Abstract
The occurrence of human enteric viruses in surface water in the Tamagawa River, Japan, was surveyed for 1 year, from April 2003 to March 2004. Sixty-four samples were collected from six sites along the river, and 500 ml of the sample was concentrated using the cation-coated filter method, which was developed in our previous study. This method showed recovery yields of 56% ± 32% (n = 37) for surface water samples inoculated with polioviruses. More than one kind of tested virus was detected in 43 (67%) of 64 samples by TaqMan PCR. Noroviruses and adenoviruses were detected in a high positive ratio; 34 (53%), 28 (44%), and 29 (45%) of 64 samples were positive for norovirus genotype 1 and genotype 2 and adenoviruses, respectively. The mean concentrations of norovirus genotype 1 or genotype 2 determined by real-time PCR were 0.087 and 0.61 genome/ml, respectively, showing much higher values in winter (0.21 genome/ml for genotype 1 and 2.3 genomes/ml for genotype 2). Enteroviruses were detected by both direct PCR (6 of 64 samples; 9%) and cell culture PCR (2 of 64 samples; 3%). Torque teno viruses, emerging hepatitis viruses, were also isolated in three samples (5%). The concentration of total coliforms and the presence of F-specific phages showed a high correlation with the presence of viruses, which suggested that the simultaneous use of total coliforms and F-specific phages as indicators of surface water may work to monitor viral contamination.
Human enteric viruses are excreted in the feces of infected patients in high concentrations and transmitted mainly by the fecal-oral route via contaminated food and water. They are estimated to cause about 30% to 90% of gastroenteritis cases worldwide (3, 20, 38).
Sewage systems have contributed to the improvement of the water quality of rivers. However, numerous types of viruses have been isolated in effluents from wastewater treatment plants (WWTPs) (7, 11, 14, 29). There are many rivers with intakes of drinking water treatment plants (DWTPs) downstream of WWTPs. Therefore, surface water, a major source of drinking water, is suspected to be contaminated with viruses derived from WWTPs. Viruses can cause diseases with an intake of low numbers (40), and it is therefore important to manage the risk of infection of viruses via tap water. The U.S. Environmental Protection Agency requires a 4-log-unit (99.99%) reduction of viruses to produce drinking water from surface water (36). This regulation should be evaluated based on the accurate assessment of the occurrence of viruses in surface water.
Cell culture was a common method to isolate viruses in water until the early 1990s (11, 15, 24). More recently, PCR has come to be a major tool for detection, and various types of viruses have been isolated in surface water by PCR, including noncultivable viruses, such as noroviruses (NVs) (4, 6, 29, 31, 32). In most of these studies, large volumes of surface water, usually more than 10 liters, were tested after concentration using filters because of the low sensitivity of detection methods. The concentration method requires beef extract to elute viruses from the filters, but the extract is known to inhibit cDNA synthesis and PCR amplification (1). In order to avoid inhibition, the PCR process should follow a method with a high recovery efficiency of viruses without any inhibitors.
In our previous study, a cation-coated filter method was developed to concentrate viruses in large volumes of freshwater, and NVs were successfully detected in 10 (10%) of 98 tap water samples in Tokyo, Japan (100 to 532 liters), by using this method, followed by TaqMan PCR (9). The mechanism of this method is based on electrostatic interactions and is expected to be effective for other types of freshwater samples, including surface water. Moreover, viruses trapped on a filter are eluted with NaOH solution (pH 10.8), which can be applied to the subsequent detection of viral genomes using PCR more effectively than using conventional elution methods.
In this study, the occurrence of various types of viruses was determined in 500 ml of surface water, much smaller volumes of water than those in some previous studies. Sixty-four surface water samples were collected from six sites along the Tamagawa River, Japan, which were applied to the detection of naturally occurring NVs, enteroviruses (EVs), and adenoviruses (AdVs) by using the cation-coated filter method, followed by TaqMan PCR. Quantitative analysis by real-time PCR was performed in order to determine the concentrations of NVs in surface water. As emerging viruses, torque teno viruses (TTVs), which were discovered as agents of unknown hepatitis in Japan in 1997 (26), were also tested, because their main route of infection is suspected to be fecal-oral (28). Total coliforms, thermotolerant coliforms, and F-specific phages were determined as indicators of viruses in order to assess the effectiveness of the method.
MATERIALS AND METHODS
Collection of surface water samples.
Sixty-four surface water samples were collected from six sites (sites A to F) along the Tamagawa River in Japan from April 2003 to March 2004. Samples at sites B to F were collected once a month, and samples at site A were collected four times (April, July, and October 2003 and January 2004). The Tamagawa River has a total length of 138 km and a catchment area of 1,240 km2, with a small WWTP and a DWTP in the upstream area, nine WWTPs in the midstream area, and three DWTPs in the downstream area, as shown in Fig. 1. Chlorination has been adopted in all of these WWTPs for the disinfection of microorganisms. The effluents from these WWTPs cover nearly half of the river water in the midstream and downstream areas. These WWTPs serve 7%, 94%, or 100% of the populations in the upstream, midstream, and downstream areas, respectively. Septic tank systems are also installed in the upstream and midstream areas.
FIG. 1.
Sampling sites, DWTPs, and WWTPs along Tamagawa River.
Water quality of surface water samples.
The water temperature and pH of the samples were determined on site immediately after sample collection. The samples were stored in plastic bottles on ice and delivered to the laboratory within 6 hours after collection. All the samples were assayed for total coliforms and thermotolerant coliforms by a double-agar-layer method using desoxycholate agar (Eiken, Tokyo, Japan) according to the protocols described by the manufacturer. For samples collected from October 2003 to March 2004, F-specific phages (13) in 10 ml of the sample were also determined by a single-agar-layer method using the host strain Salmonella enterica serovar Typhimurium WG49 (10) as described in ISO 10705 (2).
Concentration of surface water samples.
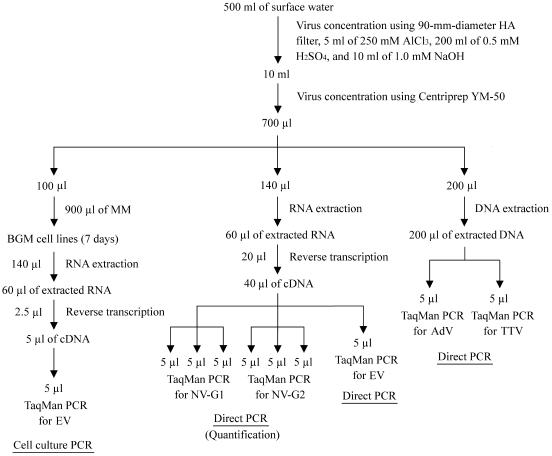
Surface water samples were tested for NVs, EVs, AdVs, and TTVs as shown in Fig. 2. Five milliliters of 250 mM AlCl3 was passed through an HA filter (0.45-μm pore size and 90-mm diameter; Millipore, Tokyo, Japan) to form a cation (Al3+)-coated filter, and then 500 ml of surface water sample was passed through the filter. The filter was rinsed with 200 ml of 0.5 mM H2SO4 (pH 3.0) to remove aluminum ions, followed by elution of viruses with 10 ml of 1.0 mM NaOH (pH 10.8). The filtrate was recovered in a tube containing 50 μl of 100 mM H2SO4 (pH 1.0) and 100 μl of 100× Tris-EDTA buffer (pH 8.0) for neutralization, followed by centrifugation using a Centriprep YM-50 (Millipore). The Centriprep YM-50 is a centrifugation unit equipped with an ultrafiltration membrane, which can achieve the high concentration efficiency needed for viruses. The filtrate was added to the Centriprep YM-50 and centrifuged according to the manufacturer's protocol; centrifugation at 2,500 rpm for 10 min, followed by removal of the sample that passed through the ultrafiltration membrane (8 ml) and further centrifugation at 2,500 rpm for 5 min to obtain a final volume of 700 μl. The final concentrated sample was stored at −20°C until further analysis.
FIG. 2.
Procedure for the detection of viruses in surface water samples.
RNA extraction and reverse transcription for detection of RNA viruses.
For the detection of NVs and EVs, RNA was extracted from 140 μl of the final concentrated sample using a QIAamp viral RNA minikit (QIAGEN, Tokyo, Japan) to obtain a final volume of 60 μl. Twenty microliters of extracted RNA was added to a reaction mixture containing 2 μl of 200-U/μl SuperScript II reverse transcriptase (Invitrogen, Tokyo, Japan), 2 μl of 100 mM dithiothreitol (Invitrogen), 8 μl of 5× first-strand buffer (Invitrogen), 1 μl of 20-U/μl RNase inhibitor (Applied Biosystems, Tokyo, Japan), 2 μl of the four 2.5 mM deoxynucleoside triphosphate stocks (Applied Biosystems), 2 μl of 50 μM random hexamers (Applied Biosystems), and 3 μl of MilliQ water (Millipore). The reaction mixture was incubated at 42°C for 60 min and at 99°C for 5 min with the GeneAmp PCR system 9600 (Applied Biosystems).
Quantification of NVs by real-time PCR.
The concentration of cDNAs of NVs was quantified by real-time PCR using the ABI PRISM 7000 sequence detection system (SDS) (Applied Biosystems) as follows.
Five microliters of each resulting cDNA sample was mixed with 45 μl of a reaction buffer containing 25 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 400 nM of each primer, and 300 nM TaqMan probe (16). The mixtures were added to a 96-well microplate (Applied Biosystems) for the detection of NVs of genotype 1 (NV-G1) and genotype 2 (NV-G2), respectively, and incubated at 50°C for 2 min, followed by 95°C for 10 min, and 50 cycles at 95°C for 15 s and at 56°C for 1 min.
In order to draw a standard curve, cDNA samples of NV-G1 and NV-G2 (107 genomes/μl each), which had been provided courtesy of Osamu Nishio (National Institute of Infectious Diseases, Tokyo, Japan), were diluted by serial 10-fold dilution.
The surface water samples and the standard samples were applied to real-time PCR at the same time, followed by analysis using the SDS software (version 1.1; Applied Biosystems) to obtain quantitative data on the concentration of NVs in a well. Three wells were used for each sample, and the average was used for the subsequent calculation. The concentration of NVs in the original surface water sample was calculated assuming that no NVs were lost during the detection processes, such as the concentration of water samples, the extraction of RNA, and the synthesis of cDNA.
Direct PCR for detection of EVs.
Five microliters of the resulting cDNA sample was mixed with 45 μl of a reaction buffer containing 25 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 400 nM of each primer (30), and 300 nM TaqMan probe (17). The primer pairs and the TaqMan probe used for the detection of EVs are shown in Table 1. Subsequently, the mixtures were added to a 96-well microplate (Applied Biosystems) for the detection of EVs. The temperature profile for the detection of EVs was 50°C for 2 min, followed by 95°C for 10 min and 50 cycles at 95°C for 15 s and at 60°C for 1 min. The ABI PRISM 7000 SDS (Applied Biosystems) was used to determine the cDNA amplification.
TABLE 1.
Oligonucleotide sequences used for detection of enteric viruses
| Virus | Functiona | Sequence (5′-3′)b | Reference |
|---|---|---|---|
| NV-G1 | S-primer | CGYTGGATGCGNTTYCATGA | 16 |
| AS-primer | CTTAGACGCCATCATCATTYAC | 16 | |
| Probe | FAM-AGATYGCGATCYCCTGTCCA-TAMRA | 16 | |
| NV-G2 | S-primer | CARGARBCNATGTTYAGRTGGATGAG | 16 |
| AS-primer | TCGACGCCATCTTCATTCACA | 16 | |
| Probe | FAM-TGGGAGGGCGATCGCAATCT-TAMRA | 16 | |
| EV | S-primer | CCTCCGGCCCCTGAATG | 30 |
| AS-primer | ACCGGATGGCCAATCCAA | 30 | |
| Probe | FAM-CCGACTACTTTGGGTGTCCGTGTTTC-TAMRA | 17 | |
| AdV | S-primer | GCCCCAGTGGTCTTACATGCACATC | 12 |
| AS-primer | GCCACGGTGGGGTTTCTAAACTT | 12 | |
| Probe | FAM-TGCACCAGACCCGGGCTCAGGTACTCCGA-TAMRA | 12 | |
| TTV | S-primer | CGGGTGCCGDAGGTGAGTTTACAC | 33 |
| AS-primer | GAGCCTTGCCCATRGCCCGGCCAG | 33 | |
| Probe | FAM-AGTCAAGGGGCAATTCGGGCTCGGGA-TAMRA | 33 |
S-primer, sense primer; AS-primer, antisense primer; Probe, TaqMan probe.
Single-letter code: B stands for G, T, or C; D stands A, G, or T; N stands for A, C, G, or T; R stands for A or G; and Y stands for C or T. Abbreviations: FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
One hundred forty microliters out of 700 μl of the final concentrated sample was applied to RNA extraction, and 2.5 μl out of 60 μl of the resulting extracted RNA was used for the detection of EVs. Therefore, the volumes tested for EVs were equivalent to 1/120 of 500 ml of the original surface water, or approximately 4.2 ml.
Cell culture PCR for detection of EVs.
One hundred microliters of the final concentrated sample was mixed with 900 μl of Eagle's minimum essential medium (MEM; Nissui Seiyaku, Tokyo, Japan) containing 1% fetal bovine serum (JRH Bioscience, Lenexa, Kans.), 1% antibiotic-antimycotic (Invitrogen, Tokyo, Japan), 1% l-glutamine (29.2 mg/ml; Invitrogen), and 2% sodium bicarbonate solution (7.5% [wt/vol]; Invitrogen), and then the mixture was added to Buffalo green monkey kidney (BGM) cells propagated in a six-well cell culture plate (Corning, Tokyo, Japan). After incubation at 37°C with 5% CO2 for 7 days, the sample was centrifuged at 1,500 rpm for 5 min, and 140 μl of the supernatant was applied to RNA extraction using the QIAamp viral RNA mini kit (QIAGEN). The reverse transcription reaction was carried out using 2.5 μl out of 60 μl of the resulting RNA, and all 5 μl of cDNA was applied to TaqMan PCR.
Direct PCR for detection of DNA viruses.
Two hundred microliters of the final concentrated sample was applied to DNA extraction using a QIAamp DNA mini kit (QIAGEN, Tokyo, Japan), and 5 μl out of 200 μl of the resulting DNA was used for the detection of AdVs and TTVs. For the detection of AdVs, 5 μl of DNA was mixed with 45 μl of a reaction buffer containing 25 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 500 nM sense primer, 500 nM antisense primer, and 400 nM TaqMan probe (12). For the detection of TTVs, 5 μl of DNA was mixed with 45 μl of a reaction buffer containing 25 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 400 nM sense primer, 400 nM antisense primer, and 300 nM TaqMan probe (33). The mixture was added to a 96-well microplate (Applied Biosystems) and incubated as follows: for AdVs, 50°C for 2 min, followed by 95°C for 10 min and 50 cycles at 95°C for 3 s, at 55°C for 10 s, and at 65°C for 1 min; for TTVs, 50°C for 2 min, followed by 95°C for 10 min and 50 cycles at 95°C for 10 s and at 62°C for 30 s. The ABI PRISM 7000 SDS (Applied Biosystems) was used to determine the DNA amplification.
Two hundred microliters out of 700 μl of the final concentrated sample was applied to DNA extraction, and 5 μl out of 200 μl of the extracted DNA was used for the detection of each DNA virus. Therefore, the volumes tested for the presence of each DNA virus were equivalent to 1/140 of 500 ml of the original surface water, or approximately 3.6 ml.
Recovery of polioviruses (PVs) from surface water using cation-coated filter method.
Type 1 PVs (LSc 2ab Sabin strain) were provided courtesy of Kazuyoshi Yano (Tokyo Metropolitan Institute of Public Health, Tokyo, Japan) and propagated in BGM cells in an incubator at 37°C with 5% CO2 (18).
Recovery yields of PVs from the surface water samples collected for virus survey using the cation-coated filter method were determined as follows. One microliter of PV stock solution (∼107 PFU/ml) was inoculated into 100 ml of the river water collected from six sites (sites A to F) in the Tamagawa River and filtered through a 47-mm-diameter HA filter (Millipore) pretreated with 2.0 ml of 250 mM AlCl3. The filter was rinsed with 50 ml of 0.5 mM H2SO4 (pH 3.0) and eluted with 5.0 ml of 1.0 mM NaOH (pH 10.8) into a tube containing 25 μl of 100 mM H2SO4 (pH 1.0) and 50 μl of 100× Tris-EDTA buffer (pH 8.0).
The concentrations of PVs in the input and eluted samples were determined by plaque assay using BGM cells propagated in six-well cell culture plates (Corning). Each sample was diluted with Eagle's MEM (Nissui Seiyaku, Tokyo, Japan) containing 1% fetal bovine serum (JRH Bioscience, Lenexa, Kans.), 1% antibiotic-antimycotic (Invitrogen, Tokyo, Japan), 1% l-glutamine, and 2% sodium bicarbonate solution, 1.0 ml of which was added to the cells in each well. After incubation for 90 to 120 min, 3.0 ml of Eagle's MEM containing 1% fetal bovine serum, 1% antibiotic-antimycotic, 1% l-glutamine (29.2 mg/ml; Invitrogen), 1.5% sodium bicarbonate solution (7.5% [wt/vol]; Invitrogen), and 1.25% agar (Nacalai Tesque, Kyoto, Japan) was overlaid and incubated for 36 to 48 h. The cells were dyed with 0.001% neutral red solution prior to plaque counting. Two replicates were tested for each dilution, and the average of the concentrations of the two replicates was used to determine the recovery yield.
RESULTS
Recovery yields of PVs using cation-coated filter method.
In total, 37 surface water samples were applied to the recovery tests using the cation-coated filter method. As shown in Table 2, recovery yields of 56% ± 32% were obtained for PVs.
TABLE 2.
Recovery yields of PVs from surface water by cation-coated filter method
| Site (no. of tested samples) | No. of PFU of PVsa
|
% Recovery of PVs (mean ± SD) | |
|---|---|---|---|
| Inoculated | Recovered | ||
| A (2) | 21,000-28,000 | 12,750-24,000 | 74 ± 13 |
| B (8) | 11,000-65,000 | 750-57,500 | 71 ± 31 |
| C (8) | 7,000-24,000 | 250-16,250 | 47 ± 38 |
| D (6) | 9,000-29,000 | 3,000-12,500 | 45 ± 19 |
| E (5) | 8,000-36,500 | 1,500-14,250 | 30 ± 14 |
| F (8) | 4,000-32,500 | 3,250-17,750 | 71 ± 29 |
| Total (37) | 4,000-65,000 | 250-57,500 | 56 ± 32 |
The concentrations of PVs were determined by plaque assay using BGM cells.
Water quality of surface water samples.
Table 3 shows the water quality parameters of the surface water samples. The concentrations of total coliforms and thermotolerant coliforms in samples at sites A and B were lower than those at sites C to F (t test; P < 0.05). The concentrations of F-specific phages ranged from 0 to 1.8 PFU/ml. F-specific phages were detected in 17 of 32 samples (53%); samples at site E showed the highest positive ratio for F-specific phages (five of six samples; 83%), while the phages were not detected in samples at site A.
TABLE 3.
Water quality of surface water samples
| Site | Water temp (°C) | pH | Total coliforms (CFU/ml) | Thermotolerant coliforms (CFU/ml) | F-specific phages (PFU/ml) |
|---|---|---|---|---|---|
| A | 2-16 | 7.8-8.3 | 1-12 | 0-3 | 0 |
| B | 2-18 | 7.5-9.3 | 0-34 | 0-2 | 0-0.1 |
| C | 8-21 | 7.1-8.4 | 27-1,300 | 1-83 | 0-0.3 |
| D | 9-22 | 6.9-7.6 | 2-1,800 | 0-64 | 0-1.8 |
| E | 6-22 | 7.0-9.2 | 12-1,500 | 0-34 | 0-0.6 |
| F | 8-25 | 6.9-9.6 | 7-1,800 | 1-22 | 0-0.9 |
Occurrence of NVs.
During a 1-year survey, NV-G1 and NV-G2 were detected in 34 (53%) and 28 (44%) of 64 samples, respectively. Either NV-G1 or NV-G2 was detected in 37 samples (58%) in total. Meanwhile, neither NV-G1 nor NV-G2 was detected in samples at sites A and B. On the other hand, 77% of 48 samples at sites C to F were positive for NVs. Out of 37 NV-positive samples, 25 samples (68%) were positive for both NV-G1 and NV-G2, 9 samples (24%) exclusively for NV-G1, and 3 samples (8%) exclusively for NV-G2.
The concentrations of NV-G1 and NV-G2 in surface water samples are shown in Fig. 3. The geometric mean concentrations of NV-G1 and NV-G2 among all NV-positive samples were 0.087 and 0.61 genome/ml, respectively. Interestingly, the NV concentrations showed a seasonal difference between winter (from December to February) and summer (from June to August). The mean concentrations of NV-G1 and NV-G2 in winter were 0.21 and 2.3 genomes/ml, respectively, while those in summer were 0.016 and 0.026 genome/ml, respectively.
FIG. 3.
Concentrations of genomes of (A) NV-G1 and (B) NV-G2 in Tamagawa River water. ×, site A; ○, site B; □, site C; ♦, site D; ▴, site E; +, site F.
Occurrence of EVs.
EVs were detected in 8 (13%) of 64 samples; 6 samples (9%) by direct PCR and 2 samples (3%) by cell culture PCR. Samples at site B in December 2003, at site C in July and August 2003, at site D in January 2004, at site E in November 2003, and at site F in March 2004 were positive for EVs when determined by direct PCR. EVs were also detected in samples at sites E and F in September 2003 when determined by cell culture PCR.
Occurrence of AdVs.
AdVs were detected in 29 (45%) of 64 samples during the survey period, as summarized in Table 4. High positive ratios of AdVs (67% to 75%) were obtained from samples at sites C, D, and E, while AdVs were not detected in samples at sites A and B. AdVs were detected most frequently in winter (10 of 16 samples; 63%), but positive ratios in the other three seasons were also high (31% to 44%).
TABLE 4.
Detection of AdVs in Tamagawa River water
| Time of collection (mo/yr) | PCR resultsa
|
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 4/03 | − | − | − | + | − | − |
| 5/03 | NS | − | + | + | − | − |
| 6/03 | NS | − | − | + | + | + |
| 7/03 | − | − | − | − | + | + |
| 8/03 | NS | − | + | + | − | − |
| 9/03 | NS | − | + | − | − | − |
| 10/03 | − | − | + | + | + | − |
| 11/03 | NS | − | + | + | + | − |
| 12/03 | NS | − | + | + | + | − |
| 1/04 | − | − | + | + | + | − |
| 2/04 | NS | − | + | + | + | + |
| 3/04 | NS | − | − | − | + | + |
NS, not sampled; +, positive for AdVs; −, negative for AdVs.
Occurrence of TTVs.
TTVs were detected in 3 (5%) of 64 samples; samples at site D in February 2004 and at site E in July 2003 and February 2004 were positive for TTVs.
Differences in occurrence of viruses among sampling sites.
More than one kind of tested virus (NV-G1, NV-G2, EVs, AdVs, and TTVs) was detected in 43 (67%) of 64 surface water samples in total. One, two, three, and four kinds of viruses were detected in 10 (23%), 12 (28%), 16 (37%), and 5 (12%) of 43 virus-positive samples, respectively. No virus was detected in samples at site A, and only 1 (8%) of 12 samples at site B was positive for viruses (EVs). Viruses were detected in 42 (88%) of 48 samples at sites C to F in total. Looking at the occurrences separately, 11 (92%), 10 (83%), 11 (92%), and 10 (83%) of 12 samples were positive for viruses at sites C, D, E, and F, respectively. There was a significant difference between the positive ratio of viruses at sites A and B and that at sites C to F (χ2 test; P < 0.01).
Relationships between viruses and microbial indicators.
The presence of each tested virus was compared with the concentration of total coliforms, the presence of thermotolerant coliforms, or the presence of F-specific phages. NVs and AdVs were detected more frequently in samples with a higher concentration of total coliforms (χ2 test; P < 0.01). That is, only 1 (8%) and 1 (8%) of 13 samples with <10 CFU/ml total coliforms was positive for NVs and AdVs, respectively, while 37 (73%) and 28 (55%) of 51 samples with >10 CFU/ml total coliforms were positive for NVs and AdVs, respectively. On the other hand, there was no significant difference between the concentration of total coliforms and the presence of EVs or TTVs (χ2 test; P > 0.05).
No significant difference was observed between the presence of thermotolerant coliforms and that of each tested virus (χ2 test; P > 0.05); NVs, EVs, AdVs, and TTVs were detected in 30 (64%), 7 (15%), 24 (51%), and 1 (21%) of 47 thermotolerant coliform-positive samples, respectively, while they were also detected in 7 (41%), 1 (6%), 5 (29%), and 1 (6%) of 17 thermotolerant coliform-negative samples, respectively.
The presence of F-specific phages was correlated with that of NVs or AdVs (χ2 test; P < 0.01), but not with that of EVs or TTVs (χ2 test; P > 0.05). NVs and AdVs were detected in 16 (94%) and 14 (82%) of 17 F-specific-phage-positive samples, respectively, while only 7 (47%) and 4 (27%) of 15 F-specific-phage-negative samples were positive for NVs and AdVs, respectively.
The relationships between the concentrations of NVs and those of indicator bacteria are shown in Fig. 3. Among the samples positive for both NVs and each indicator, a negative correlation was observed between NVs and total coliforms (correlation coefficient [r] = −0.548), while the correlation coefficients were low for thermotolerant coliforms (r = −0.248) and F-specific phages (r = −0.173).
DISCUSSION
In this study, the cation-coated filter method, which had been developed to concentrate viruses in tap water (9), was applied to the surface water in the Tamagawa River. This method was previously reported to recover PVs efficiently from MilliQ water (Millipore) and tap water; the recovery yields were 95% ± 20% (n = 10) for MilliQ water and 88% ± 16% (n = 8) for tap water (9). On the other hand, the recovery yields of PVs by the plaque assay were 56% ± 32% (n = 37) for the surface water samples in the Tamagawa River (Table 2). There was not a significant difference between the recovery yields of PVs determined by the plaque assay and those by real-time PCR (42% ± 33%; n = 22) (t test; P > 0.05).
It is well known that the recovery yields of viruses from environmental water samples are affected by many factors, such as turbidity and the concentrations of organics, which may be responsible for the unstable recovery yields of viruses observed in this study and in some previous studies (5, 8, 23, 41). However, the mean recovery yield of 56% obtained in this study still seems practical for field survey of surface water, because recovery yields of PVs from surface water by the cation-coated filter method were significantly higher than those by a conventional method using a 1MDS positively charged filer (0.2-μm pore size and 47-mm diameter; Cuno, Meriden, Conn.) and beef extract (26% ± 9%; n = 6) (t test; P < 0.01).
In the recovery tests adopted in this study, no pretreatment was done prior to the inoculation of PVs into the sample. Therefore, not only the inoculated but also the indigenous PVs might have been detected by the plaque assay. However, our results suggested that the effect of indigenous PVs may be negligible because of the low positive ratio of viable EVs by cell culture PCR (2 of 64 samples; 3%) and the high concentration of inoculated PVs in surface water (40 to 650 PFU/ml) (Table 2).
A Millipore HA filter with a 90-mm diameter was used to filter 500 ml of surface water. This filter provides 4.5 times as large a net area (43.0 cm2) as that of a filter with a 47-mm diameter (9.6 cm2). The cation-coated filter method could be applied to the concentration of viruses in 500 ml of surface water without a decrease in recovery yields, because the recovery yields were almost the same with filters with diameters of 47 and 90 mm (data not shown).
Most viruses, including PVs, are expected to carry negative charges in water at pHs observed in this study (range, 6.9 to 9.6) (22) and are easy to adsorb to the cation-coated filter due to the electrical interaction among viruses, cations, and the filter. All amounts of PVs inoculated into the surface water were trapped on an HA filter precoated with aluminum ions, while adsorption yields of PVs to an HA filter precoated with magnesium ions were 52% ± 5% (n = 3). This is probably explained by the valences of Al3+ and Mg2+; the short contact time of viruses and cations on the filter was not enough for the bivalent magnesium ion to link viruses and the filter. On the other hand, the conventional method using a 1MDS positively charged filter (Cuno) showed PV adsorption yields of 62% ± 14% (n = 6) for surface water. These results clearly demonstrate the superiority of the Al3+-coated filter for the adsorption of viruses to the filter.
Introduction of the acid rinse step with 0.5 mM H2SO4 (pH 3.0) prior to elution of PVs enhanced the recovery yields of PVs from surface water from 9% ± 13% (n = 2) to 56% ± 32% (n = 37). These results were consistent with those for MilliQ water (Millipore) (9), which indicates that the Al3+-coated filter method can be applied to the concentration of viruses from various types of freshwater samples.
Moreover, NaOH was used as an elution medium in place of beef extract, which allowed us to avoid inhibition of enzyme reactions by organic and inorganic compounds in beef extract (1). It was previously reported that the detection limit for PVs by TaqMan PCR in beef extract was a 1 log unit lower than that in MilliQ water (Millipore) (9). Thus, this study indicated that the cation-coated filter method using the NaOH elution technique has an efficiency advantage over PCR with recovery yields of viruses equivalent to those of previous concentration methods using beef extract as an elution medium.
A question remains as to whether other enteric viruses are concentrated as efficiently as PVs, since the recovery efficiency of the cation-coated filter method was evaluated only with PVs. Moreover, recovery yields of PVs inoculated into a water sample may not be equal to those of indigenous PVs. Recovery yields of other enteric viruses, such as NVs and AdVs, should be evaluated in further studies.
The Centriprep YM-50 centrifugation unit, which was used in the second-round concentration step, provided high and stable recovery yields of PVs (73% ± 9%; n = 3) (9) and of F-specific Qβ RNA coliphages (84% ± 15%; n = 4). From these results, many types of enteric viruses are expected to be concentrated with high recovery efficiencies using this centrifugation method.
Many researchers have succeeded in detecting enteric viruses in surface water, but the volumes filtered for the detection of viruses were usually very large (more than 10 liters), probably due to the low sensitivities of detection methods, especially PCR processes. In addition, some studies depend on cell culture PCR, which includes culturing in a host cell prior to PCR, to detect viruses. Therefore, information on the occurrence of noncultivable viruses, such as NVs, in surface water is very limited.
In the present study, 500 ml of surface water samples collected from six sites along the Tamagawa River were concentrated using the cation-coated filter method and applied to the detection of human enteric viruses using TaqMan PCR. In a 1-year survey, 43 (67%) of 64 surface water samples were positive for more than one kind of virus. By direct PCR, NV-G1, NV-G2, EVs, AdVs, and TTVs were detected in 34 (53%), 28 (44%), 6 (9%), 29 (45%), and 3 (5%) samples, respectively, and 2 samples (3%) were positive for EVs by cell culture PCR.
Some studies have reported that NVs are isolated more frequently in winter than in summer in surface water (29, 31). These studies perform only the positive-negative detection of NVs and do not provide any quantitative data on NV concentrations in surface water. In this study, the concentrations of NVs were quantitatively determined by real-time PCR using cDNAs of NVs as positive controls (Fig. 3). The annual mean concentration of NV-G2 (0.61 genome/ml) was 0.85 log units higher than that of NV-G1 (0.087 genome/ml). NVs were detected in the highest concentration in winter (from December to February), followed by autumn (from September to November), spring (from March to May), and summer (from June to August). The concentrations of NV-G1 and NV-G2 in winter were 1.1 log units and 2.0 log units higher than those in summer, respectively. These results agreed with the epidemiological data from the National Institute of Infectious Disease, Tokyo, Japan, where NVs were isolated mainly in winter and NV-G1 was much more frequently isolated than NV-G2 from feces of hospitalized patients in Japan.
For the procedures of the cation-coated filter method and by the Centriprep YM-50 (Millipore), the efficiency of extraction of NV RNA by the QIAamp viral RNA mini kit (QIAGEN), and the efficiency of synthesis of NV cDNA, the recovery yields of NV genomes were simply assumed to be 100% in order to calculate the concentration of NVs in the original surface water. Considering that the real genome recovery yield can be lower, many more NVs might have been in the original surface water samples than our calculation showed.
AdVs were also detected in a high positive ratio (29 of 64 samples; 45%), which agreed with the results of previous research conducted by Pina et al. in Spain (29). They reported a higher positive ratio (65%) of AdVs than our study, which may be attributable to the much larger test volumes (equivalent to 2 liters of surface water) than in our study (4.2 ml). Our method was sensitive enough to detect AdVs from smaller volumes of test water, which could be an advantage of our method over traditional methods using a positively charged filter. The abundance of AdVs in surface water may be explained by the fact that AdVs are excreted with the feces at a very high concentration (∼1011 viruses/g) (39).
EV was the only virus detected in the samples at site B. The EVs may be derived from the WWTP in the upstream area, but the reason only EVs were detected is unknown. The primers used in this study were specific for human EVs but not for bovine EVs; thus, the positive signals by PCR are expected to demonstrate the presence of human EVs. EVs were detected in two samples in September 2003 by cell culture PCR using BGM cells, which was consistent with the epidemiological data in Japan showing an epidemic of EVs from summer to autumn.
According to the U.S. Environmental Protection Agency, NVs, AdVs, and EVs are listed as viruses to be studied further regarding their occurrence as well as effective treatment and analytical methods (35). Results obtained in this study will be informative for understanding the fate of these viruses in surface water.
TTVs have been isolated in 13% (8 of 63 samples) and 2% (1 of 48 samples), respectively, of 40-ml influent and effluent samples from a WWTP in India (37). They have also been isolated in 8% (9/113) of shellfish samples in Norway (25). In this study, 3 (5%) of 64 surface water samples were positive for TTVs, and this may be the first study that detected TTVs in surface water. TTVs are prevalent in the general population, as well as in hepatitis patients, and are thought to be transmitted by the fecal-oral route (28). Therefore, it would be very important to understand the occurrence of TTVs, not only in surface water, but also in other types of water samples, such as seawater, groundwater, or tap water.
The presence or absence of each virus was tested using small volumes of surface water; 3.6 ml for EVs, 10.8 ml for NV-G1 or NV-G2, and 4.2 ml for AdVs or TTVs. Several copies of viral genomes in a PCR tube are expected to be needed for PCR detection using the primers and TaqMan probes used in this study. The detection limits are reported to be less than 10 genomes, less than 1.5 genomes, and 0.0082 to 0.082 PFU for NVs (16), AdVs (12), and PVs (9), respectively. Therefore, from the results obtained in this study, it is estimated that viruses do exist at a high concentration in surface water. In fact, the concentrations of NVs in surface water sometimes exceeded 1 genome/ml in winter (Fig. 3).
There is doubt that some genomes detected by PCR are derived from nonviable viruses. A few studies suggest that the naked viral genomes should be unstable in water and disappear in a few minutes (21, 34). On the other hand, it is known that viruses inactivated by chlorine can be detected by PCR without any specific treatment (27). We have no data on recovery yields of viral genomes in surface water by the cation-coated filter method; it is plausible that the naked viral genomes are not concentrated as efficiently as PVs. However, viruses with capsid damage might be concentrated as efficiently as intact viruses, and viruses with no infectivity might have been detected in the present study. Further study is required to clarify this possibility at the next step.
The concentration of total coliforms and the presence of F-specific phages showed a high correlation with the presence of NVs or AdVs, while the presence of thermotolerant coliforms and that of viruses were not significantly related. In addition to total coliforms, which are currently used as the indicator bacteria for surface water in Japan, F-specific phages may also work to monitor viral contamination. However, a negative correlation was found between the concentration of NVs and that of total coliforms among the samples positive for both NVs and total coliforms (Fig. 4), which implies a need for further studies of the appropriate indicators for viruses.
FIG. 4.
Relationships between NVs and (A) total coliforms, (B) thermotolerant coliforms, and (C) F-specific phages.
There are many rivers receiving effluents from WWTPs upstream with intakes of DWTPs downstream. The Tamagawa River was selected as one of the typical examples of such rivers. The occurrences of viruses and bacteria upstream were quite different from those downstream, suggesting that the effluents from WWTPs are a main source of pollution of surface water. The introduction of advanced sewage treatment systems, such as ozonation, will contribute to achieving a microbiologically safe quality of surface water.
Surface water is a main source of drinking water in Japan and is also used for recreational purposes, especially in summer. Therefore, it is important to evaluate the risk of virus infection, not only via drinking tap water but also via direct contact with surface water. Quantification of viruses in the sample is an essential step to know the risk of virus infection via water. In this study, a clear seasonal variation in the concentrations of NVs in surface water was found, showing a higher concentration in winter than in summer (Fig. 3). The risk of infection of NVs via playing in the river may not be so high in summer, while that via tap water can be an issue, especially in winter. For further study, it is necessary to accumulate much more quantitative data on viruses in surface water.
REFERENCES
- 1.Abbaszadegan, M., M. S. Huber, C. P. Gerba, and I. L. Pepper. 1993. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl. Environ. Microbiol. 59:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1995. Water quality. Detection and enumeration of bacteriophages—part 1. Enumeration of F-specific RNA bacteriophages. ISO 10705-1. International Organization for Standardization, Geneva, Switzerland.
- 3.Anonymous. 1999. Food safety standards, costs and benefits, p. 1-154. Australia-New Zealand Food Authority, Commonwealth Government of Australia, Canberra.
- 4.Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerba, C. P., S. R. Farrah, S. M. Goyal, C. Wallis, and J. L. Melnik. 1978. Concentration of enteroviruses from large volumes of tap water, treated sewage, and seawater. Appl. Environ. Microbiol. 35:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilgen, M., D. Germann, J. Lüthy, and P. Hübner. 1997. Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. Int. J. Food Microbiol. 37:189-199. [DOI] [PubMed] [Google Scholar]
- 7.Green, D. H., and G. D. Lewis. 1999. Comparative detection of enteric viruses in wastewaters, sediments and oysters by reverse transcription-PCR and cell culture. Water Res. 33:1195-1200. [Google Scholar]
- 8.Guttman-Bass, N., and J. Catalano-Sherman. 1985. Effects of humic materials on virus recovery from water. Appl. Environ. Microbiol. 49:1260-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haramoto, E., H. Katayama, and S. Ohgaki. 2004. Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Appl. Environ. Microbiol. 70:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havelaar, A. H., W. M. Hogeboom, and R. Pot. 1984. F-specific RNA bacteriophages in sewage: methodology and occurrence. Water Sci. Technol. 17:645-655. [Google Scholar]
- 11.Havelaar, A. H., M. Van Olphen, and Y. C. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in freshwater. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heim, A., C. Ebnet, G. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228-239. [DOI] [PubMed] [Google Scholar]
- 13.IAWPRC Study Group on Health Related Microbiology. 1991. Bacteriophages as model viruses in water quality control. Water Sci. Technol. 25:529-545. [Google Scholar]
- 14.Irving, L. G., and F. A. Smith. 1981. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl. Environ. Microbiol. 41:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johl, M., M. L. Kerkmann, U. Kramer, and R. Walter. 1991. Virological investigation of the River Elbe. Water Sci. Technol. 24:205-208. [Google Scholar]
- 16.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama, H., A. Shimasaki, and S. Ohgaki. 2002. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 68:1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keswick, B. H., C. P. Gerba, S. L. Secor, and I. Cech. 1982. Survival of enteric viruses and indicator bacteria in groundwater. J. Environ. Sci. Health A 17:903-912. [Google Scholar]
- 19.Reference deleted.
- 20.Kohli, E., F. Bon, P. Pothier, R. Brachet, and A. Flahault. 1999. Investigation virologique du pic épidémique de gastro-entérites de l'hiver 98-99. Virologie 3:156. [Google Scholar]
- 21.Limsawat, S., and S. Ohgaki. 1997. Fate of liberated viral RNA in wastewater determined by PCR. Appl. Environ. Microbiol. 63:2932-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lytle, C. D., and L. B. Routson. 1995. Minimized virus binding for tests of barrier materials. Appl. Environ. Microbiol. 61:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, J. F., J. Naranjo, and C. P. Gerba. 1994. Evaluation of MK filters for recovery of enteroviruses from tap water. Appl. Environ. Microbiol. 60:1974-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuura, K., S. Hasegawa, T. Nakayama, O. Morita, and H. Uetake. 1984. Viral pollution of the rivers in Toyama City. Microbiol. Immunol. 28:575-588. [DOI] [PubMed] [Google Scholar]
- 25.Myrmel, M., E. M. M. Berg, E. Rimstad, and B. Grinde. 2004. Detection of enteric viruses in shellfish from the Norwegian coast. Appl. Environ. Microbiol. 70:2678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizawa, T., H. Okamoto, K. Konishi, H. Yoshizawa, Y. Miyakawa, and M. Mayumi. 1997. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 241:92-97. [DOI] [PubMed] [Google Scholar]
- 27.Nuanualsuwan, S., and D. O. Cliver. 2002. Pretreatment to avoid positive RT-PCR results with inactivated viruses. J. Virol. Methods 104:217-225. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto, H., Y. Akahane, M. Ukita, M. Fukuda, F. Tsuda, Y. Miyakawa, and M. Mayumi. 1998. Fecal excretion of a nonenveloped DNA virus (TTV) associated with posttransfusion non-A-G hepatitis. J. Med. Virol. 56:128-132. [PubMed] [Google Scholar]
- 29.Pina, S., M. Puig, F. Lucena, J. Jofre, and R. Girones. 1998. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl. Environ. Microbiol. 64:3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh, Y. S. C., D. Wait, L. Tai, and M. D. Sobsey. 1995. Methods to remove inhibitors in sewage and other thermotolerant wastes for enterovirus detection by the polymerase chain reaction. J. Virol. Methods 54:51-66. [DOI] [PubMed] [Google Scholar]
- 31.Skraber, S., B. Gassilloud, and C. Gantzer. 2004. Comparison of coliforms and coliphages as tools for assessment of viral contamination in river water. Appl. Environ. Microbiol. 70:3644-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, M. B., N. Cox, M. A. Vrey, and W. O. K. Grabow. 2001. The occurrence of hepatitis A and astroviruses in selected river and dam waters in South Africa. Water Res. 35:2653-2660. [DOI] [PubMed] [Google Scholar]
- 33.Tokita, H., S. Murai, H. Kamitsukasa, M. Yagura, H. Harada, M. Takahashi, and H. Okamoto. 2002. High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J. Med. Virol. 67:501-509. [DOI] [PubMed] [Google Scholar]
- 34.Tsai, Y. L., B. Tran, and C. J. Palmer. 1995. Analysis of viral RNA persistence in seawater by reverse transcriptase-PCR. Appl. Environ. Microbiol. 61:363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Environmental Protection Agency. 1998. Announcement of the drinking water contaminant candidate list. Fed. Regist. 63:10273-10287. [Google Scholar]
- 36.U.S. Environmental Protection Agency. 2001. National primary drinking water standards. EPA816-F-01-007. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 37.Vaidya, S. R., S. D. Chitambar, and V. A. Arankalle. 2002. Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J. Hepatol. 37:131-136. [DOI] [PubMed] [Google Scholar]
- 38.Vinje, J., S. A. Altena, and M. Koopmans. 1997. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in The Netherlands. J. Infect. Dis. 176:1374-1378. [DOI] [PubMed] [Google Scholar]
- 39.Wadell, G. 1984. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 110:191-220. [DOI] [PubMed] [Google Scholar]
- 40.Ward, R. L., D. I. Bernstein, E. C. Young, J. R. Sherwood, D. R. Knowlton, and G. M. Schiff. 1986. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J. Infect. Dis. 154:871-880. [DOI] [PubMed] [Google Scholar]
- 41.Winona, L. J., A. W. Ommani, J. Olszewsky, J. B. Nuzzo, and K. H. Oshima. 2001. Efficient and predictable recovery of viruses from water by small scale ultrafiltration systems. Can. J. Microbiol. 47:1033-1041. [PubMed] [Google Scholar]