Abstract
Some failures in ovary function, like folliculogenesis and oogenesis, can give rise to various infertility-associated problems, including polycystic ovary syndrome (PCOS) and premature ovarian insufficiency (POI). PCOS influences 8 to 20% of women; while POI occurs in at least 1% of all women. Regrettably, the current therapies for these diseases have not sufficiently been effective, and finding a suitable strategy is still a puzzle. One of the helpful strategies for managing and treating these disorders is understanding the contributing pathogenesis and mechanisms. Recently, it has been declared that abnormal expression of microRNAs (miRNAs), as a subset of non-coding RNAs, is involved in the pathogenesis of reproductive diseases. Among the miRNAs, the roles of miRNA-21 in the pathogenesis of PCOS and POI have been highlighted in some documents; hence, the purpose of this mini-review was to summarize the evidences in conjunction with the functions of this miRNA and other effective microRNAs in the normal or abnormal functions of the ovary (i.e., PCOS and POI) with a mechanistic insight.
Keywords: MicroRNA-21, Pathogenesis, Polycystic Ovarian Syndrome, Premature Ovarian Insufficiency
Introduction
Dysfunctions in folliculogenesis and oogenesis, like failure in the formation of steroid hormones and maturation of oocytes, lead to various ovarian diseases related to infertility, such as polycystic ovarian syndrome (PCOS) and premature ovarian insufficiency (POI) (1-3). PCOS, as a prevalent endocrinopathy in the reproductive course of women, involves in 8 to 20% of women (4). This disease can be identified by detecting two of the three properties related to the Rotterdam criteria (i.e., hyperandrogenism, polycystic ovaries, and an- or oligo-ovulation) and excluding associated diseases (e.g., Cushing’s syndrome, congenital adrenal hyperplasia, hyperprolactinemia, and thyroid disease) (5, 6). Additionally, POI [another name is premature ovarian failure (POF)] influences 1% of women at young ages and it is defined by decreased estradiol (E2) expression and follicular dysplasia, in addition to increased gonadotropin expression and follicle-stimulating hormone (FSH) (7, 8).
Unfortunately, against these disorders related to folliculogenesis and oogenesis, the current therapeutic approaches have not reflected enough effectiveness; thus, discovering a therapy with high efficiency and minimum side-effects is still a challenging issue (9, 10). One of the useful ways to improve remedies and the management of diseases is knowing the pathogenic mechanisms of illnesses (11). Accumulating evidences have implicated that abnormal expression of microRNAs was linked with pathological processes of different disorders, such as reproductive diseases, metabolic disorders, cancer, cardiovascular diseases, and neurological conditions (12-16). microRNAs (miRNAs/miRs) are a subset of non-coding and single-stranded RNAs. Their lengths are approximately 18-24 nucleotides, and they can down-regulate certain gene expression in a posttranscriptional way by binding to target messenger RNA [mRNA; 3′-untranslated region (UTR)] (17, 18). The published papers have indicated that microRNAs can be involved in ovarian functions, e.g., endocrine function, folliculogenesis and modulation of steroidogenesis, as well as apoptosis and proliferation of granulosa cells (19, 20). Recently, the role of dysregulation of miR-21 in the pathogenic occurrences of ovarian function-associated diseases (i.e., PCOS and POI) has been highlighted (21- 23). Hence, in this mini-review, we aimed to discuss and review the role of this molecule and the other involved miRNAs in normal ovarian activities and pathogenic events of the mentioned diseases.
miR-21 is one of the most frequently present miRNAs in the ovary of different species, such as mouse, sheep, porcine, and bovine (24). miR-21 is divided into two types according to its strand, including miR-21 passenger strand (miR-21-3p) and miR-21 guide strand (miR-21-5p), the latter of which is the most frequent miRNA related to the RNA-induced silencing complex in granulosa cells (25, 26). It is approved that miR-21 (miR-21-5p) has a role in oocyte maturation as well as blastocyst and embryo development. It is considerably overexpressed at the time of transition from germinal vesicle to oocytes, arrested in the metaphase II (MII) stage of the meiotic division (27-29). In the research of Wright et al. (30), the results of reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR) analysis manifested that miR-21 is up-regulated about six times in oocytes and about 25 times in cumulus cells during in vitro maturation of pig oocytes. Based on the research of Han et al. (31) and Carletti et al. (32), oocyte-secreted factors (OSFs) promoted expression of miR-21 by targeting transforming growth factor-β (TGF-β) signaling, while miR-21 quenched apoptosis of cumulus and periovulatory granulosa cells by triggering the phosphatidylinositol 3-kinase (PI3K)/Akt signaling and decreasing cleaved caspase-3, respectively. The PI3K signaling pathway was described as a cell proliferation and survival regulator in the various cellular types triggered by basic fibroblast growth factor (bFGF). In addition, cleaved caspase-3 was considered a key actor of nuclear changes associated with apoptotic processes (33, 34). Furthermore, the results of Pan and Li (24) demonstrated that miR-21 enhanced porcine oocyte maturation and cumulus expansion by decreasing expression of tissue inhibitor of metalloproteinase-3 (TIMP3), as a matrix metalloproteinase inhibitor whose 3´-UTR sequence is targeted by this miRNA. Additionally, they proved a piece of evidence that the mentioned miRNA elevated levels of VERSICAN as well as the expressions of A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1) and another gene associated with cumulus expansion, HAS2, in the time of COC maturation in vitro (24, 35). Indeed, the cleaved structure of VERSICAN, as a subset of aggregating chondroitin sulfate proteoglycans, by ADAMTS1 has a crucial role in the success of the matrix remodeling of cumulus cells (36, 37). Taken together, it seems that miR-21 can be involved in the normal activities of the ovary by affecting oocyte maturation, cumulus expansion, blastocyst and embryo development, and granulosa cell viability.
Based on growing evidence, miRNAs possess a striking role in ovarian activities. In this line, Timoneda et al. (38) explored expression levels of the porcine microRNAs by the RT-qPCR method and approved that miR-25, Let-7a, and miR-106a were expressed in ovarian tissues. McBride et al. (39) assessed the expression levels of some miRNAs at different phases of follicle development, comprising small follicles, medium follicles, pre-ovulatory follicles, early and late corpora lutea, and corpus albicans. Overall, let-7a, let-7b, miR-21, and miR-125b were the most frequently expressed miRNAs at different development phases. miR-31, miR-145, and miR-199a-3p showed a significant reduction in the follicular-luteal transition and a remarkable elevation at the follicular phase. In contrast, miR-21, miR- 142-3p, and miR-503 represented a considerable increase in luteal tissues and they were normally expressed at lower levels during the follicular phases.
Different factors have function in follicle development, like TGF-β superfamily members, Smads, and activin receptor-like kinases (ALKs). Additionally, miRs can affect these agents (40-42). In this direction, it was stated that miR-224 potentiated granulosa cell proliferation by targeting Smad4, an important regulator related to follicular growth of the ovary (43). Moreover, a number of miRNAs (e.g., miR17-92 cluster and miR-183-96-182 cluster) have shown their roles in granulosa cell proliferation and differentiation as well as cell cycle transition by other mechanisms, including influencing BMPR2 and PTEN genes and FOXO1 transcription factor (44, 45). During folliculogenesis, above 99% of follicles experienced atresia, and activities of miRs in orchestrating follicle development and atresia have been illustrated (46, 47).
P-miR-1281, has-miR-936, hsa-miR-26b, hsa-miR- 10b, mmu-miR-1224, P-miR-466 g-b, hsa-miR-574-5p, P-miR-1275, R-miR-26b, hsa-miR-1275, hsa-miR-149, and has-miR-99a are among overexpressed miRNAs during this degenerative process, whilst expression of has-let-7i, R-let-7a, hsa-miR-92b, P-miR-923, has-miR- 92a, has-miR-1979, hsa-miR-1308, R-miR-739, hsamiR- 1826, ssc-miR-184, and P-miR-1826 were reduced during this occurrence (48-50). Folliculogenesis, as a highly dynamic occurrence, is linked with changes in circulating levels of ovarian hormones, and interestingly, the relationships between microRNAs and these hormones have also been scrutinized (51). As an example, Sirotkin et al. (52) indicated that 36 miRNAs (e.g., let-7b, let-7c, miR-17-3p, miR-15a, miR-92, miR-96, miR-108, miR- 134, miR-133b, miR-146, and miR-135) suppressed progesterone secretion. In contrary, 16 miRNAs (i.e., miR- 16, miR-18, miR-24, miR-25, miR-32, miR-103, miR-122, miR-125a, miR-143, miR-145, miR-147, miR-150, miR- 152, miR-153, miR-182, and miR-191) enhanced release of progesterone hormone in granulosa cells. Regarding testosterone hormone, this study determined that let-7a, let- 7b, let-7c, miR-17-3p, miR-16, miR-24, miR-26a, miR-25, miR-122, miR-108 repressed release of the aforementioned hormone. In addition, it was expressed that miR-378 influenced synthesis of estradiol hormone by binding 3′-UTR of the aromatase coding sequence (53). miRNAs can also function in ovarian activities indirectly. Hasuwa and co-workers studied effects of miR-200b and miR-429 on infertility and anovulation in female mice, and finally they found that miR-200b and miR-429 abrogated zinc-finger E-box binding homeobox 1 (ZEB1) expression in the pituitary gland, whereby expression of these miRNAs were remarkably high. Plus, miR-200b and miR-429 inhibition suppressed luteinizing hormone (LH) biosynthesis, revealing that these miRNAs facilitated ovulation indirectly by affecting the hypothalamus-pituitary-ovarian axis (54). In summing up, it looks like the normal action of the ovary is regulated or mediated by the certain miRNAs.
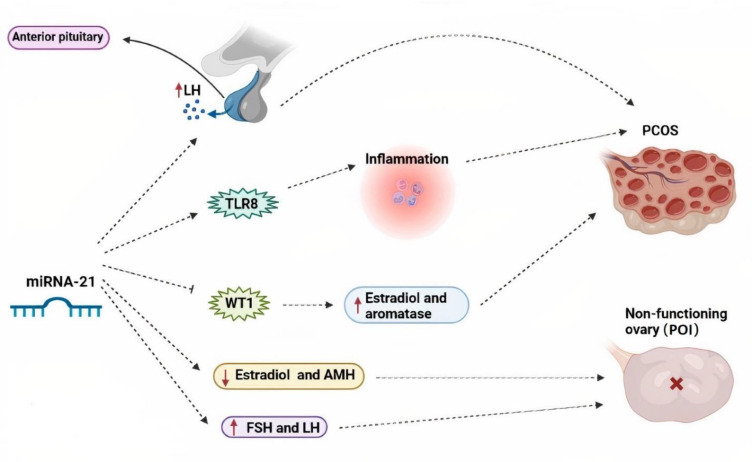
It was addressed that miR-21 expression was elevated simultaneously with an increase in LH level after, during, and before ovulation (55). Interestingly, the increment LH level, causing disruption of ovarian folliculogenesis and change in production of steroid hormones, was commonly observed in PCOS women (56). miR-21 was also upregulated in granulosa cells, blood, and follicular fluid of patients with PCOS (57). Ovarian follicular fluid was produced from theca and granulosa cells. It is known as a necessary microenvironment for oocyte development and maturation (58). According to the work performed by Yu et al. (21), miR-21 can be involved in the inflammatory events of PCOS through regulation of cell proliferation and apoptosis of granulosa cells by affecting toll-like receptor 8 (TLR8). They found that expression levels of TLR8 and miR-21 were remarkably elevated in granulosa cells of PCOS cases, in comparison with the normal granulosa cells. In this regard, miR-21 elevated mRNA translation of TLR8 and consequently enhanced release of inflammatory agents, including interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). TLR8, as a subset of the family of TLRs, is dominantly expressed in myeloid dendritic cells, macrophages, and monocytes. It has a substantial role in inflammatory reactions (59, 60). Hilker et al. (26), in another scientific endeavor, inspected miR-21-5p function in porcine granulosa cells by RT-qPCR technique. They determined that this noncoding RNA was dramatically higher in granulosa cells obtained from large antral follicles, rather than those from small antral follicles. Moreover, they revealed that miR-21-5-p curbed Wilms tumor gene (WT1) expression (Fig .1), expressed by follicular cells, via binding to the 3´-UTR sequence of WT1 in granulosa cells to potentiate estradiol synthesis and aromatase expression (26, 61). Aromatase enzyme, a converter of androgens to estradiol, was significantly stimulated in human and animal cases of PCOS (62). However, Aldakheel et al. (63) and Ren et al. (64) declared that miR-21 can be an inhibitor factor for PCOS progression through suppression of proliferation of granulosa cells by affecting SNHG7, a subclass of SMAD protein family involved in cell apoptosis and proliferation adjustment. Additionally, in this new investigation, there was a reduction in the expression levels of miR-21 in ovarian tissue samples of PCOS subjects in comparison with the normal ovarian tissue samples (63). According to the majority of documents, miR-21 dysregulation may be linked with the pathogenic occurrences of PCOS; however, more in vivo and in vitro works are offered to be carried out to express its exact role in these conditions.
Fig.1.
The possible pathogenic effects of miR-21 in the onset or progression of infertility-related disorders, like PCOS and POI. PCOS; Polycystic ovarian syndrome, POI; Premature ovarian insufficiency, FSH; Follicle-stimulating hormone, LH; Luteinizing hormone, AMH; Anti-müllerian hormone, WT1; Wilms tumor gene, and TLR8; Toll-like receptor 8.
miR-21 dysregulation has been investigated in a few studies. In the study performed by Li et al. (65) on POI patients and animal model of the disease, caused by zona pellucida glycoprotein 3 (ZP3) antigen immunization, decreased expression of this single-stranded RNA was approved. Moreover, miR-21 had direct relationship with ovarian volume, uterus size, E2 and anti-Müllerian hormone (AMH). It also had an inverse relationship with LH and FSH levels, and a number of immune indices, including anti-endometrial antibody (EMAb), anti-ovarian tissue antibody (AOAb), anti-cardiolipin antibody (ACL), anti-double-stranded DNA antibody (ds- DNA), anti-adrenal cortical antibody (ACA), anti-nuclear antibody (ANA), immunoglobulin E (IgE), IgM, IgA, IgG, serum levels of complement 3 (C3) and complement 4 (C4). Finally, these results showed that this miRNA may be related to autoimmune POI pathogenesis. In another scientific project, effects of rat mesenchymal stem cells (MSCs) transfected by miR-21 on animal models of POI were studied. In this study, POI model was induced by intraperitoneal injection of a chemotherapeutic agent, cyclophosphamide. Unlike the previous study, this research team approved that transplanting the MSCs over-expressing miR-21 had reparative impacts on POI rats by upregulating expression of the miRNA in the ovary and subsequently reducing apoptosis of granulosa cells, elevating the follicle number, ovarian weight, and E2 levels, in addition to decreasing FSH levels (66). According to these studies, it is proposed that miR-21, based on its presence in different media, may play a positive or negative role against POI; however, more and large investigations are needed to demonstrate this theory.
Dysregulative and pathogenic roles of some miRNAs in ovarian function-associated infertility have been documented. In a scientific effort, using TaqMan miRNA and Genome-wide deep sequencing assays, Sang et al. evaluated miRNA expression in human follicular fluid of PCOS cases. They observed that expression of miR-132 and miR-320 was reduced in the patient compared to the normal group. Other findings of this study implicated that miR-24, miR-132, miR-222, miR-320, and miR-520c-3p affected estradiol secretion, while miR-24, miR-483-5p, and miR- 193b influenced progesterone release in PCOS patients (67). A preliminary study explored miRNA expression profile by TaqMan RT-qPCR method on 36 PCOS women and 16 normal subjects. It was shown that circulating levels of miR-26a-5p, miR-23a-3p, miR-21-5p were upregulated, whereas miR-222-3p, miR-19b-3p, miR-376a-3p, and miR-103a-3p were downregulated in PCOS subjects rather than normal individuals. Furthermore, miR-376a-3p, miR-21-5p, and miR-103a-3p were associated with total testosterone levels (68). The actions of some microRNAs have remained a challenging issue in ovarian disorders. For example, miR-483-5p is one of the challenging miRNAs in granulosa cells (69). A scientific work revealed upregulation of miR-483-5p in the granulosa cells obtained from PCOS cases, which may reflect its role in ectopic regulation of proliferation and apoptosis in these cells by targeting Notch-3 (Notch homolog 3) gene (70). On the other hand, the other authors observed reduced expression of this miRNA in the granulosa cells of PCOS subjects which may affect insulin-like growth factor-1 (IGF-1) and subsequently potentate granulosa cell proliferation (71, 72). Concerning POI, studies have been performed by notice to both ovarian tissue and plasma samples to determine miRNAs involved in POI development. Dang and colleagues inspected expression of plasma miRNAs based on the data of the microarray platform and RT-qPCR method between women with or without POI. Eventually, this study accentuated that miR-22-3p was a protective agent for this condition and it was negatively correlated with serum levels of the FSH hormone (73). Another research team identified 20 downregulated and 63 upregulated miRNAs in the samples of ovarian tissue from the rat POI model, caused by 4-vinylcyclohexene diepoxide (VCD), than the normal group. miR-144 and miR-29a, regulators of prostaglandin secretion via affecting phospholipase A2 group IVA (PLA2G4A), were downregulated in POI tissue samples; however, several miRNAs were upregulated, such as miR-672, miR-151, miR-190, and miR-27b.a, which play roles in apoptotic process (74). Another report also demonstrated that miR-23a was upregulated in the plasma samples of POI subjects, which in turn elevated apoptosis and diminished caspase-3 and X-linked inhibitor of apoptosis protein (XIAP) levels in human granulosa cells (75). These findings revealed that abnormal function of the ovary in PCOS and POI was along with the dysregulation of many miRNAs, influencing the ovarian structure and hormone secretion (Table 1).
Table 1.
Function of miR-21 in ovarian function-related disorders, including PCOS and POI
|
| |||||
|---|---|---|---|---|---|
| Ovarian function-related diseases | Expression | Targets | Mechanisms/influences/associations | Model (human/animal) | References |
|
| |||||
| PCOS | Upregulation | TLR8 | Potentiating IL-12, TNF-α, and IFN-γ levels and granulosa cell proliferation | Human | (21) |
| PCOS | Downregulation | SNHG7 | Suppressing ovarian granulosa cell proliferation | Human | (63) |
| PCOS | Downregulation | - | Attenuating body weight and ameliorating energy expenditure | Animal | (76) |
| PCOS | Upregulation | - | Decreasing insulin resistance | Animal | (77) |
| POI | Downregulation | Positive associations with E2, AMH, ovarian volume and negative associations with LH, FSH and the number of positive immune parameters (AOAb, EMAb, ACL, ANA, ds-DNA, ACA, IgG, IgA, IgM, IgE, C3, and C4) | Animal/human | (78) | |
| POI | - | PTEN and PDCD4 | Suppressing granulosa cell apoptosis | Animal | (66) |
|
| |||||
PCOS; Polycystic ovarian syndrome, POI; Premature ovarian insufficiency, IL-12; Interleukin 12, TNF-α; Tumor necrosis factor alpha, IFN-γ; Interferon gamma, E2; Estradiol, AMH; Anti-mullerian hormone, LH; Luteinizing hormone, and FSH; Follicle-stimulating hormone.
Conclusion
It seems that miR-21 has a substantial role in processes leading to fertility, such as oocyte maturation, cumulus expansion, inhibition of cumulus and periovulatory granulosa cell apoptosis, transition from germinal vesicle to oocytes (arrested in the MII stage), blastocyst and embryo developments. However, their dysregulation may be involved in infertility-related conditions, like PCOS and POI, by different mechanisms. For example, miR-21 dysregulation by influencing TLR8, promoting secretion of inflammatory factors (e.g., IL-12, TNF-α, and IFN-γ), and inhibiting WT1 expression had a pathogenic role in PCOS. On the other hand, miR-21 malfunction exerted its negative role in POI by decreasing E2 and AMH and increasing LH and FSH levels. Thus, dysregulation of miR-21 can be associated with the pathogenic events of PCOS and POI. Despite these, multiple in vivo and in vitro investigations are required to determine pathogenic role(s) of miR-21 in these problems.
Acknowledgments
There is no financial support and conflict of interest in this study.
Author’s Contributions
R.A., H.R.-Sh.; Have made substantial contributions to Conception and Design, Acquisition of data, Analysis and Interpretation of data. H.M., F.R.T.; Have made substantial contributions to Analysis of data. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
References
- 1.Ali A, Paramanya A, Poojari P, Arslan-Acaroz D, Acaroz U, Kostić AŽ. The utilization of bee products as a holistic approach to managing polycystic ovarian syndrome-related infertility. Nutrients. 2023;15(5):1165–1165. doi: 10.3390/nu15051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafardoust S, Kazemnejad S, Darzi M, Fathi-Kazerooni M, Saffarian Z, Khalili N, et al. Intraovarian administration of autologous menstrual blood derived-mesenchymal stromal cells in women with premature ovarian failure. Arch Med Res. 2023;54(2):135–144. doi: 10.1016/j.arcmed.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Dring JC, Forma A, Chilimoniuk Z, Dobosz M, Teresiński G, Buszewicz G, et al. Essentiality of trace elements in pregnancy, fertility, and gynecologic cancers-A state-of-the-art review. Nutrients. 2021;14(1):185–185. doi: 10.3390/nu14010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emanuel RHK, Roberts J, Docherty PD, Lunt H, Campbell RE, Möller K. A review of the hormones involved in the endocrine dysfunctions of polycystic ovary syndrome and their interactions. Front Endocrinol (Lausanne) 2022;13:1017468–1017468. doi: 10.3389/fendo.2022.1017468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefagh G, Payab M, Qorbani M, Sharifi F, Sharifi Y, Ebrahimnegad Shirvani MS, et al. Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in PCOS (polycystic ovary syndrome): a systematic review and meta-analysis. Sci Rep. 2022;12(1):5770–5770. doi: 10.1038/s41598-022-09082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781–785. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 7.Fu YX, Ji J, Shan F, Li J, Hu R. Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities. Stem Cell Res Ther. 2021;12(1):161–161. doi: 10.1186/s13287-021-02212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi M, Akbari Asbagh F. Pathogenesis and causes of premature ovarian failure: an update. Int J Fertil Steril. 2011;5(2):54–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Xu JY, Fan YH, Geng JZ, Gao N, Yu FH, Xia T. Application progress of acupuncture in treatment of ovarian hypofunction. TMR Non-Drug Therapy. 2021;4(3):16–28. [Google Scholar]
- 10.Pouryousefi-Koodehi T, Shayegan S, Hashemi S, Arefnezhad R, Roghani-Shahraki H, Motedayyen H, et al. Can mesenchymal stem cells derived from adipose tissue and their conditioned medium improve ovarian functions?. A mini-review. Zygote. 2022;30(5):589–592. doi: 10.1017/S0967199422000235. [DOI] [PubMed] [Google Scholar]
- 11.Taghizabet N, Rezaei-Tazangi F, Mousavi M, Dehghani F, Zareifard N, Shabani S, et al. Endometrial cell-derived conditioned medium in combination with platelet-rich plasma promotes the development of mouse ovarian follicles. Zygote. 2023;31(1):1–7. doi: 10.1017/S096719942200020X. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi N, Manavi MS, Nazari A, Momayezi A, Faghihkhorasani F, Abdulwahid AH, et al. Nano-scale delivery systems for siRNA delivery in cancer therapy: New era of gene therapy empowered by nanotechnology. Environmental Research. 2023;4(11):36–48. doi: 10.1016/j.envres.2023.117263. [DOI] [PubMed] [Google Scholar]
- 13.Carletti MZ, Christenson LK. MicroRNA in the ovary and female reproductive tract. J Anim Sci. 2009;87(Suppl 14):E29–E38. doi: 10.2527/jas.2008-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer--a brief overview. Adv Biol Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Çakmak HA, Demir M. MicroRNA and cardiovascular diseases. Balkan Med J. 2020;37(2):60–71. doi: 10.4274/balkanmedj.galenos.2020.2020.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafi G, Aliya N, Munshi A. MicroRNA signatures in neurological disorders. Can J Neurol Sci. 2010;37(2):177–185. doi: 10.1017/s0317167100009902. [DOI] [PubMed] [Google Scholar]
- 17.Ergin K, Çetinkaya R. Regulation of microRNAs. Methods Mol Biol. 2022;2257:1–32. doi: 10.1007/978-1-0716-1170-8_1. [DOI] [PubMed] [Google Scholar]
- 18.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153(3):516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Yan G, Zhang L, Fang T, Zhang Q, Wu S, Jiang Y, et al. MicroRNA- 145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 2012;586(19):3263–3270. doi: 10.1016/j.febslet.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 20.He T, Sun Y, Zhang Y, Zhao S, Zheng Y, Hao G, et al. MicroRNA- 200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod Biol Endocrinol. 2019;17(1):68–68. doi: 10.1186/s12958-019-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Li G, He X, Lin Y, Chen Z, Lin X, et al. MicroRNA-21 regulate the cell apoptosis and cell proliferation of polycystic ovary syndrome (PCOS) granulosa cells through target toll like receptor TLR8. Bioengineered. 2021;12(1):5789–5796. doi: 10.1080/21655979.2021.1969193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldakheel FM, Abuderman AA, Alduraywish SA, Xiao Y, Guo WW. MicroRNA-21 inhibits ovarian granulosa cell proliferation by targeting SNHG7 in premature ovarian failure with polycystic ovary syndrome. J Reprod Immunol. 2021;146:103328–103328. doi: 10.1016/j.jri.2021.103328. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud EH, Fawzy A, A Elshimy RA. Serum microRNA-21 negatively relates to expression of programmed cell death-4 in patients with epithelial ovarian cancer. Asian Pac J Cancer Prev. 2018;19(1):33–38. doi: 10.22034/APJCP.2018.19.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan B, Li J. MicroRNA-21 up-regulates metalloprotease by downregulating TIMP3 during cumulus cell-oocyte complex in vitro maturation. Mol Cell Endocrinol. 2018;477:29–38. doi: 10.1016/j.mce.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Báez-Vega PM, Echevarría Vargas IM, Valiyeva F, Encarnación- Rosado J, Roman A, Flores J, et al. Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget. 2016;7(24):36321–36337. doi: 10.18632/oncotarget.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilker RE, Pan B, Zhan X, Li J. MicroRNA-21 enhances estradiol production by inhibiting WT1 expression in granulosa cells. J Mol Endocrinol. 2021;68(1):11–22. doi: 10.1530/JME-21-0162. [DOI] [PubMed] [Google Scholar]
- 27.Dehghan Z, Mohammadi-Yeganeh S, Rezaee D, Salehi M. Micro- RNA-21 is involved in oocyte maturation, blastocyst formation, and pre-implantation embryo development. Dev Biol. 2021;480:69–77. doi: 10.1016/j.ydbio.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Yang CX, Du ZQ, Wright EC, Rothschild MF, Prather RS, Ross JW. Small RNA profile of the cumulus-oocyte complex and early embryos in the pig. Biol Reprod. 2012;87(5):117–117. doi: 10.1095/biolreprod.111.096669. [DOI] [PubMed] [Google Scholar]
- 29.Bartolucci AF, Uliasz T, Peluso JJ. MicroRNA-21 as a regulator of human cumulus cell viability and its potential influence on the developmental potential of the oocyte. Biol Reprod. 2020;103(1):94–103. doi: 10.1093/biolre/ioaa058. [DOI] [PubMed] [Google Scholar]
- 30.Wright EC, Hale BJ, Yang CX, Njoka JG, Ross JW. MicroRNA-21 and PDCD4 expression during in vitro oocyte maturation in pigs. Reprod Biol Endocrinol. 2016;14:21–21. doi: 10.1186/s12958-016-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, Xue R, Yuan HJ, Wang TY, Lin J, Zhang J, et al. Micro- RNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol Reprod. 2017;96(6):1167–1180. doi: 10.1093/biolre/iox044. [DOI] [PubMed] [Google Scholar]
- 32.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83(2):286–295. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClusky LM, Barnhoorn IE, van Dyk JC, Bornman MS. Testicular apoptosis in feral Clarias gariepinus using TUNEL and cleaved caspase-3 immunohistochemistry. Ecotoxicol Environ Saf. 2008;71(1):41–46. doi: 10.1016/j.ecoenv.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Romorini L, Garate X, Neiman G, Luzzani C, Furmento VA, Guberman AS, et al. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci Rep. 2016;6:35660–35660. doi: 10.1038/srep35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83(4):549–557. doi: 10.1095/biolreprod.110.084434. [DOI] [PubMed] [Google Scholar]
- 37.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 38.Timoneda O, Balcells I, Córdoba S, Castelló A, Sánchez A. Determination of reference microRNAs for relative quantification in porcine tissues. PLoS One. 2012;7(9):e44413–e44413. doi: 10.1371/journal.pone.0044413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McBride D, Carré W, Sontakke SD, Hogg CO, Law A, Donadeu FX, et al. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction. 2012;144(2):221–233. doi: 10.1530/REP-12-0025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, He M, Zhang J, Tong Y, Chen T, Wang C, et al. Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients. Front Physiol. 2023;14:1113684–1113684. doi: 10.3389/fphys.2023.1113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian S, Zhang H, Chang HM, Klausen C, Huang HF, Jin M, et al. Activin A promotes hyaluronan production and upregulates versican expression in human granulosa cells†. Biol Reprod. 2022;107(2):458–473. doi: 10.1093/biolre/ioac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji J, Zhou Y, Li Z, Zhuang J, Ze Y, Hong F. Impairment of ovarian follicular development caused by titanium dioxide nanoparticles exposure involved in the TGF-β/BMP/Smad pathway. Environ Toxicol. 2023;38(1):185–192. doi: 10.1002/tox.23676. [DOI] [PubMed] [Google Scholar]
- 43.Yao G, Yin M, Lian J, Tian H, Liu L, Li X, et al. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24(3):540–551. doi: 10.1210/me.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreas E, Hoelker M, Neuhoff C, Tholen E, Schellander K, Tesfaye D, et al. MicroRNA 17-92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting PTEN and BMPR2 genes. Cell Tissue Res. 2016;366(1):219–230. doi: 10.1007/s00441-016-2425-7. [DOI] [PubMed] [Google Scholar]
- 45.Gebremedhn S, Salilew-Wondim D, Hoelker M, Rings F, Neuhoff C, Tholen E, et al. MicroRNA-183-96-182 cluster regulates bovine granulosa cell proliferation and cell cycle transition by coordinately targeting FOXO1. Biol Reprod. 2016;94(6):127–127. doi: 10.1095/biolreprod.115.137539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou S, Zhao A, Wu Y, Bao T, Mi Y, Zhang C. Protective effect of follicle-stimulating hormone on dna damage of chicken follicular granulosa cells by inhibiting CHK2/p53. Cells. 2022;11(8):1291–1291. doi: 10.3390/cells11081291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao H, Yang J, Xu M, Liu Z, Liu Y, Xiong Q. MicroRNA-27a-3p targeting Vangl1 and Vangl2 inhibits cell proliferation in mouse granulosa cells. Biochim Biophys Acta Gene Regul Mech. 2023;1866(1):194885–194885. doi: 10.1016/j.bbagrm.2022.194885. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Du X, Zhou J, Pan Z, Liu H, Li Q. MicroRNA-26b functions as a proapoptotic factor in porcine follicular Granulosa cells by targeting Sma-and Mad-related protein 4. Biol Reprod. 2014;91(6):146–146. doi: 10.1095/biolreprod.114.122788. [DOI] [PubMed] [Google Scholar]
- 49.Portela VM, Dirandeh E, Guerrero-Netro HM, Zamberlam G, Barreta MH, Goetten AF, et al. The role of fibroblast growth factor-18 in follicular atresia in cattle. Biol Reprod. 2015;92(1):14–14. doi: 10.1095/biolreprod.114.121376. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Fang Y, Liu Y, Yang X. MicroRNAs in ovarian function and disorders. J Ovarian Res. 2015;8:51–51. doi: 10.1186/s13048-015-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taghizabet N, Bahmanpour S, Fard NZ, Rezaei-Tazangi F, Hassanpour A, Nejad EK, et al. In vitro growth of the ovarian follicle: taking stock of advances in research. JBRA Assist Reprod. 2022;26(3):508–521. doi: 10.5935/1518-0557.20210076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirotkin AV, Ovcharenko D, Grossmann R, Lauková M, Mlyncek M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219(2):415–420. doi: 10.1002/jcp.21689. [DOI] [PubMed] [Google Scholar]
- 53.Xu S, Linher-Melville K, Yang BB, Wu D, Li J. Micro-RNA378 (miR- 378) regulates ovarian estradiol production by targeting aromatase. Endocrinology. 2011;152(10):3941–3951. doi: 10.1210/en.2011-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341(6141):71–73. doi: 10.1126/science.1237999. [DOI] [PubMed] [Google Scholar]
- 55.Tian M, Zhang X, Ye P, Tao Q, Zhang L, Ding Y, et al. MicroRNA-21 and microRNA-214 play important role in reproduction regulation during porcine estrous. Anim Sci J. 2018;89(10):1398–1405. doi: 10.1111/asj.13087. [DOI] [PubMed] [Google Scholar]
- 56.Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561–110561. doi: 10.1016/j.mce.2019.110561. [DOI] [PubMed] [Google Scholar]
- 57.Naji M, Aleyasin A, Nekoonam S, Arefian E, Mahdian R, Amidi F. Differential expression of mir-93 and mir-21 in granulosa cells and follicular fluid of polycystic ovary syndrome associating with different phenotypes. Sci Rep. 2017;7(1):14671–14671. doi: 10.1038/s41598-017-13250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sørensen AE, Wissing ML, Salö S, Englund AL, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS) Genes (Basel) 2014;5(3):684–708. doi: 10.3390/genes5030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi: 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang JP, Bowen GN, Padden C, Cerny A, Finberg RW, Newburger PE, et al. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood. 2008;112(5):2028–2034. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oréal E, Mazaud S, Picard JY, Magre S, Carré-Eusèbe D. Different patterns of anti-Müllerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn. 2002;225(3):221–232. doi: 10.1002/dvdy.10153. [DOI] [PubMed] [Google Scholar]
- 62.Bakhshalizadeh S, Amidi F, Alleyassin A, Soleimani M, Shirazi R, Shabani Nashtaei M. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst Biol Reprod Med. 2017;63(3):150–161. doi: 10.1080/19396368.2017.1296046. [DOI] [PubMed] [Google Scholar]
- 63.Aldakheel FM, Abuderman AA, Alduraywish SA, Xiao Y, Guo WW. MicroRNA-21 inhibits ovarian granulosa cell proliferation by targeting SNHG7 in premature ovarian failure with polycystic ovary syndrome. J Reprod Immunol. 2021;146:103328–103328. doi: 10.1016/j.jri.2021.103328. [DOI] [PubMed] [Google Scholar]
- 64.Ren J, Yang Y, Xue J, Xi Z, Hu L, Pan SJ, et al. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem Biophys Res Commun. 2018;496(2):712–718. doi: 10.1016/j.bbrc.2018.01.109. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Xie J, Wang Q, Cai H, Xie C, Fu X. miR-21 and pellino-1 expression profiling in autoimmune premature ovarian insufficiency. J Immunol Res. 2020;2020:3582648–3582648. doi: 10.1155/2020/3582648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu X, He Y, Wang X, Peng D, Chen X, Li X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8(1):187–187. doi: 10.1186/s13287-017-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013;98(7):3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 68.De Nardo Maffazioli G, Baracat EC, Soares JM, Carvalho KC, Maciel GAR. Evaluation of circulating microRNA profiles in Brazilian women with polycystic ovary syndrome: a preliminary study. PLoS One. 2022;17(10):e0275031–e0275031. doi: 10.1371/journal.pone.0275031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS) Gene. 2019;706:91–96. doi: 10.1016/j.gene.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 70.Xu B, Zhang YW, Tong XH, Liu YS. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol Cell Endocrinol. 2015;404:26–36. doi: 10.1016/j.mce.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 71.Shi L, Liu S, Zhao W, Shi J. miR-483-5p and miR-486-5p are downregulated in cumulus cells of metaphase II oocytes from women with polycystic ovary syndrome. Reprod Biomed Online. 2015;31(4):565–572. doi: 10.1016/j.rbmo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Xiang Y, Song Y, Li Y, Zhao D, Ma L, Tan L. miR-483 is downregulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1) Med Sci Monit. 2016;22:3383–3393. doi: 10.12659/MSM.897301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dang Y, Zhao S, Qin Y, Han T, Li W, Chen ZJ. MicroRNA-22-3p is down-regulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Steril. 2015;103(3):802–807. doi: 10.1016/j.fertnstert.2014.12.106. e1. [DOI] [PubMed] [Google Scholar]
- 74.Kuang H, Han D, Xie J, Yan Y, Li J, Ge P. Profiling of differentially expressed microRNAs in premature ovarian failure in an animal model. Gynecol Endocrinol. 2014;30(1):57–61. doi: 10.3109/09513590.2013.850659. [DOI] [PubMed] [Google Scholar]
- 75.Yang X, Zhou Y, Peng S, Wu L, Lin HY, Wang S, et al. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction. 2012;144(2):235–244. doi: 10.1530/REP-11-0371. [DOI] [PubMed] [Google Scholar]
- 76.Rezq S, Huffman AM, Syed M, Basnet J, do Carmo JM, Moak SP, et al. MicroRNA-21 modulates white adipose tissue browning and altered thermogenesis in a mouse model of polycystic ovary syndrome. J Endocr Soc. 2021;5(Suppl 1):A775–A776. [Google Scholar]
- 77.Huffman AM, Rezq S, Basnet J, Yanes Cardozo LL, Romero DG. MicroRNA-21 genetic ablation exacerbates insulin signaling dysregulation in hyperandrogenemic female mice. FASEB J. 2022;36(S1) [Google Scholar]
- 78.Li X, Xie J, Wang Q, Cai H, Xie C, Fu X. miR-21 and pellino-1 expression profiling in autoimmune premature ovarian insufficiency. J Immunol Res. 2020;2020:3582648–3582648. doi: 10.1155/2020/3582648. [DOI] [PMC free article] [PubMed] [Google Scholar]