Abstract
Background
Intermittent hypoxaemia and obstructive sleep apnoea (OSA) have been linked to lung cancer through as yet unidentified pathophysiological mechanisms. This study evaluates the effect of OSA on serum levels of biomarkers of immunosurveillance, lymphangiogenesis and intrinsic tumour cell aggressiveness in high-risk individuals screened for lung cancer and patients with established lung cancer.
Methods
Serum samples from individuals participating in a lung cancer screening cohort (SAILS study) or with newly diagnosed lung cancer (SAIL study) were analysed. All patients underwent home sleep apnoea testing. Soluble levels of programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), cytotoxic T-lymphocyte antigen-4, midkine (MDK), paraspeckle component-1 (PSPC1), transforming growth factor-β1 (TGF-β1), SMAD3, matrix metalloproteinase-2 and co-stimulus receptor of the tumour necrosis factor family of receptors (CD137) were determined by ELISA.
Results
The presence of moderate-to-severe OSA was associated with increased levels of PSPC1, MDK, PD-L1 and PD-1 in screened individuals, and with higher values of PSPC1, TGF-β1, PD-L1 and PD-1 in patients with established lung cancer. The findings correlated with nocturnal intermittent hypoxaemia indices.
Conclusion
Moderate-to-severe OSA is associated with increased expression of serum biomarkers of immune evasion, lymphangiogenesis and tumour cell aggressiveness in high-risk individuals screened for lung cancer and those with established disease.
Shareable abstract
In smokers at high risk of lung cancer and patients with lung cancer, moderate-to-severe obstructive sleep apnoea is associated with increased levels of biomarkers of immune evasion, lymphangiogenesis and intrinsic aggressiveness of tumour cells https://bit.ly/3urv8Az
Introduction
A growing body of scientific evidence supports an association between obstructive sleep apnoea (OSA) and cancer [1–3]. Although most of the evidence comes from patients with melanoma, with OSA-induced intermittent hypoxia leading to increased tumour aggressiveness and mortality [4, 5], many other tumours have also been studied [6, 7]. In particular, lung cancer, the leading cause of cancer-related mortality in developed countries [8], is also associated with OSA. A recent meta-analysis of 4 885 518 patients from four observational studies demonstrated that patients with OSA have ∼30% higher risk of lung cancer compared with those without OSA [9]. The association between OSA and lung cancer appears to be mediated by intermittent hypoxaemia [10], which doubles the risk of positive screening findings in patients enrolled in a lung cancer screening programme [11].
Although the pathogenic pathways responsible for the association between OSA and cancer have not been fully elucidated, nocturnal intermittent hypoxaemia might play a key role in a number of putative mechanisms, including immune evasion, inflammation, tumour progression and angiogenesis [12–14]. Intermittent hypoxia in patients with severe OSA interferes with the innate and adaptive immune response [15, 16], promotes angiogenesis and lymphangiogenesis [17, 18], and increases the intrinsic aggressiveness of tumour cells by inducing epithelial–mesenchymal transition (EMT) and acquisition of cancer stem cell (CSC) phenotypes [19].
Interestingly, in patients with melanoma, OSA severity correlates with serum levels of soluble programmed cell death ligand-1 (PD-L1) [20] and programmed cell death-1 (PD-1) whose binding forms the PD-L1/PD-1 immune axis, a widely studied immune checkpoint that modulates signalling pathways related to T-cell activation and is the cornerstone of current immunotherapeutic strategies. Soluble PD-L1 levels in these patients are associated with increased cancer aggressiveness and metastatic disease [20]. Furthermore, a hypoxia-dependent increase in serum levels of midkine (MDK), a recognised pro-angiogenic and pro-lymphangiogenic factor associated with increased tumour aggressiveness, has been reported in patients with OSA and melanoma [18]. Finally, OSA is also associated with melanoma aggressiveness through hypoxia-mediated overexpression of paraspeckle component-1 (PSPC1) [21]. PSPC1 functions as a master activator of metastatic reprogramming by mediating pro-metastatic autocrine signalling of transforming growth factor-β1 (TGF-β1)/SMAD2/3, inducing EMT and acquisition of CSC characteristics [22].
The existence of two cohorts of patients with either established lung cancer undergoing home sleep apnoea testing (HSAT) (SAIL study) or participating in a lung cancer screening programme (SAILS study) allowed us to investigate the behaviour of these biomarkers in the context of lung cancer. The aim of this study was to analyse the effect of OSA on serum levels of biomarkers of immunosurveillance, pro-lymphangiogenesis or tumour cell aggressiveness in patients participating in a lung cancer screening programme and in those with newly diagnosed lung cancer.
Methods
We performed a cross-sectional study including serum samples and data derived from two prospectively recruited cohorts investigating the prevalence of OSA in patients at risk for lung cancer included in a lung cancer screening programme (SAILS study; ClinicalTrials.gov: NCT02764866) [11] and in patients with newly diagnosed lung cancer (SAIL study; ClinicalTrials.gov: NCT02764866) [23].
Briefly, patients included in the SAILS study met the National Lung Screening Trial age and smoking criteria (55–75 years of age and tobacco consumption ≥30 pack-years) and had at least one additional risk factor for lung cancer: COPD, defined as post-bronchodilator forced expiratory volume in 1 s/forced vital capacity <0.70 or radiological signs of emphysema [11]. Screened individuals had no evidence or obvious symptoms of lung cancer, concurrent severe disease, unexplained weight loss or haemoptysis. Patients with newly diagnosed lung cancer were recruited for the SAIL study prior to initiating oncological or surgical treatment. In both studies, exclusion criteria included current or previous use of home oxygen therapy, known OSA, continuous positive airway pressure (CPAP) treatment or non-invasive mechanical ventilation. All participants signed an informed consent and both studies were approved by the local ethics committee (Fundación Jiménez Díaz University Hospital, Madrid, Spain; EO98/2015 and EO99/2015).
Anthropometric characteristics, including body composition, smoking habit, comorbidities, lung function tests and computed tomography scan findings were collected in all subjects. Among patients with lung cancer, tumour histology, tumour/node/metastasis (TNM) and stage at diagnosis were collected as recommended by the International Association for the Study of Lung Cancer TNM classification, eighth edition [24]. During the initial visit, fasting venous blood samples were drawn in the morning. The blood samples were centrifuged to separate the serum, and all specimens were immediately aliquoted, frozen and stored at −80 °C.
Sleep study
All patients underwent HSAT using the Nox T3 Portable Sleep Monitor (Nox Medical, Reykjavik, Iceland), which was correctly validated [25] and complies with the regulations of the American Academy of Sleep Medicine (AASM) [26]. The device includes a nasal cannula for oronasal flow and pressure recordings, thoracic and abdominal bands capable of measuring respiratory movements, a pulse oximeter, and a microphone to document snoring. All scoring and readings were conducted manually by experienced and trained personnel in accordance with AASM guidelines [26]. Apnoeas were defined as ≥90% reduction in oronasal flow lasting >10 s. Hypopnoeas were defined as 30–60% decrease in airflow lasting >10 s associated with an oxygen saturation drop of ≥3%. The apnoea–hypopnoea index (AHI) was defined as the number of apnoeas plus hypopnoeas per hour of recording. In addition, mean nocturnal arterial oxygen saturation (SaO2), minimum nocturnal SaO2, recording time with SaO2 <90% and oxygen desaturation index were used as oxygenation indices. According to the AHI, patients were divided into four groups: non-OSA (<5 events·h−1), mild OSA (5–14.9 events·h−1), moderate OSA (15–29.9 events·h−1) and severe OSA (>30 events·h−1) [27].
Determination of serum levels of biomarkers
Soluble biomarkers related to immunosurveillance (PD-1, PD-L1 and cytotoxic T-lymphocyte antigen-4 (CTLA-4)), lymphangiogenesis (MDK) and intrinsic tumour cell aggressiveness (PSPC1, transforming growth factor-β1 (TGF-β1), SMAD3, matrix metalloproteinase-2 (MMP2) and co-stimulus receptor of the tumour necrosis factor family of receptors (CD137)) were studied.
PSPC1 (MBS9324493; MyBioSource, San Diego, CA, USA), MMP2 (KHC3081; Invitrogen, Waltham, MA, USA), TGF-β1 (LEGEND MAX Free Active TGF-β1 ELISA Kit 437707; BioLegend, San Diego, CA, USA), SMAD3 (EH2148; Fine Test, Wuhan, China), PD-L1 (ab214565; Abcam, Cambridge, UK) and MDK (CSB-E08892h; https://cusabio.com/, Houston, TX, USA) concentrations were assayed using the corresponding human ELISA, following the manufacturer's standard protocol in each case. Measurements for serum samples were obtained in duplicate. The detection limits for the assays were 31.2 pg·mL−1 for PSPC1, 0.15 ng·mL−1 for MMP2, 2.3 pg·mL−1 for TGF-β1, 0.375 ng·mL−1 for SMAD3, 21.88 pg·mL−1 for PD-L1 and 7.8 pg·mL−1 for MDK. The intra-assay and inter-assay variations were <20% in the various assays. In addition, PD-1, CD137 and CTLA-4 concentration was measured using a bead-based multiplex assay panel (LEGENDplex HU Immune Checkpoint Panel 1-S/P (10-plex) w/FP; BioLegend), following the standard protocol as indicated by the manufacturer. The detection limits for each bead were 2.44 ng·mL−1 for PD-1, 12.2 ng·mL−1 for CD137 and 1.22 ng·mL−1 for CTLA-4. The intra-assay and inter-assay variations were <20% in the various assays.
Statistical analysis
Data are expressed as mean±sd, median (interquartile range (IQR)) or absolute number (percentage), according to the type and distribution of the variables. Normality was explored using the Shapiro–Wilk and skewness–kurtosis tests. Differences between subgroups were analysed using the Chi-squared or Fisher exact test (categorical variables), or t-test, Mann–Whitney test, ANOVA with Bonferroni post-hoc test or Kruskal–Wallis test (quantitative variables). For between-group comparisons, biomarker levels were adjusted for gender, age, body mass index (BMI), pack-years and presence of COPD using univariate ANOVA with general linear models. The relationship between sleep parameters with biomarker levels was evaluated using Pearson's correlation. In the combined cohort from the two studies, association between anthropometric characteristics, sleep parameters and biomarker levels with lung cancer diagnosis was analysed by bivariate and forward stepwise multiple logistic regression. All tests were two-tailed and a statistical significance level of 0.05 was retained. Analyses were performed using SPSS version 20.0 (IBM, Armonk, NY, USA).
Results
Serum samples were available in 131 out of the 236 individuals enrolled in the SAILS study and in 39 out of the 60 patients enrolled in the SAIL study. Table 1 summarises the general characteristics of subjects at high risk for lung cancer (SAILS cohort) and those with newly diagnosed lung cancer (SAIL cohort), stratified according to the presence and severity of OSA. Anthropometric characteristics, smoking, previous history of COPD and sleep parameters between the two cohorts are compared.
TABLE 1.
General characteristics of the subjects included from the two study cohorts
| SAILS cohort | SAIL cohort | SAILS versus SAIL p-value | ||||||||
| Overall (n=131) | Non-OSA (n=43) | Mild OSA (n=58) | Moderate-to-severe OSA (n=30) | p-value | Overall (n=39) | Non-OSA or mild OSA (n=19) | Moderate-to-severe OSA (n=20) | p-value | ||
| Female | 67 (51.1) | 27 (62.8) | 28 (48.3) | 12 (40.0) | 0.134 | 19 (48.7) | 13 (68.4) | 6 (30.0) | 0.038 | 0.933 |
| Age, years | 62±6 | 61±6 | 62±5 | 63±6 | 0.206 | 68±11 | 67 (55–69) | 70 (62–78) | 0.129 | 0.004 |
| BMI, kg·m−2 | 27.6±4.5 | 25.8±4.7 | 28.6±4.4¶ | 28.5±3.6# | 0.004 | 27.7±4.5 | 24.9 (21.5–27.4) | 28.8 (26.7–31.1) | 0.003 | 0.593 |
| Visceral fat, % | 11.1±4.2 | 9.0±4.4 | 12.0±3.7¶ | 12.4±3.7¶ | <0.001 | 11.4±5.8 | 7.5 (5.0–10.0) | 13.0 (11.0–18.0) | 0.002 | 0.755 |
| Free-fat mass, % | 33.2±9.8 | 31.9±10.9 | 34.7±9.5 | 32.1±8.5 | 0.286 | 33.2±9.0 | 34.5 (31.0–42.0) | 30.5 (28.5–39.0) | 0.294 | 0.966 |
| Waist/hip ratio | 0.97±0.09 | 0.94±0.09 | 0.98±0.09 | 1.01±0.07¶ | 0.007 | 0.94±0.09 | 0.88 (0.79–0.97) | 1.00 (0.95–1.04) | 0.667 | 0.428 |
| Neck perimeter, cm | 38±4 | 36±3 | 38±4¶ | 39±5¶ | <0.001 | 38±4 | 35 (34–37) | 39 (38–43) | 0.001 | 0.789 |
| Pack-years | 48±18 | 43±13 | 51±21 | 49±18 | 0.073 | 46±31 | 38 (30–60) | 49 (25–72) | 0.592 | 0.804 |
| COPD previous diagnosis | 61 (46.9) | 26 (60.5) | 20 (35.1) | 15 (50.0) | 0.116 | 18 (46.2) | 8 (42.1) | 10 (50.0) | 0.863 | 0.890 |
| AHI, events·h−1 | 10.8±11.2 | 2.7±1.5 | 9.0±2.6+ | 26.1±13.9+,## | <0.001 | 24.3±21.6 | 5.7 (3.5–11.4) | 35.1 (22.5–42.9) | <0.001 | 0.001 |
| Supine AHI, events·h−1 | 22.8±22.4 | 6.2±6.7 | 21.2±17.1+ | 49.5±20.9+,## | <0.001 | 33.8±27.3 | 8.7 (2.8–19.0) | 47.4 (31.8–72.7) | <0.001 | 0.015 |
| Desaturation index, events·h−1 | 10.5±11.2 | 2.5±1.7 | 8.7±3.2+ | 25.2±14.1+,## | <0.001 | 25.6±23.2 | 5.0 (3.4–7.8) | 35.2 (27.9–49.7) | <0.001 | 0.001 |
| tSaO2<90%, % | 23.0±30.3 | 17.2±26.7 | 25.6±34.5 | 26.7±26.4 | 0.299 | 23.6±29.9 | 1.0 (0.1–7.8) | 16.3 (4.6–42.5) | <0.001 | 0.916 |
| Mean SaO2, % | 91±2 | 91±2 | 91±3 | 90±2 | 0.327 | 91±3 | 92 (91–93) | 92 (90–93) | 0.158 | 0.824 |
| Minimum SaO2, % | 83±5 | 86±3# | 83±4 | 81±5+,§ | <0.001 | 81±8 | 86 (83–89) | 82 (77–84) | 0.002 | 0.110 |
| Central apnoea index, events·h−1 | 0.5±1.5 | 0.1±0.2 | 0.3±0.6 | 1.5±2.9+,ƒ | <0.001 | 2.5±7.3 | 0 (0–0.1) | 1.3 (0.3–2.3) | <0.001 | 0.126 |
| Snoring, % | 20.2±18.2 | 11.4±11.2 | 22.9±19.2¶ | 27.6±20.0+ | <0.001 | 18.5±17.9 | 9.7 (3.4–19.0) | 17.9 (7.5–29.7) | 0.192 | 0.631 |
Data are presented as n (%) or mean±sd, unless otherwise stated. BMI: body mass index; AHI: apnoea–hypopnoea index; SaO2: arterial oxygen saturation; tSaO2<90%: recording time with SaO2 <90%. Comparisons by ANOVA with Bonferroni post-hoc test, Mann–Whitney test or Chi-squared test with Fisher correction. #: p<0.05 versus non-OSA group; ¶: p<0.01 versus non-OSA group; +: p<0.001 versus non-OSA group (in SAILS cohort). §: p<0.05 versus mild OSA; ƒ: p<0.01 versus mild OSA; ##: p<0.001 versus mild OSA (in SAILS cohort).
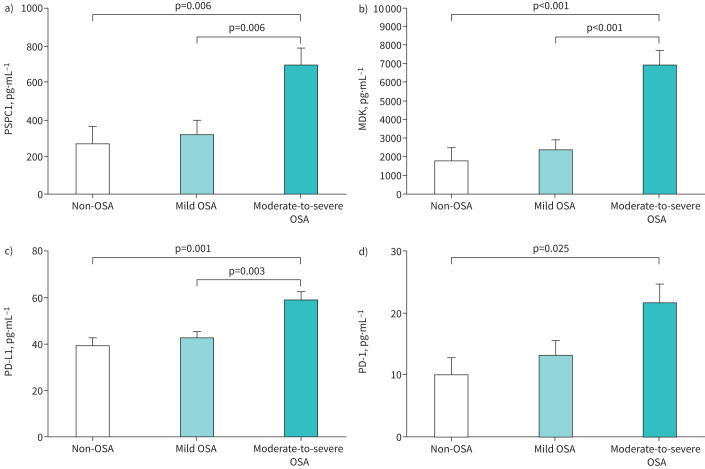
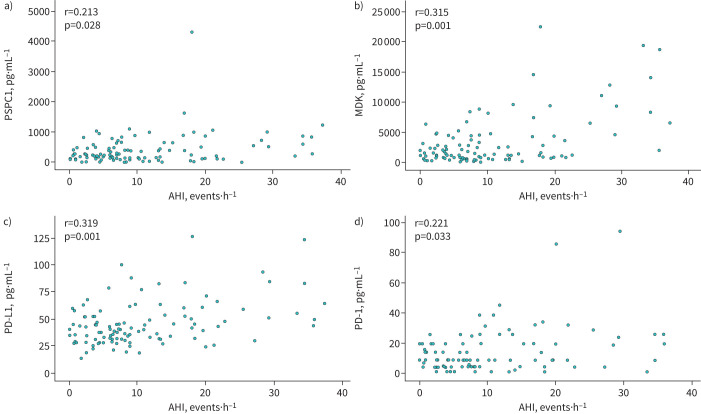
The presence of moderate-to-severe OSA was associated with higher serum levels of PSPC1, MDK and PD-L1 in screened individuals (table 2). PD-1 values were close to statistical significance and no differences were identified for the serum levels of the remaining biomarkers. After adjusting for age, sex, BMI, smoking intensity and previous COPD diagnosis, serum levels of PSPC1, MDK, PD-L1 and PD-1 remained elevated in patients with moderate-to-severe OSA with respect to subjects without OSA or with mild OSA (figure 1). Serum levels of PSPC1, MDK, PD-L1 and PD-1 were also correlated with AHI (figure 2) and indices of intermittent hypoxia, such as the desaturation index (supplementary figure S1). Similarly, minimum nocturnal oxyhaemoglobin saturation was inversely correlated with serum levels of PSPC1, MDK and PD-L1 (supplementary figure S2), while mean nocturnal oxyhaemoglobin saturation and recording time with oxyhaemoglobin saturation <90% were associated with PSPC1, TGF-β1 and PD-L1 levels (supplementary figures S3 and S4) in these high-risk individuals.
TABLE 2.
Comparison of serum levels of biomarkers between the three study subgroups of patients at high risk of lung cancer
| Non-OSA (n=43) | Mild OSA (n=57) | Moderate-to-severe OSA (n=30) | p-value | |
| TGF-β1, pg·mL−1 | 9.2 (6.6–12.4) | 8.2 (3.6–10.0) | 10.2 (5.1–14.3) | 0.112 |
| SMAD3, pg·mL−1 | 2631 (1863–4434) | 2301 (1938–4379) | 3204 (1540–6919) | 0.251 |
| CD137, pg·mL−1 | 41.9 (6.0–82.4) | 6.0 (6.0–41.9) | 41.9 (6.0–41.9) | 0.803 |
| MMP2, pg·mL−1 | 18 548 (12 820–24 748) | 17 595 (14 706–25 639) | 15 845 (14 484–17 980) | 0.507 |
| PSPC1, pg·mL−1 | 244.7 (178.6–464.4) | 215.3 (72.5–476.2) | 489.6 (113.2–856.6) | 0.029 |
| MDK, pg·mL−1 | 1536 (840–2360) | 1210 (475–2783) | 5902 (816–8337) | 0.001 |
| PD-L1, pg·mL−1 | 47.6 (27.0–59.5) | 38.6 (33.0–44.9) | 61.8 (43.1–83.0) | 0.001 |
| PD-1, pg·mL−1 | 14.7 (4.7–19.5) | 14.0 (4.0–25.5) | 28.7 (8.5–31.9) | 0.057 |
| CTLA-4, pg·mL−1 | 1.3 (0.6–2.0) | 3.9 (0.6–8.1) | 1.3 (0.6–3.9) | 0.505 |
Data are presented as median (interquartile range), unless otherwise stated. OSA: obstructive sleep apnoea; TGF-β1: transforming growth factor-β1; CD137: 4-1BB or tumour necrosis factor receptor superfamily-9; MMP2: matrix metalloproteinase-2; PSPC1: paraspeckle component-1; MDK: midkine; PD-L1: programmed cell death ligand-1; PD-1: programmed cell death-1; CTLA-4: cytotoxic T-lymphocyte antigen-4. Comparisons by Kruskal–Wallis test.
FIGURE 1.
Biomarker levels in subjects at high risk of lung cancer. Adjusted comparisons of the serum levels of soluble a) paraspeckle component-1 (PSPC1), b) midkine (MDK), c) programmed cell death ligand-1 (PD-L1) and d) programmed cell death-1 (PD-1) according to presence and severity of obstructive sleep apnoea in subjects from the SAILS cohort. Bars correspond to the mean adjusted for age, sex, body mass index, pack-years and previous COPD diagnosis. Error bars represent the standard error of the mean. p-values correspond to between-group comparisons by general linear models with post-hoc comparisons by Bonferroni test.
FIGURE 2.
Serum levels of biomarkers are related to obstructive sleep apnoea severity. Relationship between serum levels of a) paraspeckle component-1 (PSPC1), b) midkine (MDK), c) programmed cell death ligand-1 (PD-L1) and d) programmed cell death-1 (PD-1) and apnoea–hypopnoea index (AHI) in subjects from the SAILS cohort. r-values correspond to Pearson correlation coefficients.
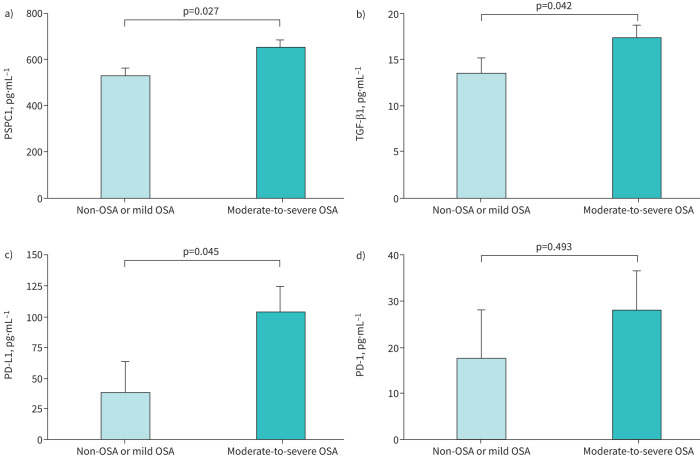
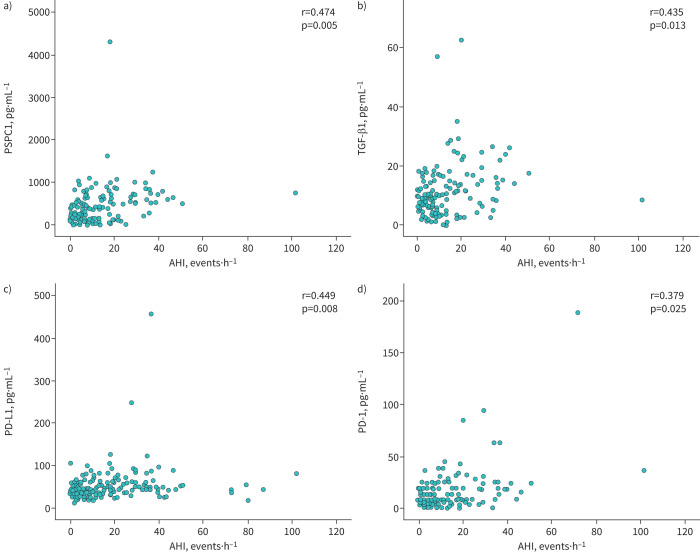
Interestingly, patients with moderate-to-severe OSA and lung cancer (SAIL cohort) also had higher serum levels of biomarkers related to immune evasion (PD-L1 and PD-1) and tumour cell aggressiveness (PSPC1 and TGF-β1) compared with cancer patients with mild or no OSA, both in the crude analysis (table 3) and after adjusting for age, sex, BMI, smoking intensity and COPD (figure 3). Furthermore, serum levels of TGF-β1, PSPC1, PD-L1 and PD-1 in lung cancer patients were related to both OSA severity (figure 4) and intermittent hypoxia, as measured by the desaturation index (supplementary figure S5). TGF-β1 levels were also related to nocturnal saturation and time spent with oxyhaemoglobin saturation <90%, while PSPC1 levels were related to nocturnal saturation (supplementary figure S6).
TABLE 3.
Comparison of serum levels of biomarkers in patients with lung cancer according to severity of obstructive sleep apnoea (OSA)
| Non-OSA or mild OSA (n=19) | Moderate-to-severe OSA (n=20) | p-value | |
| TGF-β1, pg·mL−1 | 13.2 (11.8–14.0) | 15.7 (12.4–26.5) | 0.007 |
| SMAD3, pg·mL−1 | 5087 (4624–5165) | 4918 (4581–5665) | 0.275 |
| CD137, pg·mL−1 | 82.4 (44.2–141.7) | 24.0 (6.0–41.9) | 0.774 |
| MMP2, pg·mL−1 | 17 203 (16 392–18 324) | 25 477 (23 460–29 610) | 0.115 |
| PSPC1, pg·mL−1 | 434.7 (430.3–481.3) | 639.8 (521.9–809.6) | 0.001 |
| MDK, pg·mL−1 | 4695 (4348–5226) | 4862 (4275–5857) | 0.567 |
| PD-L1, pg·mL−1 | 49.5 (41.5–51.6) | 74.8 (61.3–105.0) | 0.001 |
| PD-1, pg·mL−1 | 13.5 (13.5–25.5) | 33.9 (18.7–63.3) | 0.077 |
| CTLA-4, pg·mL−1 | 0.6 (0.6–1.3) | 10.2 (0.6–21.0) | 0.352 |
Data are presented as median (interquartile range), unless otherwise stated. TGF-β1: transforming growth factor-β1; CD137: 4-1BB or tumour necrosis factor receptor superfamily-9; MMP2: matrix metalloproteinase-2; PSPC1: paraspeckle component-1; MDK: midkine; PD-L1: programmed cell death ligand-1; PD-1: programmed cell death-1; CTLA-4: cytotoxic T-lymphocyte antigen-4. Comparisons by Mann–Whitney test.
FIGURE 3.
Biomarker levels in patients with lung cancer. Adjusted comparisons of the serum levels of soluble a) paraspeckle component-1 (PSPC1), b) transforming growth factor-β1 (TGF-β1), c) programmed cell death ligand-1 (PD-L1) and d) programmed cell death-1 (PD-1) according to presence and severity of obstructive sleep apnoea in subjects from the SAIL cohort. Bars correspond to the mean adjusted for age, sex, body mass index, pack-years and previous COPD diagnosis. Error bars represent the standard error of the mean. p-values correspond to between-group comparisons by general linear models with post-hoc comparisons by Bonferroni test.
FIGURE 4.
Serum levels of biomarkers are related to obstructive sleep apnoea severity in patients with lung cancer. Relationship between serum levels of a) paraspeckle component-1 (PSPC1), b) transforming growth factor-β1 (TGF-β1), c) programmed cell death ligand-1 (PD-L1) and d) programmed cell death-1 (PD-1) and apnoea–hypopnoea index (AHI) in subjects from the SAIL cohort. r-values correspond to Pearson correlation coefficients. 3
Finally, the pooled analysis of subjects from the SAILS and SAIL cohorts revealed differences in serum levels of several biomarkers (supplementary table S1). Inclusion of all anthropometric characteristics, sleep parameters and biomarkers that significantly differed between the two cohorts in a multiple logistic regression model revealed that only the desaturation index and serum levels of TGF-β1 were independent predictors of a lung cancer diagnosis (supplementary table S2).
Discussion
Our study shows that individuals at high risk for lung cancer with moderate-to-severe OSA have increased expression of biomarkers related to the induction of tumour aggressiveness pathways (PSPC1 and TGF-β), lymphangiogenesis (MDK) and immune evasion (PD-1/PD-L1), while patients with established lung cancer and moderate-to-severe OSA have increased expression of biomarkers related to tumour aggressiveness and immune evasion. These findings are provocative, insofar as such biomarkers may prove useful as drivers of personalised efforts to select individuals for lung cancer screening beyond conventional criteria which limit screening to heavy smokers older than 50 years. The latter only account for 50% of all patients diagnosed with lung cancer. Our findings also provide additional information on the pathogenic pathways involved in the association between OSA and lung cancer.
Angiogenesis and lymphangiogenesis are processes closely related to tumour progression. These pathways mediate tumour vascularisation, which is necessary to meet the demand for oxygen and nutrients in tumour growth and metastasis. Tumour vascularisation is orchestrated by several signalling pathways and secreted factors, including MDK. Interestingly, MDK has shown potential as a prognostic biomarker in patients with lung adenocarcinoma. A single-cell RNA sequencing study found that MDK constitutes an early biomarker which may be upregulated in early stages of the disease and may promote tumour progression [28]. Non-small cell lung cancer (NSCLC) in vitro and in vivo assays have demonstrated that hypoxaemia has a dominant role via MDK expression, promoting EMT through autocrine signalling and the migration of endothelial cells and vacuolisation via a paracrine mechanism [29]. We have shown that OSA-related intermittent hypoxia is associated with high MDK levels in patients with OSA and no cancer risk [18]. However, our study does not identify differences in MDK levels associated with OSA in patients with established lung cancer. Obviously, the limited sample size of the SAIL cohort may account for this discrepancy, not reaching the statistical power needed to detect subtle differences. However, this finding may also be due to differences in the progression of lymphangiogenesis between lung cancer and other tumours, such as melanoma, in which the role of MDK is better established. Indeed, melanoma has an inherent potential for lymph node colonisation, in which MDK acts as a systemic inducer of neolymphangiogenesis conditioning patient prognosis [30].
The elevated levels of PSPC1 and TGF-β1 point to the influence of OSA-associated intermittent hypoxia on the intrinsic characteristics of tumour cells, inducing metastasis through the activation of pathways that promote EMT and CSCs. In this context, we have previously shown that PSPC1 plays a role in TGF-β signalling that potentiates the activation of pro-metastatic pathways including EMT and CSC [22, 31]. EMT is the main feature of invasiveness and metastasis in tumour progression [32]. The mechanism linking TGF-β to EMT is associated with SMAD signalling. Activated SMAD2 and SMAD3 form complexes with SMAD4, and mediate transcriptional regulation through three families of transcription factors, resulting in repression of epithelial marker gene expression and activation and induction of EMT [33]. Furthermore, in patients with NSCLC, EMT has been associated with disease progression and poor prognosis [34]. The close relationship between the desaturation index and serum levels of PSPC1 and TGF-β1 reinforces the link between intermittent hypoxia and EMT, which has been confirmed both in vitro and in vivo [19, 35, 36].
The increased levels of PD-L1 and PD-1 in patients with moderate-to-severe OSA with or at risk for lung cancer highlight the importance of immune checkpoints in the pathogenesis and the current immunotherapeutic revolution in lung cancer. Soluble immune checkpoints are postulated as useful clinical biomarkers of survival, recurrence and treatment of multiple cancer types. In patients with NSCLC, soluble PD-L1 levels are correlated with PD-L1 protein tumour expression [37] and a poor prognosis [37, 38]. Furthermore, we have reported that soluble PD-L1 is a predictor of poor prognosis in patients with OSA and melanoma, and soluble PD-L1 levels are associated with the severity of hypoxia in patients with OSA [20]. In this regard, hypoxia-inducible factor-1α drives PD-L1 expression in monocytes [39, 40], in a severity-dependent manner [16]. In this study, our data corroborate that OSA-related hypoxaemia also plays a role in the expression of elevated soluble PD-L1 levels in both patients at high risk for lung cancer and those with established lung carcinoma.
Several studies suggest the soluble PD-1 has potential as a biomarker in tumour-specific immunity and survival in animal mouse models [41]. In our patients with lung cancer, soluble PD-1 is related to OSA severity, in agreement with a previous report that PD-1 protein expression on CD8 T-cells from OSA patients is associated with hypoxaemia [16]. Moreover, the finding that patients with small cell lung cancer with high levels of soluble PD-1 experience prolonged progression-free survival after receiving erlotinib raises the possibility that soluble PD-1 may play a role as a prognostic biomarker in lung cancer [42].
In this study we have also explored the association between OSA and other putative soluble immune checkpoints, such as CTLA-4 and CD137. However, our data are inconclusive. Soluble CTLA-4 is produced by immature dendritic cells, monocytes or regulatory T-cells (Tregs) [43], although to the best of our knowledge OSA patients do not frequently show this immature cell phenotype. Furthermore, CTLA-4 surface protein is constitutively expressed on Tregs and is essential for the generation of Tregs [44]. Our findings agree with previous reports which failed to show significant differences in CTLA-4 expression on Tregs comparing samples from patients with OSA, NSCLC or a combination of both [45]. Interestingly, Tregs are also the main cellular source of soluble CD137. Single-cell sequencing analysis suggests that CD137+ and CTLA-4+ expression on Tregs is related to lung cancer antigens [46]. Unfortunately, in our study we only had access to serum samples, so we were not able to explore the association of Tregs in this context.
The pooled analysis of biomarker levels in our study should be interpreted with caution, since the tumour process experienced by the subjects of the SAIL cohort induces the expression of several biomarkers by the tumour cells themselves in a differentiated biological environment. Our study has other potential limitations, chief among them a limited sample size. Furthermore, the determination of biomarkers in serum does not allow us to confirm the contribution of different pathogenic pathways in the development of lung cancer. Although the sleep studies were performed using a validated HSAT, only polysomnography can determine whether sleep fragmentation influences biomarker levels. At the same time, we must take into account the potential impact of certain treatments on the measurements of peripheral biomarkers within the two evaluated cohorts. Finally, this article focuses exclusively on a cross-sectional analysis, pending longitudinal follow-up of the SAILS cohort to verify the prognostic value of the biomarkers and the effect of CPAP treatment on the risk of lung cancer in OSA patients.
In conclusion, this study shows that, both in patients at high risk for lung cancer and in those with established lung cancer, moderate-to-severe OSA is associated with increased expression of serum biomarkers of immune evasion, lymphangiogenesis and tumour cell aggressiveness which may have implications for the future of screening and follow-up of lung cancer.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00777-2023.SUPPLEMENT (1.3MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This article has an editorial commentary: https://doi.org/10.1183/23120541.01050-2023
Conflict of interest: All authors have nothing to disclose.
Ethics statement: The study was approved by the local ethics committee (Fundación Jiménez Díaz University Hospital, Madrid, Spain; EO98/2015 and EO99/2015).
Support statement: This study was supported by grants from Instituto de Salud Carlos III (ISCIII) PI19/01612, PI22/01262 and P2022/BMD-7224 to F. García-Río, CP18/00028, PI19-01363 and PI22/01257 to C. Cubillos-Zapata, PI20/01416 to L.M. Seijo and G. Peces-Barba; a grant from the Spanish Thoracic Society (SEPAR) to L.M. Seijo; and co-funded by the European Union, Ayudas Luis Alvarez 2021 FIBHULP. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Sillah A, Watson NF, Gozal D, et al. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev Med Rep 2019; 15: 100886. doi: 10.1016/j.pmedr.2019.100886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013; 187: 99–105. doi: 10.1164/rccm.201209-1671OC [DOI] [PubMed] [Google Scholar]
- 3.Kendzerska T, Kapur VK. OSA-related hypoxemia and cancer risk. Chest 2020; 158: 2264–2265. doi: 10.1016/j.chest.2020.08.2046 [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Garcia MA, Campos-Rodriguez F, Nagore E, et al. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: a multicenter observational study in 443 patients. Chest 2018; 154: 1348–1358. doi: 10.1016/j.chest.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Olivas JD, Campos-Rodriguez F, Nagore E, et al. Role of sleep apnea and long-term CPAP treatment in the prognosis of patients with melanoma. A prospective multicenter study of 443 patients. Chest 2023; 164: 1551–1559. doi: 10.1016/j.chest.2023.06.012 [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Garcia MA, Campos-Rodriguez F, Almendros I, et al. Cancer and sleep apnea: cutaneous melanoma as a case study. Am J Respir Crit Care Med 2019; 200: 1345–1353. doi: 10.1164/rccm.201903-0577PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep 2016; 39: 1493–1500. doi: 10.5665/sleep.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 9.Cheong AJY, Tan BKJ, Teo YH, et al. Obstructive sleep apnea and lung cancer: a systematic review and meta-analysis. Ann Am Thorac Soc 2022; 19: 469–475. doi: 10.1513/AnnalsATS.202108-960OC [DOI] [PubMed] [Google Scholar]
- 10.Seijo LM, Pérez-Warnisher MT, Giraldo-Cadavid LF, et al. Obstructive sleep apnea and nocturnal hypoxemia are associated with an increased risk of lung cancer. Sleep Med 2019; 63: 41–45. doi: 10.1016/j.sleep.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Warnisher MT, Cabezas E, Troncoso MF, et al. Sleep disordered breathing and nocturnal hypoxemia are very prevalent in a lung cancer screening population and may condition lung cancer screening findings: results of the prospective Sleep Apnea In Lung Cancer Screening (SAILS) study. Sleep Med 2019; 54: 181–186. doi: 10.1016/j.sleep.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, You L, Nepovimova E, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol 2022; 15: 77. doi: 10.1186/s13045-022-01292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest 2022; 132: e159839. doi: 10.1172/JCI159839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korbecki J, Siminska D, Gassowska-Dobrowolska M, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci 2021; 22: 10701. doi: 10.3390/ijms221910701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-Jiménez E, Cubillos-Zapata C, Toledano V, et al. Monocytes inhibit NK activity via TGF-β in patients with obstructive sleep apnoea. Eur Respir J 2017; 49: 1602456. doi: 10.1183/13993003.02456-2016 [DOI] [PubMed] [Google Scholar]
- 16.Cubillos-Zapata C, Avendaño-Ortiz J, Hernandez-Jimenez E, et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur Respir J 2017; 50: 1700833. doi: 10.1183/13993003.00833-2017 [DOI] [PubMed] [Google Scholar]
- 17.Cubillos-Zapata C, Hernández-Jiménez E, Avendaño-Ortiz J, et al. Obstructive sleep apnea monocytes exhibit high levels of vascular endothelial growth factor secretion, augmenting tumor progression. Mediators Inflamm 2018; 2018: 7373921. doi: 10.1155/2018/7373921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubillos-Zapata C, Martínez-García M, Díaz-García E, et al. Proangiogenic factor midkine is increased in melanoma patients with sleep apnea and induces tumor cell proliferation. FASEB J 2020; 34: 16179–16190. doi: 10.1096/fj.202001247RR [DOI] [PubMed] [Google Scholar]
- 19.Díaz-García E, García-Tovar S, Casitas R, et al. Intermittent hypoxia mediates paraspeckle protein-1 upregulation in sleep apnea. Cancers 2021; 13: 3888. doi: 10.3390/cancers13153888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubillos-Zapata C, Martínez-García MA, Campos-Rodríguez F, et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur Respir J 2019; 53: 1801298. doi: 10.1183/13993003.01298-2018 [DOI] [PubMed] [Google Scholar]
- 21.Cubillos-Zapata C, Martínez-García MA, Díaz-García E, et al. Obstructive sleep apnea is related to melanoma aggressiveness through paraspeckle protein-1 upregulation. Eur Respir J 2022; 61: 2200707. doi: 10.1183/13993003.00707-2022 [DOI] [PubMed] [Google Scholar]
- 22.Yeh HW, Hsu EC, Lee SS, et al. PSPC1 mediates TGF-β1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat Cell Biol 2018; 20: 479–491. doi: 10.1038/s41556-018-0062-y [DOI] [PubMed] [Google Scholar]
- 23.Cabezas E, Pérez-Warnisher MT, Troncoso MF, et al. Sleep disordered breathing is highly prevalent in patients with lung cancer: results of the Sleep Apnea in Lung Cancer study. Respiration 2019; 97: 119–124. doi: 10.1159/000492273 [DOI] [PubMed] [Google Scholar]
- 24.Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015; 10: 990–1003. doi: 10.1097/JTO.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 25.Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep 2009; 32: 629–636. doi: 10.1093/sleep/32.5.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13: 479–504. doi: 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mediano O, González Mangado N, Montserrat JM, et al. International consensus document on obstructive sleep apnea. Arch Bronconeumol 2022; 58: 52–68. doi: 10.1016/j.arbres.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Li Z, Zhou K, et al. Deciphering cell lineage specification of human lung adenocarcinoma with single-cell RNA sequencing. Nat Commun 2021; 12: 6500. doi: 10.1038/s41467-021-26770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin DH, Jo JY, Kim SH, et al. Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers 2020; 12: 2402. doi: 10.3390/cancers12092402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmeda D, Cerezo-Wallis D, Riveiro-Falkenbach E, et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 2017; 546: 676–680. doi: 10.1038/nature22977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh HW, Jou YS. PSPC1 potentiates TGF-β-dependent metastatic dissemination. Mol Cell Oncol 2018; 5: e1472058. doi: 10.1080/23723556.2018.1472058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol 2013; 25: 76–84. doi: 10.1097/CCO.0b013e32835b6371 [DOI] [PubMed] [Google Scholar]
- 33.Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med 2016; 5: 63. doi: 10.3390/jcm5070063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmood MQ, Ward C, Muller HK, et al. Epithelial mesenchymal transition (EMT) and non-small cell lung cancer (NSCLC): a mutual association with airway disease. Med Oncol 2017; 34: 45. doi: 10.1007/s12032-017-0900-y [DOI] [PubMed] [Google Scholar]
- 35.Kuma YI, Hosomichi J, Maeda H, et al. Intermittent hypoxia induces turbinate mucosal hypertrophy via upregulating the gene expression related to inflammation and EMT in rats. Sleep Breath 2021; 25: 677–684. doi: 10.1007/s11325-020-02162-6 [DOI] [PubMed] [Google Scholar]
- 36.Gu X, Zhang J, Shi Y, et al. ESM1/HIF1α pathway modulates chronic intermittent hypoxia-induced non-small-cell lung cancer proliferation, stemness and epithelial-mesenchymal transition. Oncol Rep 2021; 45: 1226–1234. doi: 10.3892/or.2020.7913 [DOI] [PubMed] [Google Scholar]
- 37.Yang Q, Chen M, Gu J, et al. Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front Immunol 2021; 12: 665133. doi: 10.3389/fimmu.2021.665133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020; 20: 1185. doi: 10.1186/s12885-020-07690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α. J Infect Dis 2018; 217: 393–404. doi: 10.1093/infdis/jix279 [DOI] [PubMed] [Google Scholar]
- 40.Cubillos-Zapata C, Balbas-Garcia C, Avendaño-Ortiz J, et al. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology 2019; 24: 684–692. doi: 10.1111/resp.13470 [DOI] [PubMed] [Google Scholar]
- 41.Gu D, Ao X, Yang Y, et al. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer 2018; 6: 132. doi: 10.1186/s40425-018-0449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorensen SF, Demuth C, Weber B, et al. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer 2016; 100: 77–84. doi: 10.1016/j.lungcan.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 43.Ward FJ, Dahal LN, Wijesekera SK, et al. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol 2013; 43: 1274–1285. doi: 10.1002/eji.201242529 [DOI] [PubMed] [Google Scholar]
- 44.Read S, Greenwald R, Izcue A, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006; 177: 4376–4383. doi: 10.4049/jimmunol.177.7.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Lao M, Chen J, et al. Short-term prognostic effects of circulating regulatory T-cell suppressive function and vascular endothelial growth factor level in patients with non-small cell lung cancer and obstructive sleep apnea. Sleep Med 2020; 70: 88–96. doi: 10.1016/j.sleep.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 46.Yi L, Jin X, Wang J, et al. CD137 agonists targeting CD137-mediated negative regulation show enhanced antitumor efficacy in lung cancer. Front Immunol 2022; 13: 771809. doi: 10.3389/fimmu.2022.771809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00777-2023.SUPPLEMENT (1.3MB, pdf)