Abstract
Filarial nematode parasites establish long-term chronic infections in the context of an antiparasite immunity that is strongly biased toward a Th2 response. The mechanisms that lead to this Th2 bias toward filarial antigens are not clear, but one possibility is that the parasites produce molecules that have the capacity to proactively modify their immunological environment. Here we report that filarial parasites of humans secrete a homologue of the human proinflammatory cytokine macrophage migration inhibitory factor (MIF) that has the capability of modifying the activity of human monocytes/macrophages. A cDNA clone isolated from a Brugia malayi infective-stage larva expression library encoded a 12.5-kDa protein product (Bm-MIF) with 42% identity to human and murine MIF. MIF homologues were also found to be expressed in the related filarial species Wuchereria bancrofti and Onchocerca volvulus. Bm-mif was transcribed by adult and larval parasites, and the protein product was found in somatic extracts and in the parasite’s excretory-secretory products. Immunohistocytochemistry revealed that Bm-MIF was localized to cells of the hypodermis/lateral chord, the uterine wall, and larvae developing in utero. Unexpectedly, the activities of recombinant Bm-MIF and human MIF on human monocytes/macrophages were found to be similar. When placed with monocytes/macrophages in a cell migration assay, Bm-MIF inhibited random migration. When placed away from cells, Bm-MIF induced an increase in monocyte/macrophage migration that was specifically inhibited by neutralizing anti-Bm-MIF antibodies. Bm-MIF is the first demonstration that helminth parasites produce cytokine homologues that have the potential to modify host immune responses to promote parasite survival.
The parasitic nematodes Wuchereria bancrofti, Brugia malayi, and Brugia timori, the etiological agents of lymphatic filariasis in humans, infect over 120 million people worldwide. Typically, individuals become infected in early childhood through the bite of an infective mosquito, and in areas of endemicity the infection is maintained for decades. The adult parasites reside in the lumen of the lymphatics, where the females release thousands of first-stage larvae, or microfilariae (Mf), each day into the peripheral circulation. Although filariasis presents with a spectrum of clinical states, a general classification defines two major groups: microfilaremic individuals who have no discernible symptoms of infection and patients who are amicrofilaremic and have developed chronic disease. A majority of infected individuals are in the asymptomatic group. The immunity in asymptomatic/microfilaremic individuals is strongly associated with a Th2-type response with high immunoglobulin E (IgE) and IgG4 levels and eosinophilia (30, 39, 40, 54). In contrast, the immune responses of the amicrofilaremic/chronic pathology group are more of the Th1 type (30, 38). Although the specific roles that Th1 and Th2 responses play in pathology and immunity are still to be resolved, it is becoming clear that filarial nematode development in the context of a Th2 immune response conveys an advantage for parasite survival in the human host.
Among the important issues relating to parasite-host interactions is our lack of understanding of the mechanisms that result in the induction and maintenance of the type of immunity that accommodates chronic, long-term filarial infections. The ability to persist in an immunologically competent host has led to the suggestion that filarial parasites have evolved specific measures to counter immune defenses. In addition to anatomical and physical defenses such as size, motility, and the presence of a thick outer covering, the cuticle, filarial parasites produce and release as excretory-secretory (ES) products a number of molecules that have the potential to play a role in immune evasion. The proposed mechanisms for a number of these putative ES-derived immune modulators, such as proteases (60), protease inhibitors (37, 69), and antioxidant proteins (17, 36, 61), have these molecules working locally to neutralize or to interfere with the effector molecules of the innate and adaptive defense responses. Whatever impact these enzymes and enzyme inhibitors have on local effector mechanisms, it is unlikely that their actions account for the systemic immune effects that accompany filarial infections. One possible explanation for the strong bias toward Th2-type immunity seen during asymptomatic filariasis is that the parasite is able to misdirect the immune response through the presentation of epitopes that have an inherent preference for eliciting a Th2-type response (26) or by the elaboration of ligands and/or receptors that are capable of altering normal signaling between cells of the immune response. Recent reports suggest that filarial parasites of animals have the capacity to proactively shape their immunological environments (21, 68).
We report here the characterization of the first parasite-derived homologue of a human cytokine. A gene encoding a homologue of human cytokine macrophage migration inhibitory factor (MIF) was isolated as a cDNA from the human filarial parasite B. malayi. MIF was originally described as a factor that inhibited the random migration of macrophages (12, 18). With the cloning of human and mouse MIF (46, 65) and the development of new reagents to study this molecule, the scope of MIF’s biological activities has been significantly expanded. MIF is constitutively expressed by T cells, macrophages, and eosinophils (5, 16, 51). It influences T-cell (5) and NK-cell (3) activation and immunoglobulin synthesis (42) and leads to an amplification of inflammatory responses (16). MIF also plays important roles in endotoxin shock (4, 9), the response to glucocorticoid hormones (15), and the regulation of insulin secretion (64), and it has been implicated in cellular growth and differentiation events (35, 56, 66). Interestingly, MIF has been shown to have isomerase-tautomerase activity (7, 49, 50, 71), but the physiological substrate for this activity has not been identified.
The B. malayi-derived MIF homologue (Bm-MIF) reported here was found in both somatic extracts and the ES products of all of the stages developing in the vertebrate host. A recombinant form of Bm-MIF was shown to have, depending on the assay conditions, both migration inhibitory and chemotactic activities on human peripheral blood-derived monocytes/macrophages. Subsequent analysis demonstrated that mif-like genes are expressed by the related filarial parasites W. bancrofti and Onchocerca volvulus. The possible significance of parasite-derived MIF in the immunobiology of infection, immune evasion, and nematode biology are discussed.
MATERIALS AND METHODS
Isolation and sequencing.
The clone AS3ISB220 was identified from a B. malayi third-stage larval (L3) cDNA expression library (JHU93SLBmL3) as part of an expressed sequence tag (EST) sequencing initiative (11). AS3ISB220 in pBluescript (Stratagene, La Jolla, Calif.) was sequenced completely in both directions by the fluorescent dideoxy terminator method on an Applied Biosystems (Foster City, Calif.) 377 automated sequencer. The DNA and deduced amino acid sequences of clone AS3ISB220 were compared to the public protein, nucleic acid, and EST databases by using both the BLAST (1) and FASTA (47) algorithms. Motif analysis was carried out with the University of Wisconsin Genetics Computer Group suite of programs (22). Clone AS3ISB220 was designated a putative B. malayi homologue of the mammalian cytokine macrophage migration inhibitory factor (Bm-mif).
RT-PCR.
mRNA was isolated from 10,000 Mf, 1,000 L3s, 500 fourth-stage larvae (L4s), or 25 adults by the Microfast Track method (Invitrogen, San Diego, Calif.). Single-stranded cDNA was generated by reverse transcription (RT), as recommended by the manufacturer (Stratagene). PCR was carried out on appropriate dilutions of the templates by using Bm-mif-specific primers (W4598 and W4599 [see below]). The Bm-mif results were normalized to the transcriptional levels of the constitutively expressed gene, nucleoside diphosphate kinase (Bm-ndk) (28). Bm-ndk was amplified by using the primers XSL (5′-GCTCTAGAGCGGTTTAATTACCCAAGTTTGAG-3′) and W4353 (5′-GCTGAAGGCAAGGAATCT-3′). Following 20 cycles of amplification, the PCR products were resolved on an agarose gel and stained with ethidium bromide, and the gel image was digitized for densitometry analysis by using NIH Image (developed by the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). The results for each stage were expressed as a ratio of the density of the Bm-mif products to the density of the Bm-ndk products from the same template.
Genomic DNA.
Adult B. malayi nematodes were snap frozen in liquid nitrogen, ground to a powder with a mortar and pestle, and then suspended in 1 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 50 mM EDTA, 1 M NaCl, 0.5% sodium dodecyl sulfate [SDS], 100 μg of proteinase K [Boehringer Mannheim, Indianapolis, Ind.] per ml, 36 mM β-mercaptoethanol, and 25 μg of DNase-free RNase [Boehringer Mannheim]). The genomic DNA was used as a template in PCR with primers W4684 (5′-GAAGATCTATGCCATATTTTACG-3′) and W4685 (5′-GAAGATCTTTATCCCAAAGTAGATCC-3′). The resulting PCR product was purified (QIAquick; Qiagen, Chatsworth, Calif.), and both strands were sequenced to completion.
Subcloning, expression, and purification.
The sequence corresponding to the Bm-mif open reading frame (ORF) was isolated by PCR. The 5′ primer, W4598 (5′-AGATCTGCAGCTATGCCATATTTTACGATTGATAC-3′), contained a recognition site for PstI and 23 bp of Bm-mif ORF that included the codon for the initiating methionine (underlined). The 3′ primer, W4599 (5′-AAAAGCTTATCATCCCAAGTAGATCCATTAAAAGC-3′), contained a recognition site for HindIII, a stop codon, and the last 23 bp of the Bm-mif ORF (underlined). After 25 cycles of amplification, the PCR product was subcloned in frame into pRSET B (Invitrogen), which had been digested with PstI and HindIII. Recombinant plasmids were used to transform Escherichia coli BL21, and the synthesis of recombinant, histidine-tagged Bm-MIF (Bm-MIF-His) was induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 2 h at 37°C. Purified Bm-MIF-His was isolated from a nickel column (Ni-NTA Agarose; Qiagen) with elution buffer (500 mM imidazole in 20 mM Tris-HCl, [pH 7.9]). Bm-MIF-His was dialyzed against elution buffer adjusted to pH 6.0, and the protein concentration was determined by using the bicinchoninic acid assay (Pierce, Rockford, Ill.).
Bm-mif was also expressed as a non-fusion protein in pET11b (Novagen, Madison, Wis.). The Bm-mif ORF was PCR amplified from pBluescript by using the 5′ primer W4690, which contained a recognition site for NdeI and the first 15 bp of the Bm-mif ORF (underlined) (5′-GGAATTCCATATGCCATATTTTACG-3′), and the 3′ primer W4689, which contained a recognition site for NdeI, a stop codon, and the last 15 bp of the Bm-mif ORF (underlined) (5′-GGAATTCCATATGTTATCCCAAAGTAGA-3′). The PCR product was then cloned in frame into the pET11b vector. Recombinant plasmids were used to transform E. coli BL21, and recombinant Bm-MIF synthesis was induced with 0.4 mM IPTG for 1.5 h at 37°C.
The purification protocol for recombinant Bm-MIF was modified from that of Bernhagen et al. (10). Bacterial extracts were passed over a HiTrap Q anion-exchange column (Pharmacia Biotech, Piscataway, N.J.) followed by selective elution from a butyl-Sepharose hydrophobic interaction column (Pharmacia Biotech). Fractions were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (32) and silver stained with Silverstain Plus (Bio-Rad, Hercules, Calif.). Those fractions deemed to be >97% pure were pooled and processed for refolding.
Refolding.
The protocol used to generate bioactive Bm-MIF was as described for mammalian MIF (10). Briefly, protein was denatured with 10 mM dithiothreitol (DTT) and 8 M urea (pH 6.8) for 1 h at room temperature. Gradually, 10 mM DTT in TBS (20 mM Tris–150 mM NaCl [pH 6.8], prepared in tissue culture-grade water) was added until the urea was diluted to 2 M. The protein was then dialyzed overnight at 4°C against TBS with 10 mM DTT. The TBS with 10 mM DTT was gradually replaced with TBS by dialysis at 4°C. Bioactive, lipopolysaccharide (LPS)-free human MIF was prepared as described previously (10). Each preparation was tested for endotoxin levels by the Limulus amebocyte lysate chromogenic assay (BioWhittaker, Walkersville, Md.). The preparations used had <2 pg of endotoxin/μg of protein.
Antisera.
Anti-Bm-MIF-His and anti-Bm-MIF antibodies were produced in mice (27). Rabbit polyclonal anti-mouse MIF and the anti-MIF neutralizing monoclonal antibody IIID-9 have been described previously (16).
Western blots.
After being snap frozen in liquid nitrogen, parasites were ground to a fine powder, resuspended in SDS-PAGE sample buffer (0.5 M Tris [pH 6.8], 40% glycerol, 8% SDS, 4% 2-mercaptoethanol, and 0.002% bromphenol blue), incubated for 10 min at 100°C, sonicated, and centrifuged to pellet particulate material. The parasite proteins were separated by SDS-PAGE under reducing conditions on a 10 to 20% acrylamide gradient, and Western blots were prepared and immunostained as described previously (28). Extracts from O. volvulus, Ascaris suum, Dirofilaria immitis, Schistosoma mansoni, and Caenorhabditis elegans were prepared from frozen organisms.
ES products.
Mf, L4 (day 15 postinfection), and adult B. malayi organisms were obtained by lavaging the peritoneal cavity of intraperitoneally infected male gerbils (Meriones unguiculatus) and were washed and placed in 10 ml of Dulbecco modified Eagle medium (GIBCO BRL Life Technologies, Grand Island, N.Y.). The Mf, L4, and adult parasites were cultured separately at 37°C for 18 h, after which the media were collected and processed. Media containing ES products were centrifuged at high speed to remove particulate matter. After addition of 1 mM EGTA, 1 mM EDTA, 2 mM PMSF (phenylmethylsulfonyl fluoride), and 0.2 mM TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone) as protease inhibitors (all from Sigma), the ES products were concentrated with an Ultrafree-MC concentrator (Millipore) with a molecular mass cutoff of 5,000 kDa. The protein concentration was estimated by the bicinchoninic acid protein assay (Pierce).
Immunohistocytochemistry.
Adult B. malayi parasites were lavaged from the peritoneal cavity of a male gerbil at 120 days postinfection, transferred to Sorenson’s buffer (4:1 0.2 M sodium phosphate dibasic–0.2 M sodium phosphate monobasic, pH 7.4) for 1 min, and then fixed in 4% paraformaldehyde at 4°C for 16 h. The worms were processed for cryostat sectioning and immunostained with anti-Bm-MIF-His antibodies by a previously described protocol (28).
Monocyte migration assays.
The monocyte/macrophage/lymphocyte-rich fraction of blood obtained from healthy donors was isolated by centrifugation on a Percoll cushion (Pharmacia Biotech) (14). Migration assays were carried out in a Micro Chemotaxis Chamber (Neuro Probe, Cabin John, Md.) by a protocol modified from that of Schleimer et al. (52). Briefly, wells in the bottom plate were filled with 28 μl of PAGCM (110 mM NaCl, 5 mM KCl, 25 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 42 mM NaOH, 0.003% human serum albumin, 0.1% d-glucose, 1 mM MgCl2, 1 mM CaCl2) or with recombinant human MIF or recombinant Bm-MIF diluted in PAGCM. A polycarbonate, polyvinylpyrrolidone-free filter containing 5-μm pores was fitted to the bottom plate, and the top plate was secured. Cells (50 μl of 1.8 × 106 cells/ml) suspended in PAGCM were placed in the top wells. The chamber was incubated for 3 h at 37°C in a 5% CO2 humidified chamber. The filter was then processed for staining, and the cells were counted under the microscope (total magnification, ×400) with the aid of an ocular grid. Each experimental condition was replicated in three to nine wells for any one donor and repeated with cells from three or more donors. The data are expressed as the percentage of cells that migrated compared to that in the medium control, which we designated 100%.
Nucleotide sequence accession number.
The nucleotide sequence of Bm-mif has been assigned database accession no. U88035 and assigned to EST cluster BMC00238.
RESULTS
Clone AS3ISB220 was initially identified as part of an EST sequencing effort (11). The 583-bp full-length insert contained the conserved 22-nucleotide spliced leader 1 (SL1) sequence trans-spliced to the 5′ end 29 bp upstream from the initiating ATG codon (see U88035). The insert contained an ORF of 348 nucleotides and had 184 bp of 3′ untranslated sequence that included a consensus polyadenylation signal (AATAAA) 16 bp upstream from a poly(A)17 tail.
When the deduced amino acid sequence was compared to the protein sequences contained in the major databases, it was determined that clone AS3ISB220 has significant identity to the vertebrate cytokine macrophage MIF. At 115 amino acids, Bm-MIF is identical in size to the described MIF proteins from human, cow, mouse, rat, and chicken (Fig. 1A). The Bm-MIF protein sequence is 40 to 42% identical and 65 to 66% similar to the vertebrate-derived MIF sequences (Fig. 1A). In addition, Bm-MIF contains a sequence (positions 55 through 68) that conforms to the MIF family signature motif [(D/E)PCA(L/V)C(V/S)LXSIGX(I/V)G].
FIG. 1.
(A) Alignment of the amino acid sequences of MIF proteins from B. malayi (Bm-MIF [accession no. U88035]), human (hu-MIF [accession no. 1942977]), bovine (bv-MIF [accession no. 730025]), mouse (mu-MIF [accession no. 462602]), rat (ra-MIF [accession no. 1170956]), chicken (ch-MIF [accession no. 400257]), W. bancrofti (Wb-MIF [accession no. AF040629]), O. volvulus (Ov-MIF [accession no. G975442]), and C. elegans (Ce-MIF-1 [accession no. Z78012] and Ce-MIF-2 [accession no. Z71259]). Filled areas indicate those amino acids with identity to Bm-MIF. The amino acids that form the six β-strands and the two α-helices reported in the three-dimensional structure of human MIF (42) are indicated by the open and hatched boxes, respectively. The positions of the 10 invariant residues are indicated by filled diamonds. The positions where the amino acids are conserved in 8 of the 10 sequences are marked with open boxes. Amino acid positions are numbered along the left margin. Percent identity and similarity of MIF sequences to Bm-MIF are indicated at the upper right. (B) Genomic organization of the mif genes from B. malayi (Bm-mif), human (Hu-mif), mouse (Mu-mif), and C. elegans (Ce-mif-1 and Ce-mif-2). Introns are shown as open boxes, and exons are filled boxes. The size of each region (in bases) is indicated above introns and beneath exons. Vertical bars indicate the axis of the pseudo-twofold symmetry of the MIF protein (nucleotides 159 to 165 of the ORF).
The coding region of Bm-mif was labeled and used as a probe to screen a female cDNA library from the closely related filarial species W. bancrofti. The full-length W. bancrofti cDNA homologue of Bm-mif, Wb-mif, encodes 115 amino acids with 97% identity at the nucleotide level (data not shown) and 95% identity at the amino acid level to Bm-MIF (Fig. 1A).
The search of the major protein and nucleic acid databases revealed three additional nematode-derived sequences with significant identity to Bm-MIF (Fig. 1A). A gene identified as an expressed sequence from L3 of a related filarial parasite of humans, O. volvulus (Ov-mif) was found to be 26% identical and 50% similar to Bm-MIF. Two ORFs identified in the genome of the free-living nematode C. elegans appear to encode MIF homologues. The C. elegans gene C52E4.2, designated Ce-mif-1, encodes a protein of 120 amino acids that is 29% identical and 56% similar to Bm-MIF. Gene F13G3.9, designated Ce-mif-2, encodes 147 amino acids with 23% identity and 44% similarity to Bm-MIF.
An alignment of MIF protein sequences revealed that the amino acids at 24 positions were identical in at least 8 of the 10 MIF sequences, with 10 of those positions being invariant (Fig. 1A). Of particular note is the conservation of Pro at position 2, which has been shown to be critical for isomerase function in vertebrate MIF, and the highly conserved nature of the carboxy-terminal six residues, which are thought to be necessary for formation of the stable MIF homotrimer (7).
The three-dimensional structure of the human MIF monomer has two antiparallel α-helices and six β-strands that are arranged in pseudo-twofold symmetry (βαββ-βαββ) (55). When the human MIF primary sequences corresponding to these domains of major secondary structure were compared to the corresponding sequences from Bm-MIF (Fig. 1A), no apparent concentration of identical residues in these structural domains was found.
Genomic organization.
A PCR-based strategy was used to determine the genomic organization of Bm-mif. A 952-bp genomic fragment was obtained and sequenced to reveal that Bm-mif contains a single 604-bp intron at 108 bases into the ORF (Fig. 1B). The intron splice site sequences followed the GU-AG convention, with the 3′ splice site conforming to the extended 3′ splice site consensus (UUUU[C/U]AG) found in C. elegans introns (13) (see GenBank accession no. AF002699). The results of Southern blot hybridizations indicated that Bm-mif was present in single copy in the B. malayi genome (data not shown). A comparison of the genomic organization of Bm-mif with vertebrate and C. elegans mif genes demonstrated that, with the exception of the size of the first exon of Bm-mif, human mif, and murine mif, there were no interspecies similarities in intron or exon structure (Fig. 1B). In addition, the genomic organization of the MIF genes did not reflect the pseudosymmetrical domain structure of the protein. The divergent nature of the genomic organizations suggests that MIF is an ancient gene that has diverged through evolution or a gene that has arisen separately in vertebrates and nematodes and gained similarity through convergent evolution.
Transcription of Bm-mif.
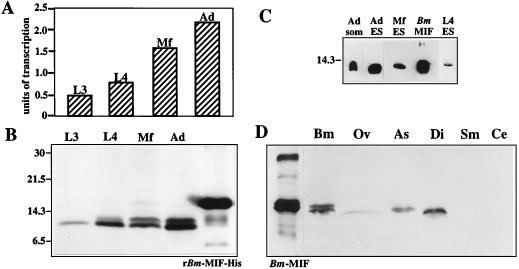
Estimates of the relative levels of transcription of Bm-mif in the various stages of B. malayi development were made by using semiquantitative RT-PCR. The amount of Bm-mif PCR product for each stage was indexed to the levels obtained from a gene known to be constitutively expressed in B. malayi, nucleoside diphosphate kinase (Bm-ndk) (28). While all stages transcribed Bm-mif, expression levels in the adult and Mf stages of development were approximately twice the levels observed in L3 and L4 parasites (Fig. 2A).
FIG. 2.
(A) Transcription of Bm-mif. Poly(A)+ mRNA was isolated from L3s, L4s, Mf, and adult (Ad) parasites, converted to single-stranded cDNA, and used as a template in PCR with primers to amplify Bm-mif or the constitutively expressed nucleoside diphosphate kinase (Bm-ndk). After 20 cycles of amplification, the Bm-ndk and Bm-mif PCR products were resolved by agarose gel electrophoresis and stained with ethidium bromide, and densitometry was carried out on a digitized image of the gel. To compare the levels of Bm-mif transcription between parasite stages, the amount of Bm-mif PCR product was indexed to the amount of Bm-ndk PCR product from the same template. Bm-mif transcription was expressed in arbitrary units. The data are representative of results from three independent repetitions. (B) Bm-MIF (Bm-MIF) in extracts from L3s L4s, Mf, and Ad parasites. Parasite proteins were separated on a 10 to 20% polyacrylamide gradient under reducing conditions, transferred to a nitrocellulose membrane, and immunostained. The positions of molecular mass standards are indicated along the left margin, in kilodaltons. (C) Bm-MIF in ES products. L4s, Mf, and Ad parasites were placed in culture for 18 h. Culture medium was concentrated, separated on a 15% polyacrylamide gel under reducing conditions, transferred to a nitrocellulose membrane, and immunostained with anti-Bm-MIF-His antibodies. Recombinant Bm-MIF (Bm MIF) and an adult somatic antigen (Ad som) extract were included as positive controls. (D) Detection of MIF-like proteins in extracts from B. malayi (Bm), O. volvulus (Ov), A. suum (As), D. immitis (Di), S. mansoni (Sm), and C. elegans (Ce). Protein extracts were separated on a 15% polyacrylamide gel under reducing conditions, transferred to a nitrocellulose membrane, and immunostained with anti-Bm-MIF-His antibodies.
Bm-MIF.
Bm-mif was expressed as a histidine-tagged fusion protein (Fig. 3). Mouse anti-Bm-MIF-His antibodies were used to immunostain Western blots containing equal amounts of protein from staged B. malayi parasites to evaluate the nature of parasite-derived Bm-MIF (Fig. 2B). Two bands were resolved in extracts of L4s, Mf, and adults, with estimated molecular masses of 12.3 and 12.8 kDa. Although only the 12.3-kDa band was resolved in extracts of L3 parasites here, both bands were resolved when more L3 protein was placed on the blot (data not shown). The presence of two potential N-linked glycosylation sites suggested that one explanation for the two bands could be differential glycosylation. However, treatment of B. malayi adult extracts with endoglycosidase F resulted in no shift in the pattern of antibody recognition, indicating that N-linked glycosylation does not account for the two immunoreactive bands (data not shown).
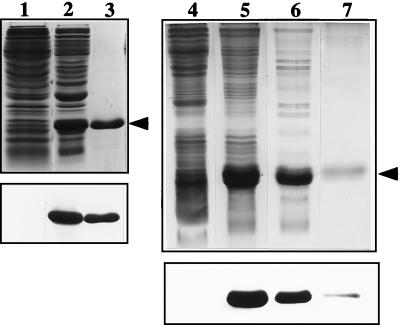
FIG. 3.
Purification of recombinant Bm-MIF. Lanes 1 to 3, pRSETB. Protein extracts from induced bacteria carrying pRSETB with no insert (lane 1) and from induced bacteria carrying pRSETB-Bm-mif (lane 2) are shown. The induced extract was passed over a nickel affinity column, and the 16.5-kDa His-tagged Bm-MIF (Bm-MIF-His) fusion protein was eluted with imidazole (lane 3). Extracts were separated on a 15% polyacrylamide gel under reducing conditions and visualized with Coommassie blue staining (top). The proteins from an identical gel were transferred to a nitrocellulose membrane and immunostained with anti-Bm-MIF-His (bottom). Lanes 4 to 7, pET11b. Protein extracts from induced bacteria carrying pET11b with no insert (lane 4) and from induced bacteria carrying the pET11b-Bm-mif construct (lane 5) are shown. The extracts of induced bacteria were passed over a MonoQ column (lane 6), and the flowthrough was placed on a butyl-Sepharose column. The 12.5-kDa Bm-MIF protein was eluted from the butyl-Sepharose with decreasing amounts of salt (lane 7). Proteins were separated on a 15% polyacrylamide gel under reducing conditions and visualized with silver staining (top). The proteins from an identical gel were transferred to a nitrocellulose membrane and immunostained with anti-Bm-MIF-His (bottom).
Adults, Mf, and L4s were placed in culture and the media were collected, concentrated, and analyzed by immunoblotting to determine if Bm-MIF was a component of the parasite’s ES products. Bm-MIF was detectable in the ES products from all of the stages tested (Fig. 2C). It was estimated that Bm-MIF makes up approximately 1.5% of the ES proteins released by adult parasites (data not shown).
Immunoblot analysis was used to determine if the anti-Bm-MIF-His antibodies could detect Bm-MIF-like molecules in extracts from other nematode and helminth parasite species. Proteins with an estimated molecular mass of 12.3 kDa were present in extracts of the parasitic species O. volvulus, A. suum, and D. immitis but not in extracts of the digenetic trematode S. mansoni (Fig. 2D). Under the conditions used here, anti-Bm-MIF-His antibodies did not detect putative MIF homologues from C. elegans.
Immunohistocytochemistry.
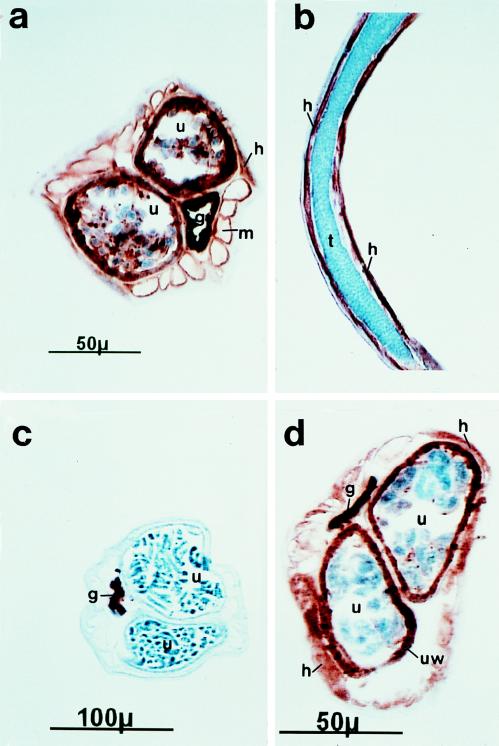
Anti-Bm-MIF-His bound strongly to the uterine lining and to the noncellular material associated with the developing embryos in sections of gravid female parasites (Fig. 4a). In addition, Bm-MIF was localized to the hypodermis and to the surface of the major body wall muscle bundles. In sections of females where more-developed embryos were evident, the embryos showed low levels of staining (Fig. 4d). In sections of male parasites, antibody staining was restricted to cells of the hypodermis/lateral chord (Fig. 4b).
FIG. 4.
Immunohistocytochemical localization of Bm-MIF in female (a, c, and d) and male (b) B. malayi organisms. Cryostat sections were immunostained with mouse anti-Bm-MIF-His antibodies (a, b, and d) or normal mouse serum (c) followed by a biotinylated horse anti-mouse antibody. Antibody binding was resolved with the Vector ABC immunostaining kit. g, intestine; h, hypodermis; m, somatic muscles; t, testis; u, uterus. Under the conditions used here, the intestine stained nonspecifically.
Migration assays.
Initial studies demonstrated that amino acid tags on either the N or the C termini of recombinant Bm-MIF resulted in the production of molecules with no bioactivity. In order to produce a recombinant protein with bioactivity, we prepared constructs to produce Bm-MIF as a non-fusion polypeptide in pET11B (Fig. 3). Prior to use in the migration assays, the purified recombinant Bm-MIF was denatured with DTT and urea and then gradually refolded to an active conformation by dialysis.
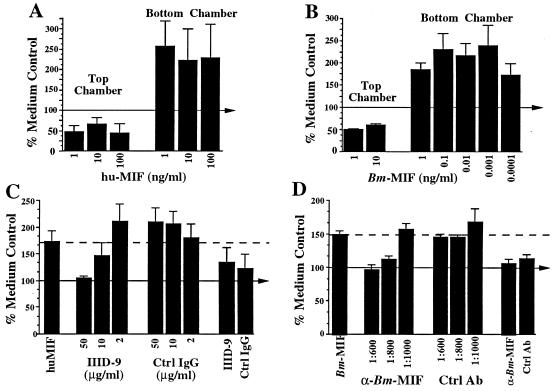
One standard for bioactivity in murine and human MIF is its ability to inhibit random migration of macrophages in an in vitro assay. Therefore, we established a migration assay to determine if Bm-MIF had any direct action on human peripheral blood monocytes/macrophages and to test the hypothesis that Bm-MIF would alter the action of human MIF. The results of migration assays produced two unexpected results. First, in our initial studies with human MIF, we found that while recombinant human MIF did inhibit random migration when placed in the top chamber with the peripheral blood monocytes/macrophages, when it was placed in the bottom chamber of the apparatus it functioned as a chemoattractant (Fig. 5A). The ability of human MIF to induce chemotaxis was specifically inhibited in a concentration-dependent fashion by an anti-human MIF monoclonal antibody (Fig. 5C). Therefore, depending on the specific circumstances, human MIF can inhibit or enhance chemotaxis of human monocytes and macrophages.
FIG. 5.
Bm-MIF (Bm-MIF) and human MIF (hu-MIF) induce changes in macrophage migration. Human peripheral blood monocytes/macrophages were placed in the top well of a microchemotaxis chamber, as outlined in Materials and Methods. The level of migration of cells exposed to human MIF (A and C), Bm-MIF (B and D), or medium was assessed after 3 h of incubation at 37°C. The level of migration of cells in medium alone was designated 100% and is indicated by the arrow in each panel. Each series of test and control treatments was carried out in triplicate on cells isolated from three to six healthy donors. Error bars represent standard errors of the means for the individual donors. (A) Human MIF at three concentrations in the top wells or the bottom wells of the chemotaxis chamber. (B) Bm-MIF at various concentrations in the top wells or the bottom wells of the chemotaxis chamber. (C) Specific inhibition of human MIF-induced migration with monoclonal antibody IIID-9. The migration induced by 1 ng of human MIF per ml in the bottom chamber is shown by the first bar and by the dashed line. IIID-9 or its corresponding isotype-matched control monoclonal antibody (Ctrl IgG) was preincubated with 1 ng of human MIF per ml for 1 h at 25°C and then placed in the bottom chamber. Reagent control reaction mixtures included cells incubated with IIID-9 or the isotype control IgG only under identical culture conditions (eighth and ninth bars, respectively). (D) Specific inhibition of Bm-MIF-induced migration of human monocytes/macrophages by anti-Bm-MIF polyclonal sera. The migration induced by 1 ng of Bm-MIF per ml in the bottom chamber of the apparatus is shown by the first bar and by the dashed line. Anti-Bm-MIF serum or preimmunization control serum (Ctrl Ab) was preincubated with 1 ng of Bm-MIF per ml for 1 h at 25°C prior to placement of the mixture in the bottom chamber. Reagent control reaction mixtures included cells incubated with anti-Bm-MIF or preimmune serum only under identical culture conditions (eighth and ninth bars, respectively).
The second unexpected result was found in migration assays using the parasite-derived MIF. Bm-MIF had an effect on human monocytes/macrophages that was nearly identical to that of human MIF. When placed in the top chamber with the cells, it inhibited migration by ∼50 to 75%, and when placed in the bottom chamber, Bm-MIF enhanced migration (Fig. 5B). This activity of Bm-MIF was also inhibited in a concentration-dependent fashion by a mouse anti-Bm-MIF antibody (Fig. 5D). It is important to note that LPS in the recombinant MIF preparation was below 2 pg/μg of protein and that, in this assay system, LPS in the bottom wells actually inhibited migration (data not shown).
To test the possibility that interactions of Bm-MIF with host-derived MIF on the same cell lead to altered activity, Bm-MIF plus human MIF were placed in the bottom wells of the migration chambers. Together, the two cytokines induced the same level of monocyte/macrophage migration as seen when the cells were exposed to only one of the molecules (data not shown).
DISCUSSION
In order to survive immune attack, pathogens have adopted a variety of strategies to evade or modify immune responses. There is an increasing appreciation that one of the approaches used by pathogens is to produce homologues of host molecules that are important in immune signaling to blunt or divert inflammation. This approach has been best documented with viruses. Poxviruses secrete chemokine-like molecules and chemokine binding factors that result in altered trafficking of infiltrating leukocytes into areas of virus infection (31, 33). Cytomegalovirus blocks the ability of the acquired and innate immune systems to recognize infected cells by interfering with the expression of host class I major histocompatibility complex molecules and deploying a virus-encoded class I-like molecule (24). A number of viruses, including Epstein-Barr virus, produce an interleukin 10 homologue that presumably functions in altering antiviral responses (25, 70). We present here the first example of a parasite-derived molecule with significant homology to a human cytokine that functions to alter the behavior of human cells.
The results of in vitro macrophage migration assays indicate that Bm-MIF is chemotactic for human cells. Assuming that Bm-MIF retains this function in vivo, it raises the question of why a parasite would release a molecule that functions in attracting cells important in immune signaling and defense. It is possible that the parasites attract host macrophages as the first step in a process that leads to alterations in their induction and/or effector functions. Bm-MIF or other ES molecules may change the levels of certain cytokines produced by antigen-presenting cells, creating an immunological environment that promotes parasite survival. This may, in part, explain the strong Th2 bias seen in a majority of chronically infected individuals (30, 38, 40, 54). Another potential reason for manipulation of the cytokine profile may be to obtain host-derived factors necessary for parasite development. Cytokines have been shown to be essential growth and reproductive cues in both protozoan (6) and helminthic parasites (2).
Although no additional parasite-derived cytokine homologues have been identified, parasites have been shown to produce factors that modify immune responses. Both Leishmania spp. and Trypanosoma cruzi release proteins that change the levels of cytokine expression of human macrophages and dendritic cells (20, 48). African trypanosomes secrete a molecule that selectively induces CD8+ T cells to secrete gamma interferon (62). Recent reports suggest that ES products from filarial parasites of animals have the capacity to proactively shape their immunological environment as well. A 62-kDa glycoprotein released by the rodent filarial parasite Acanthocheilonema viteae interferes with antigen receptor-mediated activation of B cells and T cells (21). A factor isolated from the ES products produced by the major filarial parasite of dogs, D. immitis, increases receptor expression on T and B cells, Th2 cytokine production, and IgE synthesis (68). In addition, evidence is accumulating that suggests that both protozoan (44) and helminthic (19, 29) parasites exploit the transforming growth factor β serine-threonine kinase receptor-ligand system to promote parasite survival and development.
Over 30 years ago, one of the first lymphokine activities described was a soluble factor elaborated by activated T cells that had the ability to inhibit random migration of macrophages (12, 18). With the cloning of genes encoding human and mouse MIF (46, 65), its perception as a T-cell-derived molecule that simply functions to inhibit macrophage migration has been expanded to an appreciation that the sources of MIF are diverse and its actions are complex. In addition to T cells, macrophages have been shown to be both an important target for and a major source of MIF (16). MIF is also produced by eosinophils (51), the corticotrophic cells of the anterior pituitary gland (9, 10), the β cells of the islets of Langerhans (64), the differentiating cells of the eye lens (66), and the cells of the basal layer of human epidermis and keratinocytes (53). The association of MIF with a variety of cell types in vertebrates suggests that it may carry out multiple functions.
As the cellular sources of MIF have become increasingly complex, so too have the MIF-related immune functions. MIF has direct effects on T-cell activation and antibody production (5). The T-cell-derived glycosylation inhibition factor that regulates IgE synthesis by B cells has been shown to be identical to MIF (42). MIF has been associated with the macrophage infiltrates in delayed-type hypersensitivity lesions (8), glomerulonephritis (34), rheumatoid and collagen-induced arthritis (41, 43), and acute respiratory distress syndrome (23). Preformed MIF is released from macrophages (16) and cells of the anterior pituitary (9) into the circulation in response to LPS and mediates an upregulation of tumor necrosis factor alpha production (9, 16). Glucocorticoids also induce the release of MIF from macrophages and from T cells, where it functions to override the strong suppressive action that steroids have on T-cell proliferation and cytokine production (4). MIF has been shown to inhibit NK-cell-mediated cytotoxicity by preventing the release of perforin (3). These observations strongly implicate MIF as an important factor in disease pathogenesis.
Of particular importance here is the demonstration of a role for MIF activity in the context of parasitic diseases. Macrophages secrete large amounts of MIF after phagocytosing malaria-infected erythrocytes or malaria hemazoin, and there are increased circulating levels of MIF during Plasmodium chabaudi infections in mice (41). Treatment of murine macrophages in vitro with MIF significantly enhances their ability to kill Leishmania major (45), and administration to mice in vivo significantly reduces disease (67). It is likely that host MIF also plays an important role in regulating filarial infections.
The association of vertebrate MIF with cells undergoing growth and differentiation suggests that it may play a role in normal cell biology. MIF has been identified as an important protein in rapidly differentiating cells (35, 53, 66) and in embryos (57, 63). The localization of Bm-MIF to multiple cell types in B. malayi (Fig. 3) and the presence of two MIF-like genes from the free-living nematode C. elegans suggest that the nematode-derived molecules may have important actions on nematode cells. Further work has demonstrated that both Ce-mif-1 and Ce-mif-2 are transcribed (data not shown). The presence of MIF homologues in C. elegans provides a well-defined and highly manipulatable system to characterize the roles that MIF proteins may have in the cell biology of nematode development.
MIF is distinct from other cytokines in that it also catalyzes chemical reactions. It has been shown to have tautomerase activity on at least two substrates (49, 50). Although the native cellular substrates(s) is not known, it is possible that at least part of the bioactivity of MIF is mediated through its ability to tautomerize. We have shown that Bm-MIF also has tautomerase activity (data not shown), although the level of activity is significantly lower than mammalian MIF when assayed with the currently known substrates. The tautomerase activity of MIF is dependent on the proper folding of the molecule and the presence of the conserved residues at position 2 (Pro) and at the C terminus of the molecule (7), all of which are present in Bm-MIF. Crystallographic studies of recombinant human MIF (55, 58) have shown that the monomer contains two antiparallel α-helices that pack against a four-stranded β-sheet and assemble into a barrel-shaped homotrimer with a solvent-accessible channel that runs the entire length of the threefold axis (55). The C-terminal residues are important in intersubunit interactions that lead to a stable homotrimer (7, 55). The N-terminal proline is positioned on the outside of the barrel and is believed to be the active residue during catalysis. Interestingly, mapping of the 10 invariant residues found in all members of the MIF family onto the three-dimensional structure of human MIF shows that most of these residues cluster around the N-terminal proline, forming a pocket around the N-terminal proline that resembles an enzymatic active site (59).
A parasite-derived MIF homologue that is capable of diverting or modifying important functions of the host-derived molecule could contribute significantly to the parasite’s ability to survive and replicate. Locally, this could result in diminished or qualitatively altered inflammatory responses that provide the parasite with a short-term survival advantage. Systemically, changes in normal MIF signaling may be one of the determining factors for the strong type 2 bias of the immune response observed in chronic filarial infections (30, 39, 40). A better understanding of the nature of these molecules and how they function at the level of the host cell to alter immune responsiveness will be critical for the development of effective therapies and for understanding pathogenesis in humans.
ACKNOWLEDGMENTS
This work was supported by research grants from the U.S. Public Health Service (AI-29411), the Edna McConnell Clark Foundation (EMCF 01093), and the World Health Organization (T23/181.80). Parasites and infected gerbils were supplied under the auspices of an NIAID supply contract (AI-02642), U.S./Japan Cooperative Medical Science Program.
We thank Brian Schofield for valuable assistance in carrying out immunohistocytochemistry and M. Blaxter for sequencing and bioinformatics.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amiri P, Locksley R M, Parslow T G, Sadick M, Rector E, Ritter D, McKerrow J H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 3.Apte R S, Sinha D, Mayhew E, Wistow G J, Niederkorn J Y. Role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 4.Bacher M, Meinhardt A, Lan H Y, Mu W, Metz C N, Chesney J A, Calandra T, Gemsa D, Donnelly T, Atkins R C, Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235–246. [PMC free article] [PubMed] [Google Scholar]
- 5.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakhiet M, Olsson T, Mhlanga J, Buscher P, Lycke N, van der Meide P H, Kristensson K. Human and rodent interferon-gamma as a growth factor for Trypanosoma brucei. Eur J Immunol. 1996;26:1359–1364. doi: 10.1002/eji.1830260627. [DOI] [PubMed] [Google Scholar]
- 7.Bendrat K, Al-Abed Y, Callaway D J E, Peng T, Calandra T, Metz C N, Bucala R. Biochemical and mutational investigations of the enzymatic activity of macrophage migration inhibitory factor. Biochemistry. 1997;36:15356–15362. doi: 10.1021/bi971153a. [DOI] [PubMed] [Google Scholar]
- 8.Bernhagen J, Bacher M, Calandra T, Metz C N, Doty S B, Donnelly T, Bucala R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. . (Erratum, 378:419, 1995.) [DOI] [PubMed] [Google Scholar]
- 10.Bernhagen J, Mitchell R A, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 11.Blaxter M L, Raghavan N, Ghosh I, Guiliano D, Lu W, Williams S, Slatko B, Scott A L. Genes expressed in Brugia malayi infective third stage larvae. Mol Biochem Parasitol. 1996;77:77–93. doi: 10.1016/0166-6851(96)02571-6. [DOI] [PubMed] [Google Scholar]
- 12.Bloom B R, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:514–521. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. C. elegans II. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 117–145. [PubMed] [Google Scholar]
- 14.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 15.Calandra T, Bernhagen J, Metz C N, Spiegel L A, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 16.Calandra T, Bernhagen J, Mitchell R A, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cookson E, Blaxter M L, Selkirk M E. Identification of the major soluble cuticular glycoprotein of lymphatic filarial nematode parasites (gp29) as a secretory homolog of glutathione peroxidase. Proc Natl Acad Sci USA. 1992;89:5837–5841. doi: 10.1073/pnas.89.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David J. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies S J, Pearce E J. Surface-associated serine-threonine kinase in Schistosoma mansoni. Mol Biochem Parasitol. 1995;70:33–44. doi: 10.1016/0166-6851(95)00002-i. [DOI] [PubMed] [Google Scholar]
- 20.de Diego J, Duarte M, Fresno M. Alternation of macrophage function by a Trypanosoma cruzi membrane mucin. J Immunol. 1997;159:4983–4989. [PubMed] [Google Scholar]
- 21.Deehan M R, Harnett M M, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–6111. [PubMed] [Google Scholar]
- 22.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1985;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly S C, Haslett C, Reid P T, Grant I S, Wallace W A, Metz C N, Bruce L J, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 24.Farrell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 25.Fleming S B, McCaughan C A, Andrews A E, Nash A D, Mercer A A. A homolog of interleukin-10 is encoded by the poxvirus or virus. J Virol. 1997;71:4857–4861. doi: 10.1128/jvi.71.6.4857-4861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garraud O, Nkenfou C, Bradley J E, Perler F B, Nutman T B. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol. 1995;155:1316–1325. [PubMed] [Google Scholar]
- 27.Ghosh I, Eisinger S W, Raghavan N, Scott A L. Thioredoxin peroxidases from Brugia malayi. Mol Biochem Parasitol. 1998;91:207–220. doi: 10.1016/s0166-6851(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh I, Raghavan N, FitzGerald P C, Scott A L. Nucleoside diphosphate kinase from the parasitic nematode Brugia malayi. Gene. 1995;164:261–266. doi: 10.1016/0378-1119(95)00500-6. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Escobar N, van den Biggelaar A, Maizels R. A member of the TGFβ receptor gene family in the parasitic nematode Brugia pahangi. Gene. 1997;199:101–109. doi: 10.1016/s0378-1119(97)00353-3. [DOI] [PubMed] [Google Scholar]
- 30.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite-specific energy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Investig. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krathwohl M D, Hromas R, Brown D R, Broxmeyer H E, Fife K H. Functional characterization of the C-C chemokine-like molecules encoded by molluscum contagiosum virus types 1 and 2. Proc Natl Acad Sci USA. 1997;94:9875–9880. doi: 10.1073/pnas.94.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lalani A S, McFadden G. Secreted poxvirus chemokine binding proteins. J Leukoc Biol. 1997;62:570–576. doi: 10.1002/jlb.62.5.570. [DOI] [PubMed] [Google Scholar]
- 34.Lan H Y, Mu W, Yang N, Meinhardt A, Nikolic-Paterson D J, Ng Y Y, Bacher M, Atkins R C, Bucala R. De novo renal expression of macrophage migration inhibitory factor during the development of rat crescentic glomerulonephritis. Am J Pathol. 1996;149:1119–1127. [PMC free article] [PubMed] [Google Scholar]
- 35.Lanahan A, Williams J B, Sanders L K, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu W, Egerton G L, Bianco A E, Williams S A. Thioredoxin peroxidase from Onchocerca volvulus: a major hydrogen peroxide detoxifying enzyme in filarial parasites. Mol Biochem Parasitol. 1998;91:221–235. doi: 10.1016/s0166-6851(97)00230-2. [DOI] [PubMed] [Google Scholar]
- 37.Lustigman S, Brotman B, Huima T, Prince A M, McKerrow J H. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J Biol Chem. 1992;267:17339–17346. [PubMed] [Google Scholar]
- 38.Mahanty S, Luke H E, Kumaraswami V, Narayanan P R, Vijayshekaran V, Nutman T B. Stage-specific induction of cytokines regulates the immune response in lymphatic filariasis. Exp Parasitol. 1996;84:282–290. doi: 10.1006/expr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 39.Mahanty S, Mollis S N, Ravichandran M, Abrams J S, Kumaraswami V, Jayaraman K, Ottesen E A, Nutman T B. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 40.Maizels R M, Sartono E, Kurniawan A, Partono F, Selkirk M E, Yazdanbakhsh M. T cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–56. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 41.Metz C N, Bucala R. Role of macrophage migration inhibitory factor in the regulation of the immune response. Adv Immunol. 1997;66:197–223. doi: 10.1016/s0065-2776(08)60598-2. [DOI] [PubMed] [Google Scholar]
- 42.Mikayama T, Nakano T, Gomi H, Nakagawa Y, Liu Y, Sato M, Iwamatsu H, Ishii Y, Weiser W, Ishizaka K. Molecular cloning and functional expression of a cDNA encoding glycosylation-inhibiting factor. Proc Natl Acad Sci USA. 1993;90:10056–10060. doi: 10.1073/pnas.90.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikulowska A, Metz C N, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–5517. [PubMed] [Google Scholar]
- 44.Ming M, Ewen M E, Pereira M E A. Trypanosome invasion of mammalian cells requires activation of the TGFβ signaling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- 45.Mischke R, Gessner A, Kapurniotu A, Juttner S, Kleemann R, Brunner H, Bernhagen J. Structure activity studies of the cytokine macrophage migration inhibitory factor (MIF) reveal a critical role for its carboxy terminus. FEBS Lett. 1997;414:226–232. doi: 10.1016/s0014-5793(97)01039-9. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell R A, Bacher M, Bernhagen J, Pushkarskaya T, Seldin M, Bucala R. Cloning and characterization of the gene for mouse MIF. J Immunol. 1995;154:3863–3870. [PubMed] [Google Scholar]
- 47.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst P, Skeiky Y A, Steeves M, Gervassi A, Grabstein K H, Reed S G. A Leishmania protein that modulates interleukin (IL)-12, IL-10 and tumor necrosis factor-alpha production and expression of B7-1 in human monocyte-derived antigen presenting cells. Eur J Immunol. 1997;27:2634–2642. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 49.Rosengren E, Aman P, Thelin S, Hansson C, Ahlfors S, Bjork P, Jacobsson L, Rorsman H. The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase. FEBS Lett. 1997;417:85–88. doi: 10.1016/s0014-5793(97)01261-1. [DOI] [PubMed] [Google Scholar]
- 50.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz C N, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi A G, Haslett C, Hirani N, Greening A P, Rahman I, Metz C N, Bucala R, Donnelly S C. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Investig. 1998;101:2869–2874. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schleimer R P, Freeland H S, Peters S P, Brown K E, Derse C P. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250:598–605. [PubMed] [Google Scholar]
- 53.Shimizu T, Ohkawara A, Nishihira J, Sakamoto W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immunohistochemical localization. FEBS Lett. 1996;381:199–202. doi: 10.1016/0014-5793(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 54.Steel C, Guinea A, Ottesen E A. Evidence for protective immunity to bancroftian filariasis in the Cook Islands. J Infect Dis. 1996;174:598–605. doi: 10.1093/infdis/174.3.598. [DOI] [PubMed] [Google Scholar]
- 55.Sun H W, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1996;93:5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki H, Kanagawa H, Nishihira J. Evidence for the presence of macrophage migration inhibitory factor in murine reproductive organs and early embryos. Immunol Lett. 1996;51:141–147. doi: 10.1016/0165-2478(96)02543-6. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki H, Nishihira J, Koyama Y, Kanagawa H. The role of macrophage migration inhibitory factor in pregnancy and development of murine embryos. Biochem Mol Biol Int. 1996;38:409–416. [PubMed] [Google Scholar]
- 58.Suzuki M, Sugimoto H, Nakagawa A, Tenaka I, Nishihira J, Sakai M. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- 59.Swope M, Sun H W, Blake P R, Lolis E. Direct link between cytokine activity and a catalytic site for macrophage migration inhibitory factor. EMBO J. 1998;17:3534–3541. doi: 10.1093/emboj/17.13.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamashiro W K, Rao M, Scott A L. Proteolytic cleavage of IgG and other protein substrates by Dirofilaria immitis microfilarial enzymes. J Parasitol. 1987;73:149–154. [PubMed] [Google Scholar]
- 61.Tang L, Ou X, Henkle-Dührsen K, Selkirk M E. Extracellular and cytoplasmic CuZn superoxide dismutases from Brugia lymphatic filarial nematode parasites. Infect Immun. 1994;62:961–967. doi: 10.1128/iai.62.3.961-967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaidya T, Bakhiet M, Hill K L, Olsson T, Kristenseson K, Donelson J E. The gene for a T lymphocyte triggering factor from African trypansomes. J Exp Med. 1997;180:433–438. doi: 10.1084/jem.186.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wada S, Fujimoto S, Mizue Y, Nishihira J. Macrophage migration inhibitory factor in the human ovary: presence in the follicular fluids and production by granulosa cells. Biochem Mol Biol Int. 1997;41:805–814. doi: 10.1080/15216549700201841. [DOI] [PubMed] [Google Scholar]
- 64.Waeber G, Calandra T, Roduit R, Haefliger J A, Bonny C, Thompson N, Thorens B, Temler E, Meinhardt A, Bacher M, Metz C N, Nicod P, Bucala R. Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1997;94:4782–4787. doi: 10.1073/pnas.94.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiser W Y, Temple P A, Witek-Giannotti J S, Remold H G, Clark S C, David J R. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci USA. 1989;86:7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wistow G J, Shaughnessy M P, Lee D C, Hodin J, Zelenka P S. A macrophage migration inhibitory factor is expressed in the differentiating cells of the eye lens. Proc Natl Acad Sci USA. 1993;90:1272–1275. doi: 10.1073/pnas.90.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu D, McSorley S J, Tetley L, Chatfield S, Dougan G, Chan W, Satoskar A, David J R, Liew F Y. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-α and IFN-γ administered orally via attenuated Salmonella typhimurium. J Immunol. 1998;160:1285–1289. [PubMed] [Google Scholar]
- 68.Yamaoka K A, Kolb J P, Miyasaka N, Inuo G, Fujita K. Purified excretory-secretory component of filarial parasite enhances Fc epsilon RII/CD23 expression on human splenic B and T cells and IgE synthesis while potentiating T-helper type 2-related cytokine generation from T cells. Immunology. 1994;81:507–512. [PMC free article] [PubMed] [Google Scholar]
- 69.Yenbutr P, Scott A L. Molecular cloning of a serine proteinase inhibitor from Brugia malayi. Infect Immun. 1995;63:1745–1753. doi: 10.1128/iai.63.5.1745-1753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying L, de Waal Malefyt R, Briere F, Parham C, Bridon J M, Banchereau J, Moore K W, Xu J. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J Immunol. 1997;158:604–613. [PubMed] [Google Scholar]
- 71.Zhang M, Aman P, Grubb A, Panagopoulos I, Hindemith A, Rosengren E, Rorsman H. Cloning and sequencing of a cDNA encoding rat d-dopachrome tautomerase. FEBS Lett. 1995;373:203–206. doi: 10.1016/0014-5793(95)01041-c. [DOI] [PubMed] [Google Scholar]