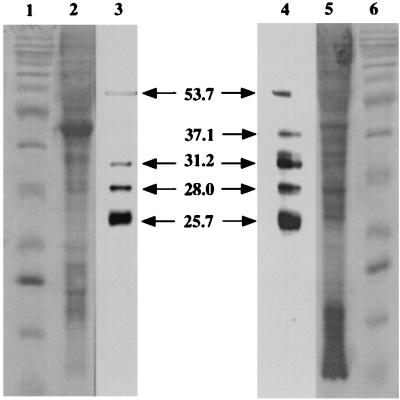
Antibodies PAB96-1 and 1G9-1C2 recognized four bands in blots of solubilized tracheal cells (molecular masses, 53.7, 31.2, 28.0, and 25.7 kDa) and five bands in blots of lung homogenates (molecular masses, 53.5, 37.1, 31.2, 28.0, and 25.7 kDa) (Fig. 5). Only a weakly staining band was seen in blots of liver and small-intestine homogenates. No bands were seen in blots of ovine BAL fluid, albumin, kidney, or spleen homogenates.
FIG. 5.
Western blot of ovine tracheal epithelium and lung homogenate. Lane 1, protein ladder (10 to 200 kDa) standard; lane 2, tracheal-cell homogenate stained with Coomassie blue; lane 3, tracheal-cell homogenate probed with antibody PAB96-1; lane 4, lung homogenate probed with antibody PAB96-1; lane 5, lung homogenate stained with Coomassie blue; lane 6, protein ladder standard. In tracheal epithelial cells, antibody PAB96-1 identified four bands with molecular masses of 53.7, 31.2, 28.0, and 25.7 kDa. In the lung, antibody PAB96-1 identified five bands with molecular masses of 53.5, 37.1, 31.2, 28.0, and 25.7 kDa. Identical results were seen with antibody 1G9-1C2 (data not shown). As controls, strips of membrane containing separated tracheal and lung proteins were incubated with preimmunized rabbit serum (1:200), preimmunized mouse serum (1:200), or peroxidase-labeled goat anti-rabbit IgG (0.5 μg/ml) or goat anti-mouse IgG (0.5 μg/ml). No reactions were seen (data not shown). In addition, preincubation of antibody PAB96-1 (5 μg/ml) or 1G9-1C2 (5 μg/ml) with 1.0 mM H-DDDDDDD-OH eliminated specific staining of all bands (data not shown). Blots were photographed (digital camera RD-175; Minolta), and the sizes of the reactive bands were determined (GelCompar, v. 4.0; Applied Maths) by extrapolation from a standard curve of the sizes of the stained bands on the protein ladder.
The majority of lung tissues collected from the slaughterhouse were normal, but a few had extensive inflammatory cell infiltration. In general, lesions consisted of infiltrates of lymphocytes and plasma cells that had features resembling those of ovine mycoplasmosis. No distinction was made between these two groups in this study, and no apparent differences in results were seen.
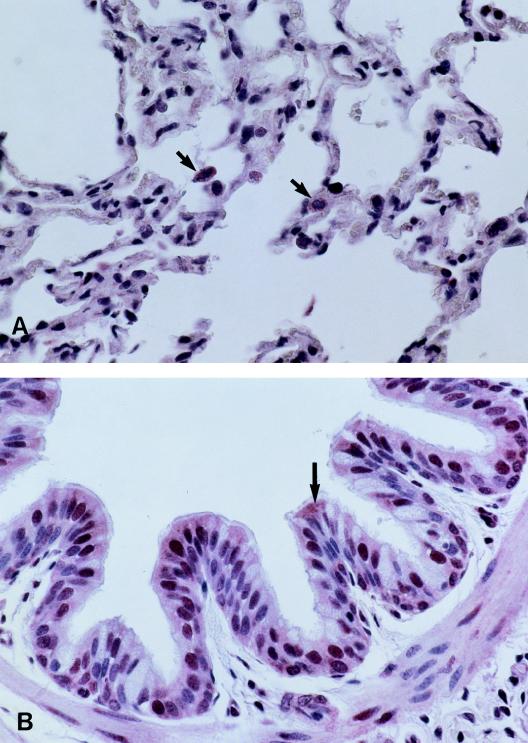
Both antibodies PAB96-1 and 1G9-1C2 identified accumulated protein in the apical cytoplasm of the bronchial and bronchiolar epithelia and in the cytoplasm of pulmonary endothelial cells and an occasional alveolar macrophage (Fig. 6). Goblet cells and epithelial cells of pulmonary alveoli were not stained. Both antibodies also reacted with the nuclear regions of many cell types. The nuclei of some bronchial and bronchiolar epithelia were brightly stained (Fig. 6B), whereas the nuclei of other cell types varied in intensity and number. These included nuclei of cells from submucosal glands of bronchi, serous cells, endothelial cells, smooth-muscle cells, alveolar macrophages, and alveolar septal cells. Control tissue sections were incubated without antibody 1G9-1C2 or with antibody 1G9-1C2 preincubated with 0.5 mM H-DDDDDDD-OH. In both of these controls, staining specific for AP could not be detected (data not shown).
FIG. 6.
Tissue sections of ovine lung, incubated with antibody 1G9-1C2, show immunocytochemically stained regions of the alveoli (A) and bronchial epithelium (B) that were not seen in these regions when tissue sections were incubated in secondary antiserum alone (data not shown). Antibody 1G9-1C2 identified accumulated protein (arrows) in the cytoplasm of pulmonary endothelial cells and an occasional alveolar macrophage (A) and in the apical cytoplasm of the bronchial and bronchiolar epithelia (B). Goblet cells and epithelial cells of pulmonary alveoli were not stained.
DISCUSSION
Antibodies specific to the C-terminal aspartic acid region of the synthetic peptide H-DDDDDDD-OH demonstrated the presence of AP in BAL fluids (by ELISA) and related epitopes in pulmonary tissues (by Western blot and immunocytochemistry). The specificity of these antibodies was confirmed by fractionating BAL fluid and epithelial-cell extracts by RP-HPLC and demonstrating the presence of H-DDDDDDD-OH or H-GDDDDDD-OH by MALDI mass spectrometry. AP, but not the larger precursor proteins, was found in BAL fluid, and both AP and the larger precursor proteins were found in epithelial-cell extracts.
While the origin of the AP is not yet known, a number of observations are consistent with the hypothesis that they are derived from precursor protein(s), paralleling the biosynthetic pathway for the cationic antimicrobial peptides (3). First, antibodies to AP recognize several larger proteins (25.7 to 53.7 kDa) in Western blots of solubilized tracheal epithelial cells and lung homogenates (Fig. 5). Second, a degenerate oligonucleotide probe of AP used to probe a Southern blot of sheep DNA identified strong hybridizing bands, all <4 kb, also suggesting that H-DDDDDDD-OH is part of a much larger gene product (Fig. 3). Third, the amino acid sequences of AP are similar to those of charge-neutralizing activation peptides of Group I serine proteases (e.g., human trypsinogen activation peptide, H-VDDDDK-OH) and contain homopolymeric regions of Asp following a Gly or Ala and terminating with Lys. Synthetic trypsinogen activation peptide and other similar fragments have antimicrobial activity (4).
A number of AP-related sequences have been found in other cellular and nuclear proteins (e.g., human B23 nucleophosmin, containing the internal sequence DEDDDDDDEEDDDEDDDDDD [GenBank accession no. X16934]). Staining of the latter protein may help explain the epithelial-cell nuclear staining we detected. However, no DNA/protein sequences encoding the heptapeptide AP have been reported in sheep or in other animal species.
Innate immune mechanisms serve to suppress or reduce microbial growth at multiple epithelial surfaces. In the respiratory tract, AP can be demonstrated readily both in respiratory epithelium and in mucosal secretions at concentrations that are antimicrobial. These findings support the hypothesis that AP not only contribute to the microenvironment on alveolar surfaces but also function as an adjunct to the well-characterized peptide and cellular host defense elements of the mammalian airway. Experiments designed to test this hypothesis in animal models of pneumonia are in progress.
ACKNOWLEDGMENTS
We thank Gwen Laird and Kim Driftmier for technical assistance.
REFERENCES
- 1.Bartlett J G. Bacteriological diagnosis of pulmonary infections. In: Sackner M A, editor. Diagnostic techniques in pulmonary disease. Vol. 16. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 707–745. [Google Scholar]
- 2.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 4.Brogden K A, Ackermann M, Huttner K M. Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrob Agents Chemother. 1997;41:1615–1617. doi: 10.1128/aac.41.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden K A, Ackermann M R, DeBey B M. Pasteurella haemolytica lipopolysaccharide-associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect Immun. 1995;63:3595–3599. doi: 10.1128/iai.63.9.3595-3599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brogden K A, De Lucca A J, Bland J, Elliott S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA. 1996;93:412–416. doi: 10.1073/pnas.93.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond G, Jones D E, Bevins C L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison R T, III, Boose D, LaForce F M. Isolation of an antibacterial peptide from human lung lavage fluid. J Infect Dis. 1985;151:1123–1129. doi: 10.1093/infdis/151.6.1123. [DOI] [PubMed] [Google Scholar]
- 9.Ganz T, Lehrer R I. Defensins. Curr Opin Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 11.Huttner K M, Lambeth M R, Burkin H R, Burkin D J, Broad T E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 12.LaForce F M, Boose D S. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect Immun. 1984;45:692–696. doi: 10.1128/iai.45.3.692-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCray P B, Jr, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 14.Parsons N J, Kwaasi A A A, Perera V Y, Patel P V, Martin P M V, Smith H. Outer membrane proteins of Neisseria gonorrhoeae associated with survival within human polymorphonuclear phagocytes. J Gen Microbiol. 1982;128:3077–3081. doi: 10.1099/00221287-128-12-3077. [DOI] [PubMed] [Google Scholar]
- 15.Rennard S I, Basset G, Lecossier D, O’Donnell M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 16.Sherman M P. Host defense in pulmonary alveoli. Annu Rev Physiol. 1992;54:331–350. doi: 10.1146/annurev.ph.54.030192.001555. [DOI] [PubMed] [Google Scholar]
- 17.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]