Abstract
The marine coccolithophorid Emiliania huxleyi is a cosmopolitan alga intensely studied in relation to global carbon cycling, biogeochemistry, marine ecology, and biomineralization processes. The biomineralization capabilities of coccolithophorids have attracted the attention of scientists interested in exploiting this ability for the development of materials science and biomedical and biotechnological applications. Although it has been well documented that biomineralization in E. huxleyi is promoted by growth under phosphate-limited conditions, the genes and proteins that govern the processes of calcification and coccolithogenesis remain unknown. Suppressive subtractive hybridization (SSH) libraries were constructed from cultures grown in phosphate-limited and phosphate-replete media as tester and driver populations for reciprocal SSH procedures. Positive clones from each of the two libraries were randomly selected, and dot blotting was performed for the analysis of expression patterns. A total of 513 clones from the phosphate-replete library and 423 clones from the phosphate-limited library were sequenced, assembled, and compared to sequences in GenBank using BLASTX. Of the 103 differentially expressed gene fragments from the phosphate-replete library, 34% showed significant homology to other known proteins, while only 23% of the 65 differentially expressed gene fragments from the phosphate-limited library showed homology to other proteins. To further assess mRNA expression, real-time RT-PCR analysis was employed and expression profiles were generated over a 14-day time course for three clones from the phosphate-replete library and five clones from the phosphate-limited library. The fragments isolated provide the basis for future cloning of full-length genes and functional analysis.
Coccolithophorids are unicellular marine algae that produce a wide variety of highly sculpted calcium carbonate cell coverings. Calcification in some coccolithophorids occurs intracellularly and is thought to begin with the conversion of calcium and bicarbonate ions into crystals of calcium carbonate that are deposited in a defined array in association with an organic matrix (35). A coccolith vesicle developing from the Golgi apparatus provides the microenvironment that promotes the formation of the crystallographically intricate coccolith structures, which are subsequently extruded from the cell to form the coccosphere (7, 20). The coccolith vesicle fuses with the plasma membrane to release the individual coccoliths. While calcification and coccolithogenesis are presumed to occur in a genetically controlled manner, the functional significance and genetic control of these complex structures and the variation in crystal shape, orientation, order, and connectivity that occurs across species are poorly understood
The size and morphology of the calcite structures are as diverse as the coccolithophorids that produce them. Some of the calcite elements are simple rhombohedral units, while others appear as ornate oval platelets, extended spicules, or elaborate coronets. The mechanisms behind the natural nanofabrication of the highly sculpted calcium carbonate coccoliths are increasingly being investigated. Scanning electron and atomic force microscopy have defined many of the structural features of various types of coccoliths (13, 41). Ecophysiological and environmental studies have related biomineralization to nutrient status, light, cell growth and division, and the life cycle of coccolithophorids (28). Biochemical analysis has led to the isolation of several macromolecules associated with the organic matrix (5, 20, 22, 34), while more recently, expressed sequence tags (ESTs) have been used to catalog genes and proteins expressed during calcification (36, 37). Despite these efforts, our understanding of the mechanisms governing biomineralization in coccolithophorids is very limited. This limitation has historically been due to the lack of molecular and genetic approaches for addressing questions regarding the biology of these globally significant marine algae.
Emiliania huxleyi (Fig. 1) is the most prominent coccolithophorid and is rapidly gaining status as the model organism for studying the biology of these organisms. E. huxleyi is found throughout the world's oceans, forming massive blooms sometimes covering 100,000 square kilometers (3, 14). In the laboratory, cells can be grown in batch cultures and manipulated with ease (17, 25, 30). In addition, the genome of E. huxleyi strain 1516 is presently being sequenced in a collaborative effort between our laboratory and the Department of Energy. Although the amount of research on biomineralization in E. huxleyi is growing, research aimed at identification of genes and proteins involved in calcification is still in its infancy. To date, only one protein, designated GPA, has been implicated in the process of biomineralization in E. huxleyi (5). GPA, which is rich in glutamic acid, proline, and alanine, contains a Ca2+ motif and was isolated from intracellular polysaccharide fractions. GPA is thought to play a role in coccolith formation by controlling either crystal nucleation and growth or Ca2+ transport.
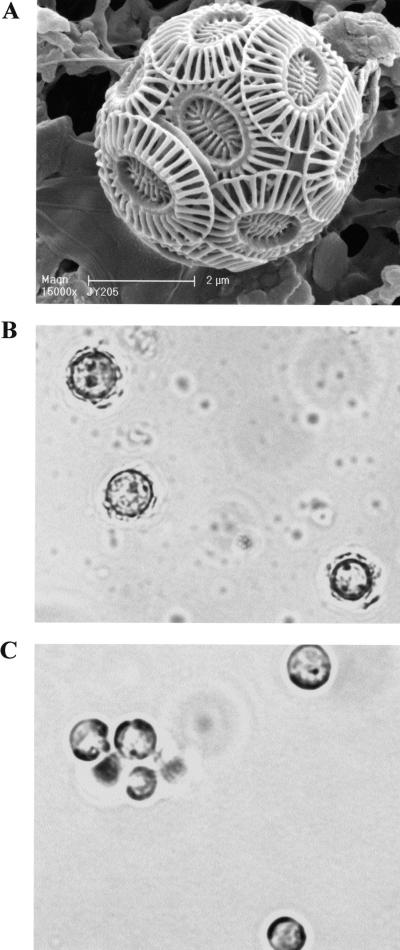
FIG. 1.
(A) Scanning electron micrograph of coccolith-bearing cell of E. huxleyi CCMP1516 showing overlapping coccoliths (magnification, ×15,000; courtesy of J. R. Young). (B and C) Light microscopy images of CCMP 1516 cells showing (B) up-regulation of calcification in phosphate-limited (f/50) medium and (C) down-regulation in phosphate-replete (f/2) medium. Magnification, ×2,000.
Until this study, biomineralization in E. huxleyi had only been investigated using physiological and morphological techniques, and no studies have been done employing molecular approaches for evaluating differential gene expression patterns in calcifying versus noncalcifying cells. To understand the molecular processes governing biomineralization in E. huxleyi, relevant subsets of differentially expressed genes must be identified, cloned, and studied in detail. In this report, we have used suppressive subtractive hybridization (SSH) to identify E. huxleyi genes expressed when cells are grown in phosphate-limited media, a condition known to promote biomineralization in E. huxleyi (1, 23, 25). SSH, developed by Diatchenko and colleagues (8), is a sophisticated technique that combines normalization and cDNA subtraction to enrich and isolate differentially expressed genes. SSH has been widely applied in plants for the identification of disease-related (6), developmentally associated (21), tissue-specific (16), and other differentially expressed genes (18, 19, 38). More recently, SSH-based methods have been employed with algae (12, 43).
There is a growing body of evidence indicating that the processes of coccolith production and calcification are closely related to phosphate metabolism in E. huxleyi (1, 23, 24, 32, 33). Not only are E. huxleyi blooms in the open ocean found predominately in oligotrophic regions, where phosphorus and nitrate are limited (32), but in the laboratory, Paasche and Bruback (24) and van Bleijswijk et al. (33) reported an increase in the ratio of carbon deposited in coccoliths to carbon incorporated in photosynthesis when cells are starved of phosphorus. Paasche (23) further demonstrated increases in the number of coccoliths and in the molar Ca2+/C content of the cultures and of the coccoliths in cells grown in batch culture and/or in chemostats under phosphate-limited conditions. Andersen (1) showed that coccoltih formation could be reversibly induced in naked E. huxleyi cells, also known as N cells, by growing them in a phosphorus-deficient medium. While the mechanisms remain to be defined, and whether it is a direct or indirect effect remains to be determined, it is clear that phosphate limitation is linked to biomineralization and coccolith production in E. huxleyi.
Mutant strains that fail to calcify regardless of nutrient status are also available; however, these noncalcifying phenotypes may be caused by a point mutation in a single gene. Hence, our rationale for this study was to employ cells similar to those described by Andersen (1): cells that cease to produce coccoliths when grown under normal growth conditions and calcify when starved of phosphorus. This strategy afforded greater potential in terms of obtaining information regarding genes (and proteins) involved in coccolith production and calcification than any experimental approach previously employed. In the present investigation for SSH analysis, E. huxleyi strain 1516 was grown under noncalcifying conditions in phosphate-replete medium and compared to cells grown under calcifying conditions in phosphate-limited medium.
During the course of this investigation, we identified 168 gene sequences that were differentially expressed by comparing E. huxleyi cultures grown in phosphate-limited and phosphate replete media using reciprocal SSH. Of these transcripts, 63 were specific to the phosphate-limited conditions that promote calcification, while 105 were specific to the phosphate-replete conditions that retard calcification. The differential expression pattern of a subset of these genes over a 14-day time course is discussed based on real-time RT-PCR analysis. Together, these results provide novel information for developing molecular genetic approaches for addressing important questions on the biology of coccolithophorids, including the biomineralization and coccolithogenesis processes in these organisms.
MATERIALS AND METHODS
Strains and growth conditions.
E. huxleyi strain 1516 was obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton and cultured in either f/2 (phosphate replete; 41.7 μM NaH2PO4 · H2O) or f/50 (phosphate limited; 1.7 μM NaH2PO4 · H2O) artificial seawater medium as described previously (11, 17). Cells were grown in batch culture by inoculating ∼5 × 107 cells into 1 liter of medium in 4-liter flasks. Cultures were grown photoautotrophically at 17 to 18°C under cool white fluorescent light (660 μmol · m−2 · s−2) under a discontinuous light (12 h dark, 12 h light) cycle.
RNA isolation.
RNA was isolated from 3 liters of late-log-phase cultures (8 days after inoculation) grown in both f/2 and f/50 cultures. Dissolution of calcium carbonate coccolith material was accomplished prior to RNA extraction by lowering the pH of the culture with HCl (0.1 N) to 5.0 for 2 min and then rapidly readjusting the pH to 8.0 with NaOH (1 N). Cultures grown in both f/2 and f/50 media were treated in this manner. A standard guanidinium isothiocyanate procedure (31) was used to isolate total RNA. Briefly, cells were lysed by grinding them in liquid nitrogen with a mortar and pestle. Membranes were further disrupted, and the activity of ribonucleases was inhibited when the cell material was resuspended in extraction buffer (4 M guanidinium isothiocyanate, 25 mM sodium citrate, 0.5% Sarkosyl, 0.1 M β-mercaptoethanol). To separate RNA from other cellular components, a phenol extraction was performed, followed by an isopropanol precipitation with 3 M sodium acetate (pH 5.2). A second precipitation using 2 M lithium chloride was carried out to further purify the RNA. The concentration and purity of the RNA concentration were determined spectrophotometrically by measuring the absorbance at 260 and 280 nm, and the integrity of the RNA was assessed using denaturing agarose gel electrophoresis.
cDNA synthesis and suppressive subtractive hybridization.
Reciprocal SSH was performed by Evrogen, JSC (Moscow, Russia) using the method of Diatchenko and colleagues (8). Amplification of double-stranded cDNA was performed using 1 μg of total RNA isolated from cells grown in phosphate-replete and phosphate-limited media, using CLONTECH's SMART reverse transcriptase template-switching approach. The SMART Oligo II and CDS primers (Table 1) were used to synthesize and anchor first-strand cDNA, which was then used for PCR amplification with the SMART PCR primer (Table 1). Eighteen PCR cycles (each cycle included 95°C for 7 s, 65°C for 20 s, and 72°C for 3 min) were performed. The SMART-amplified cDNA samples were digested with RsaI to obtain shorter blunt-ended fragments for SSH procedures.
TABLE 1.
Oligonucleotides used for cDNA synthesis and SSH
| Oligonucleotide | Sequence |
|---|---|
| SMART Oligo II | 5′-AAGCAGTGGTATCAACGCAGAGTACGCGGG-3′ |
| CDS primer | 5′-AAGCAGTGGTATCAACGCAGAGTA-d(T)30-3′ |
| SMART PCR primer | 5′-AAGCAGTGGTATCAACGCAGAGT-3′ |
| Adapter 1 | 5′-CTAATACGACTCACTATAGGGCTCGAGCGGCCGCCGGGCAGGT-3′ |
| 3′-GGCCCGTCCA-5′ | |
| PCR primer 1 | 5′-CTAATACGACTCACTATAGGGC-3′ |
| Nested primer 1 | 5′-TCGAGCGGCCGCCCGGGCAGGT-3′ |
| Nested primer 2R | 5′-AGCGTGGTCGCGGCCGAGGT-3′ |
Subtractive hybridizations were performed using the SSH method in both directions (f/2 versus f/50 and f/50 versus f/2) as described previously (8, 9). For isolation of cDNAs associated with coccolith morphology, the cDNA from the phosphate-limited (f/50) E. huxleyi cells was used as the “tester,” and the cDNA from the phosphate-replete (f/2) cells was used as the “driver.” For isolation of cDNAs associated with non-coccolith-bearing morphology, the cDNA from the phosphate-replete (f/2) cells was used as the “tester,” and the cDNA from the phosphate-limited (f/50) cells was used as the “driver.” For each direction, two tester populations were created separately by ligating suppression adapters 1 and 2R (Table 1) to the blunt-ended RsaI-digested cDNA synthesis products. The two tester populations were mixed with 30× driver excess (driver cDNA had no adaptors) in two separate tubes, denatured, and allowed to renature. After this first hybridization, the two samples were mixed and hybridized together. The subtracted cDNA was then amplified in a primary PCR consisting of 26 cycles with primer 1 (Table 1). A secondary PCR of 11 cycles was then performed using the nested primers 1 and 2R (Table 1).
Subtracted library construction.
Two subtracted cDNA samples enriched with differentially expressed sequences (f/2 specific and f/50 specific) obtained by secondary PCR were used for library construction. In each case, approximately 40 ng of purified cDNA was cloned into the pAL16 vector and used for Escherichia coli transformation. For both libraries, a white:blue colony ratio of 60:40 was obtained, and of the white colonies, no less than 90% contained plasmids with inserts.
Dot blot differential screening of clones.
One 96-well plate of randomly picked white clones from the tester f/2-specific library and one 96-well plate of randomly picked white clones from the driver f/50-specific library were used for differential dot blot screening. The clones were grown in LB medium containing ampicillin (75 μg/ml) for 6 h at 37°C. PCR amplification of plasmid inserts was accomplished using 1 μl of culture and plasmid-specific primers. Approximately 100 ng of PCR-amplified insert DNA was arrayed in a 96-well format onto duplicated nylon membranes and hybridized with 32P-labeled f/2- and f/50-subtracted cDNA probes.
Virtual Northern blots.
Virtual Northern blots were prepared using 2.5 μg of SMART-amplified f/2- and f/50-unsubtracted cDNAs. The cDNA was resolved on a 1% agarose gel, processed, and blotted onto Hybond-N membranes (Roche Diagnostics, Mannheim, Germany). Individual cDNA clones generated from the SSH procedures were labeled using 32P and used as probes. Membranes were hybridized and washed according to standard procedures.
DNA sequencing.
The Phi29 DNA polymerase-based rolling-circle amplification method was used to prepare sequencing templates from randomly picked f/2- and f/50-specific clones. Single-pass sequencing was performed by Windsor Pond Associates (Chicago, Ill.) using the dideoxy chain termination and dye termination chemistry (Applied Biosystems). Analysis was completed on an ABI 3100 fluorescence automated sequencer.
Data handling and analysis.
Expressed sequence tags from both f/2- and f/50-specific libraries were edited and assembled using the programs PHRED, CROSS_MATCH, and CONSED from PHRAP (P. Green [http://bozeman.mbt.washington.edu/]). Contaminating vector sequences were removed, and sequences having a PHRED quality score of at least 20 and a minimum size of 300 bases were taken for further analysis. Sequences from both libraries were grouped into classes of identical overlapping sequences (contigs) using PHRAP and were viewed to assess the accuracy of assembly using CONSED. For the assembly of contigs, PHRAP parameters were set at a minimum match of 20 with a default score of 30. CROSS_MATCH parameters included a minimum match of 10 and score of 20.
To identify potential homologues to the E. huxleyi genes, searches were performed against the nonredundant protein database in GenBank (National Center for Biotechnology Information) using BLASTX with the BLOSUM62 matrix. For all other parameters, such as filtering and word size, BLASTX default settings were employed. Similarities to known proteins were considered significant when database searches returned matches with e values of less than 1.0 × 10−2.
Measuring temporal expression of select clones by real-time RT-PCR analysis.
The mRNA expression levels of five of the f/50- and three of the f/2-specific clones were measured over a 14-day time course. Cells were grown in f/50 and f/2 media, and total RNA was extracted from cultures at 4, 7, 10, and 14 days postinoculation. First-strand cDNA synthesis was performed using an Omniscript cDNA synthesis kit (QIAGEN Inc., Valencia, CA). For cDNA synthesis, 2 μg of total RNA from each time point was used as a template in a 20-μl reaction mixture. Each reaction mixture contained 2 μg template RNA, 1× buffer reverse transcriptase (RT), 0.5 mM deoxynucleoside triphosphate, 1 μM oligo(dT) primer, 10 U of RNase inhibitor, and 4 U of Omniscript reverse transcriptase. The reaction mixture was incubated for 60 min at 37°C. cDNA was diluted 1:100, and 5 μl of the dilution was used in a SYBR Green RT-PCR.
Real-time PCR experiments were carried out using SYBER Green chemistry for amplicon detection. The SYBER Green assays were performed on the iCycler iQ System (Bio-Rad). Amplification of target genes, along with a template-minus control, was performed in triplicate in a 96-well plate. Each 25-μl reaction mixture contained 5 μl cDNA, 7.1 μl 2× SYBER Green Master Mix, and 0.24 μM (each) of forward and reverse primers. The cycling conditions for amplification included a 10-min polymerase activation at 95°C followed by 40 cycles of 95°C for 10 seconds, 60°C for 30 seconds, and 82°C for 30 seconds. Fluorescence was measured at 82°C at each cycle. The cycle threshold (CT) value is inversely proportional to the quantity of cDNA present in the reaction mixture at that particular cycle. After PCR amplification, melt curves were generated by denaturing the sample for 1 min at 95°C, cooling it to 55°C for 1 min, and then ramping the temperature 0.5°C every 10 seconds beginning at 55°C until a final temperature of 95°C was reached. Real-time PCR data were plotted using Sigma Plot version 8.0 by taking the mean of three replicates per time point. The relative difference in expression between f/2 and f/50 cDNA clones was measured using the Basic ΔCT method as recommended by the Bio-Rad iCycler manufacturer (Bio-Rad Laboratories). Change between f/2 and f/50 cDNA clones was calculated as 2ΔCT. Primers were designed using Primer Express software version 1.0 (PE Applied Biosystems) and are listed in Table 2. Results from the sequence detection software were exported as tab-delimited text files and imported into Microsoft Excel for further analysis.
TABLE 2.
Primers used for amplification of transcripts by real-time RT-PCR
| Primer name | Primer sequence (5′-3′)a | Primer %GC | Amplicon size (bp) | Amplicon Tmb
|
|
|---|---|---|---|---|---|
| expected | observed | ||||
| F/2Contig26 | For: CACCATCAATACATCGTCGGAT | 45 | 82 | 78 | 81.5 |
| Rev: CTGGATCATAATCACGCACGAA | 45 | ||||
| F/2Contig34 | For: TTTGTCGAACTTCCGCATGA | 45 | 78 | 83 | 85.5 |
| Rev: TCGTTGGCGCACATACTGA | 53 | ||||
| F/2Contig22 | For: AGGCCATCTACAACCACTACAAACC | 48 | 77 | 81 | 83 |
| Rev: CCTCATGCAATTCGTGTTCCA | 48 | ||||
| F/50Contig29 | For: AGTTGAAGTGCTGCCCTGGTT | 52 | 97 | 82 | 85.2 |
| Rev: TCGTAGACAGTCCGTGGTTCCT | 55 | ||||
| F/50Contig31 | For: CCTAGGACAGTTCATCCAAACGA | 48 | 96 | 79 | 83.5 |
| Rev: GCAAAGCACAAGGACTCATCAA | 45 | ||||
| F/50Contig18 | For: GACTTCGCCAGCTACAATCCA | 52 | 84 | 81 | 84 |
| Rev: GGCGAACTTTGCCATATCCTT | 48 | ||||
| F/50Contig36 | For: GTGTCACGCCCTCTCAATCAGT | 55 | 76 | 83 | 85 |
| Rev: TGTTCGCCAGAATCGTTTCAC | 48 | ||||
| F/50Contig33 | For: CCATCCTGCAGTCCTTGATACC | 55 | 84 | 82 | 85.5 |
| Rev: AGATGCCGTTTGCGCTTT | 50 | ||||
For, forward; Rev, reverse.
Tm, melting temperature.
RESULTS
Differential screening of subtracted libraries.
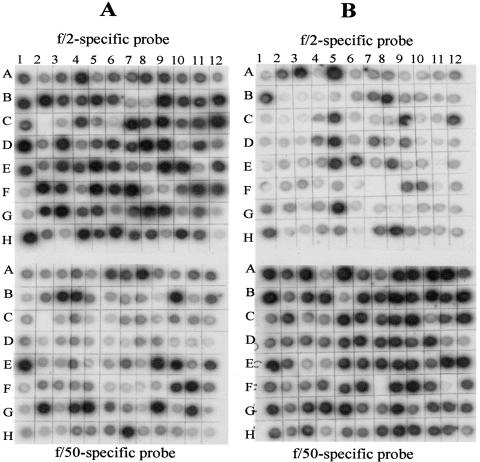
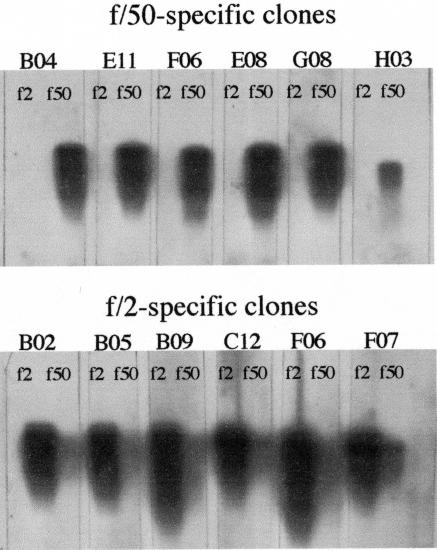
Figure 1 shows the effect of phosphate levels on calcification in E. huxleyi CCMP 1516 cells grown under phosphate-replete (f/2 medium) (Fig. 1B) versus phosphate-limited (f/50 medium) (Fig. 1C) conditions. The dramatic difference in the amount of calcification occurring in response to the phosphate concentration permitted us to employ this as a tool for looking at gene expression differences using SSH. Following construction of our reciprocal SSH libraries, dot blotting and virtual Northern blots were employed to screen the differential cDNA fragments from the two libraries (Fig. 2 and 3). Differential screen results yielded 44 f/50-specific clones (Fig. 2A) and 41 f/2-specific clones (Fig. 2B), indicating that both libraries contain about 40% positive clones. Additional clones from each of the two libraries exhibited various degrees of more subtle differential expression that may warrant further investigation. To verify differential screen results, virtual Northern blots were also performed using six of the dot blot clones from each subtracted library. Results from the virtual Northern blots are shown in Fig. 3 top (f/50-specific clones) and bottom (f/2-specific clones). Differential expression of all 12 genes from the subtracted libraries was confirmed by our virtual Northern blot results.
FIG. 2.
Dot blot showing differential screening results of subtracted library clones from a phosphate-limited (f/50 tester) (A) and a phosphate-replete (f/2 tester) (B) plate. Clones on each plate were differentially screened with tester-specific and driver-specific cDNA probes.
FIG. 3.
Virtual Northern blot analysis of differential clones obtained from phosphate-limited (f/50) and phosphate-replete (f/2) subtracted libraries (see text for details).
Sequence assembly and annotation.
In order to obtain more sequence information in the form of longer reads, we sequenced and assembled several hundred clones from each library. Table 3 describes the general characteristics of the ESTs that were sequenced from each of the two SSH libraries. Of the 423 ESTs sequenced from the phosphate-limited library, 27 were singletons and the remainder were assembled into 38 contiguous sequences (contigs) with an overall EST redundancy of 93% (calculated as the number of ESTs in contigs divided by the number of ESTs). Of the 513 ESTs sequenced from the phosphate-replete library, 53 were singletons and the remainder were assembled into 50 contigs. The overall EST redundancy for the phosphate-replete library was 90%. Assembled sequences from each library were annotated by BLASTX search (e value ≤ 10−2) against the nonredundant protein database from GenBank. Overall, 23% and 34% of the identified genes from the phosphate-limited and phosphate-replete libraries, respectively, encode polypeptides with similarity to proteins with known functions in GenBank. The remaining sequences fell into the unclassified category of proteins with unknown functions (expressed as putative or hypothetical proteins) or with no GenBank match.
TABLE 3.
Comparative analysis of E. huxleyi 1516 f/2 and f/50 cDNA libraries
| Descriptive category | Phosphate limiteda | Nutrient repleteb |
|---|---|---|
| No. of clones sequenced | 423 | 513 |
| No. of contigs | 38 | 50 |
| No. of singletons | 27 | 53 |
| No. of unigenes | 65 | 103 |
| No. of EST matches with e value of >1 × 10−2 | 37 | 19 |
| No. of EST matches with e value of <1 × 10−2 | 15 | 35 |
| No. of EST with no GenBank matchc | 13 | 49 |
RNA extracted from E. huxleyi 1516 grown in f/50 medium (ca. 1.5 μM phosphate) as driver.
RNA extracted from E. huxleyi 1516 grown in f/2 medium (ca. 36 μM phosphate) as driver.
Searches yielding e values of >10−2.
A complete list of the ESTs and their GenBank homologues for each of the SSH libraries is presented in Tables 4 and 5. The vast majority of the most abundant transcripts in the phosphate-limited data set were of unknown function, while transcripts encoding ribosomal proteins and RNA polymerase subunits were abundant in the phosphate-replete data set. Transcripts encoding proteins involved in photosynthesis, such as the photosystem I subunits and the chloroplast ATP synthase subunit proteins, were also present among the most abundant clusters in the phosphate-replete data set.
TABLE 4.
BLASTX results of most prevalent transcripts from f/2-specific SSH librarya
| SeqID | Putative ID | e value | Reads | Length |
|---|---|---|---|---|
| Sig1 | Unknown | >0.001 | 1 | 865 |
| Sig2 | ORF497, homologous to Porphyra ORF491 (Odontella sinensis) | 2.47E-28 | 1 | 797 |
| Sig3 | Unknown | >0.001 | 1 | 828 |
| Sig4 | Unknown | >0.001 | 1 | 543 |
| Sig5 | Unknown | >0.001 | 1 | 479 |
| Sig6 | Apocytochrome f, chloroplast precursor | 7.499E-13 | 1 | 866 |
| Sig7 | RNA polymerase beta apos; apos; subunit (Cyanidium caldarium) | 3.593E-09 | 1 | 628 |
| Sig8 | Pentose-5-phosphate-3-epimerase (Pseudomonas fluorescens PfO-1) | 6.42E-16 | 1 | 804 |
| Con2 | Ribosomal protein S9 (Crocosphaera watsonii WH 8501) | 1.36E-29 | 1 | 960 |
| Con3 | RNA polymerase beta prime subunit (Gloeobacter violaceus PCC 7421) | 4.26E-38 | 1 | 913 |
| Con4 | Ribosomal protein S3 (Nostoc punctiforme) | 1.09E-31 | 1 | 803 |
| Con5 | Ribosomal protein S3 (Porphyra purpurea) | 3.29E-28 | 1 | 807 |
| Con6 | Ribosomal protein S3 (Porphyra purpurea) | 1.72E-16 | 1 | 916 |
| Con7 | Unknown | >0.001 | 1 | 801 |
| Con8 | Orf122-(Chlorobium tepidum) | 5.686E-10 | 1 | 1,002 |
| Con9 | Unknown | >0.001 | 2 | 322 |
| Con10 | Hypothetical protein (Trichodesmium erythraeum IMS101) | 3.08E-40 | 2 | 634 |
| Con11 | Unknown | >0.001 | 2 | 433 |
| Con12 | Unknown | >0.001 | 2 | 416 |
| Con14 | Ribosomal protein S2 (Guillardia theta) | 4.21E-24 | 2 | 863 |
| Con15 | Unknown | >0.001 | 2 | 1,174 |
| Con16 | Ribosomal protein S4 (Nephroselmis olivacea) | 5.61E-26 | 2 | 447 |
| Con17 | Unknown | >0.001 | 2 | 419 |
| Con18 | Hypothetical protein (Bacillus megaterium) | 8.79E-17 | 2 | 396 |
| Con19 | Unknown | >0.001 | 2 | 392 |
| Con20 | Unknown | >0.001 | 2 | 405 |
| Con21 | Unknown | >0.001 | 2 | 645 |
| Con22 | Unknown | >0.001 | 2 | 455 |
| Con23 | Signal transduction histidine kinase (Crocosphaera watsonii WH 8501) | 1.93E-30 | 2 | 781 |
| Con24 | Unknown | >0.001 | 2 | 301 |
| Con25 | Elongation factor Tu GTP binding domain (Bacillus anthracis A2012) | 3.32E-33 | 2 | 897 |
| Con26 | Photosystem I P700 chlorophyll a apoprotein A2 (Emiliania huxleyi) | 1.76E-74 | 2 | 616 |
| Con27 | Hypothetical protein (Plasmodium yoelii yoelii) | 5.41E-19 | 3 | 961 |
| Con28 | ATP synthase delta chain, chloroplast | 1.37E-30 | 3 | 572 |
| Con29 | Unknown | >0.001 | 3 | 402 |
| Con30 | ABC transporter (Cyanophora paradoxa) | 2.18E-49 | 3 | 621 |
| Con31 | Ribosomal protein S9 (Crocosphaera watsonii WH 8501) | 1.18E-32 | 3 | 587 |
| Con32 | Hypothetical protein (Nostoc sp. strain PCC 7120) | 3.50E-18 | 3 | 869 |
| Con33 | 50S ribosomal protein L3 (Prochlorococcus marinus) | 1.08E-19 | 3 | 537 |
| Con34 | Unknown | >0.001 | 4 | 1,021 |
| Con35 | Hypothetical chloroplast RF7 (Guillardia theta) | 6.85E-04 | 5 | 364 |
| Con36 | Hypothetical protein Avar020180 (Anabaena variabilis ATCC 29413) | 0.0001666 | 5 | 514 |
| Con37 | Preprotein translocase subunit Sec Y (Porphyra purpurea) | 8.27E-20 | 6 | 866 |
| Con38 | COG0087: ribosomal protein L3 (Trichodesmium erythraeum IMS101) | 1.79E-11 | 7 | 465 |
| Con39 | Chloroplast ATP synthase a chain precursor (ATPase subunit IV) | 1.54E-76 | 7 | 745 |
| Con40 | Subunit epsilon of ATPase (Ochrosphaera neapolitana) | 8.68E-30 | 7 | 1,020 |
| Con41 | Photosystem 1 subunit III (Guillardia theta) | 1.11E-39 | 8 | 1,027 |
| Con42 | Photosystem 1 subunit XI (Guillardia theta) | 2.82E-35 | 8 | 1,152 |
| Con43 | Predicted protein (Methanosarcina acetivorans strain C2A) | 1.02895 | 10 | 619 |
| Con44 | Orf122 (Chlorobium tepidum) | 8.41E-19 | 10 | 971 |
| Con45 | Ribosomal protein S2 (Guillardia theta) | 1.41E-56 | 10 | 566 |
| Con46 | Hypothetical protein TC0128 (imported)—Chlamydia muridarum (strain Nigg) | 0.116837 | 12 | 692 |
| Con47 | COG0092: ribosomal protein S3 (Nostoc punctiforme) | 6.21E-31 | 19 | 651 |
| Con48 | Hypothetical protein (Chlamydophila pneumoniae AR39) | 2.60643 | 56 | 1,570 |
| Con49 | COG0092: ribosomal protein S3 (Anabaena variabilis ATCC 29413) | 1.27E-40 | 71 | 1,128 |
| Con50 | COG0048: ribosomal protein S12 (Crocosphaera watsonii WH 8501) | 1.81E-37 | 151 | 1,766 |
BLASTX results following PHRAP assembly are listed as follows: SeqID (sequence identification number) for singletons (Sig) and contigs (Con), Reads (numbers of EST fragments per assembled sequence), and Length (average sequence length in base pairs).
TABLE 5.
BLASTX results of most prevalent transcripts from f/50-specific SSH librarya
| SeqID | Putative ID | e value | Reads | Length |
|---|---|---|---|---|
| Sig1 | Unknown | >0.001 | 1 | 740 |
| Sig2 | Unknown | >0.001 | 1 | 1,108 |
| Sig3 | Subunit beta of ATPase (Ochrosphaera neapolitana) | 1.93E-44 | 1 | 612 |
| Sig4 | Unknown | >0.001 | 1 | 663 |
| Sig5 | Hypothetical protein (Plasmodium yoelii yoelii) | 1.4E-14 | 1 | 668 |
| Sig6 | Unknown | >0.001 | 1 | 781 |
| Sig7 | Unknown | >0.001 | 1 | 1,378 |
| Sig8 | Unknown | >0.001 | 1 | 537 |
| Sig9 | NADH dehydrogenase subunit 1 (Emiliania huxleyi) | 6.56E-40 | 1 | 746 |
| Sig10 | Unknown | >0.001 | 1 | 479 |
| Sig11 | Unknown | >0.001 | 1 | 604 |
| Sig12 | Unknown | >0.001 | 1 | 721 |
| Sig13 | Unknown | >0.001 | 1 | 407 |
| Sig14 | Unknown | >0.001 | 1 | 596 |
| Sig15 | Translation elongation factor Tu (Chromobacterium violaceum) | 1.51E-52 | 1 | 802 |
| Sig16 | Unknown | >0.001 | 1 | 790 |
| Sig17 | Unknown | >0.001 | 1 | 831 |
| Sig18 | Unknown | >0.001 | 1 | 849 |
| Uni1 | Unknown | >0.001 | 1 | 785 |
| Uni2 | Unknown | >0.001 | 1 | 893 |
| Uni4 | Unknown | >0.001 | 1 | 571 |
| Uni5 | Unknown | >0.001 | 1 | 661 |
| Uni6 | Unknown | >0.001 | 1 | 655 |
| Uni7 | 347L (Invertebrate iridescent virus 6) | 2.6E-07 | 1 | 559 |
| Con8 | Cytochrome c oxidase subunit 2 (Emiliania huxleyi) | 8.43E-31 | 2 | 587 |
| Con9 | Unknown | >0.001 | 2 | 242 |
| Con10 | Photosystem I P700 apoprotein A2 (Guillardia theta) | 5.21E-16 | 2 | 503 |
| Con11 | Unknown | >0.001 | 2 | 426 |
| Con12 | Unknown | >0.001 | 2 | 328 |
| Con13 | Unknown | >0.001 | 2 | 433 |
| Con14 | Unknown | >0.001 | 2 | 775 |
| Con15 | Unknown | >0.001 | 2 | 766 |
| Con17 | Unknown | >0.001 | 3 | 718 |
| Con18 | Unknown | >0.001 | 3 | 287 |
| Con19 | Unknown | >0.001 | 3 | 506 |
| Con20 | Unknown | >0.001 | 4 | 510 |
| Con21 | Unknown (Vibrio vulnificus CMCP6) | 1.9E-05 | 4 | 509 |
| Con22 | Unknown | >0.001 | 4 | 358 |
| Con23 | Unknown | >0.001 | 4 | 528 |
| Con24 | Unknown | >0.001 | 5 | 170 |
| Con25 | Unknown | >0.001 | 5 | 348 |
| Con26 | Unknown | >0.001 | 8 | 640 |
| Con27 | Ribosomal protein S3 (Porphyra purpurea) | 6.32E-17 | 8 | 636 |
| Con28 | Unknown | >0.001 | 8 | 596 |
| Con29 | Unknown | >0.001 | 16 | 787 |
| Con30 | Photosystem II reaction center protein D1 (Emiliania huxleyi) | 5.39E-37 | 18 | 841 |
| Con31 | 347L (Invertebrate iridescent virus 6) | 4.15E-06 | 18 | 1,279 |
| Con32 | Unknown | >0.001 | 18 | 422 |
| Con33 | Hypothetical protein (Nostoc punctiforme) | 8.19E-06 | 19 | 1,170 |
| Con35 | Putative senescence-associated protein (Pisum sativum) | 9.65E-23 | 25 | 1,014 |
| Con36 | Unknown | >0.001 | 29 | 1,980 |
| Con37 | Unknown | >0.001 | 42 | 968 |
| Con38 | Hypothetical protein | 0.00022 | 105 | 2,391 |
BLASTX results following PHRAP assembly are listed as follows: SeqID (sequence identification number) for singletons (Sig) and contigs (Con), Reads (numbers of EST fragments per assembled sequence), and Length (average sequence length in base pairs).
Temporal expression of selected genes by real-time RT-PCR analysis.
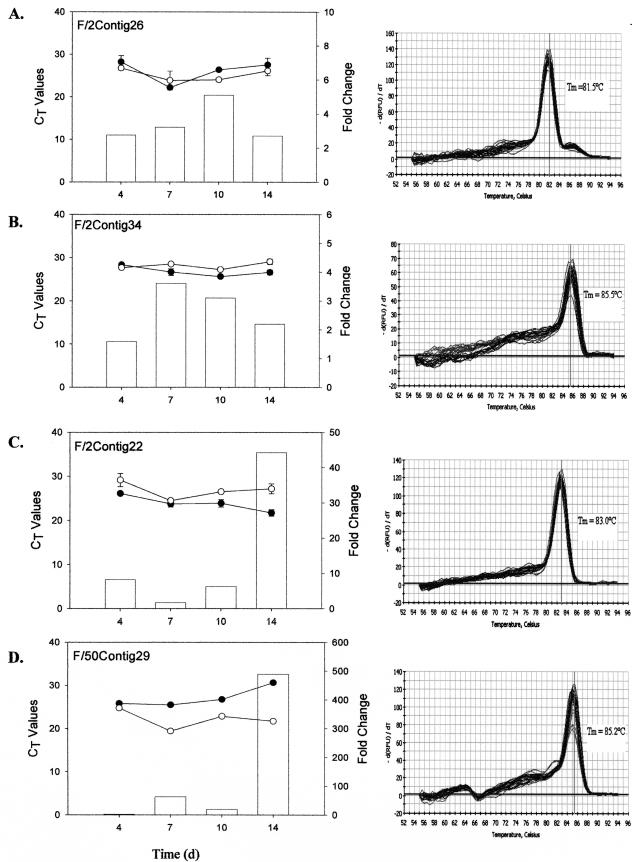
Real-time RT-PCR was conducted to verify expression of a small subset of eight differentially expressed genes using independently prepared RNA that was extracted from cultures of f/2- and f/50-grown cells 4, 7, 10, and 14 days after inoculation. The 14-day time course was intended to determine transcripts (cDNA clones) that may be directly involved in biomineralization, as calcification is known to occur most notably during the late log and stationary phases of growth (2), which generally occur between 7 and 14 days of growth (17).
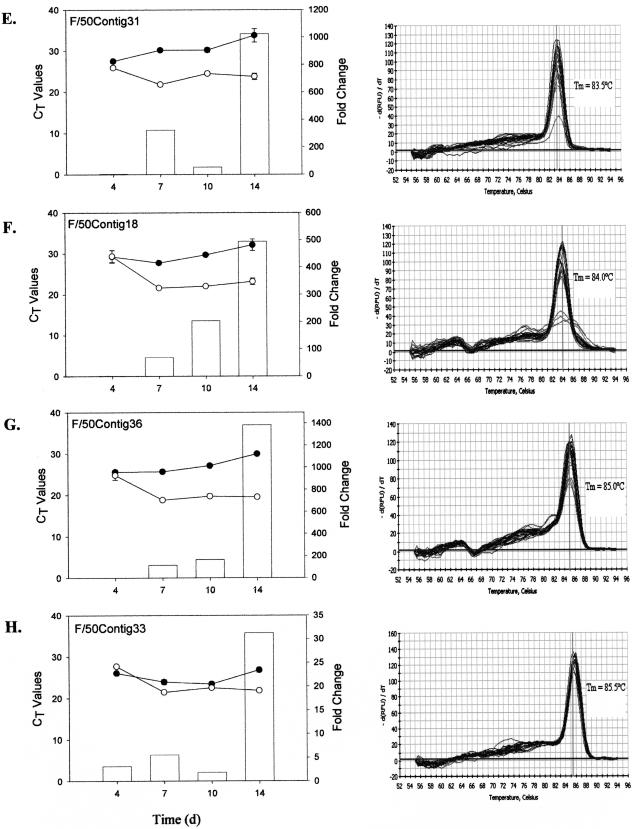
The amplification profiles (along with their respective standard deviations) and dissociation curves for each of the selected genes, three from the phosphate-replete library and five from the phosphate-limited library, are shown in Fig. 4. While contigs 18, 29, 31, and 36 from the f/50 library showed differential mRNA expression in cultures grown in phosphate-limited compared to phosphate-replete media from day 4 onward, none of the transcripts were induced immediately. Maximal differences in expression were reached 14 days following inoculation. In all cases, with the exception of contig 18, contig-specific expression was detectable under both growth conditions. By day 14, changes in gene expression for contigs 31 and 35 were 3 orders of magnitude greater for cells grown in phosphate-limited versus phosphate-replete media (Fig. 4E and G). Changes of more than 2 orders of magnitude were noted for contigs 29 and 18 at day 14 (Fig. 4D and F). Contig 18-specific expression, however, was barely detectable in cells grown in phosphate-replete medium. More modest changes in gene expression were evident for contig 33 from the f/50 library (Fig. 4H).
FIG. 4.
Real-time RT-PCR results showing temporal expression patterns of individual cDNA transcripts from the f/2-specific (A to C) and f/50-specific (D to H) SSH libraries over a 14-day period. Total RNA was isolated from E. huxleyi cells grown in either f/2 (•) or f/50 (○) medium. mRNA transcript abundance is inversely proportional to the cycle threshold (CT) value. Change (n-fold; bars) at each time point was calculated as 2ΔCt and expressed as the relative difference in expression in f/50 versus f/2. The data represent the mean of three replicates per time point, and standard deviations are expressed as error bars. d, days.
Real-time RT-PCR also confirmed candidates from the f/2 library whose down-regulation during phosphate limitation was revealed via SSH procedures. For example, the estimated change for contig 34 across the 14-day time course highlights the ability of SSH to identify genes that are expressed differentially, but at low levels. Down-regulation of this transcript in the phosphate-deficient cells varied between 1.5- and 3.6-fold (Fig. 4B). Transcript levels for one of the P700 reaction center proteins exhibited an interesting expression pattern across the 14 days. The transcript was down-regulated at 4 days (∼2-fold) under phosphate-limited conditions but then appeared to be up-regulated (∼3- to 5-fold) during the later stages of growth (days 10 to 14) (Fig. 4A). The dissociation curves for all samples showed a single peak at the expected temperature, indicating target-specific amplification.
DISCUSSION
PCR-based suppressive subtractive hybridization was used successfully in the present investigation to detect differences in gene expression in coccolith-bearing E. huxleyi cells grown in phosphate-limited medium and non-coccolith-bearing cells grown in phosphate-replete medium. A total of 168 differentially expressed transcripts were identified, some of which may be related to phosphate starvation and some of which presumably are involved in calcification and coccolithogenesis. The high redundancy rates of 93% and 90% that were obtained after sequencing 423 clones from the phosphate-limited and 513 clones from the phosphate-replete libraries, respectively, may be a function of the SSH methodology. Alternatively, these rates may indicate that the potential for novel gene discovery under these conditions is nearly exhausted, suggesting that only a small number of genes, perhaps less than 100, are required for biomineralization in E. huxleyi.
SSH is a powerful technology that enriches for differentially expressed genes, but it is by no means perfect. It is difficult to control for sequence-dependent factors, such as cDNA synthesis and PCR amplification efficiencies. Construction of leaky libraries in which the same transcript is present in the two reciprocal libraries can also be problematic. While the reciprocal libraries constructed in the present investigation were mutually exclusive, with membership in each library specific to the particular growth conditions, overrepresentation of specific clones was noted in both libraries. SSH includes a normalization step to overcome the problem of differences in mRNA abundance (8). All differentially regulated clones are expected to be equally represented after normalization. Overrepresentation of clones, such as the various ribosomal proteins, Orf122, and hypothetical protein TC0128 in the phosphate-replete library and the photosystem II reaction center protein D1, the putative senescence-associated protein, and the different hypothetical (contigs 38 and 32), unknown proteins in the phosphate-deficient library, is an artifact.
Nearly half of the clones recovered were estimated to be specific by dot blot hybridization, being either completely absent or substantially different in the two different cell types. Further tests of a smaller subset of select gene sequences using Northern blots and/or real-time RT-PCR corroborated some of the more distinctly different gene expression profiles, such as those of contigs 31 and 36 from the f/50 SSH library. Representation of quantitatively different cDNAs in the SSH libraries is also apparent, as several gene sequences present in the phosphate-replete SSH library, including the ribosomal proteins and photosynthesis transcripts, are fundamental housekeeping genes. The differential expression data of these particular genes support previous findings that suggest that calcification and coccolithogenesis are stimulated under conditions that result in reduced cell growth and increased cell size and are inhibited under conditions that favor cell growth and a decrease in cell size (4, 26, 28, 29).
The SSH method applied here has also proved to be effective in uncovering some of the distinguishing, albeit less abundant, transcripts from coccolith-bearing and non-coccolith-bearing E. huxleyi cells. This is evidenced by the fact that combined, 70% of the ESTs identified in this investigation (49% from the phosphate-limited and 21% from the phosphate-replete libraries) were not represented in our previously described EST data sets (36, 37) that were derived from nonnormalized cDNA libraries. The effectiveness of subtraction was also demonstrated by the real-time RT-PCR data, in which the expression of eight genes tested over a 14-day time course experiment validated results obtained from these libraries. The suppressive subtraction was successful in detecting small differences in the expression of highly expressed, as well as less abundant, transcripts. These results demonstrate the importance of SSH-based EST sequencing as an approach complementary to existing EST resources for functional genomics research on marine coccolithophorids.
The most distinguishing feature of the SSH library prepared from the coccolith-bearing cells derived from the phosphate-limited media is the large number of transcripts that show no significant homology to sequences in GenBank. We obtained and sequenced the corresponding cDNA clones for six of the eight SSH clones that were subjected to real-time RT-PCR analysis. Since contig 26 showed significant homology to the P700 chlorophyll a apoprotein A2 from E. huxleyi, it was not characterized any further, and since contig 29 from the f/50 SSH lacked a correlate in our previously described unigene set of 4,561 sequences from E. huxleyi (36), the clone and full-length sequence could not be readily obtained. In an attempt to characterize the six other sequences, we used NCBI's BLASTX and ORF-finder to identify the most likely open reading frame and employed the Protein Prediction Meta Server available through Columbia University to search for sequence motifs, domains, low-complexity regions, and protein localization signals. Secondary structure, transmembrane regions, solvent accessibility, disulfide bonds, and posttranslation modifications were also predicted.
Only a few biomineralization proteins have been identified in marine organisms. Some of these are proteins such as lustrin and perlucin from the mollusk shell; nacre and pearlin from the nacreous layer of the oyster; and MS130, SpP16, and SpP19 from the sea urchin. While these particular proteins show little sequence homology to each other or any other known biomineralization proteins (15, 27, 39), they do share a number of chemical and physical features. They generally are small acidic proteins or glycoproteins that are rich in aspartic acid or glutamic acid (10) and manifest prominent repeat sequences (40, 42). They also tend to exhibit extended or elastomeric conformations possessing little or no distinct secondary structure (42). Unfortunately, none of the gene products we examined showed homology to previously identified biomineralization proteins, nor were they noticeably acidic, nor did they contain obvious amino acid repeat elements. This result is not surprising given the limited number of biomineralization proteins identified to date and the lack of understanding of the details of the structure and function of these proteins in relation to biomineralization processes.
Contig 22 from the SSH f/2 library, which showed little differential expression until day 14, when it was up-regulated over 40-fold, exhibited no significant homology to any protein deposited in GenBank and showed no distinct motif or domain matches. The transcript is predicted to be an extracellular or cell wall protein and appears to contain three dominant alpha helices. Two of the f/50 SSH contigs that showed marked up-regulation—contig 31, which was expressed at a level 1,000-fold greater, and contig 18, which was expressed at a level 500-fold greater—in cells under phosphate-limited conditions on day 14 relative to phosphate-replete conditions also returned no significant GenBank BLASTX hits and exhibited no distinct motif or domain match. Although the cellular location of the protein correlates cannot be predicted, both proteins are expected to be membrane bound. Of the three alpha helices that can be discerned for each of the two proteins, one in contig 31 and two in contig 18 have a high probability of being transmembrane helices.
The sequences of three transcripts exhibited significant homology to proteins in GenBank. The sequence of one of the transcripts from the f/2 SSH library was up-regulated slightly (1.5- to 4-fold) across the 14-day time course in the non-coccolith-bearing cells and showed significant homology (BLASTX; 2E-07) to a hypothetical protein of unknown function from Leptospira interrogans. The protein, which may reside in the nucleus (PSORT), features two prominent alpha-helical regions interspersed with short stretches of beta-sheets but shows no other outstanding characteristics. The most likely protein product encoded by contig 33 from the f/50 SSH library showed significant homology to a hypothetical protein from Nostoc (1E-14), with more distant homology to bacterial and fungal methylase enzymes (1E-7). This particular transcript was marginally up-regulated (2- to 5-fold) in coccolith-bearing cells during the first 10 days of growth but by day 14 was significantly up-regulated (30-fold).
One of the more interesting sequences was that of contig 36 from the f/50 SSH library, which at day 14 was expressed in the phosphate-limited coccolith-bearing cells at a level that was 1,400-fold greater than in the non-coccolith-bearing cells maintained in phosphate-replete medium. The full-length sequence of the corresponding cDNA clone showed significant homology (BLASTX) to a number of ligand-gated anion channels, including that of the AVR-15 protein from Caenorhabditis elegans (4E-7), a glutamate-gated chloride channel from Haemonchus contortus (9E-06), and rat gamma-aminobutyric acid A receptor (1E-04). Four strong transmembrane helices likely to form the ion channel were predicted (using bioinformatics tools such as TMHMM, TMpred, and RPS BLAST), along with a distinct arginine-rich region outside the membrane that may represent the ligand binding domain. Our laboratory is currently trying to develop tools to determine the membrane location of the contig 36 gene product with the goal of evaluating the physiological consequences of blocking the channel or of knocking out expression of the gene to elucidate its physiological role. These data may indicate whether it is a highly selective ion channel or an indiscriminate activated channel that functions in low-affinity nutrient uptake, signaling, and/or ion transport.
The expression profiles of the phosphate-limited contigs 31, 18, 36, and 29 are intriguing, with progressively greater differential expression occurring during the later stages of growth. This is consistent with what we know about biomineralization and growth of E. huxleyi in batch culture (2). The effects of phosphate limitation, however, are also expected to become progressively more severe over time, and hence, we cannot unequivocally relate the differential expression patterns of these genes to biomineralization (4, 20, 27, 30).
Based upon what is presently known of biomineralization proteins, one cannot logically argue that any of the aforementioned proteins is directly involved in calcium carbonate biomineralization. However, the differential expression data support the possibility that these proteins may be peripherally involved in the processes of biomineralization and coccolithogenesis. Perhaps of greater significance is the fact that the data presented in this study provide novel and important information to the research community for developing molecular and biochemical approaches to determine the metabolic roles of these genes and gene products differentially expressed under calcifying conditions in E. huxleyi.
In summary, our results support the value of SSH-based EST sequencing to complement existing EST resources for functional genomics research in marine coccolithophorid algae. We have detected 168 differentially expressed transcripts in comparing coccolith-bearing and non-coccolith-bearing E. huxleyi cells maintained in phosphate-limited and phosphate-replete media, respectively, by screening SSH libraries. The specific expression of a subset of these genes was confirmed by a combination of dot blotting, virtual Northern blotting, and real-time RT-PCR. Given the fact that methods for genetic transformation and mutagenesis have not been developed for E. huxleyi, or for any coccolithophorid, additional full-length cDNA cloning, combined with more detailed functional studies using temporal cDNA microarray analysis or RNA interference methodologies, will help us to understand the molecular mechanisms underlying biomineralization and coccolithogenesis.
Acknowledgments
We thank Jeremy Young (Department of Palaeontology, The Natural History Museum, London, United Kingdom) for kindly providing the scanning electron micrograph of E. huxleyi. We also thank Patrick Quinn for his helpful input on the manuscript.
This work was supported by a grant from the National Institutes of Health (GM 059833).
REFERENCES
- 1.Andersen, O. K. 1981. Coccolith formation and calcification in an N-cell culture of Emiliania huxleyi during phosphorus-limited growth in batch and chemostat cultures. Ph.D. thesis. University of Oslo, Oslo, Norway.
- 2.Balch, W. M., P. M. Holligan, and K. A. Kilpatrick. 1992. Calcification, photosynthesis and growth of the bloom-forming coccolithophore Emiliania huxleyi. Cont. Shelf Res. 12:1353-1374. [Google Scholar]
- 3.Brown, C. W., and J. A. Yoder. 1994. Coccolithophorid blooms in the global ocean. J. Geophys. Res. 99:7467-7482. [Google Scholar]
- 4.Buitenhuis, E. T., H. J. W. de Baar, and M. J. W. Veldhuis. 1999. Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species. J. Phycol. 35:949-959. [Google Scholar]
- 5.Corstjens, P. L. A. M., A. van der Kooij, C. Linschooten, G.-J. Brouwers, P. Westbroek, and E. W. de Vrind-de Jong. 1998. GPA, a calcium-binding protein in the coccolithophorid Emilianis huxleyi (Prymnesiophyceae). J. Phycol. 34:622-630. [Google Scholar]
- 6.Cramer, R. A., and C. B. Lawrence. 2004. Identification of Alternaria brassicicola genes expressed in planta during pathogenesis of Arabidopsis thaliana. Fungal Genet. Biol. 41:115-128. [DOI] [PubMed] [Google Scholar]
- 7.de Vrind-de Jong, E. W., and J. P. M. de Vrind. 1997. Algal deposition of carbonates and silicates, p. 267-307. In J. F. Banfield and K. H. Nealson (ed.), Geomicrobiology: interactions between microbes and minerals. Mineralogical Society of America, Washington, D.C.
- 8.Diatchenko, L., Y.-F. C. Lau, A. P. Campbell, A. Chenchik, F. Moqadam, B. Huang, S. Lukyanov, K. Lukyanov, N. Gurskaya, E. D. Sverdlov, and P. D. Siebert. 1996. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc. Natl. Acad. Sci. USA 93:6025-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diatchenko, L., S. Lukyanov, Y. Lau, and P. Siebert. 1999. Suppression subtractive hybridization: a versatile method for identifying differentially expressed genes. Methods Enzymol. 303:349-380. [DOI] [PubMed] [Google Scholar]
- 10.Gotliv, B.-A., L. Addadi, and S. Weiner. 2003. Mollusk shell acidic proteins: in search of individual functions. Chembiochem. 4:522-529. [DOI] [PubMed] [Google Scholar]
- 11.Guillard, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29-60. In W. L. Smith and M. H. Chanley (ed.), Culture of marine invertebrate animals. Plenum Press, New York, N.Y.
- 12.Happe, T., and A. Kaminski. 2002. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 269:1022-1032. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen, K., S. L. S. Stipp, J. R. Young, and P. R. Bown. 2003. Tailoring calcite: nanoscale AFM of coccolith biocrystals. Am. Mineralogist 88:2040-2044. [Google Scholar]
- 14.Holligan, P. M., E. Fernandez, J. Aiken, W. M. Balch, P. Boyd, P. H. Burkill, M. Finch, S. B. Groom, G. Malin, K. Muller, D. A. Purdie, C. Robinson, C. Trees, S. M. Turner, and P. van de Wal. 1993. A biogeochemical study of the coccolithophore Emiliania huxleyi in the North Atlantic. Global Biogeochem. Cycles 7:879-900. [Google Scholar]
- 15.Illies, M. R., M. T. Peeler, A. M. Dechtiaruk, and C. A. Ettensohn. 2002. Identification and developmental expression of new biomineralization proteins in the sea urchin Stronglyocentrotus purpuratus. Dev. Genes Evol. 212:419-431. [DOI] [PubMed] [Google Scholar]
- 16.Kim, M., S. Kim, S. Kim, and B. D. Ki. 2001. Isolation of cDNA clones differentially accumulated in the placenta of pungent pepper by suppression subtractive hybridization. Mol. Cells 11:213-219. [PubMed] [Google Scholar]
- 17.Laguna, R., J. Romo, B. A. Read, and T. M. Wahlund. 2001. Induction of phase variation events in the life cycle of the marine coccolithophorid Emiliania huxleyi. Appl. Environ. Microbiol. 67:3824-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao, H., F. L. Wong, T. H. Phang, M. Y. Cheung, W. Y. Li, G. Shao, X. Yan, and H. M. Lam. 2003. GmPAP3, a novel purple acid phosphatase-like gene in soybean induced by NaCl stress but not phosphorus deficiency. Gene 318:103-111. [DOI] [PubMed] [Google Scholar]
- 19.Mahalingam, R., A. Gomez-Buitrago, N. Eckardt, N. Shah, A. Guevara-Garcia, P. Day, R. Raina, and N. V. Fedoroff. 2003. Characterizing the stress/defense transcriptome of Arabidopsis. Genome Biol. 4:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh, M. E. 2003. Regulation of CaCO3 formation in coccolithophores. Comp. Biochem. Physiol. 136:743-754. [DOI] [PubMed] [Google Scholar]
- 21.Moyano, E., I. Portero-Robles, N. Medina-Escobar, V. Valpuesta, J. Munoz-Blanco, and J. L. Caballero. 1998. A fruit-specific putative dihydroflavonol 4-reductase gene is differentially expressed in strawberry during the ripening process. Plant Physiol. 117:711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozaki, N., S. Sakuda, and H. Nagasawa. 2001. Isolation and some characterization of an acidic polysaccharide with anti-calcification activity from coccoliths of a marine alga, Pleurochrysis carterae. Biosci. Biotechnol. Biochem. 65:2330-2333. [DOI] [PubMed] [Google Scholar]
- 23.Paasche, E. 1998. Roles of nitrogen and phosphorus in coccolith formation in Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 33:33-42. [Google Scholar]
- 24.Paasche, E., and S. Bruback. 1994. Enhanced calcification in the coccolithophorid Emiliania huxleyi (Haptophyceae) under phosphorous limitation. Phycologia 33:324-330. [Google Scholar]
- 25.Palenik, B., and S. E. Henson. 1997. The use of amides and other organic nitrogen sources by the phytoplankton Emiliania huxleyi. Limnol. Oceanogr. 42:1544-1551. [Google Scholar]
- 26.Sekino, K., and Y. Shiraiwa. 1994. Accumulation and utilization of dissolved inorganic carbon by a marine unicellular coccolithophorid, Emiliania huxleyi. Plant Cell Physiol. 35:353-361. [Google Scholar]
- 27.Shen, X., A. M. Belcher, P. K. Hansma, G. D. Stucky, and D. E. Morse. 1997. Molecular cloning and characterization of lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufescens. J. Biol. Chem. 272:32472-32481. [DOI] [PubMed] [Google Scholar]
- 28.Shiraiwa, Y. 2003. Physiological regulation of carbon fixation in the photosynthesis and calcification of coccolithophorids. Comp. Biochem. Physiol. B 136:775-783. [DOI] [PubMed] [Google Scholar]
- 29.Sikes, C. S., R. D. Roer, and K. M. Wilbur. 1980. Photosynthesis and coccolith formation: inorganic carbon sources and net inorganic reaction deposition. Limnol. Oceanogr. 25:248-261. [Google Scholar]
- 30.Stoll, H. M., P. Ziveri, M. Geisen, I. Probert, and J. R. Young. 2002. Potential and limitations of Sr/Ca ratios in coccolith carbonate: new perspectives from cultures and monospecific samples from sediments. Phil. Trans. Ser. A 360:719-747. [DOI] [PubMed] [Google Scholar]
- 31.Strommer, J., R. Gregerson, and M. Vayda. 1993. Isolation and characterization of plant mRNA, p. 49-66. In B. R. Glick and J. E. Thompson (ed.), Methods in plant molecular biology and biotechnology, 1st ed. CRC Press, Boca Raton, Fla.
- 32.Tyrrell, T., and A. H. Taylor. 1996. A modeling study of Emiliania huxleyi in the NE Atlantic. J. Mar. Syst. 9:83-112. [Google Scholar]
- 33.van Bleijswijk, J. D. L., and M. J. W. Velduis. 1995. In situ gross growth rates of Emiliania huxleyi in enclosures with different phosphate loadings revealed by diel changes in DNA content. Mar. Ecol. Prog. Ser. 121:271-277. [Google Scholar]
- 34.Van Der Wal, P., E. W. de Jong, P. Westbroek, W. C. de Bruijin, and A. A. Muller-Stapel. 1983. Ultrastructural polysaccharide localization in calcifying and naked cells of the coccolithophorid Emiliania huxleyi. Protoplasma 118:157-168. [Google Scholar]
- 35.van Emburg, P. R. 1989. Coccolith formation in Emiliania huxleyi. Ph.D. thesis. Leiden University, Leiden, The Netherlands.
- 36.Wahlund, T. M., A. R. Hadaegh, R. Clark, B. Nguyen, M. Fanelli, and B. A. Read. 2004. Analysis of expressed sequence tags from calcifying cells of the marine coccolithophorid, Emiliania huxleyi. Mar. Biotechnol. 6:278-290. [DOI] [PubMed] [Google Scholar]
- 37.Wahlund, T. M., X. Zhang, and B. A. Read. 2004. EST expression profiles from calcifying and non-calcifying cultures of Emiliania huxleyi. J. Micropaleontol. 51:145-155. [Google Scholar]
- 38.Wang, W., P. Wu, M. Xia, Z. Wu, Q. Chen, and F. Liu. 2002. Identification of genes enriched in rice roots of the local nitrate treatment and their expression patterns in split-root treatment. Gene 297:93-102. [DOI] [PubMed] [Google Scholar]
- 39.Weiss, I. M., S. Kaufmann, K. Mann, and M. Fritz. 2000. Purification and characterization of perlucin and perlustrin, two new proteins from the shell of the mollusk, Haliotis laevigata. Biochem. Biophys. Res. Commun. 267:17-21. [DOI] [PubMed] [Google Scholar]
- 40.Wustman, B. A., R. Santos, B. Zhang, and S. J. Evans. 2002. Identification of a “glycine-loop”-like coiled structure in the 34 aa pro, gly, met repeat domain of the biomineral-associated protein, PM27. Biopolymers 65:362-372. [DOI] [PubMed] [Google Scholar]
- 41.Young, J. R., S. A. Davis, P. R. Bown, and S. Mann. 1999. Coccolith ultrastructure and biomineralisation. J. Struct. Biol. 126:195-215. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, B., G. Xu, and J. S. Evans. 2000. Model peptide studies of sequence repeats derived from the intracrystalline biomineralization protein, SM50. II. Pro, asn-rich tandem repeats. Biopolymers 54:464-475. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, X. N., H. Wang, Z. C. Qu, M. M. Ye, and D. L. Shen. 2002. Cloning and prokaryotic expression of a salt-induced cDNA encoding a chloroplastic fructose-1,6-diphosphate aldolase in Dunaliella salina (Chlorophyta). DNA Seq. 13:195-202. [DOI] [PubMed] [Google Scholar]