Abstract
The group II intron Ll.ltrB is found within the ltrB relaxase gene of the conjugative element pRS01 in Lactococcus lactis. Precise splicing of the intron is essential for pRS01 transfer. The transcription regulation and in vivo splicing activity of Ll.ltrB have not been investigated thoroughly in L. lactis in the natural pRS01 context. We developed absolute quantitative real-time reverse transcription-PCR assays to quantify RNA levels of the 5′ exon (ltrBE1) and the spliced relaxase (ltrB) and intron-encoded protein (ltrA) genes, as well as Ll.ltrB splicing activity under different physiological conditions. The mRNA levels for the ATP-binding protein OppD were assayed for comparison to the ltrB transcripts. The oppD mRNA ranged from 10- to 10,000-fold higher than ltrB region genes. ltrBE1 expression was growth-phase dependent. The mRNA level of ltrA was almost constant during all growth phases and in all media tested. Ll.ltrB in vivo splicing activity ranged from (6.5 ± 2.1)% to (22.1 ± 8.0)%. Acid challenge significantly decreased both ltrB region mRNA levels and intron splicing activity. The presence of recipient cells, different mating environments, and temperature stress had no significant effects on expression and splicing. Western blotting showed that the level of LtrB protein expressed from an intronless ltrB gene was much higher (about 20-fold) than the level of protein expressed from an intron-containing construct. Interestingly, LtrB protein showed a tendency to function in cis on its oriT target. The low level of ltrB transcript and relatively inefficient splicing of the intron may limit Ll.ltrB mobility and dissemination in nature.
Group II introns are large catalytic RNAs found in many bacteria and in organelles of plants, fungi, and algae (20). Some group II introns can also function as mobile genetic elements, capable of inserting into intronless alleles (homing) or into ectopic sites (transposition) at lower frequencies (20). Although sequence conservation is limited, all group II introns fold into a conserved secondary structure composed of six stem-loop domains (domains I through VI) (29). Domains I, V, and VI form the catalytic core and play essential roles in intron splicing, while domains II and III are involved in intron folding (29). Domain IV is looped out from the main structure. In some group II introns, domain IV contains an open reading frame whose gene product is essential for group II intron splicing and mobility. Group II introns splice via two transesterification reactions in vitro, with the intron spliced out as a lariat structure (20). This splicing reaction mechanism is similar to that of eukaryotic pre-mRNA splicing, which is catalyzed by trans-acting snRNAs and splicing factors.
Ll.ltrB was the first bacterial group II intron identified to show full splicing function in vivo. It was found in the conjugative plasmid pRS01 of Lactococcus lactis strain ML3 and in the nearly identical chromosomal sex factor from L. lactis subsp. lactis 712 (22, 36). Ll.ltrB splits the putative relaxase gene, ltrB, into two exons, ltrBE1 and ltrBE2 (22). The LtrB protein is predicted to nick the plasmid at its transfer origin, initiating single-stranded DNA transfer from the donor cell to the recipient cell during conjugation. Efficient splicing of Ll.ltrB is required for the conjugative transfer of pRS01 (6). Ll.ltrB encodes a protein, LtrA, which is required for the efficient splicing and mobility of this intron (19, 42). Like other intron-encoded proteins, LtrA has multiple functions, including reverse transcriptase, DNA endonuclease, and maturase activities (19). LtrA binds to the intron RNA and induces conformational changes important for the ribozyme activity (18). The primary binding site of LtrA is a stem-loop region in domain IV, which overlaps with the ribosome binding site of the ltrA gene (37). While the binding of LtrA to the intron RNA is necessary for splicing and mobility, it also represses ltrA translation (37).
The 48.4-kb plasmid pRS01 is a broad-host-range conjugative element in L. lactis. It mediates the transfer of lactose utilization ability between L. lactis ML3 and a recipient by cointegration with a nonconjugative Lac plasmid pSK08 (1). The copy number of pRS01 is less than 1 molecule per cell, and it likely integrates reversibly into the chromosome (1). A 15-kb region containing genes responsible for pRS01 transfer by analyzing the random cointegrates between pRS01 and pSK08 (1) was identified. Later studies found that plasmid pTRK28 can form stable cointegrates with pRS01 via insertion sequence element-mediated recombination (32). Further genetic mapping of pRS01::pTRK28 cointegrates identified four regions involved in conjugative plasmid transfer: Tra1, Tra2, Tra3, and Tra4 (23). Tra3 and Tra4 correspond to transfer regions identified previously, which encode proteins required for mating pair formation. Tra1 and Tra2 encompass the transfer origin (oriT), the ltrB locus, and several other genes: ltrC, ltrD, ltrE (21, 22), and ltrF (Fig. 1A) (15). The ltrC, ltrD, ltrE, and ltrF gene products may be involved in relaxosome formation during conjugation. The G/C ratio of the Ll.ltrB intron (36%) and the codon usage for ltrA are similar to those of the L. lactis genes, indicating that the intron insertion was not a recent event (22). We have found recently that the Tra1 and Tra2 region genes are transcribed in an operon, which suggests that the Ll.ltrB intron might be part of a long RNA transcript (Y. Chen, J. Klein, and G. Dunny, unpublished data). Previously, an internal promoter upstream of ltrA inside the intron was identified (42). The transcription of the ltrA promoter might extend into ltrBE2 (42).
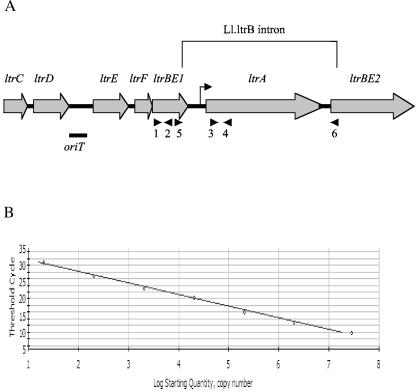
FIG. 1.
(A) Map of the Tra1 and Tra2 region of pRS01 (adapted from reference 15). The two exons of the interrupted ltrB gene (ltrBE1 and ltrBE2), the Ll.ltrB intron, and the intron-encoded protein gene ltrA are indicated. The ltrC, ltrD, ltrE, and ltrF gene products may be involved in relaxosome formation during conjugation. The transfer origin of pRS01 is between ltrD and ltrE, which is indicated by a bar under the map. A bent arrow indicates the internal ltrA promoter inside the intron. Arrows under the map indicate primers used in the real-time RT-PCR assay. Primer pair 1-2 was used to amplify ltrBE1, 3-4 to amplify ltrA, and 5-6 to amplify ligated relaxase gene ltrB. Primer 1, RTPCRltrBE1L4; primer 2, RTPCRltrBE1R3; primer 3, RTPCRltrAL1; primer 4, RTPCRltrAR1; primer 5, RTPCRltrBE1; primer 6, RTltrBE2. (B) The standard curve generated from plasmid DNA containing the ligated relaxase gene ltrB by using primers 5 and 6. The threshold cycle is the point at which the reaction fluorescent intensity is above the background level.
Most of the research on Ll.ltrB has focused on intron RNA structure (24) and LtrA protein function (34, 37), as well as on Ll.ltrB mobility mechanisms (4) and genetic engineering applications (12). Very little is known about the regulation of the Ll.ltrB intron transcription and splicing in vivo or how such regulation might affect conjugation. In this study, we developed quantitative real-time reverse transcription (RT)-PCR assays to analyze the expression and splicing activity of Ll.ltrB in its natural host, pRS01. We analyzed Ll.ltrB splicing activity (ltrB mRNA relative to ltrBE1 mRNA) and mRNA levels of ltrB region transcripts, 5′ exon ltrBE1, ltrA, and ligated relaxase gene ltrB, under various physiological conditions. We show that ltrB region genes are constitutively expressed at low levels. Ll.ltrB in vivo splicing efficiency is relatively low under all the conditions tested. Furthermore, we show that the amount of LtrB protein expressed from pRS01 in its natural context is undetectable by Western blotting.
MATERIALS AND METHODS
Bacterial strains.
Lactococcus lactis strain ML3 was used in all physiological experiments. L. lactis LM2301 was used as a recipient strain in all mating experiments. L. lactis MMS370 is a recombination deficient strain (2) and was used in LtrB protein expression and quantitative conjugation experiments. Escherichia coli DH5α and BL21(DE3) (Novagen) were used in DNA cloning experiments. E. coli strains were cultured in Luria broth and grown at 37°C with shaking. L. lactis strains were cultured in M17 medium (38) supplemented with 0.5% glucose (GM17 medium, pH 7.0) (except where otherwise indicated) at 30°C without shaking. Antibiotics were added at the following final concentrations: carbenicillin at 50 μg/ml, spectinomycin at 50 μg/ml, and kanamycin at 40 μg/ml for E. coli, and spectinomycin at 300 μg/ml, rifampin at 100 μg/ml, erythromycin at 10 μg/ml, and fusidic acid at 25 μg/ml for L. lactis.
Growth conditions.
To investigate the roles of medium and growth phase on the expression of ltrB region, L. lactis ML3 was cultured in either M17 medium, M17 medium supplemented with 0.5% lactose (LM17 medium), GM17 medium, or 10% skim milk (buffered with β-glycerophosphate by adding 19 g/liter; the pH was adjusted to 7.0 by using HCl). Overnight cultures were diluted 1:100 in fresh medium and incubated at 30°C. After 120 min of growth, 15 ml of culture was collected. Ten milliliters of culture was collected after 210 min and after 290 min of growth. Five milliliters of culture was collected after 480 min of growth. Collected cells were centrifuged at 4°C and quickly frozen on dry ice. Growth was monitored by measuring the optical density at 600 nm (OD600). Serial dilutions of cells were also plated on M17 medium, LM17 medium, GM17 medium, and skim milk plates for viable cell counts. To read the OD600 for cells growing in skim milk, 2 volumes of 0.2% EDTA (pH 12) were added to samples to dissociate the casein micelles immediately before the optical density reading (14).
To investigate the role of mating environment on the expression of ltrB locus, overnight L. lactis ML3 and LM2301 cultures grown in GM17 medium or 10% skim milk were diluted 1:100 into fresh media and grown until the OD600 reached 0.5. For broth mating, equal volumes (15 ml) of ML3 and LM2301 cells were mixed and mated for 1 h. Five milliliters of mating mixture was centrifuged at 4°C and quickly frozen on dry ice. L. lactis ML3 was used as a control. For agar plate mating, 30 ml of exponential-phase ML3 and LM2301 cultures were harvested and resuspended in 300 μl of 10 mM potassium phosphate-buffered saline, pH 7.2. One hundred microliters of ML3 and LM2301 cells were mixed, plated on agar plates, and incubated for 1 h. As a control, 100 μl of ML3 cells was plated on agar plates. Cells were washed off the plates, collected by centrifugation at 4°C, and quickly frozen on dry ice.
To investigate the effects of low pH on ltrB region expression, overnight L. lactis ML3 cultures grown in GM17 medium (pH 7.0) were diluted 1:100 in fresh GM17 medium (pH 7.0). Twenty microliters of exponential-phase (OD600 = 0.5) cells were harvested and washed twice with 20 ml of 0.9% NaCl. During the second wash, cells were divided into two aliquots. The pellet was resuspended in GM17 medium at either pH 5.0 or pH 7.0 and grown for 30 min. Cells were harvested and quickly frozen on dry ice. The M17 medium used in this experiment was made from polypeptide (5 g/liter), phytopeptide (5 g/liter), yeast extract (2.5 g/liter), beef extract (3 g/liter), ascorbic acid (0.5 g/liter), and 1 M magnesium sulfide (1 ml/liter). β-Glycerophosphate was added to the medium, and the pH was adjusted to 7.0 or 5.0 as previously described.
To investigate ltrB region gene expression levels in response to temperature stress, overnight L. lactis ML3 cultures grown in LM17 medium at 30°C were diluted 1:100 in fresh LM17 medium. The cells were grown at 21°C to an optical density of 0.4 at 600 nm. Heat stress was applied by transferring half of the culture to 38°C. Samples were taken after 15 min and 30 min from both 21°C and 38°C cultures.
Primer design and generation of recombinant DNA external standard curves.
Primers were designed by using Vector NTI and Primer 3 software. For ltrBE1, a fragment of 136 bp was amplified with RTPCRltrBE1L4 (5′-GTGCTTGGTCATCACCTCATCCA-3′) and RTPCRltrBE1R3 (5′-CGACGTGGGTTGCAATCACAA-3′); for ltrA, a fragment of 147 bp was amplified with RTPCRltrAL1 (5′-GGTATGCGGACGACTTCATTA-3′) and RTPCRltrAR1 (5′-CGGGTTGACTGCTATGTGTGA-3′); and for ltrB, a fragment of 214 bp was amplified with RTPCRltrBE1 (5′-CAGGTGGCGAATATGAATTTGTG-3′) and RTltrBE2 (5′-TTTGCGCCATAACGTGAAGA-3′). The relative positions of the primers are indicated in Fig. 1A. For chromosomal gene oppD, a fragment of 145 bp was amplified with OppDL1 (5′-CAGGGTCTGTGCCTTCTCTG-3′) and OppDR1 (5′-GCCTGACCTCGAACAAAATG-3′), based on the sequence of the L. lactis IL-1403 oppD gene (AE006414).
The pCOM9 plasmid was constructed by cloning the ltrB region into pDL281 (42). pCOM-ex is a derivative of pCOM9 containing an intronless allele of ltrB (42). The RT-PCR product of OppDL1 and OppDR1 was cloned in the pGEM-T easy vector (Promega) according to the manufacturer's instructions to generate pGEMOppD. Recombinant plasmids pCOM9, pCOM-ex, and pGEMOppD were linearized with a unique cutting restriction enzyme digestion (NcoI or SphI; New England Biolabs). Linear double-stranded plasmids were quantified by multiple (n = 5) optical measurements in three different dilutions at OD260. Plasmids were diluted from 6 × 109 single-stranded molecules/μl down to 6 single-stranded molecules/μl. Derived plasmid dilutions were aliquoted and frozen at −20°C.
RNA isolation and cDNA synthesis.
RNA was isolated from L. lactis cells collected under various physiological conditions, as described above. RNA was extracted by using the RedFast RNA kit (Bio101) according to the manufacturer's instructions, except that two phenol-chloroform-isoamyl alcohol (25:24:1) extractions were performed. The RNA concentration was quantified by measuring the absorbance at OD260. The residual DNA contamination was removed by the following procedure. RNA was treated with RQ1 DNase (2.5 U/μg RNA) (Promega) for 30 min and precipitated with 0.5 volume of 7.5 M LiCl at −20°C for 1 h. RNA was pelleted, washed with 70% ethanol, and treated with RQ1 DNase for 30 min. DNase-treated RNA was further purified by using the RNeasy mini kit (QIAGEN). The integrity of the RNA samples was checked by electrophoresis in 1.5% agarose gels.
For reverse transcription, 1.5 μg of DNase-treated RNA was denatured at 65°C for 5 min in the presence of 2 μg of pd(N)6 random hexamer (Amersham Biosciences) and then cooled on ice. Superscript II buffer (5×) (Invitrogen), 1 mM dithiothreitol, 0.5 mM dNTPs, and 5 U RNase inhibitor (Promega) were added to the samples in a final volume of 38 μl. The reaction mixture was divided into two aliquots. Two hundred units of Superscript II enzyme (Invitrogen) was added to one aliquot. As a negative control, 1 μl diethyl pyrocarbonate-treated H2O was added to another aliquot. Reaction mixtures were incubated at 42°C for 60 min and the Superscript II was inactivated by incubation at 75°C for 15 min. After cDNA synthesis, 20 μl of diethyl pyrocarbonate-treated H2O was added to samples to give a final volume of 60 μl.
SYBR green quantitative PCR.
Gene quantification was performed with the iCycler system (Bio-Rad). The reaction mixture (25 μl) contained the following components: 12.5 μl of SYBR green PCR master mix (QIAGEN), 0.2 μM of each specific oligonucleotide primer, and 2.4 μl of the cDNA sample. PCR conditions were optimized for the four primer sets. Gradient annealing was used to determine the optimal annealing temperature for all the primer sets. The following real-time PCR cycling conditions were used for all quantifications: 15 min at 95°C, followed by 40 repeats of 15 s at 95°C, 15 s at 54.5°C, and 20 s at 72°C. A melting curve analysis was performed in all PCR runs to check for primer dimers. During each run, standard dilutions of known quantities of recombinant plasmid DNA were included to generate standard curves and to permit gene quantification. RNA samples that were not treated with reverse transcriptase were included to check for genomic DNA contamination. Negative controls that contained no template were also included. For each RNA preparation, the measurement of gene expression was assayed in triplicate in each PCR run. At least two independent RNA preparations were tested for each physiological condition.
For statistical analysis, Student's t test was used to determine the significance between gene expression levels. A P value of <0.05 was considered to be significant.
Quantitative conjugation assay.
Mating assays between MMS370pM1014 carrying pCOM9 or pCOMT and LM2301 were done as previously described (42).
Analysis of LtrB protein.
The ltrB gene was cloned into pET28b(+) (Novagen) to generate pJK18. E. coli strain BL21 (DE3) (Novagen) carrying pJK18 was grown to exponential phase, and expression of LtrB was induced with IPTG (isopropyl-ā-d-thiogalactopyranoside) according to the manufacturer's instructions. The induced protein was found in the insoluble fraction of cell extracts. The inclusion bodies were collected as described in the Novagen pET system manual. Collected inclusion bodies were boiled and then loaded in 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Bands containing LtrB protein, which migrated at the predicted mobility of LtrB and were present only in induced culture expressing the cloned gene, were cut out and homogenized by passing through syringes with 18-gauge needles followed by syringes with 20-, 21-, 22-, and 23-gauge needles. The final protein-acrylamide mixture was used to immunize a New Zealand White rabbit. Subsequent injections were done in a similar way at 3- to 6-week intervals. Test bleedings were carried out 8 to 10 days after each injection. The antiserum used in this study was obtained 8 days after the fourth injection. The Western blot analysis was done as previously described (42).
RESULTS
Development of quantitative real-time RT-PCR assays.
To measure the 5′ exon (ltrBE1), the ligated relaxase message (ltrB), and the intron-encoded protein gene (ltrA) mRNA levels in L. lactis, we developed absolute quantitative real-time RT-PCR assays for each of these messages. Quantification was based on external standard curves generated from recombinant plasmid DNA containing the gene of interest. The chromosomal oppD gene (39) was used for comparison with ltrB region gene expression levels. The oppD gene encodes a subunit of the oligopeptide transport system, and its expression has been shown to be regulated by carbon and nitrogen availability in Borrelia burgdorferi (40). Specificity of the primers was determined by melting-curve analysis and gel electrophoresis. In order to compare data generated by different primer pairs, it is important that the primer pairs have equal amplification efficiencies. To measure efficiency, standard curves for ltrBE1, ltrA, ltrB, and oppD primer pairs were generated by regression analysis of 10-fold serial dilutions of recombinant plasmid DNA. Figure 1B shows a representative standard curve. Real-time PCR efficiencies were calculated from slopes of the standard curves based on the equation E = 10(−1/slope) (30). Investigated transcripts showed high PCR efficiency rates, between 1.91 and 1.97 (Table 1). Standard curves were generated with high precision and linearity (Pearson correlation coefficient r > 0.99) over 7 orders of magnitude. Intra-assay and interassay variations were below 4.2% (Table 1), which indicated high reproducibility and low test variability. Because the accuracy of a real-time PCR assay depends on identical amplification efficiencies for both the native target and the standard curve, we also tested PCR efficiencies by using cDNA templates generated from in vitro-transcribed total L. lactis ML3 RNA. Similar efficiencies were obtained (Table 1).
TABLE 1.
SYBR green real-time PCR assay characteristicsa
| Parameter | Assay value for indicated gene
|
|||
|---|---|---|---|---|
| ltrBE1 | ltrB | ltrA | oppD | |
| PCR efficiency determined by usingb: | ||||
| Recombinant plasmid DNA | 1.97 | 1.96 | 1.91 | 1.96 |
| cDNA templates | 1.92 | 1.95 | 1.98 | 1.94 |
| Melting temp (°C) | 78.5 | 79.0 | 77.0 | 78.0 |
| Variationc (%) | ||||
| Intra-assay | 1.7 | 1.3 | 1.6 | 3.9 |
| Interassay | 3.0 | 3.9 | 2.7 | 4.2 |
PCR efficiency (E) is calculated according to the following equation: E = 10(−1/slope). The maximal efficiency of PCR is 2 where every PCR product is replicated every cycle.
PCR efficiency was determined by using recombinant plasmid DNA containing target genes as templates and by using cDNA templates generated from in vitro-transcribed total L. lactis ML3 RNA.
Intra-assay variations were determined in three repeats within one real-time PCR run. Interassay variations were determined in four different experiment runs in 4 days. Intra-assay and interassay variations were determined by using recombinant plasmid DNA containing target genes as templates.
Expression of ltrB region genes during exponential and stationary growth in different media.
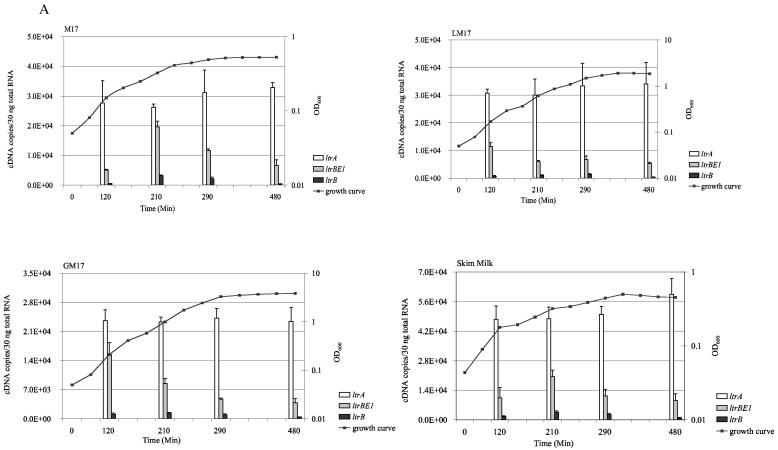
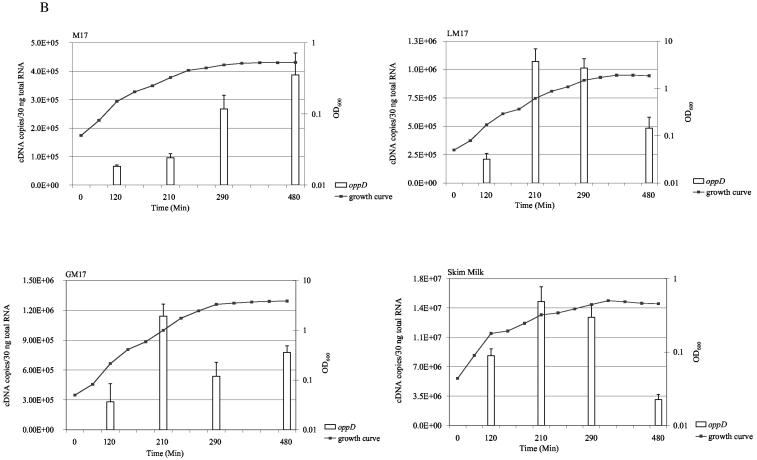
We first measured the levels of ltrB region transcripts in different media and in different growth phases. Total RNA was extracted from early-, mid-, and late-exponential-phase and stationary-phase L. lactis ML3 cultures growing in the following media: M17 medium, GM17 medium, LM17 medium, and 10% skim milk. Aliquots of total RNA were treated with DNase and subjected to reverse transcription. We compared the use of sequence-specific priming or random priming for each target gene. Similar results were obtained with both priming methods. Therefore, we used random hexamers to generate cDNA in all the quantification analysis. The resulting cDNA was amplified by using SYBR green real-time PCR. Genomic DNA contamination was determined by amplifying RNA samples that were not treated with reverse transcriptase. By using the DNase treatment method described in this report, we found there were more than 10 amplification cycles of difference between untreated samples and those treated with reverse transcriptase. This indicated that DNA contamination was less than 0.1% of input RNA. In each PCR run, standard curves were generated by amplifying known quantities of recombinant plasmids containing target genes, and these were used to deduce mRNA levels in the samples. The results are summarized in Fig. 2. The ltrB region genes were constitutively expressed at very low levels in all the media tested. In contrast, oppD mRNA was about 10- to 10,000-fold higher. The ltrA mRNA level was the highest among the ltrB region genes. Depending on the media and growth phases, the amount of ltrA mRNA was about 1.5- to 7-fold greater than that of ltrBE1 mRNA and 9- to 90-fold greater than that of ltrB mRNA. ltrA expression levels were almost constant during all growth phases and in all the media tested. Expression of ltrBE1 and ltrB were growth-phase dependent. Peak expression of ltrBE1 was reached during early exponential phase in LM17 and GM17 media, while expression was highest during mid-exponential phase in M17 medium and skim milk. Expression of ltrB was highest during exponential phase in all media tested. Medium composition did not influence ltrB region mRNA levels significantly, but we observed an increase of almost 10-fold in oppD expression in skim milk compared to the levels observed with M17, LM17, and GM17 media.
FIG. 2.
Expression of ltrBE1, ltrB, ltrA, and oppD genes during the exponential- and stationary-growth phases in the following media: M17 medium, LM17 medium (M17 medium supplemented with 0.5% lactose), GM17 medium (M17 medium supplemented with 0.5% glucose), and 10% skim milk. The left y axes and solid bars represent gene expression levels. The right y axes and solid lines show measurements of growth (as optical densities at 600 nm). Each measurement was repeated at least twice using independent RNA preparations.
Expression of ltrB region genes under mating conditions.
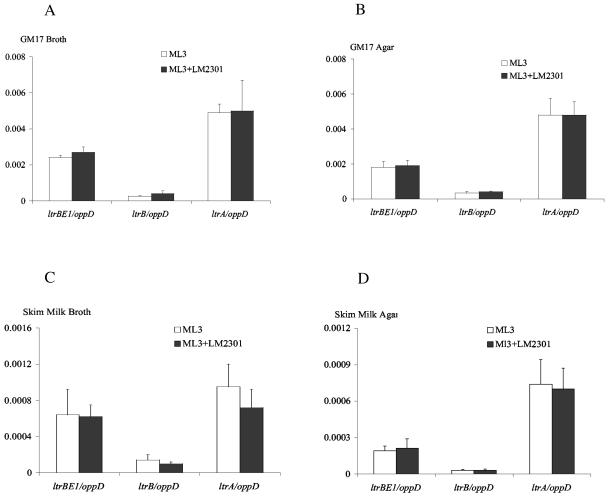
In several conjugation systems, the presence of recipient cells has been shown to regulate the expression of conjugative transfer genes (41). The physical environment (broth mating or solid-surface mating) also influences plasmid transfer frequency (27). We determined the mRNA levels of ltrBE1, ltrB, and ltrA under different mating conditions by the quantitative real-time RT-PCR assays. Equal volumes of mid-exponential-growth-phase donor L. lactis ML3 cells and plasmid-free recipient LM2301 cells were mixed and mated for 1 h in broth or on agar plates containing GM17 medium or skim milk. ML3 donor cells alone were used as nonmating controls. The results are summarized in Fig. 3. The oppD mRNA levels were the same under nonmating and mating conditions. The ltrB region gene mRNA levels were normalized relative to oppD mRNA under the same mating conditions. The results showed that the expression levels of ltrB region genes were almost the same under mating and nonmating conditions. In addition, there was no significant difference in ltrB region mRNA levels between broth mating and agar plate mating. We also determined ltrB region gene expression levels in cells that mated for 30 min, 2 h, and 4 h in GM17 medium liquid mating, and we observed no significant differences (data not shown). As we observed in the previous experiment, the level of ltrA expression was the highest among those of the ltrB region genes. The results suggest that the presence of recipient cells and the mating environment have little effect on the expression of ltrB region genes.
FIG. 3.
Expression of ltrBE1, ltrB, and ltrA mRNA levels under different mating conditions. L. lactis ML3 and plasmid-free LM2301 cultures were grown to exponential phase. Equal volumes of donor and recipient cells were mixed and mated at 30°C for 1 h under the following conditions: GM17-broth (A), GM17-solid agar (B), skim milk-broth (C), and skim milk-solid agar (D). ltrBE1, ltrB, and ltrA mRNA levels are expressed relative to oppD mRNA under the same mating conditions. L. lactis ML3 cells were used as nonmating controls. Each measurement was repeated at least twice using independent RNA preparations.
Expression of ltrB region genes after exposure to low pH and temperature stress.
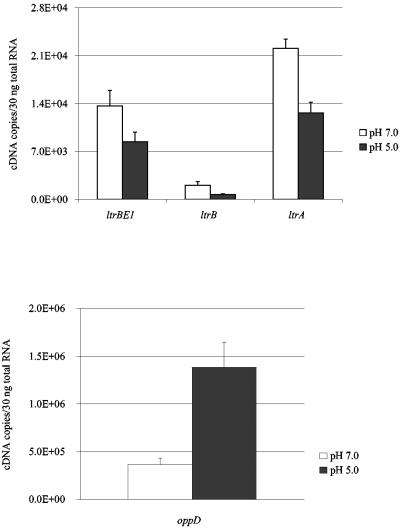
A distinctive feature of L. lactis is acid production during growth, which results in acidification of the medium and, ultimately, growth arrest. Low pH has been shown to induce the expression of a small set of chromosome proteins in L. lactis (9). Since pH is an important physiological factor for pRS01 in its natural environment, we examined the effects of acid challenge on the expression levels of ltrB region transcripts and Ll.ltrB splicing activity. Mid-exponential-phase L. lactis ML3 cells grown in GM17 medium at pH 7.0 were switched to GM17 medium at pH 5.0 and further cultured for 30 min. ML3 cells grown in GM17 medium (pH 7.0) were used as controls. The results are summarized in Fig. 4. The mRNA levels of ltrB region genes were all reduced at pH 5.0 compared to pH 7.0. The expression of ltrBE1 was decreased 1.6-fold (P = 0.003), that of ltrA decreased 1.8-fold (P = 0.0014), and that of ltrB decreased 3.0-fold (P = 4.12 × 10−6). Interestingly, mRNA levels of oppD were increased 2.7-fold (P = 2.7 × 10−4) at pH 5.0, suggesting that oppD was induced by acid challenge.
FIG. 4.
Effect of acid challenge on the expression of ltrBE1, ltrB, ltrA, and oppD genes. L. lactis ML3 was cultured in GM17 medium (pH 7.0) until the OD600 reached 0.5. The culture was divided into two aliquots. After two washes, cell pellet was resuspended in GM17 medium at either pH 5.0 or pH 7.0 and grown for 30 min. Open bars, gene expression at pH 7.0; dark bars, gene expression at pH 5.0. Each measurement was repeated at least twice using independent RNA preparations.
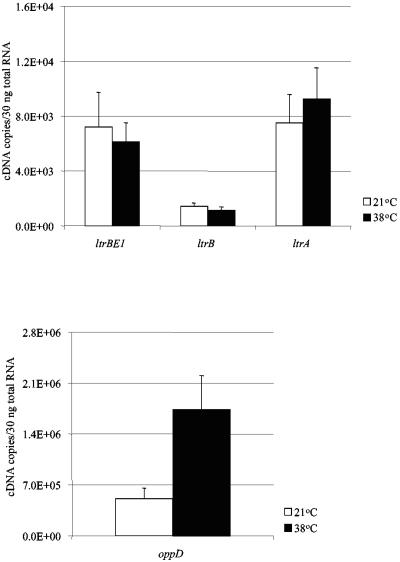
During dairy product production and in the natural environment, L. lactis encounters various temperatures. We analyzed ltrB region gene expression levels when exponential growing ML3 cells were shifted from 21°C to 38°C (Fig. 5). When cells were grown at 21°C, the expression levels of ltrB region genes and the oppD gene were similar to those of cells grown at 30°C. After heat shock at 38°C for 30 min, there were no significant changes in the ltrB region gene expression levels. However, oppD gene mRNA level was increased 3.4-fold (P = 0.001). We also determined mRNA levels after heat shock at 38°C for 15 min. No significant differences were observed for both ltrB region genes and the oppD gene compared to those in cells grown at 21°C.
FIG. 5.
Effect of temperature stress on the expression of ltrBE1, ltrB, ltrA, and oppD genes. L. lactis ML3 was cultured in LM17 medium at 21°C until the OD600 reached 0.4. Heat stress was applied by transferring half of the culture to 38°C for 30 min. Open bars, gene expression at 21°C; dark bars, gene expression at 38°C. Each measurement was repeated at least twice using independent RNA preparations.
Ll.ltrB in vivo splicing activity.
To determine the splicing efficiency of Ll.ltrB in L. lactis ML3 cells, we calculated the percentage of ltrB mRNA in comparison to ltrBE1 mRNA under all the conditions tested previously. The results are summarized in Table 2, Table 3, and Table 4. Splicing activity was growth-phase dependent, with mid-exponential and late exponential phases showing the highest splicing activity, which was between (15.9 ± 2.2)% and (21.6 ± 7.7)%. During early exponential phase and stationary phase, the splicing activity was relatively low, ranging from (6.5 ± 2.1)% to (14.6 ± 2.1)%. Medium composition had no significant effect on splicing activity. Ll.ltrB showed similar splicing activities in the presence and absence of the recipient cells in different mating environments and media. Temperature stress had little effect on intron splicing activity. The splicing activity decreased 1.8-fold when medium pH dropped to 5.0. Under all the conditions tested, the Ll.ltrB intron splicing activity ranged from (6.5 ± 2.1)% to (22.1 ± 8.0)%. These results indicate that Ll.ltrB splicing activity is low in its natural host, pRS01.
TABLE 2.
Ll.ltrB splicing activity in L. lactis in different media
| Growth phase | % Splicinga ± SD in indicated medium
|
|||
|---|---|---|---|---|
| M17 | LM17 | GM17 | Skim milk | |
| Early exponential | 9.9 ± 1.1 | 7.0 ± 2.0 | 6.7 ± 2.8 | 14.6 ± 2.1 |
| Mid exponential | 15.9 ± 2.2 | 17.3 ± 7.5 | 16.4 ± 7.3 | 17.9 ± 4.1 |
| Late exponential | 18.3 ± 5.2 | 19.7 ± 8.1 | 19.9 ± 5.7 | 21.6 ± 7.7 |
| Stationary | 6.5 ± 2.1 | 8.4 ± 1.0 | 10.9 ± 3.0 | 9.9 ± 4.3 |
The percentage of Ll.ltrB splicing is defined by the following equation: (ltrB mRNA/ltrBE1 mRNA) × 100.
TABLE 3.
Ll.ltrB splicing activity in L. lactis under different mating conditions
| Mating condition | % Splicing during mating ± SDa
|
|
|---|---|---|
| Donor | Donor and recipient | |
| GM17 | ||
| Broth | 11.4 ± 3.8 | 14.5 ± 5.8 |
| Solid agar | 18.2 ± 5.2 | 21.0 ± 3.2 |
| Skim milk | ||
| Broth | 22.1 ± 8.0 | 15.4 ± 3.5 |
| Solid agar | 16.3 ± 3.1 | 13.7 ± 7.6 |
The percentage of L1.ltrB splicing is defined by the following equation: (ltrB mRNA/ltrBE1 mRNA) × 100. The donor was L. lactis ML3 and the recipient was plasmid-free L. lactis LM2301.
TABLE 4.
Ll.ltrB splicing activity in L. lactis under different physiological conditionsa
| Condition | % Splicing ± SD |
|---|---|
| pH | |
| 7.0 | 14.8 ± 2.4 |
| 5.0 | 8.1 ± 1.8 |
| Temp (°C)b | |
| 21 | 20.2 ± 7.6 |
| 38 | 18.7 ± 5.7 |
The percentage of L1.ltrB splicing is defined by the following equation: (ltrB mRNA/ltrBE1 mRNA) × 100.
Cells were transferred from 21°C to 38°C and grown for a further 30 min.
LtrB protein expression and cis acting on oriT DNA in L. lactis.
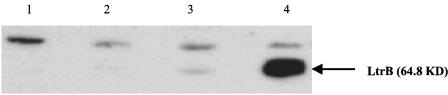
The low-level expression of the ltrB region and inefficient splicing of Ll.ltrB suggested that the amount of relaxase LtrB protein might also be expressed at very low levels in L. lactis ML3. To investigate LtrB protein expression, we generated a polyclonal antibody against LtrB. Protein expression from an intronless ltrB gene and an intron-containing ltrB gene was tested by Western blotting (Fig. 6). pCOM9 is a multicopy plasmid containing the ltrB region of pRS01 on a shuttle vector (pDL281), while pCOM-ex contains an intronless ltrB gene. The amount of LtrB expressed from pCOM-ex was about 20-fold greater than the amount of protein expressed from pCOM9. In ML3 cells, LtrB protein was below the detection limit of the antibody (data not shown). To examine the possibility that LtrB protein might show a tendency to function in cis, we carried out conjugation assays with donor strains carrying pairs of plasmids expressing various transfer components. In the presence of pCOM9, the transfer frequency of pM1014, which is a conjugation-defective derivative of pRS01, was about 1.2 × 10−1 transconjugants/donor, while the mobilization frequency of pCOM9 was about 1.3 × 10−4 transconjugants/donor. When the oriT region of pRS01 was cloned in pCOM9 (resulting in plasmid pCOMT), the pM1014 transfer frequency decreased to 3.7 × 10−2 transconjugants/donor. The mobilization frequency of pCOMT increased to 1.0 transconjugants/donor. These results indicate that pRS01 requires only a minimal amount of relaxase protein for conjugative transfer and the relaxase protein may bind preferentially to cis-oriT DNA.
FIG. 6.
Western blot analysis of LtrB protein expression in L. lactis harboring different plasmids. Total cell extracts were separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with antibody raised against whole LtrB protein. L. lactis strains and plasmid contents are as follows: lane 1, L. lactis plasmid-free strain MMS370; lane 2, MMS370 containing plasmid pM2036 (a cointegrate between pRS01 and pTRK28; the insertion site of pTRK28 is downstream of ltrB region); lane 3, MMS370 containing plasmid pCOM9; and lane 4, MMS370 containing plasmid pCOM-ex. The arrow indicates the LtrB protein.
DISCUSSION
The unique feature of the pRS01 conjugative system is the presence of the group II intron Ll.ltrB in the ltrB relaxase gene (22). Precise splicing of Ll.ltrB is essential for pRS01 conjugative transfer. While transcription activity of the relaxase gene determines the level of Ll.ltrB intron RNA, the presence of the intron may influence the transcription or translation of the ltrB region, and the conjugative ability of pRS01. In addition, it is important to investigate the ltrB region transcription and Ll.ltrB splicing regulation in pRS01 in its natural context (low copy number). In several conjugation systems, genes involved in conjugative plasmid transfer are controlled by complex regulatory circuits (41). Physiological conditions have been shown to regulate transfer gene expression. Growth phase, temperature, and intracellular cyclic AMP levels affect transfer gene expression in F-like plasmids (41). Ti plasmid transfer is influenced by cell density and nutrient availability (10). Studies with the IncP system and F-like plasmids have shown that global regulators and local circuits both regulate relaxase expression (41). Despite early discovery, relatively little is known about pRS01 transfer regulation. In this study, we designed and optimized absolute quantitative real-time RT-PCR assays to study gene expression in L. lactis. Applying this approach, we analyzed the mRNA levels of the ltrB region genes and the splicing efficiency of group II intron Ll.ltrB in pRS01 under various physiological conditions.
We analyzed ltrB region transcript levels and Ll.ltrB splicing activity in four different media, M17 medium, LM17 medium, GM17 medium, and skim milk, at different growth phases. We found that, in comparison with those of oppD, the amounts of ltrB region mRNAs were relatively small in the four media tested. Low expression of transfer genes was observed in IncP and F-like conjugative systems, which has been suggested to reduce the metabolic burden on the bacterial host (41). There was no significant difference in gene expression among the different media tested, suggesting that medium composition differences are not involved in ltrB region regulation. In contrast, oppD mRNA increased almost 10-fold when L. lactis was grown in skim milk, indicating a strong medium-dependent regulation. Previous studies have shown that the oligopeptide transport system plays a crucial role during L. lactis growth in skim milk (13), which is consistent with our results. The amount of ltrA transcript was greater than ltrBE1 and ltrB, due to the internal promoter upstream of ltrA. ltrBE1 and ltrB showed growth-phase-dependent regulation, while ltrA levels were constant throughout growth. The different gene expression patterns of ltrBE1 and ltrA indicate that ltrA and ltrB promoters are differentially regulated. We found that Ll.ltrB spliced at low efficiency in vivo, ranging from (6.5 ± 2.1)% to (22.1 ± 8.0)%. Splicing activity was medium independent and growth-phase dependent. The highest splicing activity was during the mid-exponential and late exponential growth phases. Previous experiments showed that Ll.ltrB spliced efficiently under in vitro conditions (33). Since ltrBE1 and ltrB messages showed similar levels of stability (data not shown), the low splicing efficiency we calculated was not due to faster degradation of ltrB message. Although ltrA mRNA was abundant during all growth phases, the limitation of LtrA protein may account for the low splicing efficiency of Ll.ltrB, due to the autoregulation of ltrA translation (37). In addition, transcription and translation of the ltrB region might interfere with Ll.ltrB splicing. It has been shown that translation across the 5′-splice site interfered with splicing of the group I intron IA2 in the nrdB gene of bacteriophage T4 (25). On the other hand, ribosomes may facilitate intron RNA folding and have been shown to be necessary for efficient splicing of the T4 phage thymidylate synthase intron in vivo (35). LtrA binds to a region in the intron that overlaps with the ribosome binding site of ltrA (37), suggesting competition between translation and splicing. It might be interesting to further investigate the role of ribosomes in Ll.ltrB splicing in vivo.
Few studies have examined the influence of cell physiology and environmental conditions on transfer gene expression. On solid-surface matings, the Ti plasmid pTiC58 transfer frequency was three- to fourfold higher than those conducted in broth (28). However, there was no significant difference in tra gene expression between mating on solid agar and mating in broth medium (28). The mating pair formation genes (mpfA) of plasmid pCg1 of Pseudomonas putida Cg1 were expressed constitutively both in broth and on solid media (27). Similar to the cases with pTiC58 and pCg1, we found that the mating environment had little effect on ltrB region gene expression and Ll.ltrB splicing. In addition, there was no significant difference in the ltrB region transcript levels and Ll.ltrB splicing activities in the presence and absence of recipient cells. We also found that acid stress significantly reduced ltrB region gene expression and Ll.ltrB splicing in pRS01, while temperature stress had no effect.
Since the discovery of the first bacterial group II intron in 1993 (8), more than 30 full-length group II introns and 9 partial group II introns had been identified in eubacteria by 2002 (5). A majority of these introns were identified through sequence analysis as a result of microbial genome sequencing projects (5). Recently, group II introns were also discovered in archaea (11). Several bacterial group II introns have been demonstrated to splice in vivo: Ll.ltrB from L. lactis, B.a.I1 and B.a.I2 from Bacillus anthracis (31), and RmInt1 from Sinorhizobium meliloti (17). The Avi.groEL intron from Azotobacter vinelandii inserts within the termination codon of the essential groEL gene (7). Avi.groEL could self-splice in vitro, but no intron RNA transcript was detected under physiological conditions or in response to heat shock which induces high expression of the groEL gene (7). RNase protection mapping suggests that active transcription termination within the intron domain Ia aborts the expression of the Avi.groEL intron (7). Although Ll.ltrB is inside an essential gene for conjugation, our data show that Ll.ltrB is transcribed at low levels in pRS01, and the in vivo splicing efficiency is also low. Since the majority of bacterial group II introns insert between genes or directly after Rho-independent terminators (5), it is possible that most of these bacterial group II introns may not be transcribed or may be transcribed in small amounts in the cell. In contrast, organelle group II introns often insert in essential genes, like NAD dehydrogenase, ribulose-bisphosphate carboxylase, and subunits of cytochrome oxidase, which require efficient transcription and splicing (5).
Ll.ltrB has been shown to be able to splice and to be mobile in E. coli (19), eukaryotic cells (12), and Enterococcus faecalis (3) when the intron was cloned into high-copy-number expression plasmids. Based on these studies, it was suggested that Ll.ltrB could spread efficiently throughout populations by homing and transposition. However, in these studies, the intron RNA and LtrA protein were greatly overexpressed compared to the case with the pRS01 system. Although pRS01 is a broad-host-range plasmid, Ll.ltrB distribution is limited to pRS01 and the nearly identical sex factor from L. lactis subsp. lactis 712 in natural populations. In addition, these two identical introns represent a single insertion in a common strain (16). Only one group II intron, which is 99% identical to Ll.ltrB, has been found in the putative relaxase gene of L. lactis naturally occurring plasmid pAH90 (26). This intron might have originated from Ll.ltrB insertion in pAH90. The small amount of Ll.ltrB intron RNA and inefficient in vivo splicing may be barriers that limit Ll.ltrB spread in the natural environment.
Acknowledgments
This work was supported by a National Institutes of Health grant (GM58279) to G.M.D.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1984. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J. Bacteriol. 158:954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. G., and L. L. McKay. 1983. Isolation of a recombination-deficient mutant of Streptococcus lactis ML3. J. Bacteriol. 155:930-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belhocine, K., I. Plante, and B. Cousineau. 2004. Conjugation mediates transfer of the Ll.ltrB group II intron between different bacterial species. Mol. Microbiol. 51:1459-1469. [DOI] [PubMed] [Google Scholar]
- 4.Cousineau, B., D. Smith, S. Lawrence-Cavanagh, J. E. Mueller, J. Yang, D. Mills, D. Manias, G. Dunny, A. M. Lambowitz, and M. Belfort. 1998. Retrohoming of a bacterial group II intron: mobility via complete reverse splicing, independent of homologous DNA recombination. Cell 94:451-462. [DOI] [PubMed] [Google Scholar]
- 5.Dai, L., and S. Zimmerly. 2002. Compilation and analysis of group II intron insertions in bacterial genomes: evidence for retroelement behavior. Nucleic Acids Res. 30:1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunny, G. M., and L. L. McKay. 1999. Group II introns and expression of conjugative transfer functions in lactic acid bacteria. Antonie Leeuwenhoek 76:77-88. [PubMed] [Google Scholar]
- 7.Ferat, J. L., M. Le Gouar, and F. Michel. 2003. A group II intron has invaded the genus Azotobacter and is inserted within the termination codon of the essential groEL gene. Mol. Microbiol. 49:1407-1423. [DOI] [PubMed] [Google Scholar]
- 8.Ferat, J. L., and F. Michel. 1993. Group II self-splicing introns in bacteria. Nature 364:358-361. [DOI] [PubMed] [Google Scholar]
- 9.Frees, D., F. K. Vogensen, and H. Ingmer. 2003. Identification of proteins induced at low pH in Lactococcus lactis. Int. J. Food Microbiol. 87:293-300. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. Conway de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, H., M. Karberg, M. Long, J. P. Jones III, B. Sullenger, and A. M. Lambowitz. 2000. Group II introns designed to insert into therapeutically relevant DNA target sites in human cells. Science 289:452-457. [DOI] [PubMed] [Google Scholar]
- 13.Juillard, V., D. Le Bars, E. R. Kunji, W. N. Konings, J. C. Gripon, and J. Richard. 1995. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl. Environ. Microbiol. 61:3024-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanasaki, M., S. Breheny, A. J. Hillier, and G. R. Jago. 1975. Effect of temperature on the growth and acid production of lactic acid bacteria. I. A rapid method for the estimation of bacterial populations in milk. Aust. J. Dairy Technol. 30:142-144. [Google Scholar]
- 15.Klein, J. R., Y. Chen, D. A. Manias, J. Zhuo, L. Zhou, C. L. Peebles, and G. M. Dunny. 2004. A conjugation-based system for genetic analysis of group II intron splicing in Lactococcus lactis. J. Bacteriol. 186:1991-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourgeois, P., M. L. Daveran-Mingot, and P. Ritzenthaler. 2000. Genome plasticity among related Lactococcus strains: identification of genetic events associated with macrorestriction polymorphisms. J. Bacteriol. 182:2481-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Abarca, F., S. Zekri, and N. Toro. 1998. Characterization and splicing in vivo of a Sinorhizobium meliloti group II intron associated with particular insertion sequences of the IS630-Tc1/IS3 retroposon superfamily. Mol. Microbiol. 28:1295-1306. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura, M., J. W. Noah, and A. M. Lambowitz. 2001. Mechanism of maturase-promoted group II intron splicing. EMBO J. 20:7259-7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuura, M., R. Saldanha, H. Ma, H. Wank, J. Yang, G. Mohr, S. Cavanagh, G. M. Dunny, M. Belfort, and A. M. Lambowitz. 1997. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes Dev. 11:2910-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel, F., and J. L. Ferat. 1995. Structure and activities of group II introns. Annu. Rev. Biochem. 64:435-461. [DOI] [PubMed] [Google Scholar]
- 21.Mills, D. A., T. G. Phister, G. M. Dunny, and L. L. McKay. 1998. An origin of transfer (oriT) on the conjugative element pRS01 from Lactococcus lactis subsp. lactis ML3. Appl. Environ. Microbiol. 64:1541-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills, D. A., L. L. McKay, and G. M. Dunny. 1996. Splicing of a group II intron involved in the conjugative transfer of pRS01 in lactococci. J. Bacteriol. 178:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills, D. A., C. K. Choi, G. M. Dunny, and L. L. McKay. 1994. Genetic analysis of regions of the Lactococcus lactis subsp. lactis plasmid pRS01 involved in conjugative transfer. Appl. Environ. Microbiol. 60:4413-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noah, J. W., and A. M. Lambowitz. 2003. Effects of maturase binding and Mg2+ concentration on group II intron RNA folding investigated by UV cross-linking. Biochemistry 42:12466-12480. [DOI] [PubMed] [Google Scholar]
- 25.Ohman-Heden, M., A. Ahgren-Stalhandske, S. Hahne, and B. M. Sjoberg. 1993. Translation across the 5′-splice site interferes with autocatalytic splicing. Mol. Microbiol. 7:975-982. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan, D., R. P. Ross, D. P. Twomey, G. F. Fitzgerald, C. Hill, and A. Coffey. 2001. Naturally occurring lactococcal plasmid pAH90 links bacteriophage resistance and mobility functions to a food-grade selectable marker. Appl. Environ. Microbiol. 67:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, W., C. O. Jeon, A. M. Hohnstock-Ashe, S. C. Winans, G. J. Zylstra, and E. L. Madsen. 2003. Identification and characterization of the conjugal transfer region of the pCg1 plasmid from naphthalene-degrading Pseudomonas putida Cg1. Appl. Environ. Microbiol. 69:3263-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piper, K. R., and S. K. Farrand. 1999. Conjugal transfer but not quorum-dependent tra gene induction of pTiC58 requires a solid surface. Appl. Environ. Microbiol. 65:2798-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin, P. Z., and A. M. Pyle. 1998. The architectural organization and mechanistic function of group II intron structural elements. Curr. Opin. Struct. Biol. 8:301-308. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen, R. 2001. Quantification on the LightCycler, p. 21-34. In S. Meuer, C. Wittwer, and K. Nakagawara (ed.), Rapid cycle real-time PCR, methods and applications. Springer Press, Heidelberg, Germany.
- 31.Robart, A. R., N. K. Montgomery, K. L. Smith, and S. Zimmerly. 2004. Principles of 3′ splice site selection and alternative splicing for an unusual group II intron from Bacillus anthracis. RNA 10:854-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero, D. A., and T. R. Klaenhammer. 1991. Construction of an IS946-based composite transposon in Lactococcus lactis subsp. lactis. J. Bacteriol. 173:7599-7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldanha, R., B. Chen, H. Wank, M. Matsuura, J. Edwards, and A. M. Lambowitz. 1999. RNA and protein catalysis in group II intron splicing and mobility reactions using purified components. Biochemistry 38:9069-9083. [DOI] [PubMed] [Google Scholar]
- 34.San Filippo, J., and A. M. Lambowitz. 2002. Characterization of the C-terminal DNA-binding/DNA endonuclease region of a group II intron-encoded protein. J. Mol. Biol. 324:933-951. [DOI] [PubMed] [Google Scholar]
- 35.Semrad, K., and R. Schroeder. 1998. A ribosomal function is necessary for efficient splicing of the T4 phage thymidylate synthase intron in vivo. Genes Dev. 12:1327-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shearman, C., J. J. Godon, and M. Gasson. 1996. Splicing of a group II intron in a functional transfer gene of Lactococcus lactis. Mol. Microbiol. 21:45-53. [DOI] [PubMed] [Google Scholar]
- 37.Singh, R. N., R. J. Saldanha, L. M. D'Souza, and A. M. Lambowitz. 2002. Binding of a group II intron-encoded reverse transcriptase/maturase to its high affinity intron RNA binding site involves sequence-specific recognition and autoregulates translation. J. Mol. Biol. 318:287-303. [DOI] [PubMed] [Google Scholar]
- 38.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tynkkynen, S., G. Buist, E. Kunji, J. Kok, B. Poolman, G. Venema, and A. Haandrikman. 1993. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J. Bacteriol. 175:7523-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, X. G., B. Lin, J. M. Kidder, S. Telford, and L. T. Hu. 2002. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J. Bacteriol. 184:6198-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zatyka, M., and C. M. Thomas. 1998. Control of genes for conjugative transfer of plasmids and other mobile elements. FEMS Microbiol. Rev. 21:291-319. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, L., D. A. Manias, and G. M. Dunny. 2000. Regulation of intron function: efficient splicing in vivo of a bacterial group II intron requires a functional promoter within the intron. Mol. Microbiol. 37:639-651. [DOI] [PubMed] [Google Scholar]