Abstract
Background
Multiple sclerosis (MS) is an immune‐mediated disease of the central nervous system and a leading cause of disability in young and middle‐aged adults. Mycophenolate mofetil (MMF) is an immunosuppressive agent that has been used for the prevention of allograft rejection after renal, cardiac, or liver transplant and in patients with autoimmune diseases such as active relapsing‐remitting (RRMS) and progressive MS.
Objectives
To assess the efficacy and safety of MMF for preventing disease activity in patients with RRMS.
Search methods
We searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Specialised Register (January 14, 2013). We searched three Chinese databases (January 2013) and checked reference lists of identified trials. We contacted authors and pharmaceutical companies to ask for additional information. We applied no language restrictions.
Selection criteria
We included randomized controlled trials with a follow‐up of at least 12 months that compared MMF as monotherapy or in combination with other treatments versus placebo, another drug, or the same cointervention as the treated group.
Data collection and analysis
Two review authors independently selected the trials for inclusion, assessed trial quality, and extracted data.
Main results
One included study involving 26 participants with new‐onset RRMS investigated the efficacy and safety of MMF (13 participants) versus placebo in interferon β‐1a–treated participants. It was assessed to be at high risk of bias, and had a small numbers of participants receiving treatment with short‐term duration. There was inadequate information provided by the study to determine the effect of MMF in reducing relapses, preventing disability progression, or developing new T2‐ or new gadolinium (Gd)‐enhanced lesions on magnetic resonance imaging (MRI) after a 12‐month follow‐up period. No data were available at 24 months. No serious adverse effects were reported. All participants in the MMF‐treated group suffered from gastrointestinal upset, but none of them discontinued therapy as a result.
Authors' conclusions
The evidence we found from one small study was insufficient to determine the effects of MMF as an add‐on therapy for interferon β‐1a in new‐onset RRMS participants.
Keywords: Humans; Adjuvants, Immunologic; Adjuvants, Immunologic/therapeutic use; Immunosuppressive Agents; Immunosuppressive Agents/adverse effects; Immunosuppressive Agents/therapeutic use; Interferon beta‐1a; Interferon‐beta; Interferon‐beta/therapeutic use; Multiple Sclerosis, Relapsing‐Remitting; Multiple Sclerosis, Relapsing‐Remitting/drug therapy; Mycophenolic Acid; Mycophenolic Acid/adverse effects; Mycophenolic Acid/analogs & derivatives; Mycophenolic Acid/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Mycophenolate mofetil for relapsing‐remitting multiple sclerosis
Multiple sclerosis (MS) is an immune‐mediated disease of the central nervous system. Preliminary data show that mycophenolate mofetil (MMF), an immunosuppressive agent, might be beneficial for MS patients. The authors of this review evaluated the efficacy and safety of MMF in patients with relapsing‐remitting MS. Only one small study met the inclusion criteria, and it compared MMF versus placebo in 26 interferon β‐1a–treated patients. The results showed no evidence favoring MMF in reducing relapses or preventing disability progression after a 12‐month follow‐up period. No data were available at 24 months. All patients receiving MMF suffered from gastrointestinal upset, and one had a transient diarrhea, but no serious adverse effects were reported.
Background
Description of the condition
Multiple sclerosis (MS) is an immune‐mediated demyelinating disease of the central nervous system and a leading cause of disability in young and middle‐aged adults (Koch‐Henriksen 2010). It affects approximately 400,000 Americans and 2.5 million people worldwide (Vollmer 2007), with an overall incidence rate of 3.6 cases per 100,000 person‐years in women and 2.0 in men (Alonso 2008). Different clinical types of MS are distinguishable. About 80% of patients have a disease course characterized by relapses and remissions (relapsing‐remitting MS [RRMS]), and the remaining patients have primary or transitional progressive MS (PPMS) and experience a progressive decline in neurological function from the onset. People with MS and their families are affected by their disabling condition, and society carries a substantial economic burden. In the United States, annual costs of drugs and other medical care per patient are estimated to average USD 47,215 (Kobelt 2006).
Description of the intervention
Mycophenolate mofetil (MMF) is an expensive immunosuppressive agent that has been used in the past few years for the prevention of allograft rejection after renal, cardiac, or liver transplant (Villarroel 2009). It is increasingly used in the treatment of autoimmune diseases such as MS because of its potential efficacy and safety (Etemadifar 2011; Frohman 2010; Remington 2010; Vermersch 2007). MMF, a prodrug of mycophenolic acid (MPA), is a compound that was synthesized to improve the bioavailability of MPA. After oral or intravenous administration, MMF is rapidly converted to MPA in the plasma, resulting in a bioavailability of MPA of 94% (Lipsky 1996). MPA is rapidly conjugated to a glucuronide that is excreted in the urine; its plasma elimination half‐life is approximately 17.9 hours (Zwerner 2007). Generally, MMF is well tolerated; the most frequently reported adverse effects have been gastrointestinal (abdominal pain, vomiting, diarrhea) or related to the hematopoietic system (anemia and leukopenia) (Hood 1997; Kulkarni 2004; Lipsky 1996). Hematopoietic adverse effects have been reported in about 10% of study participants (Kulkarni 2004). In addition, MMF has been reported on occasion to increase the risk of certain malignancies (Hood 1997; Kulkarni 2004). The recommended oral dose of MMF ranges from 1.0 to 1.5 g/d twice daily.
How the intervention might work
MMF is a selective inhibitor of inosine 5'‐monophosphate dehydrogenase type II, an important enzyme that is responsible for the de novo synthesis of the purine nucleotide guanine within activated T and B lymphocytes and macrophages (Allison 2000; Frohman 2004; Zwerner 2007). Therefore, it exerts its immunomodulatory activities through inhibition of both T and B lymphocyte proliferation, reducing the synthesis of antibodies. MPA, the active metabolite of MMF, has been shown to prevent the production of interferon‐gamma (IFN‐γ), lipopolysaccharide (LPS)‐induced interleukin‐6 (IL‐6), and nitric oxide (NO) (Frohman 2004; Miljkovic 2002), which may lead to decreased inflammation and cytotoxic effects to the central nervous system. Hence, MMF may be useful in the treatment of patients with MS.
Why it is important to do this review
MMF has been increasingly used for the treatment of patients with RRMS and has shown some promising therapeutic effects (Etemadifar 2011; Frohman 2004; Frohman 2010; Remington 2010; Vermersch 2007), but it has not been systematically reviewed. The aim of this review was to investigate efficacy and safety in randomized trials evaluating MMF for patients with RRMS,
Objectives
To assess the efficacy and safety of MMF for preventing disease activity in patients with RRMS.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and randomized cross‐over trials were included. RCTs with follow‐up less than or equal to six months were excluded.
Types of participants
Patients of all age groups and genders with clinically definite RRMS according to Poser criteria (Poser 1983) or original or revised McDonald criteria (McDonald 2001; Polman 2005; Polman 2011) were eligible for inclusion.
Types of interventions
MMF as monotherapy or in combination with other treatment versus placebo or another active agent, or the same cointervention as was used in the the MMF group.
Types of outcome measures
Primary outcomes
Proportions of participants who had relapses at 12 and 24 months after randomization.
Proportions of participants who experienced disease progression, as measured by the Expanded Disability Status Scale (EDSS) (Kurtzke 1983), at 12 and 24 months after randomization. Progression is defined as an increase of 0.5 point of disability for participants with baseline EDSS greater than or equal to 5.5, and 1.0 point of disability for those with basal EDSS less than or equal to 5.0, sustained for six months.
Secondary outcomes
Proportions of participants who developed new T2‐ or new Gd‐enhanced lesions (GELs) on magnetic resonance imaging (MRI) at 12 and 24 months after randomization.
Numbers of new T2‐enhanced lesions or GELs on MRI at 12 and 24 months after randomization.
Proportions of participants who experienced any of the following side effects were evaluated: abdominal pain, vomiting, diarrhea, anemia, leukopenia, and infection, or other severe adverse effects.
Search methods for identification of studies
Electronic searches
The Review Group's Trials Search Co‐ordinator searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group Trials Register (January 14, 2013), which, among other sources, contains the following.
The Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1).
MEDLINE (PubMed) (1966 to January 14, 2013).
EMBASE (EMBASE.com) (1974 to January 14, 2013).
CINAHL (EBSCO host) (1981 to January 14, 2013).
LILACS (Bireme) (1982 to January 14, 2013).
PEDro (1990 to January 14, 2013).
Clinical trial registries (http://clinicaltrials.gov).
Information on the Review Group's Trials Register and details of search strategies used to identify trials can be found in the "Specialised register" section within the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group's module.
The keywords used in the search strategy are listed in Appendix 1.
Additional databases were searched by the review authors.
The following three Chinese databases were searched using the search terms (duofaxingyinghua) AND ((matimaikaofenzhi) OR (maikaofensuan)).
China Biological Medicine Database (CBM‐disc) (1979 to January 20, 2013).
Chinese National Knowledge Infrastructure Database (CNKI) at www.cnki.net (1979 to January 20, 2013).
VIP Chinese Science and Technique Journals Database (1979 to January 20, 2013).
Searching other resources
To identify other relevant study data, we:
contacted authors of published studies when data reported were incomplete;
screened reference lists of review articles and primary studies identified; and
contacted the pharmaceutical company (Roche Pharmaceutical Co., Ltd.) that produces MMF to identify further published or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (XY and HJ) independently read titles and abstracts of all references identified by the search and excluded irrelevant references. The full texts of the remaining studies were obtained, and the same two review authors independently evaluated each trial for inclusion. Disagreements were resolved by discussion among all review authors.
Data extraction and management
Two review authors (XY and HJ) independently extracted the following data using a predefined data extraction form.
Participants: diagnostic criteria, number in each group, age, gender, baseline clinical characteristics, and withdrawals or losses to follow‐up.
Methods: study design, randomization, allocation concealment, blinding, and intention‐to‐treat analysis.
Interventions: details of MMF and control intervention, such as dosage and duration, treatment period, and cointerventions.
Outcomes: primary and secondary outcomes and adverse effects.
Other: country and setting, publication year, and sources of funding.
Disagreements were resolved by discussion among all review authors.
Assessment of risk of bias in included studies
Two review authors (XY and HJ) independently assessed the risk of bias (RoB) of included studies using the Q Cochrane Collaboration criteria (Higgins 2011). These included random sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, and other bias. The RoB of each study was explicitly judged on each criterion, and each study was classified as having "low," "high," or "unclear" risk of bias. Disagreements were resolved by discussion among all review authors.
Measures of treatment effect
We did not perform a meta‐analysis because only one study was included in this review. If new data become available in the future, we will present the results for dichotomous outcomes using risk ratios (RRs) with 95% confidence intervals (CIs), and for continuous outcomes, mean differences (MDs) with 95% CIs.
Unit of analysis issues
The unit of data extraction, evaluation, and analysis was the primary randomized trial.
Dealing with missing data
Review authors contacted study authors to request additional data not reported in the included study; however, we received no response.
Assessment of heterogeneity
As only one study was included in this review, we did not assess heterogeneity. If further studies will be available in future updates, we will assess statistical heterogeneity by calculating the Chi² test and the I² statistic. If the I² statistic will be greater than 50%, which indicates substantial heterogeneity (Higgins 2011), potential sources of heterogeneity will include different baseline characteristics of participants (diagnostic criteria, age, gender, disease duration), interventions (administration method, dosage and duration, control intervention, cointerventions), and study designs (allocation concealment, blinding methods, incomplete outcome data). We will investigate the distribution of these characteristics and will carry out a subgroup analysis for efficacy outcomes at each time point.
Assessment of reporting biases
If sufficient studies will be identified in the future, potential biases of reporting will be assessed by using funnel plots and visual inspection for asymmetry according to the approach outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
Review Manager 5.2 software (RevMan 2013) will be used to synthesize the results. If sufficient studies are identified in future updates of the review, the selection of a fixed‐effect model or a random‐effects model will be based on results of the Chi² test and the I² statistic for heterogeneity (Higgins 2011). If the I² statistic indicates substantial statistical heterogeneity, we will explore potential causes of heterogeneity first, to determine whether a subgroup analyses is needed. If the substantial heterogeneity still cannot be explained, we will adopt a random‐effects model. If the I² statistic indicates no significant statistical heterogeneity, we will use a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
If we identify a sufficient number of studies in future updates, the following subgroup analyses will be performed.
Treatment dose and duration.
Cointerventions.
Types of control groups.
Sensitivity analysis
The RoB ("low," "high," or "unclear" risk of bias) of included studies will be taken into account in the interpretation of evidence.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
A flow chart describes the results of the electronic search (Figure 1). Twenty‐seven references were identified. After screening titles and abstracts, we excluded 19 references, as they were not relevant. Of the remaining eight references, we excluded five studies (seven references) (Frohman 2004; Frohman 2010; NCT00618527; Remington 2010; Vermersch 2007); the reasons for exclusion were carefully recorded in the Characteristics of excluded studies table. One study (NCT00324506) was completed in June 2013, but only partial short‐term results were published (Frohman 2010). One study (Etemadifar 2011) was included in the review.
1.
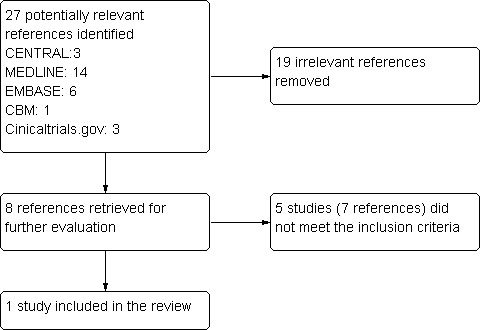
Study flow diagram.
Included studies
A randomized, blinded, placebo‐controlled study (Etemadifar 2011) enrolled a total of 26 participants with RRMS. This study investigated the efficacy and safety of oral MMF 2000 mg daily versus placebo in IFN‐β‐1a–treated participants. All 26 participants completed the 12‐month follow‐up period and were included in the final analysis. The primary outcome was the number of new T2 lesions and new GELs noted on MRI evaluation; secondary outcomes included relapse rates, changes in EDSS score, and adverse effects. Details of the study are outlined in the Characteristics of included studies table.
Excluded studies
Five studies were excluded for the following reasons: Frohman 2004 was a retrospective review; Vermersch 2007 was a case series without a control group; Remington 2010 enrolled both participants with early RRMS and those with clinically isolated syndrome (CIS), and no separate data were available; Frohman 2010 had a short‐term (six out of 12 months as planned) follow‐up study of MMF compared with IFN‐β in participants with RRMS; NCT00618527 was an experimental study that evaluated mRNA for levels of the MxA gene, a marker of IFN bioactivity, as measured after treatment.
One study awaiting classification (NCT00324506) was completed in June 2013, but only partial short‐term results were published (Frohman 2010). In the second‐phase evaluation (12 months after the beginning of the study), MRIs and clinical examinations are planned for participants treated with active combination therapy consisting of both MMF and IFN‐β‐1a.
Risk of bias in included studies
See overall results of risk of bias (RoB) assessments as summarized in Characteristics of included studies, Figure 2, and Figure 3.
2.
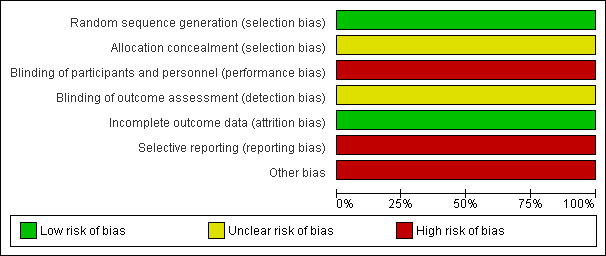
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
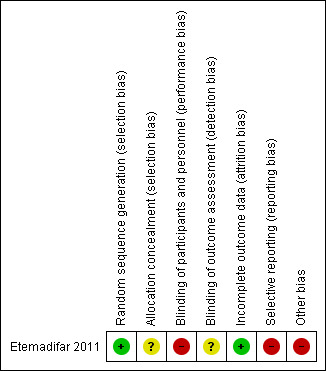
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of allocation concealment was not described. It is unclear whether all potentially eligible patients were approached for participation, and whether the results reflect a continuous series of participants or a selected series.
Blinding
This study did not blind clinical outcome assessors or treating personnel to the study arm allocation. In addition, no description was provided of whether MMF and placebo with identical appearance and packaging were provided.
Incomplete outcome data
All participants completed the 12‐month follow‐up period and were included in the final analysis. We judged this study to be at low risk of attrition bias.
Selective reporting
All specified primary and secondary outcomes were reported. However, the interval of confirmation used to assess sustained disability progression was not reported. Poor reporting of adverse effects was another major limitation of the study.
Other potential sources of bias
Sample size calculation was not clearly reported. Small sample size led to an inadequately powered trial. No description was provided of the source of the MMF and placebo (eg, sponsored by a pharmaceutical company or other).
Effects of interventions
MMF plus IFN versus placebo plus IFN
Primary outcomes
In light of the fact that only a single trial was suitable for inclusion, no meta‐analysis was performed; instead, outcome measures reported in the trial were described.
1. Proportions of participants who had relapses at 12‐month and 24 months after randomization.
One participant in the placebo group had a relapse compared with no participants in the MMF group. No data were available at 24 months.
2. Proportions of participants who experienced disease progression at 12‐month and 24 months after randomization.
Three participants in each group experienced more than one‐point progression in EDSS at 12‐month follow‐up. The study authors reported no significant differences between groups in EDSS scores at 12‐month follow‐up. No data were available at 24 months.
Secondary outcomes
1. Proportions of participants who developed new T2‐ or new Gd‐enhancing lesions at 12‐month and 24 months after randomization.
No significant differences between groups were noted in the proportion of participants developing new T2‐ or new Gd‐enhanced lesions at 12‐month follow‐up (Etemadifar 2011). No data were available at 24 months.
2. Numbers of new T2‐ or new Gd‐enhanced lesions at 12‐month and 24 months after randomization.
Differences between the two groups in the number of new T2 lesions seen on MRI were not statistically significant. Two participants in the placebo group had Gd enhancement, but no participants in the MMF group experienced this finding (Etemadifar 2011). No data were available at 24 months.
3. Proportions of participants who experienced any of the following side effects: abdominal pain, vomiting, diarrhea, anemia, leukopenia, and infection, or other severe adverse effects.
Adverse effects were briefly reported. In the MMF‐treated group, all participants suffered from gastrointestinal upset of varying degrees, and one participant had a transient diarrhea; however, no participants needed to discontinue the drug (Etemadifar 2011).
Discussion
Summary of main results
This systematic review assessed the efficacy and safety of MMF in participants with RRMS. Only one study (Etemadifar 2011) comparing oral MMF 2000 mg daily versus placebo in IFN‐β‐1a–treated participants with RRMS was eligible for inclusion.There was inadequate information provided by the study to determine the effect of MMF in reducing relapses, preventing disability progression, or developing new T2‐ or new gadolinium (Gd)‐enhanced lesions on magnetic resonance imaging (MRI) after a 12‐month follow‐up period. No data were available at 24 months.
It is worth noting that both MMF plus IFN and placebo plus IFN groups reported a very low risk of relapse at the end of the 12‐month follow‐up, and only one participant in the placebo group had a relapse. A reasonable explanation for the low relapse rate might be that the investigators included participants with new‐onset RRMS with a short disease duration (mean of five months in the MMF group and 3.6 months in the placebo group) and few relapses (one of 13 participants in the MMF group and two of 13 in the placebo group had relapses in the two years before enrollment) (Etemadifar 2011).
Overall completeness and applicability of evidence
The findings presented in this review originated from a single high risk study with a small number of participants with RRMS in short‐term treatment. Moreover, another important limitation involved the characteristics of the enrolled participants, whose condition was new‐onset and almost without relapse in the previous two years. For this reason, the validity of this review is limited. Furthermore, no evidence is available on MMF monotherapy for RRMS, and evidence about the long‐term efficacy of MMF is still lacking.
Quality of the evidence
The included study (Etemadifar 2011) was a randomized controlled trial with serious limitations that deserve consideration. The sample size was small, leading to an inadequately powered trial. Treating personnel and likely the assessor of clinical outcomes were unblinded, leading to performance and detection biases. The interval of confirmation used to assess sustained disability progression was not reported. Poor reporting of adverse effects was another major limitation of the study. No information was provided on the source of the investigated drugs, and conflicts of interest could not be excluded. All of the above mentioned limitations make the results unconvincing.
Potential biases in the review process
No potential biases were reported by the review authors in the review process.
Agreements and disagreements with other studies or reviews
The efficacy and safety of MMF for the treatment of patients with RRMS have never been systematically reviewed.
Authors' conclusions
Implications for practice.
The evidence we found from one small study was insufficient to determine the effects of MMF as an add‐on therapy for interferon β‐1a in new‐onset RRMS participants. The study had serious methodological limitations; therefore, no definitive conclusions can be drawn.
Implications for research.
High‐quality, large‐scale RCTs are needed to assess the efficacy and safety of MMF monotherapy or as add‐on treatment for patients with RRMS. Future studies should carefully select participants and outcome measures and should have a follow‐up duration of at least two years.
Acknowledgements
We would like to thank the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group and the peer reviewers for their help in developing this review.
Appendices
Appendix 1. Keywords
{mycophenolic acid morpholinoethylester } OR {mycophenolate mofetil hydrochloride} OR {sodium mycophenolate} OR {myfortic} OR {rs 61443} OR {RS‐61443} OR {cellcept} OR {mycophenolate sodium} OR {mycophenolate mofetil} OR {Mycophenolate‐mofetil}
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Etemadifar 2011.
| Methods | Randomized, blinded, placebo‐controlled design was mentioned in the report. Random list generated was using a random number table. We could obtain no additional information through correspondence with the study authors | |
| Participants | Setting: Isfahan MS Society, Iran N = 26; MMF group = 13, F/M = 8/5, mean age = 31.3 ± 8.1 years, disease duration = 5.0 ± 4.5 months; placebo group = 13, F/M = 9/4, mean age = 29.6 ± 6.7 years, disease duration = 3.6 ± 3.2 months Inclusion criteria: clinically definite RRMS, age 18 to 55 years, disease duration < two years, baseline EDSS < 6.0 Exclusion criteria: use of immunomodulatory or immunosuppressive drugs, relapse within two months, pregnant, abnormal blood tests, other major medical illnesses (eg, cancer, significant gastrointestinal disease, immunodeficiency, other autoimmune diseases) Characteristics of participants at baseline: similar |
|
| Interventions | Intervention group: IFN‐β‐1a and oral MMF started at 500 mg daily for one week, then increased by 500 mg per week until a target dose of 2000 mg daily; after this, dose was continued for 12 months Control group: IFN‐β‐1a and placebo; same dose and administration method as in the intervention group for 12 months | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generated by software |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treating personnel were not blinded. No description on whether MMF and placebo with identical appearance and packaging were provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blinded. Blinding is reported only for "Two radiologists, blinded to the treatment arms, [who] were responsible for MRI evaluations" but is not reported for assessors of clinical outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | All specified primary and secondary outcomes were reported. The interval of confirmation to assess sustained disability progression was not reported. Poor reporting of adverse effects was another major limitation of the study |
| Other bias | High risk | Sample size calculation was not clearly reported. Small sample size led to an inadequately powered trial No description of the source of MMF and placebo (eg, sponsored by a pharmaceutical company or other) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Frohman 2004 | Retrospective review |
| Frohman 2010 | Six‐month follow‐up study without our specified 12‐month follow‐up outcome measures |
| NCT00618527 | Experimental study that was "completed" in May 2012 (as reported on clinicaltrials.gov). Outcome measures did not fulfil our inclusion criteria |
| Remington 2010 | Study authors enrolled both participants with early RRMS and those with clinically isolated syndrome (CIS). Details of RRMS and CIS were not available |
| Vermersch 2007 | Case series study of 30 participants with RRMS treated with MMF in combination with IFN without a control group |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00324506.
| Methods | Randomized, open‐label, parallel‐group multicenter study to determine the safety/efficacy of mycophenolate mofetil in mono and combination therapy with interferon‐β‐1a in patients with relapsing remitting multiple sclerosis |
| Participants | Sixty participants (20 participants at each recruiting center) with RRMS |
| Interventions | Mycophenolate mofetil in mono and combination therapy with interferon‐β‐1a |
| Outcomes | Primary objective of this safety/mechanistic study is to determine the safety and tolerability of oral mycophenolate when compared with weekly intramuscular interferon‐β‐1a in RRMS. Safety will be assessed by virtue of changes on MRI. Secondary outcome measures included changes in exacerbation frequency, incidence of exacerbation in treated groups, changes in level of sustained disability, and changes in quality of life measures and in assessment of fatigue |
| Notes | Primary completion date: June 2009 (final data collection date for primary outcome measure). This study has been completed. Last updated: June 17, 2013 |
Contributions of authors
All the authors listed contributed to this review.
Declarations of interest
None known.
New
References
References to studies included in this review
Etemadifar 2011 {published data only}
- Etemadifar M, Kazemi M, Chitsaz A, Hekmatnia A, Tayari N, Ghazavi A, et al. Mycophenolate mofetil in combination with interferon beta‐1a in the treatment of relapsing‐remitting multiple sclerosis: a preliminary study. Journal of Research in Medical Sciences 2011;16(1):1‐5. [PUBMED: 21448376] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Frohman 2004 {published data only}
- Frohman EM, Brannon K, Racke MK, Hawker K. Mycophenolate mofetil in multiple sclerosis. Clinical Neuropharmacology 2004;27(2):80‐3. [PUBMED: 15252268] [DOI] [PubMed] [Google Scholar]
Frohman 2010 {published data only}
- Frohman EM, Cutter G, Remington G, Gao H, Rossman H, Weinstock‐Guttman B, et al. A randomized, blinded, parallel‐group, pilot trial of mycophenolate mofetil (CellCept) compared with interferon beta‐1a (Avonex) in patients with relapsing‐remitting multiple sclerosis. Therapeutic Advances in Neurological Disorders 2010;3(1):15‐28. [PUBMED: 21180633] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00618527 {published data only}
- NCT00618527. Combination therapy using mycophenolate mofetil (CellCept) and human interferon beta1a (Rebif) in early treatment of multiple sclerosis. http://clinicaltrials.gov/ct2/show/NCT00618527 (accessed 11 January 2013).
Remington 2010 {published data only}
- NCT00223301. A one‐year prospective, randomized, placebo‐controlled, double‐blind, phase II/III safety trial of combination therapy with IFN beta‐1a (Avonex) and mycophenolate mofetil (Cellcept) in early multiple sclerosis. http://clinicaltrials.gov/ct2/show/NCT00223301 (accessed 11 January 2013).
- Remington GM, Treadaway K, Frohman T, Salter A, Stuve O, Racke MK, et al. A one‐year prospective, randomized, placebo‐controlled, quadruple‐blinded, phase II safety pilot trial of combination therapy with interferon beta‐1a and mycophenolate mofetil in early relapsing‐remitting multiple sclerosis (TIME MS). Therapeutic Advances in Neurological Disorders 2010;3(1):3‐13. [PUBMED: 21180632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermersch 2007 {published data only}
- Vermersch P, Waucquier N, Michelin E, Bourteel H, Stojkovic T, Ferriby D, et al. Combination of IFN beta‐1a (Avonex) and mycophenolate mofetil (Cellcept) in multiple sclerosis. European Journal of Neurology 2007;14(1):85‐9. [PUBMED: 17222119] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00324506 {published and unpublished data}
- NCT00324506. A randomized, open‐label, parallel‐group multicenter study to determine the safety/efficacy of mycophenolate mofetil in mono & combination therapy with interferon beta 1a in patients with relapsing remitting multiple sclerosis. http://clinicaltrials.gov/ct2/show/study/NCT00324506 (accessed 11 January 2013).
Additional references
Allison 2000
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47(2‐3):85‐118. [PUBMED: 10878285] [DOI] [PubMed] [Google Scholar]
Alonso 2008
- Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71(2):129‐35. [PUBMED: 18606967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hood 1997
- Hood KA, Zarembski DG. Mycophenolate mofetil: a unique immunosuppressive agent. American Journal of Health‐System Pharmacy 1997;54(3):285‐94. [PUBMED: 9028422] [DOI] [PubMed] [Google Scholar]
Kobelt 2006
- Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross‐sectional study in the United States. Neurology 2006;66(11):1696‐702. [PUBMED: 16769943] [DOI] [PubMed] [Google Scholar]
Koch‐Henriksen 2010
- Koch‐Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurology 2010;9(5):520‐32. [PUBMED: 20398859] [DOI] [PubMed] [Google Scholar]
Kulkarni 2004
- Kulkarni AA, Shah BV. Mycophenolate mofetil: a promising immunosuppressive agent. The Journal of the Association of Physicians of India 2004;52:33‐8. [PUBMED: 15633716] [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. [PUBMED: 6685237] [DOI] [PubMed] [Google Scholar]
Lipsky 1996
- Lipsky JJ. Mycophenolate mofetil. Lancet 1996;348(9038):1357‐9. [PUBMED: 8918281] [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Annals of Neurology 2001;50(1):121‐7. [PUBMED: 11456302] [DOI] [PubMed] [Google Scholar]
Miljkovic 2002
- Miljkovic Dj, Samardzic T, Drakulic D, Stosic‐Grujicic S, Trajkovic V. Immunosuppressants leflunomide and mycophenolic acid inhibit fibroblast IL‐6 production by distinct mechanisms. Cytokine 2002;19(4):181‐6. [PUBMED: 12297111] [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840‐6. [PUBMED: 16283615] [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292‐302. [PUBMED: 21387374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [PUBMED: 6847134] [DOI] [PubMed] [Google Scholar]
RevMan 2013 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Villarroel 2009
- Villarroel MC, Hidalgo M, Jimeno A. Mycophenolate mofetil: an update. Drugs of Today 2009;45(7):521‐32. [PUBMED: 19834629] [DOI] [PubMed] [Google Scholar]
Vollmer 2007
- Vollmer T. The natural history of relapses in multiple sclerosis. Journal of the Neurological Sciences 2007;256 Suppl 1:5‐13. [PUBMED: 17346747] [DOI] [PubMed] [Google Scholar]
Zwerner 2007
- Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatologic Therapy 2007;20(4):229‐38. [PUBMED: 17970888] [DOI] [PubMed] [Google Scholar]


