Abstract
Fifty-nine erm(B)-positive Enterococcus faecium strains isolated from pigs, broilers, and humans were typed using multilocus sequence typing (MLST), and the coding sequence of the erm(B) gene was determined. Identical erm(B) gene sequences were detected in genetically unrelated isolates. Furthermore, genetically indistinguishable strains were found to contain different erm(B) alleles. This may suggest that horizontal exchange of the erm(B) gene between animal and human E. faecium strains or the existence of a common reservoir of erm(B) genes might be more important than direct transmission of resistant strains.
Macrolide, lincosamides, and streptogramin (MLS) antibiotics may be important as alternative therapy for treatment of enterococcal infections in humans (19, 21). Acquired resistance to these antibiotics has frequently been described in enterococci originating from humans as well as from animals (4, 17, 21). The erm(B) gene conferring cross-resistance against MLS antibiotics is very common among human, porcine, and poultry enterococci (1, 12, 17, 21, 22).
Two different routes of antimicrobial drug resistance transfer from animal to humans are of importance, a direct and an indirect way of transfer. Direct transmission of resistance occurs when resistant zoonotic bacteria infect humans. In the indirect way, resistant bacteria originating from animals transfer their resistance genes horizontally to the human bacterial population. This occurs when animal-derived strains are able to survive, at least temporarily, in human sites, primarily the gut (1, 7, 9, 15).
This study was undertaken to obtain better insights into transmission dynamics of macrolide resistance genes between human and animal Enterococcus faecium strains, focusing on the hospital environment and pigs and broilers, respectively. The possible linkage to other genes was likewise investigated. For this purpose, macrolide- and lincosamides-resistant, erm(B)-positive E. faecium strains isolated from pigs, broilers, and humans were typed using multilocus sequence typing (MLST), and the coding sequence of their erm(B) gene was determined. Furthermore, the presence of the vanA and aphA-3 genes, encoding vancomycin and aminoglycoside resistance, respectively, was determined to study genetic linkage of multiple resistance genes in macrolide resistant E. faecium strains.
A total of 59 erm(B)-positive E. faecium strains isolated from humans (19 strains), pigs (19 strains), pork carcasses (4 strains), and broilers (17 strains) were used in this study (Table 1). Twelve human isolates (H) were obtained from different patients in one hospital. The other human strains were isolated from seven different nonhospitalized (NH) (hemodialysis) patients and were collected in the same hospital. The porcine isolates all originated from different farms and slaughterhouses. The poultry isolates originated from nine different farms (Farm A to I). The isolates were identified as E. faecium using a PCR described by Kariyama et al. (14). The detection of the erm(B) gene was performed with a PCR described by Martel et al. (16).
TABLE 1.
erm(B) allele and MLST data of the 59 E. faecium isolated recovered from humans, pigs, and broilersa
| erm(B) allele | Strain | Host | Source | Farm | MLST allelic profile
|
ST | CC | Genogroup | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| adk | atpA | ddl | gyd | gdh | purK | pstS | ||||||||
| erm(B-1) | HE28 | Human (NH) | Stool | 5 | 5 | 3 | 1 | 1 | 9 | 1 | 37 | 5 | A | |
| AME31 | Pig | Colon | 5 | 3 | 5 | 1 | 1 | 3 | 1 | 139 | D138-139 | D | ||
| HE3 | Human (NH) | Stool | 5 | 4 | 2 | 1 | 1 | 9 | 1 | 6 | 5 | A | ||
| P2 | Pig | Colon | 5 | 5 | 2 | 1 | 1 | 3 | 1 | 184 | 5 | A | ||
| VZ200 | Pig | Carcass | 5 | 3 | 2 | 1 | 1 | 9 | 1 | 140 | 5 | A | ||
| erm(B-2) | K331 | Broiler | Cloaca | F | 1 | 5 | 2 | 1 | 6 | 6 | 7 | 9 | 9 | B |
| HE2 | Human (H) | Urine | 1 | 7 | 1 | 5 | 1 | 1 | 1 | 18 | D18-186 | C1 | ||
| AME4 | Pig | Colon | 1 | 15 | 1 | 1 | 1 | 2 | 1 | 121 | S | C | ||
| HE35 | Human (NH) | Stool | 1 | 9 | 3 | 1 | 1 | 6 | 1 | 25 | 9 | C | ||
| P10 | Pig | Colon | 1 | 3 | 3 | 1 | 1 | 2 | 1 | 32 | S | C | ||
| K44 | Broiler | Cloaca | B | 1 | 5 | 2 | 1 | 24 | 14 | 39 | 197 | S | B | |
| erm(B-3) | P8 | Pig | Colon | 5 | 4 | 2 | 1 | 1 | 9 | 1 | 6 | 5 | A | |
| erm(B-4) | K40 | Broiler | Cloaca | B | 1 | 5 | 2 | 1 | 7 | 6 | 7 | 194 | 9 | B |
| K413 | Broiler | Cloaca | G | 1 | 2 | 2 | 1 | 1 | 30 | 11 | 199 | S | B | |
| P7 | Pig | Colon | 5 | 5 | 2 | 1 | 1 | 9 | 1 | 5 | 5 | A | ||
| AME129 | Pig | Colon | 5 | 5 | 2 | 1 | 1 | 9 | 1 | 5 | 5 | A | ||
| AME61 | Pig | Colon | 1 | 29 | 2 | 1 | 12 | 6 | 6 | 143 | S | B | ||
| HE47 | Human (H) | Blood | 1 | 9 | 2 | 1 | 1 | 6 | 1 | 26 | 9 | B | ||
| SH48 | Pig | Carcass | 5 | 28 | 2 | 2 | 1 | 12 | 34 | 144 | S | NA | ||
| WV235 | Pig | Carcass | 8 | 5 | 4 | 1 | 12 | 6 | 1 | 142 | S | NA | ||
| LO122 | Pig | Carcass | 5 | 5 | 5 | 1 | 1 | 9 | 1 | 141 | 5 | A | ||
| erm(B-5) | K283 | Broiler | Cloaca | I | 1 | 9 | 2 | 1 | 1 | 3 | 33 | 200 | S | B |
| K57 | Broiler | Cloaca | A | 1 | 5 | 2 | 1 | 6 | 6 | 7 | 9 | 9 | B | |
| K61 | Broiler | Cloaca | A | 1 | 5 | 2 | 1 | 6 | 6 | 7 | 9 | 9 | B | |
| P3 | Pig | Colon | 1 | 31 | 1 | 1 | 1 | 1 | 1 | 187 | 17 | C1 | ||
| AME136 | Pig | Colon | 5 | 3 | 5 | 1 | 1 | 3 | 14 | 138 | D138-139 | D | ||
| AME46 | Pig | Colon | 1 | 4 | 5 | 1 | 1 | 3 | 1 | 29 | D29-123 | B | ||
| HE15 | Human (NH) | Stool | 1 | 2 | 3 | 1 | 1 | 26 | 1 | 136 | S | C | ||
| HE29 | Human (NH) | Stool | 1 | 2 | 3 | 1 | 1 | 26 | 1 | 136 | S | C | ||
| HE41 | Human (H) | Stool | 5 | 5 | 2 | 1 | 1 | 9 | 20 | 185 | 5 | A | ||
| HE55 | Human (NH) | Stool | 1 | 4 | 5 | 1 | 9 | 3 | 20 | 183 | S | B | ||
| P5 | Pig | Colon | 1 | 5 | 5 | 1 | 1 | 3 | 1 | 123 | D29-123 | B | ||
| HE43 | Human (H) | Urine | 1 | 7 | 2 | 5 | 1 | 1 | 1 | 186 | D18-186 | C1 | ||
| K180 | Broiler | Cloaca | C | 1 | 5 | 2 | 1 | 1 | 6 | 7 | 8 | 9 | B | |
| AME30 | Pig | Colon | 5 | 4 | 2 | 1 | 1 | 9 | 1 | 6 | 5 | A | ||
| V7A | Pig | Colon | 5 | 4 | 2 | 1 | 12 | 9 | 20 | 149 | S | A | ||
| erm(B-6) | P1 | Pig | Colon | 5 | 5 | 2 | 1 | 1 | 9 | 1 | 5 | 5 | A | |
| erm(B-7) | P9 | Pig | Colon | 5 | 5 | 2 | 1 | 1 | 9 | 1 | 5 | 5 | A | |
| erm(B-8) | K106 | Broiler | Cloaca | E | 1 | 16 | 2 | 2 | 6 | 6 | 7 | 198 | S | B |
| K176 | Broiler | Cloaca | D | 1 | 5 | 2 | 1 | 7 | 6 | 7 | 194 | 9 | B | |
| K311 | Broiler | Cloaca | I | 1 | 9 | 2 | 1 | 6 | 3 | 7 | 82 | 9 | B | |
| K318 | Broiler | Cloaca | F | 1 | 5 | 2 | 1 | 6 | 6 | 36 | 196 | 9 | B | |
| K29 | Broiler | Cloaca | B | 1 | 5 | 2 | 1 | 7 | 6 | 7 | 194 | 9 | B | |
| K396 | Broiler | Cloaca | F | 1 | 9 | 2 | 1 | 1 | 6 | 7 | 195 | 9 | B | |
| K431 | Broiler | Cloaca | G | 1 | 9 | 2 | 1 | 7 | 6 | 1 | 124 | 9 | B | |
| K351 | Broiler | Cloaca | G | 1 | 5 | 2 | 1 | 7 | 6 | 7 | 194 | 9 | B | |
| erm(B-9) | HE46 | Human (H) | Sterile wound | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 16 | 17 | C1 | |
| HE10 | Human (H) | Abdominal fluid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | 17 | C1 | ||
| HE40 | Human (NH) | Urine | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | 17 | C1 | ||
| HE42 | Human (H) | Urine | 1 | 1 | 1 | 1 | 1 | 1 | 33 | 181 | 17 | C1 | ||
| HE44 | Human (H) | Urine | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 16 | 17 | C1 | ||
| HE45 | Human (H) | Urine | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 16 | 17 | C1 | ||
| HE48 | Human (H) | Blood | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 | 17 | C1 | ||
| HE49 | Human (H) | Urine | 1 | 1 | 1 | 1 | 1 | 1 | 19 | 182 | 17 | C1 | ||
| AME81 | Pig | Colon | 1 | 7 | 1 | 5 | 1 | 1 | 1 | 18 | D18-186 | C1 | ||
| erm(B-10) | P6 | Pig | Colon | 1 | 3 | 3 | 1 | 1 | 2 | 1 | 32 | S | C | |
| HE22 | Human (H) | Wound fluid | 1 | 7 | 1 | 1 | 1 | 1 | 11 | 19 | S | C1 | ||
| K294 | Broiler | Cloaca | H | 1 | 9 | 2 | 1 | 6 | 6 | 7 | 157 | 9 | B | |
| erm(B-11) | P4 | Pig | Colon | 1 | 15 | 2 | 1 | 12 | 6 | 1 | 49 | S | B | |
NA, not assigned; H, hospitalized; NH, nonhospitalized; ST, sequence type; CC, clonal complexes were identified using the eBURST algorithm (8) with stringent (6 of 7 shared alleles) group definition with 1,000 bootstrap replicates. Genogroup was determined by amplified fragment length polymorphism (AFLP) analysis (10).
MLST was performed as described by Homan et al. (10). The eBURST program was used to assess the genetic relationships of genotypes, to assign isolates to genetic complexes, and to study patterns of evolutionary descent of isolates within a complex (8). Sequence types were also compared with a database of MLST data from 139 epidemiologically unlinked E. faecium isolates from humans and livestock in The Netherlands, Australia, United Kingdom, United States, and France (http://efaecium.mlst.net) (10).
The coding sequence of the erm(B) gene was amplified using primers ORR1, 5′-ATGAACAAAAATATAAAATATT-3′, and ORR2, 5′-TTATTTCCTCCCGTTAAA-3′. The amplicon was sequenced using primers E1, 5′GAAAAGA/GTACTCAACCAAATA3′; E2, 5′AGTAACGGTACTTAAATTGTTTAC3′; E3, 5′CCATACCACAGATGTTCCAG3′; and E4, 5′AGATAGATGTCAGACGCACG3′ (18).
The primers and the PCR running conditions for the detection of the resistance genes aphA-3 and vanA were as described elsewhere (5, 20). To determine whether the erm(B) gene was linked with the vat(E) gene, a PCR was used with primers described by Jensen et al. (13).
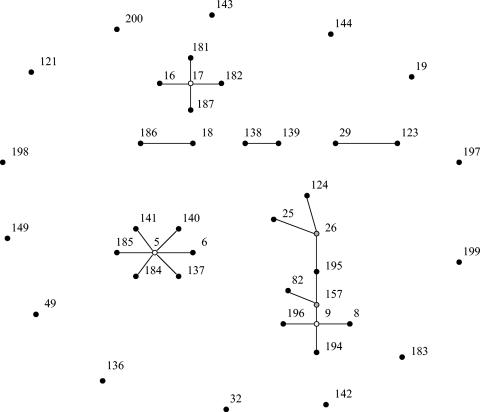
Application of MLST revealed 42 different sequence types (STs) among the 59 isolates. eBURST analysis clustered the STs into three major complexes, three minor complexes, and 14 singletons (Fig. 1) (Table 1). Clonal complex (CC) 9 with presumed ancestral type ST-9 consists of 10 STs, representing 13 isolates from broilers and 2 isolates from humans (one H and one NH) and could be considered a poultry-specific CC. In contrast, CC5, with ST-5 as its presumed founder, could be classified as a pig-specific CC, since it consists of seven STs representing nine isolates from pigs and three isolates from humans (two NH and one H). The majority of isolates from hospitalized patients grouped in CC17, with ST-17 as the presumed founder. Complex 17 has been recognized as a clinically relevant genetic complex harboring hospital outbreak and human clinical isolates from various countries on five continents (R. Willems et al., unpublished data). Also, one isolate from a nonhospitalized person and one isolate recovered from the colon of a pig grouped in CC17.
FIG. 1.
Clustering of 42 sequence types, representing 59 isolates, with eBURST (8). This algorithm identifies the founder of a complex or genogroup of related sequence types and subsequent patterns of evolutionary descent. The primary founder of a complex, indicated with a white dot, is defined as the ST with the largest number of single-locus variants (SLVs). In larger complexes there may be secondary founders of additional lineages that have a number of SLVs of their own. These secondary founders are indicated in gray. Numbers correspond to ST numbers. The area of each circle corresponds to the number of isolates of the ST. All complexes (major and minor) and singletons (STs not belonging to any of the identified complexes) are shown.
Grouping of the 59 isolates with 139 previously typed isolates (10) showed that all poultry isolates grouped in genogroup B, which predominantly contains poultry isolates, while 83% of the isolates recovered from hospitalized patients clustered in the ‘epidemic’ genogroup C1. Isolates from pigs and nonhospitalized persons were more dispersed over different genogroups (Table 1).
Sequence alignment and unweighted-pair group method with arithmetic mean clustering resulted in 11 allelic variations of the erm(B) gene, designated erm(B-1) through erm(B-11), which are more than 98.6% identical. The erm(B-3), erm(B-6), erm(B-7), and erm(B-11) sequences were found in one porcine isolate only. The erm(B-8) sequence was found in eight isolates, all originating from broilers, representing six different STs, of which five belonged to CC9, suggesting an evolutionary link between these seven broiler isolates (Fig. 1) (Table 1). The erm(B-1) and erm(B-9) sequences were detected in human isolates as well as in isolates originating from pigs. The erm(B-9) allele was predominantly found in isolates from hospitalized patients in CC17. erm(B-2), erm(B-4), erm(B-5), and erm(B-10) were found in genetically unrelated isolates from humans, pigs, and broilers. Interestingly, in three more cases, a human- and animal-derived isolate carried an identical erm(B) gene and belonged to the same CC. The sequences erm(B-2), erm(B-4), erm(B-8), erm(B-9), and erm(B-10) were 100% identical to the erm(B) sequences in GenBank entries of E. faecalis plasmid pAMβ-1 (Y00116), E. faecalis plasmid pRE25 (X92945), a genetic element containing erm(B) and vat(E) (AF242872), E. faecium plasmid pUW786 (AF516335), and transposon Tn917 (M11180), respectively (2, 13, 24, 26, 30).
The kanamycin resistance gene aphA-3 was detected in 18 human, 10 porcine, and 3 broiler isolates. All isolates that carried the erm(B-9) allele contained the aphA-3 gene. The glycopeptide resistance gene vanA was found in 13 human and 2 broiler isolates. This gene was detected in the four human isolates (HE42, HE44, HE45, and HE46) containing the erm(B-9) allele. Three of these isolates belonged to the same clone (ST-16) and all belonged to the same CC17. In 10 broiler isolates, including all isolates containing the erm(B-8) allele, the erm(B) gene was linked with the vat(E) gene. Such linkage was not detected in the other isolates.
MLST of a heterogeneous set of animal- and human-derived E. faecium isolates revealed that isolates from pigs and nonhospitalized humans are genetically more diverse than isolates from broilers and hospitalized humans. These results are in agreement with other studies (3, 32).
Similarly, there is also a greater homology among the broiler erm(B) sequences, whereas more heterogeneity is seen among the porcine erm(B) sequences. In the eight broiler isolates containing the erm(B-8) allele, the erm(B) gene was linked with the vat(E) gene, confirming the wide distribution of this link among E. faecium poultry isolates found in other studies (13, 29). In contrast to Werner et al. (29), this link was not found among isolates from humans.
Although no porcine, broiler, and human strains with identical STs as well as identical erm(B) sequences were detected, the clustering of animal and human strains in four cases with identical erm(B) genes in clonal complexes indicates that these animal and human strains originate from the same founder, which may suggest an epidemiological link between these isolates. The human isolate HE3 and the porcine isolate VZ200 are single-locus variants (differing in one locus only) and contain an identical erm(B) gene. This might indicate a direct transmission of a resistant isolate between animals and humans and is in agreement with other studies (6, 28).
Several human isolates tested in the present studies belonged to the same clone. This might indicate intrahospital spread of these strains because the human isolates were collected in the same hospital. Also, two porcine isolates and three broiler isolates belonged to one clone. This is most probably due to interfarm spread, since these isolates originated from different farms. Broiler isolates K57 and K61 belong to the same clone and originated from the same farm, indicating clonal spread within a flock.
One porcine isolate (P3) belonged to CC17 that thus far contained no animal isolates. This may indicate a spread of a human strain to a pig. A human-to-animal transmission has also been described by Seguin et al. (25).
Within the three major complexes and two of the minor complexes, different erm(B) sequences were detected. In several cases, these alleles were identical to a described erm(B) sequence carried by a conjugative plasmid or transposon. This suggests that these strains may have acquired the erm(B) gene from different sources by horizontal transfer. This is in agreement with Simjee et al. and Descheemaeker et al. (6, 27).
In addition, identical erm(B) alleles were found in porcine, broiler, and human isolates belonging to different complexes. This also points towards horizontal transfer of the erm(B) gene in this case between genetically different E. faecium isolates from pigs, broilers, and humans or the existence of a common reservoir of erm(B) genes. This is in agreement with previous studies (6, 11, 23, 31).
The majority of the isolates belonging to CC17 carried the erm(B-9) allele. This sequence is identical to the erm(B) gene sequence found by Werner et al. (30) in a multiresistance gene cluster linking vanA, erm(B), and aadE-sat4-aphA-3 on plasmid pUW786 in a clinical isolate of E. faecium (30). In four isolates containing the erm(B-9) allele, vanA as well as aphA-3 were also detected, suggesting the presence of pUW786. This plasmid might be responsible for the spread of this erm(B) allele in strains belonging to closely related STs that circulate in hospitals. The presence of erm(B-9) in the remaining isolates might be due to other mobile elements containing this allele. One may speculate on the independent evolution of the erm(B) gene through mutations after introduction in a hospital strain followed by horizontal spread of this allele between hospital strains. This is in agreement with Willems et al. (31).
In conclusion, identical erm(B) sequences were found in genetically linked but also in genetically unrelated isolates from animals and humans. Furthermore, different erm(B) alleles were found in genetically indistinguishable isolates. This might indicate that resistance exchange between animals and humans possibly is due to direct transmission of resistant E. faecium strains, but horizontal exchange of the erm(B) gene between E. faecium isolates from animals and humans or the existence of a common reservoir of erm(B) genes might be more important.
Nucleotide sequence accession numbers.
The six alleles not described previously were deposited in GenBank under accession numbers AY827541 [erm(B-1)], AY827542 [erm(B-3)], AY827543 [erm(B-5)], AY827544 [erm(B-6)], AY827545 [erm(B-7)], and AY827546 [erm(B-11)].
Acknowledgments
The technical assistance of Sofie De Bruyckere and Marga van Santen-Verheuvel is gratefully appreciated. We thank L. A. Devriese for helpful suggestions and informative discussions. Human E. faecium strains were kindly provided by H. Goossens, Department of Medical Microbiology, University of Antwerp.
This work was supported by the Federal Public Service Public Health, Food Chain Safety and Environment (DG4), Brussels, Belgium.
REFERENCES
- 1.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 2.Brehm, J., G. Salmond, and N. Minton. 1987. Sequence of adenine methylase gene of the Streptococcus faecalis plasmid pAM beta 1. Nucleic Acids Res. 15:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruinsna, N., R. J. L. Willems, A. E. van den Bogaard, M. van Santen-Verheuvel, N. London, C. Driessen, and E. E. Stobberingh. 2002. Different levels of genetic homogeneity in vancomycin-resistant and -susceptible Enterococcus faecium isolates from different human and animal sources analyzed by amplified-fragment length polymorphism. Antimicrob. Agents Chemother. 46:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butaye, P., L. A. Devriese, and F. Haesebrouck. 2001. Differences in antibiotic patterns of Enterococcus faecalis and Enterococcus faecium strains isolated from farm and pet animals. Antimicrob. Agents Chemother. 45:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derbise, A., S. Aubert, and N. El Solh. 1997. Mapping the regions carrying the three contiguous antibiotic resistance genes aadE, satA, and aphA-3 in the genomes of staphylococci. Antimicrob. Agents Chemother. 41:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Descheemaeker, P. R. M., S. Chapelle, L. A. Devriese, P. Butaye, P. Vandamme, and H. Goossens. 1999. Comparison of glycopeptide-resistant Enterococcus faecium isolates and glycopeptide resistance genes of human and animal origins. Antimicrob. Agents Chemother. 43:2032-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donabedian, S. M., L. A. Thal, E. Hershberger, M. B. Perri, J. W. Chow, P. Bartlett, R. Jones, K. Joyce, S. Rossiter, K. Gay, J. Johnson, C. Mackinson, E. Debess, J. Madden, F. Angulo, and M. J. Zervos. 2003. Molecular characterization of Gentamicin-resistant enterococci in the United States: evidence of spread from animals to humans through food. J. Clin. Microbiol. 41:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes, J. R., L. L. English, P. J. Carter, T. Proescholdt, K. Y. Lee, D. D. Wagner, and D. G. White. 2003. Prevalence of antimicrobial resistance of Enterococcus species isolated from retail meats. Appl. Environm. Microbiol. 69:7153-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. A. van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen, L. B., P. Ahrens, L. Dons, R. N. Jones, A. M. Hammerum, and F. M. Aarestrup. 1998. Molecular analysis of Tn1546 in Enterococcus faecium from animals and humans. J. Clin. Microbiol. 36:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen, L. B., N. Frimodt-Moller, and F. M. Aarestrup. 1999. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol. Lett. 170:151-158. [DOI] [PubMed] [Google Scholar]
- 13.Jensen, L. B., A. M. Hammerum, and F. M. Aarestrup. 2000. Linkage of vat(E) and erm(B) in streptogramin-resistant Enterococcus faecium isolates from Europe. Antimicrob. Agents Chemother. 44:2231-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kariyama, R., R. Mitsuhata, J. W. Chow, D. B. Clewell, and H. Kumon. 2000. Simple and reliable multiplex PCR assay for surveillance isolates of vancomycin-resistant enterococci. J. Clin. Microbiol. 38:3092-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klare, I., C. Konstabel, D. Badstubner, G. Werner, and W. Witte. 2003. Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int. J. Food Microbiol. 88:269-290. [DOI] [PubMed] [Google Scholar]
- 16.Martel, A., M. Baele, L. A. Devriese, H. Goossens, H. J. Wisselink, A. Decostere, and F. Haesebrouck. 2001. Prevalence and mechanism of resistance against macrolides and lincosamides in Streptococcus suis isolates. Vet. Microbiol. 83:287-297. [DOI] [PubMed] [Google Scholar]
- 17.Martel, A., L. A. Devriese, A. Decostere, and F. Haesebrouck. 2003. Presence of macrolide resistance genes in streptococci and enterococci isolated from pigs and pork carcasses. Int. J. Food Microbiol. 84:27-32. [DOI] [PubMed] [Google Scholar]
- 18.Martel, A. 2004. Ph.D. thesis. Seizing the macrolide resistance in the porcine Gram-positive flora and its implications for human health. Ghent University, Belgium.
- 19.Molander, A., and G. Dahlén. 2003. Evaluation of the antimicrobial potential of tetracycline or erythromycin mixed with calcium hydroxide as intracanal dressing against Enterococcus faecalis in vivo. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 96:744-750. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Hernandez, X., S. Méndez-Alvarez, and F. Claverie-Martin. 2002. A PCR assay for rapid detection of vancomycin-resistant enterococci. Diagn. Microbiol. Infect. Dis. 42:273-277. [DOI] [PubMed] [Google Scholar]
- 21.Portillo, A., F. Ruiz-Larrea, M. Zarazaga, A. Alfonso, J. L. Martinez, and C. Torres. 2000. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemother. 44:967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schouten, M. A., R. J. L. Willems, W. A. G. Kraak, J. Top, J. A. A. Hoogkamp-Korstanje, and A. Voss. 2001. Molecular analysis of Tn1546-like elements in vancomycin-resistant enterococci isolated from patients in Europe shows geographic transposon type clustering. Antimicrob. Agents Chemother. 45:986-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz, F. V., V. Perreten, and M. Teuber. 2000. Sequence of the 50kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170-187. [DOI] [PubMed] [Google Scholar]
- 25.Seguin, J. C., R. D. Walker, J. P. Caron, W. E. Kloos, C. G. George, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 1999. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J. Clin. Microbiol. 37:1459-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw, J. H., and D. B. Clewell. 1985. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J. Bacteriol. 164:782-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simjee, S., D. G. White, D. D. Wagner, J. Meng, S. Qaiyumi, S. Zhao, and P. F. McDermott. 2002. Identification of vat(E) in Enterococcus faecalis isolates from retail poultry and its transferability to Enterococcus faecium. Antimicrob. Agents Chemother. 46:3823-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stobberingh, E., A. van den Bogaard, N. London, C. Driessen, J. Top, and R. Willems. 1999. Enterococci with glycopeptide resistance in turkey, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of The Netherlands: evidence for transmission of vancomycin resistance from animals to humans? Antimicrob. Agents Chemother. 43:2215-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner, G., B. Hildebrandt, I. Klare, and W. Witte. 2000. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int. J. Med. Microbiol. 290:543-548. [DOI] [PubMed] [Google Scholar]
- 30.Werner, G., B. Hildebrandt, and W. Witte. 2003. Linkage of erm(B) and aadE-sat4-aphA-3 in multiple-resistant Enterococcus faecium isolates of different ecological origins. Microb. Drug Resist. 1(9 Suppl.):S9-S16. [DOI] [PubMed] [Google Scholar]
- 31.Willems, R. J. L., J. Top, N. Van Den Braak, A. van Belkum, D. J. Mevius, G. Hendriks, M. van Santen-Verheuvel, and J. D. A. van Embden. 1999. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob. Agents Chemother. 43:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van den Bogaard, and J. D. A. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]