Abstract
Dietary doses of 2,500 ppm ZnO-Zn reduced bacterial activity (ATP accumulation) in digesta from the gastrointestinal tracts of newly weaned piglets compared to that in animals receiving 100 ppm ZnO-Zn. The amounts of lactic acid bacteria (MRS counts) and lactobacilli (Rogosa counts) were reduced, whereas coliforms (MacConkey counts) and enterococci (Slanetz counts, red colonies) were more numerous in animals receiving the high ZnO dose. Based on 16S rRNA gene sequencing, the colonies on MRS were dominated by three phylotypes, tentatively identified as Lactobacillus amylovorus (OTU171), Lactobacillus reuteri (OTU173), and Streptococcus alactolyticus (OTU180). The colonies on Rogosa plates were dominated by the two Lactobacillus phylotypes only. Terminal restriction fragment length polymorphism analysis supported the observations of three phylotypes of lactic acid bacteria dominating in piglets receiving the low ZnO dose and of coliforms and enterococci dominating in piglets receiving the high ZnO dose. Dietary doses of 175 ppm CuSO4-Cu also reduced MRS and Rogosa counts of stomach contents, but for these animals, the numbers of coliforms were reduced in the cecum and the colon. The influence of ZnO on the gastrointestinal microbiota resembles the working mechanism suggested for some growth-promoting antibiotics, namely, the suppression of gram-positive commensals rather than potentially pathogenic gram-negative organisms. Reduced fermentation of digestible nutrients in the proximal part of the gastrointestinal tract may render more energy available for the host animal and contribute to the growth-promoting effect of high dietary ZnO doses. Dietary CuSO4 inhibited the coliforms and thus potential pathogens as well, but overall the observed effect of CuSO4 was limited compared to that of ZnO.
In Denmark, pig producers voluntarily abolished all use of antimicrobial growth promoters (AGPs) in pig feed starting in the year 2000. In the European Union, most AGPs have been banned and the rest will be withdrawn by the end of 2005. The main negative consequence of the Danish initiative has been compromised thriving, poorer growth performance, and an increased incidence of diarrhea among piglets within the first 1 to 2 weeks after weaning. Consequently, in order to cope with or address these problems, there is an urgent need to find and evaluate alternatives to AGPs as well as to shed light on the mechanisms of interactions between host animals, the commensal microbiota, and potential pathogens.
Zinc and copper are essential trace elements for prokaryotic and eukaryotic cellular functions, acting as components of a range of enzymes such as alkaline phosphatase and terminal oxidases, respectively (4). The basic physiological needs for these trace elements in pigs imply a need for dietary amendments, typically in the range of 50 to 100 ppm Zn and about 10 ppm Cu depending on the animal's age (30, 45). However, recent studies indicated that young piglets have more need for zinc for 1 to 2 weeks after weaning (9), and growth-promoting properties of elevated levels of dietary zinc, in particular ZnO, and copper, usually CuSO4, during the postweaning period have been reported in several studies (5, 7, 24, 41, 43, 44; H. D. Poulsen, Abstr. 40th Annu. Meet. Eur. Assoc. Anim. Prod., abstr. P5.14, 1989). The effect has been connected to improved feed intake by piglets (6, 19) as well as to an improved physiological zinc status (amount of zinc in plasma) of piglets. It is thus suggested that both additives prevent the development of a physiological zinc deficiency (8, 11, 20, 43). On the other hand, the reported effects of ZnO and CuSO4 on the gastrointestinal microbiota are sparse and have mainly been based on analyses of fecal samples. High doses of dietary ZnO have thus been shown to support a large diversity of coliforms in weaned piglets (31), to reduce the susceptibility of pigs to Escherichia coli infection (41), and to reduce fecal counts of lactobacilli and enterococci during the postweaning period, but only temporarily (6, 29). Previously, we observed a clear decrease in the total number of anaerobes and lactobacilli and an increase in the number of coliforms in ileum samples from animals fed a ZnO-amended diet (25). The use of CuSO4 in pig diets was reported to specifically reduce the number of streptococci in fecal samples (17). Furthermore, CuSO4 amendment of the diet for growing pigs reduced the number of ureolytic bacteria as a group, of which streptococci made up approximately 75% (50). The number of lactobacilli has also been shown to decrease in pigs fed copper-amended diets (26, 32), whereas coliforms remain unaffected or even tend to increase in numbers (17, 26).
The present study was conducted to examine the effects of elevated dietary doses of ZnO and CuSO4 on the composition and activity of the commensal indigenous microbiota in samples taken from segments representing the entire gastrointestinal tracts of newly weaned piglets.
MATERIALS AND METHODS
Diets.
Diets were based on a Danish standard starter feed mixture with the following composition (in grams per kilogram): 330 g of barley, 325.8 g of wheat, 170 g of dehulled toasted soybean meal, 100 g of fish meal, 50 g of animal fat, 9 g of monocalcium phosphate, 5.9 g of calcium carbonate, 4.8 g of l-lysine (40%), 2.5 g of sodium chloride, and 2 g of vitamin mixture. The vitamin mixture contained (in international units per gram or milligrams per kilogram): 2,200 IU of vitamin A, 500 IU of vitamin D3, 30,000 mg of vitamin E, 1,100 mg of vitamin K3, 1,100 mg of vitamin B1, 2,000 mg of vitamin B2, 1,650 mg of vitamin B6, 5,500 mg of d-pantothenic acid, 11,000 mg of niacin, 27.5 mg of biotin, 11 mg of vitamin B12, 25,000 mg of FeSO4 · 7H2O, 13,860 mg of MnO, 20 mg of CuSO4 · 7H2O, 99 mg of KI, and 150 mg of Na2SeO3. The diet was supplemented with the following four combinations of zinc as ZnO and copper as CuSO4 (in milligrams per kilogram or ppm): (i) 100 mg of Zn, 0 mg of Cu (low Zn, low Cu); (ii) 100 mg of Zn, 175 mg of Cu (low Zn, high Cu); (iii) 2,500 mg of Zn, 0 mg of Cu (high Zn, low Cu); and (iv) 2,500 mg of Zn, 175 mg of Cu (high Zn, high Cu).
Chemical analysis of feed components.
The chemical composition of the feed was analyzed by standard methods for determining the contents of zinc and copper (43).
Animals.
The study included 32 Danish Landrace × Yorkshire piglets (eight sets of four littermates) separated into four groups of eight animals each (one littermate per group). The animals were weaned at the age of 28 days (live body weight, approximately 8 kg) and thereafter housed individually with free access to water. The pigs were fed the experimental diets ad libitum, but the light was turned off from 10 p.m. to 7 a.m. Feed intake and body weights were recorded on day 7 and day 14 postweaning. On day 14 after weaning, the animals were sacrificed at 10 a.m. (3 h after the light was turned on) by an intraperitoneal overdose of sodium pentobarbital. At slaughter, the live body weight of each animal was approximately 10 kg.
Sample handling.
Gastrointestinal tracts were immediately removed from the body cavities of the slaughtered piglets and divided into the following eight segments: the stomach, three segments of the small intestine of equal lengths, the cecum, and three segments of the colon, including the rectum, of equal lengths. All digesta were immediately removed from the segments for determinations of the total content and for further processing under anoxic conditions. The dry matter content was determined by freeze-drying of subsamples. The majority of the response parameters were analyzed only for digesta from the stomach (St), the distal segment of the small intestine (Si3), the cecum (Cae), and the mid-segment of the colon (Co2).
Enumeration of bacteria and yeasts.
Digesta samples (approximately 10 g) were immediately transferred to infusion bottles containing 90 ml of a prereduced salt broth (22). The suspensions were transferred to sterile, CO2-flushed stomacher bags and homogenized for 2 min in a stomacher blender (Seward Medical, London, United Kingdom). Serial dilutions were made anaerobically in prereduced salt medium by the technique of Miller and Wolin (40). Total counts of anaerobic bacteria were determined on rumen fluid-glucose-cellobiose (RFGC) agar by the anaerobic roll tube technique, replacing the headspace air with CO2 and incubating at 38°C for 7 days (23, 40). Lactic acid bacteria were quantified by spread plating on deMan, Rogosa, and Sharp (MRS) agar (Merck) and incubation in anaerobic jars (Anaerocult A; Merck) at 38°C for 2 days. Anaerocult A chemically binds oxygen and generates equimolar amounts of CO2 within the first hour of incubation. Lactobacilli were selectively cultured on Rogosa (Merck) agar (38°C, anaerobic jars, 2 days), coliform bacteria were cultured on McConkey (Merck) agar (38°C, aerobic growth, 1 day), and yeasts were cultured on malt extract-chloramphenicol (MC) agar (38°C, aerobic growth, 2 days). The MC agar contained (per liter) yeast extract (3 g; Merck), malt extract (3 g; Merck), peptone from meat peptic digest (5 g; Merck), d(+)=glucose (10 g; Merck), and agar-agar (15 g; Merck); after autoclaving, 10 ml of 0.5% chloramphenicol in 96% ethanol was added. Enterococci were enumerated as dark red colonies appearing on Slanetz-Barkley (Merck) agar (38°C, aerobic growth, 2 days).
Identification of culturable bacteria.
Two experiments were performed to identify the bacteria forming colonies on RFGC, MRS, and Rogosa agar. In both cases, fecal samples were taken directly from the rectums of animals fed the control diet. The samples were handled anaerobically as described above. For the first experiment, the rectal samples were incubated in parallel on RFGC agar (anaerobic roll tube technique) and MRS agar plates (anaerobic jars). For the second experiment, the rectal samples were spread plated on MRS and Rogosa agar. The tubes and plates were incubated as described above. Bacterial cells were then picked randomly from colonies growing in the tubes and on the agar plates and were cultured in rumen fluid-glucose-cellobiose broth. The purity of the cultures was evaluated by microscopy. Chromosomal DNAs were extracted from the cultures, and partial sequencing (approximately 1,450 bp) of the 16S rRNA gene was performed basically as described by Mikkelsen et al. (39), using the forward primer 5′-AGRGTTTGATYMTGGCTCAG and the reverse primer 5′-GGYTACCTTGTTACGACTT. These primers were modified versions of the S-D-Bact-0008-a-S-20 and S-*-Univ-1510-a-A-19 primers used by Leser et al. (36), degenerated for better targeting of lactic acid bacteria. The 16S rRNA gene sequences were finally compared to GenBank sequences by use of the NCBI BLAST tool. The isolates were tentatively identified by the best matching sequences of bacterial strains as well as of clones from the porcine bacterial clone library of Leser et al. (36). The latter are referred to as operational taxonomic units (OTUs).
T-RFLP analysis.
Frozen digesta samples (2 g) were thawed, added to 18 ml of phosphate-buffered saline (pH 7.2), and homogenized for 2 min with a stomacher lab blender (Interscience, St. Nom, France). The supernatants (0.5 ml) were transferred to sterile Eppendorf tubes, 5 μl of lysozyme (10 mg/ml) was added, and the mixtures were incubated at 37°C for 30 min. Five microliters each of proteinase K and RNase (10 mg/ml) was then added to each suspension, the mixtures were vortexed and incubated at 65°C for 1 h, and subsequently, 50 μl of 24% sodium dodecyl sulfate was added and the suspension was vortexed and incubated at 65°C for another 10 min. The samples were transferred to 2-ml bead beater vials containing 0.5 g of glass beads (0.18-mm diameter), and lysis of the bacterial cells was completed by shaking the samples for 2 min in a minibead beater (Biospec Products Inc., Bartlesville, Okla.). After centrifugation (5,500 × g for 10 min), the supernatant (550 μl) was transferred to Geneclean Turbo purification columns (Qbiogene, Inc., Carlsbad, Calif.), and the DNA was extracted according to the manufacturer's protocol. PCRs were performed on a Hybaid PCR Express apparatus. The reaction mixtures (50 μl) contained 0.2 μM of each primer (DNA Technology A/S, Aarhus, Denmark), 0.2 mM of each deoxynucleoside triphosphate (BioLabs, Inc.), 1.0 U DyNAzyme II DNA polymerase supplied with 10× PCR buffer (Finnzymes OY, Finland), and approximately 50 ng of template DNA. Amplification was performed under the following conditions: 1 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 57°C, and 45 s at 72°C; and 10 min at 72°C. The 16S rRNA genes were amplified by use of the 6-carboxyfluorescein-labeled forward primer S-D-Bact-0008-a-S-20 (5′-6-FAM-AGAGTTTGATCMTGGCTCAG) and the reverse primer PH1522 (5′-AAGGAGGTGATCCAGCCGCA). Four replicate PCRs in 50-μl reaction mixtures were performed for each sample. The PCR products of the replicates were pooled, purified with a QIAquick PCR purification kit (QIAGEN GmbH, Hilden, Germany), and eluted in a final volume of 50 μl. The purified PCR products were verified by electrophoresis on 1% agarose gels, and samples (10 μl) were digested overnight at 37°C with 20 U of the restriction endonuclease HhaI (New England BioLabs) in 20-μl reaction mixtures. The fluorescently labeled terminal restriction fragments (T-RFs) were analyzed by electrophoresis in an automatic sequence analyzer (ABI PRISM 377; PE Biosystems) using the GeneScan mode. The T-RF samples (2 μl) were mixed with 2.5 μl of loading buffer and 0.5 μl of a standard size marker. Determinations of the sizes of T-RFs in the range of 50 to 700 bp were performed with the size standard GS-1000 ROX (PE Biosystems). Before loading of the T-RFs onto a denaturing polyacrylamide gel, the T-RF mixtures were denatured at 95°C for 2 min. The electrophoresis settings were 3,000 V and 60 mA for 7 h with filter set F, and after electrophoresis, the lengths of the T-RFs were determined by using GeneScan software (PE Biosystems). The peaks of the T-RF length polymorphism (T-RFLP) electropherograms were compared to those for T-RFs obtained by in silico digestion of 16S rRNA gene sequences of GenBank strains as well as those for sequences obtained from our own culture collection of approximately 130 different porcine gastrointestinal bacterial strains.
ATP, lactate, and SCFA.
For ATP analysis, digesta samples (approximately 5 g) were extracted with 10 ml PCA-EDTA (2 M cold perchloric acid in 10 mM EDTA) and stored at −80°C until further analysis. The content of ATP in the digesta was quantified by the luciferin-luciferase method of Jensen and Jørgensen (27). The concentrations of lactate, succinate, and short-chain fatty acids (SCFA; defined here as acetate, butyrate, isobutyrate, propionate, valerate, and isovalerate) were determined by gas chromatography (GC)-flame ionization detection analysis as described by Jensen et al. (28).
Potential urease activity.
Potential urease activity was measured as the in vitro production rate of ammonia in agitated digesta suspensions supplemented with urea. Assuming that there is no substrate limitation, the determined ammonia production rate is therefore considered proportional to the urease concentration of the sample. In short, 2.5 g digesta was suspended in 97.5 ml urease test buffer (0.12 M NaCl, 0.02 M KH2PO4) and mixed in a stomacher for 2 min. The supernatant (40 ml) was transferred to a 120-ml infusion bottle. The samples were incubated with agitation in a water bath at 37°C for 5 min, and 1 ml of 40% (wt/vol) urea was then added to each bottle. One-milliliter samples were retrieved from the assay suspension after 0, 50, and 100 min, transferred to Eppendorf tubes, and heated at 90°C for 5 min to stop the reaction. The samples were then stored at −18°C for later analysis of the ammonia content. Ammonia was measured photochemically by a reaction with salicylate and free chlorine in the presence of sodium nitroprusside as a catalyst by a modification of the method described by Verdouw et al. (51). In short, 0.5 ml of assay suspension was mixed with 0.4 ml sodium salicylate, potassium sodium tartrate, trisodium citrate solution (0.62 M C7H5NaO3, 35.4 mM C4H4KNaO6, 0.34 M C6H5Na3O7), 0.08 ml sodium hypochlorite solution (2.25% NaClO, 1.13 M NaOH), and 0.08 ml sodium nitroprusside solution {1.68 mM Na2[Fe(CN)5NO]} in a microcuvette. The cuvettes were sealed with Parafilm, mixed, and incubated at room temperature for 45 min. The absorbance at 650 nm was read on a spectrophotometer (LKB Ultraspec II).
Data presentation.
Unless otherwise stated, all results are expressed on the basis of digesta wet weight rather than dry weight to illustrate actual in situ concentrations of, e.g., lactic acid and SCFA in the gastrointestinal tract. However, normalizing the results according to the dry matter content of the digesta did not change the patterns observed in the present study (data not shown).
Statistical analysis.
The effects of ZnO and CuSO4 on the various parameters measured in various segments of the gastrointestinal tract were analyzed by the general linear model procedure of SAS (46). The following model was used:
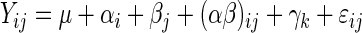 |
where Yij is the dependent variable, μ is the overall mean, αi is the effect of copper (i = 1, 2), βj is the effect of zinc (j = 1, 2), (αβ)ij is the effect of the interaction between copper and zinc, γj is the effect of the litter (j = 1, …, 8), and ɛ [∼(0,σ2ɛ)] represents the unexplained random error.
Analyses were performed with SAS for Windows, version 6.12 (SAS Institute, Cary, N.C.). When the interaction between ZnO and CuSO4 was significant, differences between means were compared by Tukey's least significant difference. A significant difference was declared for P values of <0.05. The data are presented as least significant means (LSMeans) ± standard deviations, and the number of replicates is stated for each set of data.
RESULTS
Diet composition.
Chemical analyses showed that the four diets contained (mean values ± standard deviations; n = 6) 170 ± 29, 178 ± 29, 2,550 ± 423, and 2,610 ± 239 mg of Zn kg−1 dry weight and 31 ± 3, 191 ± 44, 33 ± 3, and 190 ± 25 mg of Cu kg−1 dry weight. The concentrations reflect the contributions of the intrinsic pools of the basic diet (approximately 60 mg of Zn kg−1 and 30 mg of Cu kg−1) and the specific supplements of the two metals. In the text, we will refer to the two concentration levels of zinc and copper as low or high when presenting and discussing the data.
Animal performance.
Feed intake and daily weight gains (daily gain) were numerically higher for piglets fed the high level of dietary zinc oxide (feed intake, 3.90 versus 3.59 kg; daily gain, 169 versus 156 g). Feed intake and daily gain were numerically lower for piglets fed the high level of dietary copper than those fed the low level (feed intake, 3.64 versus 3.86 kg; daily gain, 156 versus 170 g). However, the observed effects on feed intake and daily gain were not statistically significant in any case. Diarrhea was observed temporarily for four of the included piglets, including one piglet on diet i, one on diet ii, and two on diet iv. The included animals were part of a larger growth performance experiment that will be described and reported separately.
Digesta content.
The total content of the digesta was higher for animals receiving the high zinc oxide dose, but statistically significant differences were only observed for the distal small intestine and the cecum (P ≤ 0.02) (Table 1). The addition of CuSO4 had no significant influence on the digesta content (P ≥ 0.11).
TABLE 1.
Physicochemical characteristics of digesta in the gastrointestinal tracts of piglets fed experimental diets for 2 weeks
| Characteristic or segment of gastrointestinal tract | Value with indicated treatmenta
|
SEM | Effect of treatment (P value)
|
Interaction (P value) of Zn and Cu | ||||
|---|---|---|---|---|---|---|---|---|
| 100 ppm Zn
|
2,500 ppm Zn
|
|||||||
| 0 ppm Cu | 175 ppm Cu | 0 ppm Cu | 175 ppm Cu | Zn | Cu | |||
| Digesta contents (g [wet wt]) | ||||||||
| Stomach | 332 | 348 | 392 | 306 | 41 | 0.83 | 0.41 | 0.24 |
| Distal small intestine | 260b | 272b | 475c | 317b | 30 | <0.001 | 0.03 | 0.01 |
| Cecum | 82 | 74 | 123 | 92 | 11 | 0.02 | 0.11 | 0.34 |
| Mid-colon | 163 | 167 | 218 | 196 | 22 | 0.09 | 0.70 | 0.57 |
| Dry matter (% of wet wt) | ||||||||
| Stomach | 27.4 | 25.1 | 21.7 | 18.9 | 1.9 | <0.001 | 0.21 | 0.89 |
| Distal small intestine | 14.0b | 10.4c | 11.9b,c | 14.4b | 1.1 | 0.38 | 0.63 | 0.01 |
| Cecum | 10.1 | 10.7 | 15.5 | 14.8 | 0.9 | <0.0001 | 0.91 | 0.51 |
| Mid-colon | 16.5 | 16.1 | 21.9 | 19.4 | 1.9 | 0.04 | 0.45 | 0.60 |
| pH | ||||||||
| Stomach | 3.7 | 3.8 | 3.9 | 4.0 | 0.3 | 0.54 | 0.73 | 0.86 |
| Distal small intestine | 6.7 | 6.8 | 7.5 | 7.3 | 0.1 | <0.0001 | 0.90 | 0.25 |
| Cecum | 5.8 | 5.8 | 6.3 | 6.2 | 0.1 | <0.01 | 0.81 | 0.53 |
| Mid-colon | 6.6 | 6.5 | 6.4 | 6.5 | 0.1 | 0.48 | 0.92 | 0.55 |
Data are presented as LSMeans (n = 8). For the control group (100 ppm Zn, 0 ppm Cu), n = 7. In cases of interactions between treatments (PZn×Cu < 0.05), significant differences (P < 0.05) between means in the same row are indicated by different superscripts (b or c).
Physicochemical characteristics of digesta.
The dry matter (DM) content of the stomach digesta decreased significantly (P < 0.01), from approximately 25% to 20%, in animals receiving the high dose of ZnO compared to the control group (Table 1). In the distal parts of the gut, on the other hand, the high ZnO dose resulted in a significant increase in DM, from 10% to 15% in the cecum (P < 0.0001) and from 15% to 20% in the colon (P = 0.04). The addition of CuSO4 to the feed had no significant influence on the DM content in any part of the gastrointestinal tract (P ≥ 0.21).
pH.
A significant increase in the pH of the digesta was observed for the ileums (P < 0.0001) and ceca (P ≤ 0.01) of the animals receiving the high dose of ZnO, whereas no differences were observed for the stomach (P = 0.54) and colon (P = 0.48) (Table 1). No differences in pH were observed after the addition of CuSO4 to the feed (P ≥ 0.73).
Counts of bacteria and yeast.
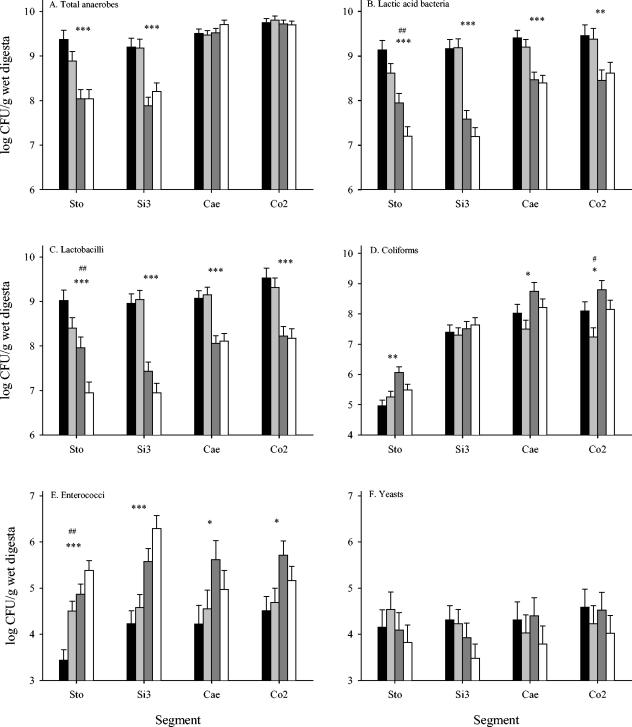
An approximately 10-fold reduction in the total counts of anaerobes was observed for the stomachs and ileums (P < 0.0001) of the animals receiving the high dose of ZnO, while no effect was observed for the cecum and colon (P ≥ 0.22) (Fig. 1A). The addition of CuSO4 to the feed had no significant effect on the total counts of anaerobes in any of the gut segments (P ≥ 0.28). Feeding animals the high dose of ZnO reduced the MRS counts (lactic acid bacteria) (P < 0.01) and Rogosa counts (lactobacilli) (P < 0.0001) for all segments of the gastrointestinal tract (Fig. 1B and C, respectively). A significant reduction in the number of both lactic acid bacteria (P < 0.01) and lactobacilli (P < 0.01) was also observed for the stomachs of animals receiving CuSO4. Thus, for the stomach, we observed an additive effect of a combined amendment of CuSO4 and a high dose of ZnO on the numbers of lactic acid bacteria and lactobacilli. The numbers of coliform bacteria in the stomach, cecum, and colon were significantly higher (P ≤ 0.02) for pigs receiving the high ZnO dose, whereas no effect of Zn (P = 0.36) was observed for the ileum (Fig. 1D). The addition of CuSO4 to the feed reduced the numbers of coliform bacteria in the cecum and the colon, but the reduction was only significant for the latter segment (P = 0.09 and P = 0.02, respectively). The enterococci followed somewhat the same patterns as the coliforms (Fig. 1E). Thus, the high ZnO dose resulted in significantly higher levels of enterococci throughout the gastrointestinal tract. In the stomach, significantly larger numbers of enterococci (P < 0.01) were found for piglets receiving the high CuSO4 dose. Counts of yeast ranged between 3.5 and 4.6 (log CFU per g digesta) and were not significantly affected by the ZnO or CuSO4 amendment in any segment of the gastrointestinal tract (Fig. 1F).
FIG. 1.
Counts of selected groups of bacteria and yeast (as indicated in each graph), determined as CFU in digesta from four segments of the gastrointestinal tracts of pigs. The pigs were fed diets supplemented with the following combinations of Zn as ZnO and Cu as CuSO4: 100 ppm Zn, 0 ppm Cu (black bars); 100 ppm Zn, 175 ppm Cu (light gray bars); 2,500 ppm Zn, 0 ppm Cu (dark gray bars); and 2,500 ppm Zn, 175 ppm Cu (white bars). The results are presented as LSMeans ± standard errors of the means (n = 8). The levels of significance of main effects were as follows: *, PZn < 0.05; **, PZn < 0.01; ***, PZn < 0.001; #, PCu < 0.05; ##, PCu < 0.01.
Characterization of bacteria growing on selective media.
Based on 16S rRNA gene sequencing and comparisons with GenBank strains and porcine bacterial clones (36), tentative identifications (based on the most sequence similarity) of the bacteria growing on rumen fluid-glucose-cellobiose agar in Hungate tubes, on MRS agar, and on Rogosa agar were performed with two experiments (Table 2). The first experiment clearly showed that a diverse population of bacteria was capable of growing on RFGC agar and that the 100 isolates analyzed covered at least 40 different phylotypes, but 17% of the isolates were identified as Streptococcus alactolyticus (OTU180). On the other hand, the 98 isolates obtained from the MRS agar plates (MRS isolates) were covered almost entirely by six phylotypes of lactic acid bacteria (67% lactobacilli, 29% streptococci, and 1% enterococci), although three colonies of staphylococci were obtained as well. Three phylotypes alone, tentatively identified as Lactobacillus amylovorus (OTU171), Lactobacillus reuteri (OTU173), and Streptococcus alactolyticus (OTU180), covered 93% of the MRS isolates. Strikingly, however, the most dominant phylotype among the MRS isolates, Lactobacillus amylovorus (OTU171), was not recovered from and thus apparently not capable of growing on RFGC agar. In the second experiment, we characterized totals of 58 and 62 isolates from MRS and Rogosa agar, respectively. Overall, the MRS isolates showed a similar picture as that obtained with the first experiment and were again covered by six phylotypes of lactic acid bacteria. Although the six phylotypes were not all identical to those from the first experiment, the three dominant types were again L. amylovorus (OTU171), L. reuteri (OTU173), and S. alactolyticus (OTU180). The isolates obtained from Rogosa agar were clearly dominated by lactobacilli, with 94% of the isolates being distributed among only four Lactobacillus species. However, two isolates of enterococci and two isolates of staphylococci were found as well. More importantly, however, isolates of the otherwise dominating phylotype Streptococcus alactolyticus were not recovered from Rogosa agar plates. The Rogosa medium thus seems suitable for the selective growth of lactobacilli from digesta and fecal samples of pigs and has also been used for this purpose by others (49).
TABLE 2.
Tentative identities (16S rRNA gene similarities) and frequencies of isolates randomly picked from colonies appearing on RFGC, MRS, or Rogosa agar after incubation of rectal samples from piglets fed control diets
| Best 16S rRNA gene GenBank matcha (species or clone) | Corresponding porcine cloneb | T-RFc (bp) | Frequency (%) on indicated medium
|
|||
|---|---|---|---|---|---|---|
| Expt 1d
|
Expt 2e
|
|||||
| RFGC | MRS | MRS | Rogosa | |||
| Lactobacillus amylovorus (AY944408) | OTU171 | 596 | 42 | 62 | 53 | |
| Lactobacillus reuteri (X76328) | OTU173 | 406 | 3 | 22 | 14 | 31 |
| Streptococcus alactolyticus (AJ201899) | OTU180 | 581 | 17 | 29 | 10 | |
| Lactobacillus ruminis (M58828) | OTU329 | 1,123 | 3 | 9 | 8 | |
| Lactobacillus mucosae (AF126738) | OTU174 | 267 | 2 | 3 | 2 | |
| Megasphaera elsdenii (U95027) | OTU196 | 591 | 7 | |||
| Mitsuokella multiacida (X81878) | OTU191 | 390 | 7 | |||
| Catenibacterium mitsuokai (AB030221) | OTU376 | 211 | 6 | |||
| Mitsuokella jalaludinii (AF479674) | OTU191 | 390 | 6 | |||
| Mitsuokella multiacida (X81878) | OTU191 | 390 | 5 | |||
| Enterococcus gallinarum (AJ301833) | OTU328 | 218 | 3 | |||
| Lactobacillus vitulinus (M23727) | OTU046 | 75 | 3 | |||
| Roseburia intestinalis (AJ312385) | OTU048 | 190 | 3 | |||
| Staphylococcus epidermis (AY030340) | OTU181 | 238 | 3 | |||
| Staphylococcus hominis (L37601) | OTU181 | 239 | 3 | |||
| Bifidobacterium boum (D86190) | OTU325 | 181 | 2 | |||
| Clostridium celerecrescens (AJ295659) | OTU75 | 190 | 2 | |||
| Enterococcus hirae (AJ420799) | OTU178 | 217 | 1 | 1 | ||
| Eubacterium formicigenerans (L34619) | OTU89 | 1,091 | 2 | |||
| Lactobacillus acidophilus johnsonii (AJ002575) | OTU170 | 1,131 | 2 | |||
| mpn-isolate group 2 (AY028442) | OTU022 | 102 | 2 | |||
| Prevotella bryantii (AJ006457) | OTU016 | 102 | 2 | |||
| Ruminococcus obeum (X85101) | OTU83 | 189 | 2 | |||
| Ruminococcus torques (L76604) | OTU88 | 1,089 | 2 | |||
| UB adh43 (AF132272)f | OTU16 | 104 | 2 | |||
| UB HuCB5 (AJ408989)f | OTU184 | 37 | 2 | |||
| URB 4C3d-9 (AB034082)f | OTU265 | 582 | 2 | |||
| Faecalibacterium prausnitzii (AJ413954) | OTU107 | 37 | 1 | |||
| Lactobacillus delbreuckii (AY050172) | OTU172 | 222 | 1 | |||
| Staphylococcus warneri (L37603) | OTU181 | 237 | 1 | |||
| Uncultured bacterium clonesg | 17 | |||||
The bacterial species and clones are listed together with their GenBank accession numbers. The 16S rRNA gene similarities between isolates and the listed species/clones were higher than 97% unless otherwise stated. Hence, the use of bold characters indicates that the best GenBank match showed <97% similarity.
Operational taxonomic units (GenBank accession numbers AF371468 to AF371949) from the porcine bacterium clone library of Leser et al. (36).
Length of 16S rRNA gene terminal restriction fragments after in silico digestion of GenBank sequences with HhaI, using E. coli position 8 as the 5′ primer start.
Frequencies are based on 100 and 98 colonies picked from rumen agar tubes and MRS agar plates, respectively.
Frequencies are based on 58 and 62 colonies picked from MRS and Rogosa agar plates, respectively.
UB, uncultured bacterium; URB, uncultured rumen bacterium.
Single isolates matching (>97% similarity) different uncultured bacterial clones.
For the present study, we did not isolate and characterize bacteria from colonies growing on Slanetz-Bartley agar plates. However, earlier observations showed that lactobacilli identified as Lactobacillus acidophilus, L. amylovorus, and L. reuteri as well as isolates identified as S. alactolyticus grew on Slanetz-Bartley medium when digesta samples from pigs were incubated in this medium (data not shown). All of these bacteria formed white colonies. Colonies of enterococci, on the other hand, showed a range of coloration from dark red (typically Enterococcus faecalis) to light red (typically Enterococcus faecium) due to reduction of the included 2,3,5-triphenyl tetrazolium chloride to a formazan compound (15, 47). In order to avoid false-positive results, we chose for the present study to count only the dark red colonies as indicators of enterococci. Slanetz-Bartley agar was in this way believed to be applicable to semiselective culturing of enterococci in digesta and feces from pigs, as also anticipated by others (1, 2).
T-RFLP analysis.
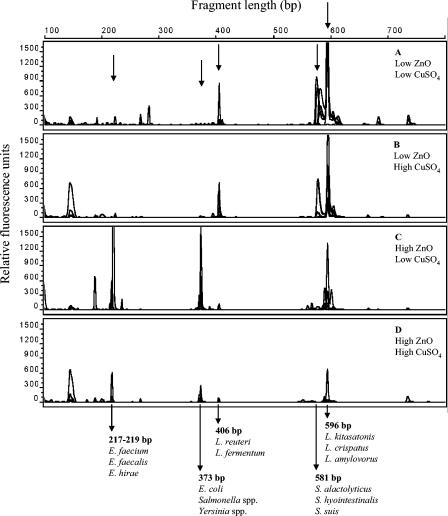
Our T-RFLP analysis of digesta samples showed patterns dominated by relatively few major peaks or T-RFs (Fig. 2). Comparisons by in silico digestion of 16S rRNA sequences of strains from GenBank as well as of strains from a local porcine bacterial culture collection resulted in tentative identifications of some of the dominating T-RFs. Thus, T-RFs of 217 to 219 bp in our analysis uniquely represented enterococci such as E. faecium, Enterococcus hirae, and E. faecalis; a T-RF of 373 bp represented strains of Escherichia, Salmonella, and Yersinia; a T-RF of 406 bp represented two species of Lactobacillus, namely, L. reuteri and L. fermentum; and a T-RF of 581 bp represented streptococci such as S. alactolyticus, Streptococcus hyointestinalis, and Streptococcus suis. T-RFs of 594 to 604 bp represented, in our analysis, at least 12 species of lactobacilli, including L. amylovorus and Lactobacillus crispatus (596 bp). The T-RFLP patterns of cecum samples indicated that samples from animals receiving the low ZnO dose were dominated by T-RFs representing lactic acid bacteria such as L. reuteri, L. amylovorus, and S. alactolyticus (Fig. 2A and B). These T-RFs were clearly less dominant in digesta samples from animals receiving the high ZnO dose, for which peaks representing enterococci and other organisms, e.g., E. coli, showed up instead (Fig. 2C and D).
FIG. 2.
T-RFLP profiles obtained from DNAs extracted from cecum digesta. The panels represent the four dietary treatments. (A) 100 ppm Zn, 0 ppm Cu; (B) 100 ppm Zn, 175 ppm Cu; (C) 2,500 ppm Zn, 0 ppm Cu; (D) 2,500 ppm Zn, 175 ppm Cu. Each panel consists of an overlay of the profiles obtained from six individual animals. Sizes in bp and tentative identifications of selected fragments are indicated.
ATP, lactate, succinate, and short-chain fatty acids.
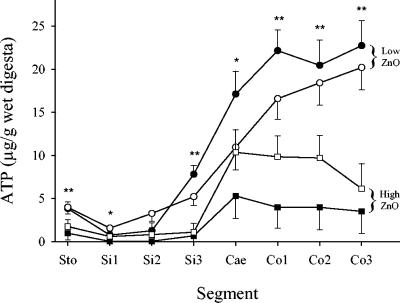
Significantly lower concentrations of ATP (P ≤ 0.03) were measured in digesta from seven of eight segments of the gastrointestinal tracts of pigs receiving the high ZnO dose (Fig. 3, squares) than in those from pigs receiving the low ZnO dose (Fig. 3, circles). The ATP levels in digesta from animals receiving CuSO4-amended diets (Fig. 1, white bars) did not differ significantly (P ≥ 0.07) from those in digesta from animals receiving diets without CuSO4 amendment (Fig. 3, closed symbols).
FIG. 3.
Concentrations of ATP in digesta from eight segments of the gastrointestinal tracts of pigs fed diets supplemented with the following combinations of Zn as ZnO and Cu as CuSO4: 100 ppm Zn, 0 ppm Cu (•); 100 ppm Zn, 175 ppm Cu (○); 2,500 ppm Zn, 0 ppm Cu (▪); 2,500 ppm Zn, 175 ppm Cu (□). The results are presented as LSMeans ± standard errors of the means (n = 6). The levels of significance of main effects were as follows: *, PZn < 0.05; **, PZn < 0.01. There was no significant effect of Cu (PCu ≥ 0.07).
The concentrations of bacterially produced short-chain fatty acids were significantly reduced in all segments of the gastrointestinal tracts (P ≤ 0.03) of animals receiving the high ZnO dose (Table 3). The addition of CuSO4 to the feed had no significant effect on the SCFA content of the digesta (P ≥ 0.11). Concentrations of lactate and succinate in digesta were also reduced significantly in the stomachs and distal small intestines of pigs fed the high ZnO dose (P ≤ 0.05). However, in the cecum and colon, where lactate and succinate are usually not detectable, a significant (P ≤ 0.02) accumulation of both acids was observed for the animals receiving the high ZnO dose. The addition of CuSO4 to the feed had no significant (P ≥ 0.13) effect on lactate and succinate accumulation in the digesta, but it counteracted the effect of ZnO in ceca and colons of piglets fed the high ZnO dose.
TABLE 3.
Response parameters of microbial activity along the gastrointestinal tracts of piglets fed experimental diets for 2 weeks
| Parameter or segment of gastrointestinal tract | Value for indicated treatmenta
|
SEM | Effect of treatment (P value)
|
Interaction (P value) of Zn and Cu | ||||
|---|---|---|---|---|---|---|---|---|
| 100 ppm Zn
|
2,500 ppm Zn
|
|||||||
| 0 ppm Cu | 175 ppm Cu | 0 ppm Cu | 175 ppm Cu | Zn | Cu | |||
| SCFA concn (μmol/g wet digesta) | ||||||||
| Stomach | 31.1 | 18.3 | 10.4 | 8.1 | 4.4 | <0.01 | 0.11 | 0.25 |
| Distal small intestine | 11.2 | 8.5 | 6.5 | 7.5 | 1.2 | 0.03 | 0.47 | 0.14 |
| Cecum | 134.9 | 124.3 | 57.4 | 100.0 | 14.4 | <0.01 | 0.29 | 0.09 |
| Mid-colon | 121.0 | 123.8 | 57.0 | 88.3 | 15.1 | <0.01 | 0.28 | 0.37 |
| Lactic acid concn (μmol/g wet digesta) | ||||||||
| Stomach | 40.5 | 50.4 | 19.5 | 16.2 | 6.5 | <0.001 | 0.63 | 0.34 |
| Distal small intestine | 51.1 | 60.1 | 7.8 | 14.8 | 5.6 | <0.0001 | 0.18 | 0.87 |
| Cecum | 2.8 | 5.0 | 33.8 | 10.0 | 6.7 | 0.02 | 0.13 | 0.07 |
| Mid-colon | 0.3 | 0.4 | 19.9 | 8.3 | 4.5 | <0.01 | 0.23 | 0.23 |
| Succinic acid concn (μmol/g wet digesta) | ||||||||
| Stomach | 2.1b,c | 3.5b | 2.2b,c | 1.2c | 0.5 | 0.05 | 0.70 | 0.04 |
| Distal small intestine | 1.2 | 1.1 | 0.3 | 0.5 | 0.4 | 0.05 | 0.94 | 0.69 |
| Cecum | 0.0 | 0.0 | 7.2 | 2.9 | 1.6 | <0.01 | 0.20 | 0.19 |
| Mid-colon | 0.5 | 0.0 | 8.0 | 3.3 | 1.9 | 0.01 | 0.21 | 0.30 |
| Urease activity (μmol NH4+/g wet digesta/h) | ||||||||
| Stomach | ND | ND | ND | ND | ||||
| Distal small intestine | ND | ND | ND | ND | ||||
| Cecum | 376 | 294 | 170 | 186 | 82 | 0.11 | 0.72 | 0.60 |
| Mid-colon | 878 | 1,021 | 279 | 399 | 149 | <0.01 | 0.43 | 0.95 |
Data are presented as LSMeans (n = 8). For the control group (100 ppm Zn, 0 ppm Cu), n = 7. ND, not detectable. In cases of interactions between treatments (PZn×Cu < 0.05), significant differences (P < 0.05) between means of the same row are indicated by different superscripts (b or c).
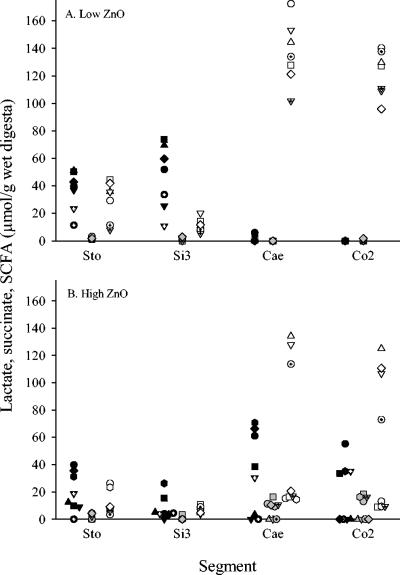
A data plot of lactate, succinate, and SCFA concentrations for individual animals illustrates that lactate and succinate never accumulated to any significant degree in ceca and colons of animals receiving the low ZnO dose (Fig. 4A). Furthermore, for all animals receiving the low dose of ZnO, SCFA always reached levels of 100 μmol/g digesta or more in the cecum and colon, as illustrated in Fig. 4A. Figure 3 only includes data for animals fed diets without the CuSO4 addition, but the pattern was identical for animals receiving the high dietary dose of CuSO4. The data set further shows that for the pigs receiving the high ZnO dose, high lactate and succinate accumulation levels were closely linked to a low level of SCFA accumulation in the cecum and the colon (Fig. 4B). Thus, for four animals from this group (Fig. 4B, circles, squares, dotted triangles, and hexagons), the latter pattern was observed for both the cecum and the colon, whereas for one animal (diamonds), it was observed for the cecum only. For three of the animals receiving the high ZnO dose (Fig. 4B, triangles, inverted triangles, and dotted circles), lactate and succinate accumulation was not observed in either the cecum or the colon and SCFA accumulated to high levels similar to those observed for the control group. However, compared to the control group, very low levels of lactate accumulation were observed in the stomachs and especially the ileums of these animals. The close linking of high lactate and succinate accumulation to low SCFA accumulation in the cecum and the colon was also observed for animals receiving the high ZnO dose in combination with CuSO4 (data not shown).
FIG. 4.
Concentrations of lactate (black symbols), succinate (gray symbols), and SCFA (white symbols) in digesta from four segments of the gastrointestinal tracts of pigs fed diets supplemented with either 100 ppm ZnO-Zn (A) (n = 7 animals) or 2,500 ppm ZnO-Zn (B) (n = 8 animals). No CuSO4 was added to the diets of these animals. The results are presented for individual animals, with each represented by a unique symbol in the graphs.
Potential urease activity.
The addition of the high ZnO dose to the feed tended to reduce the potential urease activity in the cecum (P = 0.11) and did so significantly in the colon samples (P < 0.01) (Table 2). The addition of CuSO4 had no effect on the potential urease activity (P ≥ 0.43). The potential urease activity was below the detection limit for the stomach and the ileum and showed a high degree of variation for the cecum and the colon between individual animals. A similar pattern was previously observed for slaughter pigs (21).
DISCUSSION
The observations of a numerically larger feed intake, daily gain, and content of digesta for piglets receiving the high ZnO dose are in good accordance with observations from animal performance studies (11, 12, 20). In contrast to other studies (5, 7), however, our observations indicate a negative effect of CuSO4 amendment on animal performance. It should, however, be emphasized that effects on animal performance can only be evaluated thoroughly by including a larger number of animals than we did in the present study.
The decrease in the dry matter content of stomach digesta from animals receiving the high ZnO dose could have been due to a higher water intake. Moreover, the significantly larger values for the dry matter contents of cecum and colon digesta observed for animals receiving the high dose of ZnO could reflect more efficient water retention due to ZnO-induced resistance of the animals to secretory disorders (9).
The increased pH levels observed in digesta from the distal small intestines and ceca of the animals receiving the high ZnO dose most probably reflected the overall decrease in the concentration of organic acids (lactate, succinate, and SCFA) in these segments. However, pancreatic bicarbonate secretion is also known to be influenced by factors such as the lactate concentration of the stomach contents (26). The low organic acid concentration was not reflected in the pH level in the stomach and colon, which for the stomach may have been due to the fact that the secretion of HCl is an important part of pH control. Clearly, though, feeding high dietary doses of ZnO and/or CuSO4 did not promote one of the conditions often associated with an optimal gut ecosystem, namely, reduced pH levels in the stomach and distal small intestine (26).
Bacterial counts indicated that major groups of lactic acid bacteria and potentially phylotypes such as L. amylovorus (OTU171), L. reuteri (OTU173), and S. alactolyticus (OTU180), which dominated in animals receiving the low ZnO dose, were reduced in animals receiving the high ZnO dose, whereas the numbers of coliforms increased throughout the gastrointestinal tracts of the latter group. It should be emphasized that identification cannot be based on 16S rRNA gene similarity alone, and therefore the species names reported should be considered tentative. Other studies have, however, identified OTU171-type lactobacilli as dominating in pigs, and they were listed as belonging to a Lactobacillus amylovorus-like phylotype as well (35, 36). Though low in most segments, the counts of enterococci were observed to increase significantly in animals receiving the high ZnO dose, indicating that not all types of lactic acid bacteria were inhibited by this treatment. T-RFLP data should be interpreted with caution (37), but they clearly supported the observation of a shift in the gastrointestinal bacterial population when the high ZnO dose was fed to piglets. Lactic acid bacteria (MRS and Rogosa counts) were further reduced in the stomachs of animals receiving the diet amended with a high dose of CuSO4. It is thus striking that the major effect of the two additives was to reduce groups of commensal lactic acid bacteria which are considered important for stabilizing the porcine gut ecosystem (35). Our observation of a lowered number of lactobacilli at 2 weeks postweaning in animals receiving the high ZnO dose is, however, in good accordance with the results of a recent study reporting lower fecal counts of lactobacilli 19 days after weaning of animals receiving a diet amended with ZnO and avilamycin (6). However, the authors of that study were not able to distinguish between the effects of the two additives.
Reductions in bacterial activity, as determined by the concentration of ATP, in the digesta of animals receiving the high ZnO dose most likely reflected the smaller number of bacteria present in the digesta of these animals. Thus, the ATP data support the picture of a major influence of the high ZnO dose on the function of the gastrointestinal tract microbiota, whereas no influence of CuSO4 was reflected in this parameter. Besides the promotion of feed intake, these data show that feeding high dietary ZnO doses may render more energy available for the host animal by suppressing the commensal gut microbiota. This has also been suggested as one of the working mechanisms behind the effect of growth-promoting antibiotics (13, 18). It should be emphasized, though, that this might not be only a question of available energy. The lactic acid bacteria may also utilize essential feed components such as amino acids and may influence fat digestibility by hydrolyzing bile salts (33, 34).
The reduced level and impaired activity of lactic acid bacteria in the proximal part of the gastrointestinal tract may have resulted in a higher flow of readily fermentable substrates to the cecum and the proximal part of the colon. Substrate availability can have a dramatic influence on bacterial fermentation biochemistry, and under certain conditions, e.g., an electron donor excess, the reoxidation of NADH becomes the speed-limiting step of fermentative energy formation (38). To comply with the immediate need for oxidation power, pyruvate reduction is directed towards lactate and/or succinate formation rather than the formation of SCFA and other fermentation products (38). In the present study, the accumulation of lactate and succinate in the cecum and colon was indeed directly linked to low concentrations of SCFA in the digesta, and conversely, when lactate and succinate were not detectable, we always observed high concentrations of SCFA. This pattern could illustrate the time factor involved. Thus, lactate and succinate accumulation in the cecum and colon may be seen as a response to a temporary excess of a readily fermentable substrate, e.g., in relation to feed intake. When the substrate pool is reduced, lactate and succinate are further fermented to SCFA. The different patterns of organic acid accumulation in the animals receiving the high ZnO dose were most likely due to the fact that the piglets were fed ad libitum and thus may reflect a difference in the time lapse from the last feed intake to slaughter, even though all of the animals were sacrificed 3 h after the light was turned on, when they usually all started to eat. The accumulation of lactate or succinate is a well-described phenomenon connected to abnormal fermentation in the large intestine, such as in cases of inflammatory bowel diseases and short bowel syndrome in humans (42) and dyspepsia in pigs (48), but fecal lactate accumulation has also been observed in relation to acute changes in the diets of weaning piglets (16). It has not been reported if and how a temporary accumulation of lactate and succinate in the cecum and colon in itself influences animal physiology, but we did not observe any effects, e.g., compromised growth performance, in connection to this in the present study.
Urease is expressed in many bacteria and mediates the hydrolysis of urea to ammonia, which is a bacterial nitrogen source but may further enable the bacterial cell to establish a pH-buffered zone around itself as protection against the acidic environment in, e.g., the stomach (3). Ammonia can, however, be directly toxic to the epithelial cells of the gut and can put an energetic burden on the host animal by increasing the turnover of epithelial cells. Moreover, ammonia may inhibit butyrate metabolism in colonocytes (14). The observed decrease in the urease levels in digesta from ceca and colons of animals receiving the high ZnO dose may therefore also contribute to the beneficial effect of ZnO on animal health and performance.
The data from the present study clearly illustrate that elevated doses of dietary ZnO and CuSO4 reduced the sizes of major groups of bacteria among the porcine gastrointestinal commensals, namely, the lactobacilli and streptococci. The reduced level of these commensals in the proximal part of the gastrointestinal tract may benefit the host animal by allocating more feed components for its growth performance. Furthermore, feeding the animals high dietary ZnO doses resulted in an altered pattern of organic acid accumulation, with lower levels of lactate and succinate in the stomach and small intestine and an accumulation of these compounds in the cecum and colon. How this influences the physiology of the animals needs further elucidation in detail, since lactic acid produced in the stomach is normally considered a part of the natural defense mechanism of the host, whereas lactate accumulation in the large intestine has mainly been observed in connection with various disorders. CuSO4 reduced the number of coliforms in the large intestine, which may be a part of other mechanisms, such as the suppression of specific pathogens and induced resistance of the animal to pathogen adhesion and invasion as well as pathogen-produced toxins (10). If and how mechanisms like this compensate for the overall decrease in the population of lactic acid bacteria should be examined further.
Acknowledgments
This study was supported by The Research Secretariat of The Danish Ministry of Agriculture, Food, and Fisheries and by The National Committee for Pig Production, The Danish Bacon and Meat Council.
We thank Karin Durup, Mona Dinesen, Trine Poulsen, Thomas Rebsdorf, and Carsten Berthelsen for excellent practical and technical assistance.
REFERENCES
- 1.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., F. Bager, N. E. Jensen, M. Madsen, A. Meyling, and H. C. Wegener. 1998. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS 106:606-622. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, M. J. 1997. The versatility of Helicobacter pylori in the adaptation to the human stomach. J. Physiol. Pharmacol. 48:307-314. [PubMed] [Google Scholar]
- 4.Blencowe, D. K., and A. P. Morby. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 27:291-311. [DOI] [PubMed] [Google Scholar]
- 5.Braude, R. 1967. Copper as a stimulant in pig feeding (cuprum pro pecunia). World Rev. Anim. Prod. 3:69-82. [Google Scholar]
- 6.Broom, L. J., H. M. Miller, K. G. Kerr, and P. Toplis. 2003. Removal of both zinc oxide and avilamycin from the post-weaning piglet diet: consequences for performance through to slaughter. Anim. Sci. 77:79-84. [Google Scholar]
- 7.Bunch, R. J., V. C. Speer, V. W. Hays, J. H. Hawbaker, and D. C. Catron. 1961. Effects of copper sulfate, copper oxide and chlortetracycline on baby pig performance. J. Anim. Sci. 20:723. [Google Scholar]
- 8.Carlson, D. 2003. The physiological role of dietary zinc and copper in weaned piglets, with emphasis on zinc and intestinal mucosal function. Ph.D. thesis. The Royal Veterinary and Agricultural University, Copenhagen, Denmark.
- 9.Carlson, D., and H. D. Poulsen. 2003. Dietary zinc enhances gastrointestinal function in pigs after weaning, p. 229-231. In R. O. Ball (ed.), Proceedings of the 9th International Symposium on Digestive Physiology in Pigs, vol. 2. University of Alberta, Edmonton, Alberta, Canada. [Google Scholar]
- 10.Carlson, D., H. D. Poulsen, and J. Sehested. 2004. Influence of weaning and effect of post weaning dietary zinc and copper on electrophysiological response to glucose, theophylline and 5-HT in piglet small intestinal mucosa. Comp. Biochem. Physiol. A 137:757-765. [DOI] [PubMed] [Google Scholar]
- 11.Carlson, M. S., G. M. Hill, and J. E. Link. 1999. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J. Anim. Sci. 77:1199-1207. [DOI] [PubMed] [Google Scholar]
- 12.Case, C. L., and M. S. Carlson. 2002. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 80:1917-1924. [DOI] [PubMed] [Google Scholar]
- 13.Collier, C. T., M. R. Smiricky-Tjardes, D. M. Albin, J. E. Wubben, V. M. Gabert, B. Deplancke, D. Bane, D. B. Anderson, and H. R. Gaskins. 2003. Molecular ecological analysis of porcine ileal microbiota responses to antimicrobial growth promoters. J. Anim. Sci. 81:3035-3045. [DOI] [PubMed] [Google Scholar]
- 14.Darcy-Vrillon, B., C. Cherbuy, and M. T. Morel. 1996. Short chain fatty acids and glucose metabolism in isolated pig colonocytes: modulation by NH4+. Mol. Cell. Biochem. 156:154-161. [DOI] [PubMed] [Google Scholar]
- 15.Devriese, L. A., B. Pot, L. Van Damme, K. Kersters, and F. Haesebrouck. 1995. Identification of Enterococcus species isolated from foods of animal origin. Int. J. Food Microbiol. 26:187-197. [DOI] [PubMed] [Google Scholar]
- 16.Etheridge, R. D., R. W. Seerley, and T. L. Huber. 1984. The effect of diet on fecal moisture, osmolarity of fecal extracts, products of bacterial fermentation and loss of minerals in feces of weaned pigs. J. Anim. Sci. 58:1403-1411. [DOI] [PubMed] [Google Scholar]
- 17.Fuller, R., L. G. M. Newland, C. A. E. Briggs, R. Braude, and K. G. Mitchell. 1960. The normal intestinal flora of the pig. IV. The effect of dietary supplements of penicillin, chlortetracycline or copper sulphate on the faecal flora. J. Appl. Bacteriol. 23:195-205. [Google Scholar]
- 18.Gaskins, H. R., C. T. Collier, and D. B. Anderson. 2002. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 13:29-42. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, J. D., and D. H. Baker. 1993. Growth and plasma zinc responses of young pigs fed pharmacological levels of zinc. J. Anim. Sci. 71:3020-3024. [DOI] [PubMed] [Google Scholar]
- 20.Hill, G. M., D. C. Mahan, S. D. Carter, G. L. Cromwell, R. C. Ewan, R. L. Harrold, A. J. Lewis, P. S. Miller, G. C. Shurson, and T. L. Veum. 2001. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J. Anim. Sci. 79:934-941. [DOI] [PubMed] [Google Scholar]
- 21.Højberg, O., N. Canibe, B. Knudsen, and B. B. Jensen. 2003. Potential rates of fermentation in digesta from the gastrointestinal tract of pigs: effect of feeding fermented liquid feed. Appl. Environ. Microbiol. 69:408-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holdeman, L. V., E. P. Cato, and W. E. C. Moore. 1977. Anaerobe laboratory manual. Virginia Polytechnic Institute and State University, Blacksburg, Va.
- 23.Holdeman, L. V., and W. E. C. Moore. 1972. Roll-tube techniques for anaerobic bacteria. Am. J. Clin. Nutr. 25:1314-1317. [DOI] [PubMed] [Google Scholar]
- 24.Holm, A. 1988. Escherichia coli-betinget fravænningsdiarré hos grise: zinkoxid tilsat foder som antibakterielt princip (Escherichia coli-associated diarrhea in weaner pigs: zinc oxide added to the feed as a preventive measure). Dansk Veterinaertidsskrift 71:1118-1126. [Google Scholar]
- 25.Jensen, B. B. 1987. Tarmfloraen, zinkoxid og colidiarré hos svin (Intestinal microflora, zinc oxide and coli enteritis in pigs). Landbonyt 41(August):5-10. [Google Scholar]
- 26.Jensen, B. B. 1998. The impact of feed additives on the microbial ecology of the gut in young pigs. J. Anim. Feed Sci. 7:45-64. [Google Scholar]
- 27.Jensen, B. B., and H. Jørgensen. 1994. Effect of dietary fibre on microbial activity and microbial gas production in various regions of the gastrointestinal tracts of pigs. Appl. Environ. Microbiol. 60:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen, M. T., R. P. Cox, and B. B. Jensen. 1995. Microbial production of skatole in the hind gut of pigs given different diets and its relation to skatole deposition in backfat. Anim. Sci. 61:293-304. [Google Scholar]
- 29.Jensen-Waern, M., L. Melin, R. Lindberg, A. Johannisson, L. Petersson, and P. Wallgren. 1998. Dietary zinc oxide in weaned pigs—effects on performance, tissue concentrations, morphology, neutrophil functions and fecal microflora. Res. Vet. Sci. 64:225-231. [DOI] [PubMed] [Google Scholar]
- 30.Jondreville, C., P. S. Revy, A. Jaffrezic, and J. Y. Dourmad. 2002. Le cuivre dans l'alimentation du porc: oligo-element essential, facteur de croissance et risque potentiel pour l'homme et l'environement. INRA Prod. Anim. 15:247-265. [Google Scholar]
- 31.Katouli, M., L. Melin, M. Jensen-Waern, P. Wallgren, and R. Möllby. 1999. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J. Appl. Microbiol. 87:564-573. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg, T. F., V. W. Hays, D. V. Catron, L. Y. Quinn, and V. C. Speer. 1964. Effect of dietary chemotherapeutics on the performance and fecal flora of baby pigs. J. Anim. Sci. 25:1102-1106. [Google Scholar]
- 33.Knarreborg, A., R. M. Engberg, S. K. Jensen, and B. B. Jensen. 2002. Quantitative determination of bile salt hydrolase activity in bacteria isolated from the small intestine of chickens. Appl. Environ. Microbiol. 68:6425-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5918-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantinov, S. R., A. Awati, H. Smidt, B. A. Williams, A. D. L. Akkermans, and W. M. de Vos. 2004. Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl. Environ. Microbiol. 70:3821-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Møller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lueders, T., and M. W. Friedrich. 2003. Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl. Environ. Microbiol. 69:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macfarlane, S., and G. T. Macfarlane. 2003. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 62:67-72. [DOI] [PubMed] [Google Scholar]
- 39.Mikkelsen, L. L., C. Bendixen, M. Jakobsen, and B. B. Jensen. 2003. Enumeration of bifidobacteria in gastrointestinal samples from piglets. Appl. Environ. Microbiol. 69:654-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mores, N., J. Christani, I. A. Piffer, W. Barioni, Jr., and G. M. M. Lima. 1998. Efeito do oxido de zinco no controle da diarreia pos-desmame em leitoes infectados experimentalmente com Escherichia coli (Effects of zinc oxide on postweaning diarrhea control in pigs experimentally infected with. E. coli). Arq. Brasil. Med. Vet. Zootec. 50:513-523. [Google Scholar]
- 42.Mortensen, P. B., and M. R. Clausen. 1996. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. 31:132s-148s. [DOI] [PubMed] [Google Scholar]
- 43.Poulsen, H. D. 1995. Zinc oxide for weanling piglets. Acta Agric. Scand. 45:159-167. [Google Scholar]
- 44.Poulsen, H. D. 1998. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J. Anim. Feed Sci. 7:135-142. [Google Scholar]
- 45.Revy, P. S., C. Jondreville, J. Y. Dourmad, and Y. Nys. 2003. Le zinc dans l'alimentation du porc: oligo-élément essentiel et risque potentiel pour l'environnement. INRA Prod. Anim. 16:3-18. [Google Scholar]
- 46.SAS. 1989. User's guide: statistics, version 6, 4th ed. SAS Institute Inc., Cary, N.C.
- 47.Slanetz, L. W., M. S. Bent, and C. H. Bartley. 1955. Use of the membrane filter technique to enumerate enterococci in water. Public Health Rep. 70:67-72. [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukahara, T., and K. Ushida. 2001. Organic acid profiles in feces of pigs with pathogenic or non-pathogenic diarrhea. J. Vet. Med. Sci. 63:1351-1354. [DOI] [PubMed] [Google Scholar]
- 49.van Winsen, R. L., B. A. P. Urlings, L. J. A. Lipman, J. M. A. Snijders, D. Keuzenkamp, J. H. M. Verheiijden, and F. van Knapen. 2001. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl. Environ. Microbiol. 67:3071-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varel, V. H., I. M. Robinson, and W. G. Pond. 1987. Effect of dietary copper sulfate, aureo SP250, or clinoptilolite on ureolytic bacteria found in the pig large intestine. Appl. Environ. Microbiol. 53:2009-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verdouw, H., C. J. A. Van Echteld, and E. M. J. Dekkers. 1977. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 12:399-402. [Google Scholar]