Abstract
A healthy intestinal microbiota is considered to be important for priming of the infants' mucosal and systemic immunity. Breast-fed infants typically have an intestinal microbiota dominated by different Bifidobacterium species. It has been described that allergic infants have different levels of specific Bifidobacterium species than healthy infants. For the accurate quantification of Bifidobacterium adolescentis, Bifidobacterium angulatum, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium catenulatum, Bifidobacterium dentium, Bifidobacterium infantis, and Bifidobacterium longum in fecal samples, duplex 5′ nuclease assays were developed. The assays, targeting rRNA gene intergenic spacer regions, were validated and compared with conventional PCR and fluorescent in situ hybridization methods. The 5′ nuclease assays were subsequently used to determine the relative amounts of different Bifidobacterium species in fecal samples from infants receiving a standard formula or a standard formula supplemented with galacto- and fructo-oligosaccharides (OSF). A breast-fed group was studied in parallel as a reference. The results showed a significant increase in the total amount of fecal bifidobacteria (54.8% ± 9.8% to 73.4% ± 4.0%) in infants receiving the prebiotic formula (OSF), with a diversity of Bifidobacterium species similar to breast-fed infants. The intestinal microbiota of infants who received a standard formula seems to resemble a more adult-like distribution of bifidobacteria and contains relatively more B. catenulatum and B. adolescentis (2.71% ± 1.92% and 8.11% ± 4.12%, respectively, versus 0.15% ± 0.11% and 1.38% ± 0.98% for the OSF group). In conclusion, the specific prebiotic infant formula used induces a fecal microbiota that closely resembles the microbiota of breast-fed infants also at the level of the different Bifidobacterium species.
Generally, the intestinal microbiota of breast-fed infants is primarily composed of lactic acid bacteria, like bifidobacteria and lactobacilli. The microbiota of formula-fed infants is, on the other hand, more diverse and in general contains more Bacteroides, Clostridium, and Enterobacteriaceae (3, 13, 18, 23). The intestinal microbiota may be modified temporarily by nutritional changes in the diet or by the consumption of pro- or prebiotics (9, 14). Prebiotics are defined as nondigestible food ingredients that selectively stimulate the growth and/or activity of one or more bacterial species in the colon and thereby beneficially affect the host (16). For infant formulas, a specific mixture of galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS) has been described that can serve as a growth factor for bifidobacteria, similar to the human milk oligosaccharides in breast milk (4, 6, 47). The number of bifidobacteria in infants receiving a formula containing GOS/FOS was shown to be elevated in comparison to that of infants receiving a standard formula, but it is unclear whether the prebiotics stimulate specific Bifidobacterium species (5, 33, 42). In healthy breast-fed infants, many Bifidobacterium species are found, with the most dominant species being Bifidobacterium infantis, Bifidobacterium breve, and Bifidobacterium longum (29, 32). In adults, the total level of bifidobacteria is generally lower, and Bifidobacterium adolescentis is one of the more abundant species. Recently, it was shown that the levels of B. adolescentis are also relatively high in allergic infants (19, 21, 37, 39). To test whether GOS/FOS stimulate different Bifidobacterium species similar to human milk, it is relevant to quantitatively determine bifidobacteria at the species level. For this purpose, species-specific duplex 5′ nuclease assays (quantitative real-time PCR) were developed.
Currently, Bifidobacterium species-specific PCR and a Bifidobacterium species PCR-enzyme-linked immunosorbent assay are mainly used to semiquantify Bifidobacterium species. These methods use the plateau phase of the PCR (11, 15, 29, 32, 40), and such determinations have important limitations, like diminishing effects of differences in PCR product abundance and a constant maximum level of PCR products with varying amounts of starting DNA (30, 34, 38). The assumption that a higher level of PCR product in the plateau phase means the presence of a higher initial amount of DNA is not always valid, for example, when the different amplification efficiencies are not the same (28). The formation of species-specific amplicons during the PCR can also be followed by using DNA binding dyes like SYBR green (31, 45). The major disadvantages of this method are that nonspecific PCR products will also be detected and that only one specific reaction can be quantified (8).
In this study, duplex 5′ nuclease assays targeted at the intergenic spacer of the 16S-23S rRNA genes were developed for Bifidobacterium adolescentis, Bifidobacterium angulatum, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium catenulatum, Bifidobacterium dentium, Bifidobacterium longum, and Bifidobacterium infantis. The assays are based on the addition of a TaqMan probe to conventional PCR (20), which makes it possible to follow the complete PCR process and overcome the limitations correlated with detection in the plateau phase of PCR. Furthermore, a high specificity is accomplished by the use of specific primers and probes instead of SYBR green.
These newly developed assays were used to study the different Bifidobacterium species in breast-fed (BF) infants and infants receiving a standard formula (SF) or a standard formula supplemented with a specific prebiotic GOS/FOS mixture (OSF).
MATERIALS AND METHODS
Study design and sample collection.
The study was a double blind, placebo-controlled multicenter trial with two intervention groups (24). Fully formula-fed term infants, aged 28 to 90 days, were recruited from four hospitals in Germany. After enrollment, the infants were randomly allocated to one of two formula groups: a group that received an infant formula supplemented with 0.8 g/100 ml GOS/FOS in a 9 to 1 ratio (OSF group) and a group that received a standard infant formula (SF group). A group of exclusively breast-fed infants was studied in parallel and used as a reference (BF group). Within 2 days after study enrollment and at the end of the study period (6 weeks), fecal samples were collected and frozen at −20°C (for details of the study, see reference 24). For the present analyses, fecal samples from 10 infants were randomly selected from each study group and analyzed to determine the levels of the different Bifidobacterium species.
Bacterial strains and culture conditions.
The bacterial strains used to design and validate the assays for the relative quantification of the different Bifidobacterium species are listed in Table 1.
TABLE 1.
Bacterial strains used in this study
| Organism | Straina |
|---|---|
| Bifidobacterium strains | |
| B. adolescentis | ATCC 15703T |
| ATCC 15705 | |
| B. angulatum | DSM 20098T |
| B. animalis | ATCC 25527T |
| DSM 10140 | |
| B. bifidum | DSM 20456T |
| NCIMB 8810 | |
| B. boum | ATCC 27917T |
| B. breve | ATCC 15700T |
| DSM 20091 | |
| LMG 11613 | |
| B. catenulatum | ATCC 27539T |
| ATCC 27675 | |
| B. dentium | ATCC 27534T |
| B. gallicum | DSM 20093T |
| B. gallinarum | ATCC 33777T |
| B. infantis | LMG 8811T |
| B. inopinatum | DSM 10107T |
| B. longum | ATCC 15707T |
| B. magnum | ATCC 27540T |
| B. pseudocatenulatum | DSM 20438T |
| B. pseudolongum | ATCC 25526T |
| B. suis | ATCC 27533T |
| Other strains | |
| Bacillus cereus | ATCC 11778 |
| Bacteroides fragilis | LMG 10263T |
| Brevibacterium casei | ATCC 35513T |
| Clostridium difficile | ATCC 9689T |
| Enterococcus faecalis | DSM 20478T |
| Escherichia coli | ATCC 35218 |
| Gardnerella vaginalis | ATCC 14018T |
| Lactobacillus acidophilus | ATCC 4356T |
| Lactobacillus brevis | LMG 18022 |
| Lactobacillus bulgaricus | ATCC 11842T |
| Lactobacillus casei | ATCC 393T |
| DSM 20011T | |
| Lactobacillus fermentum | DSM 20052T |
| Lactobacillus plantarum | DSM 20174T |
| Lactobacillus reuteri | LMG 9213T |
| Lactobacillus rhamnosus | ATCC 53103 |
| Listeria monocytogenes | ATCC 7644 |
| Pediococcus acidilactici | DSM 20284T |
| Propionibacterium avidum | DSM 4901 |
| Pseudomonas aeruginosa | DSM 1117 |
| Saccharomyces cerevisiae | DSM 2548 |
| Salmonella enterica Typhimurium | ATCC 14028 |
| Staphylococcus aureus | ATCC 29213 |
ATCC, American Type Culture Collection; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Germany; LMG, Laboratory for Microbiology, University of Gent, Belgium; NCIMB, National Collections of Industrial and Marine Bacteria, United Kingdom.
All bifidobacteria, lactobacilli, propionibacteria, Saccharomyces, enterococci, and pediococci strains were cultured in Mann Rogosa Sharp (MRS) broth (Oxoid, Basingstoke, United Kingdom) at 37°C under anaerobic conditions.
Gut commensals and pathogens, like Bacteroides fragilis and Pseudomonas aeruginosa, were cultured in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) at 37°C, and Bacillus cereus, Brevibacterium casei, and Listeria monocytogenes were cultured at 30°C. Overnight cultures were stored at −20°C until further processing.Gardnerella vaginalis was cultured on Columbia blood agar base (Oxoid, Basingstoke, United Kingdom) supplemented with 5% defibrinated rabbit blood (BioTrading Benelux BV, Mijdrecht, The Netherlands) and Gardnerella vaginalis selective supplement (Oxoid, Basingstoke, United Kingdom) in a microaerophilic environment at 37°C. Cells were swabbed from the plate, resuspended in 1 ml of sterile water, and stored at −20°C until further processing.
DNA extraction.
For DNA extraction, the frozen cultures were thawed in ice water. Cells were harvested at 4°C (20 min at 3,500 × g) and washed with 1 ml of TES (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 50 mM NaCl). Cell pellets were resuspended in 1 ml of THMS (30 mM Tris-HCl [pH 8.0], 3 mM MgCl2, 25% [wt/vol] sucrose) and treated enzymatically as described previously (46). After phenol-chloroform extraction, the DNA samples were treated with 25 μg/ml RNase A (Roche Diagnostics, Mannheim, Germany) for 30 min at 37°C, precipitated, resuspended in 100 μl milli-Q, and stored at −20°C.
DNA was isolated from feces by thawing 1 ml of homogenized feces in ice water, followed by centrifugation for 1 min at 1,000 × g to remove debris and large particles. Supernatants were transferred to a new tube and centrifuged for 5 min at 10,000 × g. Pellets were resuspended in 1 ml of TN150 (10 mM Tris-HCl [pH 8.0], 10 mM EDTA) and transferred to sterile tubes containing 0.3 g of zirconium beads (diameter, 0.1 mm; BioSpec Products). To these suspensions, 150 μl of TE-buffered phenol (pH 7.5) was added and the samples were placed in a mini-bead beater (BioSpec Products) for 3 min at 5,000 rpm. After cooling on ice and phenol-chloroform extraction, the DNA was precipitated and resuspended in 100 μl milli-Q and stored at −20°C (46).
Species-specific qualitative PCR analysis.
PCRs were carried out on a PTC-200 Peltier Thermal Cycler (Biozym, Landgraaf, The Netherlands) as described previously (32). Amplification products were checked by agarose gel electrophoresis and ethidium bromide staining.
Species-specific quantitative real-time PCR.
To develop primers and probes for the 5′ nuclease assays, sequences of the 16S-23S intergenic spacer region of the different Bifidobacterium species were retrieved from the GenBank, EMBL, and DDBJ databases. Accession numbers are as follows: B. adolescentis, U09511 (26), U09512 (26), U09513 (26), and U09514 (26); B. angulatum, U09515 (26); Bifidobacterium animalis, AY225132 (44), L36967 (26), and U09858 (26); B. asteroides, U09516 (26); B. breve, AJ245850 (7), U09518 (26), U09519 (26), U09520 (26), and U09521 (26); B. bifidum, U09517 (26) and U09831 (26); B. catenulatum, U09522 (26); B. choerinum, L36968 (26); B. coryneforme, U09523 (26); B. cuniculi, U09790 (26); B. dentium, U10434 (26); B. indicum, U09791 (26); B. infantis, AJ245851 (7), U09525 (26), U09527 (26), and U09792 (26); B. longum, AJ245849 (7) and U09832 (26); B. pseudolongum, U09524 (26) and U09879 (26); B. magnum, U09878 (26); B. thermophilum, U09528 (26). All retrieved sequences were aligned using DNASIS for Windows v2.5 (Hitachi Software Engineering Co., Ltd., Wembley, United Kingdom), and the overall conserved regions of these sequences were used to design primers and probes for the detection of the Bifidobacterium genus. To increase the specificity (and the sensitivity) of the assays, TaqMan minor groove binding probes were used. Species-specific sequences were used to design primers and probes for B. adolescentis, B. angulatum, B. breve, B. bifidum, B. catenulatum, B. dentium, B. infantis, and B. longum (including B. pseudolongum due to a high similarity between the species).
The primers and probes were designed with the help of Primer Express 1.5a (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). All primers and probes have a GC content of 30 to 80% and do not contain three or more successive identical nucleotides. The melting temperature of the probe was between 68°C and 70°C, whereas the primers had a melting temperature 10°C below the melting temperature of the probe. Furthermore, the probes have no G at the 5′ end and the strand with more C than G bases was selected for probe design. The developed primers do not contain more than two G and/or C bases in the 5 nucleotides at the 3′ end, and the PCR amplicons have a maximum length of 150 base pairs. All primers and probes were tested for specificity using the Basic Local Alignment Search Tool (BLAST) (1).
The oligonucleotide probe designed for the detection of the genus Bifidobacterium is labeled with the 5′ reporter dye VIC and the 3′ quencher NFQ-MGB (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). The different bifidobacterial species are detected with oligonucleotide probes labeled with the 5′ reporter dye 6-carboxyfluorescein and the 3′ quencher NFQ-MGB (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands).
An already described universal probe and primer set was used for the determination of the total bacterial load (35). The universal probe is labeled with the 5′ reporter dye 6-carboxyfluorescein and the 3′ quencher dye 6-carboxytetramethyl-rhodamine.
All primer and probe concentrations for performing duplex 5′ nuclease assays were optimized, which is necessary due to potential competition between the primers and the probes. The specificities of the optimized duplex 5′ nuclease assays were tested using the strains listed in Table 1. The assays were performed with a 25-μl PCR amplification mixture containing 12.5 μl TaqMan universal master mix (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands), optimized concentrations of the primers and probes, and 2.5 μl DNA isolated from the bacterial strains. The temperature profile for the amplification consisted of 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C (ABI Prism 7700; Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). The sensitivity of these duplex 5′ nuclease assays was compared to that of conventional PCR by testing dilution series of specific monocultures with both techniques. For the detection limit of the assay in CFU per milliliter, monocultures were also plated on MRS agar and incubated anaerobically for 24 h at 37°C.
The relative amount of the different Bifidobacterium species was calculated according to the method of Liu and Saint (27). The efficiency of each amplification curve was calculated separately and used to determine the initial amount of DNA. Finally, the obtained ratios between the initial amounts of DNA were normalized against a monoculture of the same species, which was set at 100%.
The coefficients of variation (CV) within each duplex 5′ nuclease assay were determined by testing DNA isolated from feces spiked with a monoculture. This was performed 10 times for determination of the reproducibility and three times in quadruplicate for repeatability.
FISH.
The total number of bacteria and the percentage of bifidobacteria were determined by fluorescence in situ hybridization (FISH) as described previously, with some slight modifications (18, 25). Stool samples were thawed in ice water, diluted 10× (wt/vol) in phosphate-buffered saline (PBS; pH 7.4), and homogenized for 10 min using a stomacher (IUL Instruments, Barcelona, Spain). Aliquots with 1 ml of fecal sample were fixed overnight at 4°C with 3 ml freshly prepared 4% (wt/vol) paraformaldehyde in PBS. Samples were stored at −20°C until further processing.
To each separate well of a gelatin-coated object slide (8 square-shaped wells [1 cm2/well]; CBN labsuppliers, Drachten, The Netherlands), 10 μl of the fixed stool samples was applied. Slides were air dried and subsequently dehydrated in 96% ethanol for 10 min. Hybridization was performed overnight in a dark moist chamber at 50°C with 10 ng/μl of the Cy3-labeled Bifidobacterium-specific 16S rRNA gene-targeted oligonucleotide probe (Bif164, CATCCGGCATTACCACCC) in preheated (50°C) hybridization buffer (20 mM Tris-HCl, 0.9 M NaCl, 0.1% sodium dodecyl sulfate [pH 7.2]). After hybridization, the samples were washed for 30 min in 50 ml preheated washing buffer (20 mM Tris-HCl, 0.9 M NaCl [pH 7.2]) and shortly rinsed in milli-Q. Samples were incubated with 0.25 ng/μl 4′,6-diamidino-2-phenylindole (DAPI) in PBS for 5 min at room temperature. After DAPI staining, the samples were briefly rinsed in milli-Q, dried, and mounted with Vectashield (Vector Laboratories).
Samples were analyzed using an Olympus AX70 epifluorescence microscope equipped with an F-View II charge-coupled device 12-bit high-resolution monochrome camera (Soft Imaging System GmbH, Münster, Germany). The percentage of bifidobacteria was determined at 25 randomly chosen positions on each well by counting all cells using a DAPI filter set (SP100; Chroma Technology Corp.) and by counting the bifidobacteria by using a Cy3 filter set (41007; Chroma Technology Corp.).
Data analyses.
For statistical analysis of the results, the software package SPSS for Windows (version 12.0.1; SPSS, Inc.) was used. All values were checked for normality by visual inspection of the normal probability plots. Differences in the percentage of Bifidobacterium species between the start and end of the intervention period as well as differences between the breast- and/or formula-fed groups were tested with paired-sample t tests. If the P value was <0.05, the difference was considered statistically significant. Although statistical analyses were performed to compare the breast-fed group with the formula groups, it has to be kept in mind that it is not possible to double-blindly assign subjects to a breast-fed group, and consequently, no complete randomization was obtained.
RESULTS
Species-specific quantitative real-time PCR.
The sequences of the designed primers and probes for the duplex 5′ nuclease assays are given in Table 2, and the optimized concentrations of the primers and probes are shown in Table 3.
TABLE 2.
Primers and probes used in the duplex 5′ nuclease assays
| Target | Primer or probe | Sequence (5′ → 3′) | Tm (°C) | % GC | BLAST identification no. or reference | Amplicon length (bp) |
|---|---|---|---|---|---|---|
| B. adolescentis | F_adol_IS | ATA GTG GAC GCG AGC AAG AGA | 59 | 52 | 1015335678-6465-18906 | 71 |
| R_adol_IS | TTG AAG AGT TTG GCG AAA TCG | 59 | 43 | 1015335740-7519-1624 | ||
| P_adol_IS | CTG AAA GAA CGT TTC TTT TTa | 69 | 30 | 1015335863-95222-17207 | ||
| B. angulatum | F_angul_IS | TGG TGG TTT GAG AAC TGG ATA GTG | 59 | 46 | 1015336044-12581-14600 | 117 |
| R_angul_IS | TCG ACG AAC AAC AAT AAA CAA AAC A | 59 | 32 | 1015336147-14351-29932 | ||
| P_angul_IS | AAG GCC AAA GCC TC | 70 | 57 | 1015488648-5575-2104 | ||
| B. bifidum | F_bif_IS | GTT GAT TTC GCC GGA CTC TTC | 60 | 52 | 1015336612-215666-12828 | 105 |
| R_bif_IS | GCA AGC CTA TCG CGC AAA | 60 | 56 | 1015336668-22451-30731 | ||
| P_bif_IS | AAC TCC GCT GGC AAC A | 70 | 56 | 1015336773-24053-3416 | ||
| B. breve | F_breve_IS | GTG GTG GCT TGA GAA CTG GAT AG | 59 | 52 | 1015243936-11550-20833 | 118 |
| R_breve_IS | CAA AAC GAT CGA AAC AAA CAC TAA A | 58 | 32 | 1015244110-13595-29514 | ||
| P_breve_IS | TGA TTC CTC GTT CTT GCT GT | 69 | 45 | 1015244238-15062-16853 | ||
| B. catenulatum | F_cate_IS | GTG GAC GCG AGC AAT GC | 58 | 65 | 1015335268-99-20718 | 67 |
| R_cate_IS | AAT AGA GCC TGG CGA AAT CG | 58 | 50 | 1015335364-1571-12175 | ||
| P_cate_IS | AAG CAA ACG ATG ACA TCA | 68 | 39 | 1015335455-2899-17859 | ||
| B. dentium | F_dent_IS | CCG CCA CCC ACA GTC T | 59 | 71 | 1015399643-15856-19947 | 150 |
| R_dent_IS | AGC AAA GGG AAA CAC CAT GTT T | 59 | 41 | 1015399751-16991-11210 | ||
| P_dent_IS | ACG CGT CCA ACG GA | 70 | 64 | 1015399833-18158-5198 | ||
| B. infantis | F_inf_IS | CGC GAG CAA AAC AAT GGT Ta | 58 | 47 | 1037961234-06371-14364 | 76 |
| R_inf_IS | AAC GAT CGA AAC GAA CAA TAG AGT T | 58 | 36 | 1037961263-06691-25461 | ||
| P_inf_IS | TTC GAA ATC AAC AGC AAA Aa | 69 | 32 | 1037961294-06967-17477 | ||
| B. longum | F_long_IS | TGG AAG ACG TCG TTG GCT TT | 59 | 50 | 1015323391-27595-22257 | 109 |
| R_long_IS | ATC GCG CCA GGC AAA Aa | 58 | 56 | 1015323469-28673-23147 | ||
| P_long_IS | CGC ACC CAC CGC A | 68 | 77 | 1015488566-4529-13934 | ||
| All bifidobacteria | F_allbif_IS | GGG ATG CTG GTG TGG AAG AGA | 60 | 57 | 1015399960-19603-31240 | 231a |
| R_allbif_IS | TGC TCG CGT CCA CTA TCC AGT | 60 | 57 | 1015400076-20827-17418 | ||
| P_allbif_IS | TCA AAC CAC CAC GCG CCA | 70 | 61 | 1015400166-21749-18424 | ||
| All bacteria | F_eub | TCC TAC GGG AGG CAG CAG T | 59 | Reference 35 | 466a | |
| R_eub | GGA CTA CCA GGG TAT CTA ATC CTG TT | 58 | ||||
| P_eub | CGT ATT ACC GCG GCT GCT GGC AC | 70 |
In these cases, concessions to the probe and primer design had to be made (more than three consecutive nucleotides are the same or amplicon length is greater then 150 bp).
TABLE 3.
Optimized primer and probe concentrations for the duplex 5′ nuclease assays
| Target | 5′ Nuclease assay | Concn (nM) of:
|
||
|---|---|---|---|---|
| Forward primer | Reverse primer | Probe | ||
| B. adolescentis | B. adolescentis | 300 | 150 | 100 |
| All bifidobacteria | 300 | 600 | 100 | |
| B. angulatum | B. angulatum | 900 | 900 | 200 |
| All bifidobacteria | 300 | 300 | 50 | |
| B. bifidum | B. bifidum | 600 | 600 | 200 |
| All bifidobacteria | 300 | 300 | 100 | |
| B. breve | B. breve | 300 | 300 | 100 |
| All bifidobacteria | 450 | 450 | 150 | |
| B. catenulatum | B. catenulatum | 300 | 300 | 100 |
| All bifidobacteria | 600 | 600 | 100 | |
| B. dentium | B. dentium | 900 | 900 | 200 |
| All bifidobacteria | 300 | 300 | 50 | |
| B. infantis | B. infantis | 300 | 300 | 100 |
| All bifidobacteria | 900 | 900 | 100 | |
| B. longum | B. longum | 300 | 300 | 100 |
| All bifidobacteria | 600 | 600 | 200 | |
| All bifidobacteria | All bifidobacteria | 450 | 450 | 100 |
| All bacteria | 900 | 900 | 200 | |
All of the duplex 5′ nuclease assays were specific for the Bifidobacterium species for which they were developed, except the assay for B. catenulatum, which also detects Bifidobacterium pseudocatenulatum. The 5′ nuclease assay for detection of the genus Bifidobacterium detected all Bifidobacterium species tested and was negative for all other species like Propionibacterium spp., Gardnerella spp., or Lactobacillus spp. To further test the specificity, fecal samples were plated on MRS and DNA extracted from the different colonies was tested in the different duplex nuclease assays. For all 23 of the colonies that were positive in the duplex nuclease assays, the identity could be confirmed by double-stranded 16S rRNA gene sequencing.
Samples treated with RNase gave the same amplification plots as untreated samples, indicating that contaminating RNA does not disturb the assays. Samples treated with DNase did not give any amplification products, as expected. The CV values for reproducibility and repeatability of the different assays are shown in Table 4.
TABLE 4.
Coefficients of variation for reproducibility and repeatability of duplex 5′ nuclease assays
| Target | CV for:
|
|
|---|---|---|
| Reproducibility | Repeatability | |
| B. adolescentis | 0.051 | 0.056 |
| B. angulatum | 0.195 | 0.209 |
| B. bifidum | 0.117 | 0.112 |
| B. breve | 0.021 | 0.041 |
| B. catenulatum | 0.094 | 0.148 |
| B. dentium | 0.127 | 0.114 |
| B. infantis | 0.023 | 0.023 |
| B. longum | 0.091 | 0.082 |
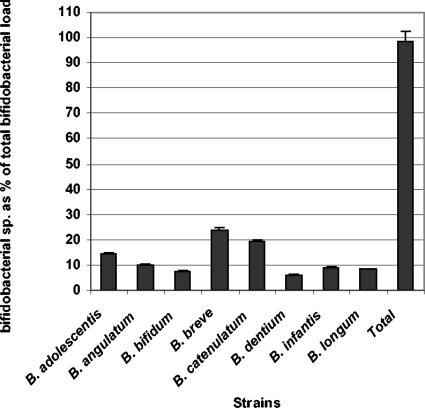
To test competition between the different Bifidobacterium species, a random mix of B. adolescentis, B. angulatum, B. breve, B. bifidum, B. catenulatum, B. dentium, B. infantis, and B. longum monocultures was prepared and tested with the different assays. The sum of the different species adds up to almost 100%, indicating that the different assays do not interfere (Fig. 1).
FIG. 1.
Relative quantification of a mix of 8 different bifidobacterial cultures. “Total” indicates the sum of the different species (98.64% ± 1.67%). Bars represent standard errors.
Comparison with conventional techniques.
The duplex 5′ nuclease assays were compared with conventional PCR methods to determine the sensitivity of the assays and to check for false positives or false negatives. Overall, the 5′ nuclease assays were at least 100-fold more sensitive than the conventional PCR assays, and samples positive in the conventional PCR were always positive in the 5′ nuclease assays.
Dilution series of the different monocultures were enumerated by conventional plating techniques and with the duplex 5′ nuclease assays, and the detection limit of the nuclease assays was found to be around 0.15 CFU/ml.
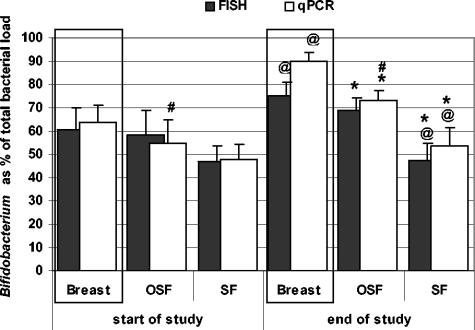
There are no statistically significant differences in the percentages of bifidobacteria determined by previously described FISH methods or by the newly developed quantitative real-time PCR assays (Fig. 2).
FIG. 2.
Bifidobacteria as percentage of total bacterial load determined by FISH and real-time PCR (qPCR) in fecal samples of BF infants and infants who received SF or OSF. Bars represent standard errors. @, significant difference (P < 0.05) between the BF and SF groups; *, significant difference (P < 0.05) between the OSF and SF groups; #, significant increase (P < 0.05) during the study period.
Bifidobacteria in fecal samples from the intervention study.
The developed 5′ nuclease assays were used to determine the levels of the different Bifidobacterium species in fecal samples of BF infants and infants receiving SF or OSF.
The number of bifidobacteria as a percentage of the total bacteria is shown in Fig. 2. At the study start, the percentages of bifidobacteria in the BF, OSF, and SF groups were not statistically different (63.3% ± 7.7%, 54.8% ± 9.8%, and 48.0% ± 6.9%, respectively). At the study end, after a 6-week intervention period, the percentages of bifidobacteria in the BF group (90.0% ± 3.6%; P = 0.015) and OSF group (73.4% ± 4.0%; P = 0.047) were significantly higher than in the SF group (53.4% ± 7.9%). Furthermore, there was a significant increase of the percentage of bifidobacteria during the study period in the OSF group (54.8% ± 9.8% versus 73.4% ± 4.0% [P = 0.041]).
The percentages of the different Bifidobacterium species are given in Table 5. A large variety of Bifidobacterium species is present in all fecal samples of all infants in the BF, SF, and OSF groups. At the start of the study, the most dominant species in all groups was B. infantis, followed by B. breve and B. longum. For infants in the BF and OSF groups, the percentages of these species were relatively constant, whereas infants in the SF group showed a significant decrease in the percentage of B. breve during the study period from 10.70% ± 2.65% to 3.94% ± 3.42% (P = 0.017).
TABLE 5.
Bifidobacterium species as percentages of total bifidobacterial load in fecal samples of infants receiving BF, SF, or OSF as determined by different duplex 5′ nuclease assays
| Organism | % (SE) of total bifidobacterial load in fecal samples of infants by feeding regimen at:
|
|||||
|---|---|---|---|---|---|---|
| Start of study (n = 10)
|
End of study (n = 10)
|
|||||
| BF | OSF | SF | BF | OSF | SF | |
| B. adolescentis | 3.70 (0.85)a | 3.42 (1.17)a | 3.24 (2.65) | 0.30 (0.29)a | 0.15 (0.11)a | 2.71 (1.92) |
| B. angulatum | 0.80 (0.50) | 0.54 (0.30) | 0.30 (0.23) | 1.00 (1.00) | 0.07 (0.07) | <0.00 (0.00) |
| B. bifidum | 0.04 (0.04) | 0.40 (0.28) | 0.02 (0.02) | <0.00 (0.00) | <0.00 (0.00) | <0.00 (0.01) |
| B. breve | 13.05 (3.23) | 11.27 (3.67) | 10.70 (2.65)a | 11.74 (3.04) | 6.44 (2.96) | 3.94 (3.42)a |
| B. catenulatum | 1.82 (0.89) | 2.27 (1.52) | 2.14 (0.96) | 1.90 (0.61)b | 1.38 (0.98)c | 8.11 (4.12)b,c |
| B. dentium | <0.00 (0.00) | <0.00 (0.00) | <0.00 (0.00) | <0.00 (0.00) | <0.00 (0.00) | <0.00 (0.00) |
| B. infantis | 24.17 (5.42) | 26.30 (5.17) | 23.34 (3.95) | 32.03 (5.97) | 32.41 (6.44) | 33.95 (6.35) |
| B. longum | 6.21 (2.72) | 7.67 (4.90) | 6.54 (4.21) | 7.34 (4.38) | 5.25 (3.40) | 5.94 (3.05) |
| All others | 50.21 (17.08) | 48.13 (18.22) | 53.74 (16.83) | 45.69 (15.29) | 54.3 (13.96) | 45.35 (18.87) |
Significant decrease (P < 0.05) during the study period.
Significant difference (P < 0.05) between the BF and SF groups.
Significant difference (P < 0.05) between the OSF and SF groups.
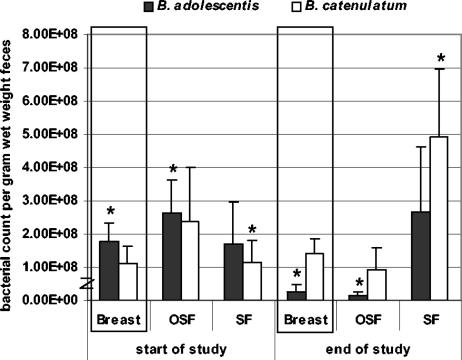
The numbers of the different bifidobacterium species per gram (wet weight) of feces were determined from the total bacterial count as determined by FISH, and the relative percentages are given in Table 5. The numbers of B. adolescentis and B. catenulatum per gram (wet weight) of feces are shown in Fig. 3. The BF group showed a significant decrease of B. adolescentis during the intervention period (1.79E08 ± 5.58E07 versus 2.53E07 ± 2.39E07 per gram [wet weight] of feces [P = 0.017]). A significant decrease was also found for B. adolescentis in the OSF group (2.62E08 ± 1.02E08 versus 1.49E07 ± 1.21E07 bacteria per gram [wet weight] of feces [P = 0.040]) but not in the SF group. The SF group, on the other hand, showed a significant increase of B. catenulatum (1.13E08 ± 6.79E07 versus 4.94E08 ± 2.02E08 bacteria per gram [wet weight] of feces [P = 0.048]), which was not observed in the OSF and BF groups.
FIG. 3.
B. adolescentis and B. catenulatum counts, as determined with a combination of FISH and the duplex 5′ nuclease assays, in feces of BF infants and infants who received OSF or SF. Bars represent standard errors. *, significant difference (P < 0.05) during the study period.
DISCUSSION
To study the distribution of Bifidobacterium species in the fecal samples from infants, duplex 5′ nuclease assays were designed, optimized, and validated. With these newly developed assays, it could be demonstrated that the Bifidobacterium species in infants receiving OSF closely resembles the Bifidobacterium species in breast-fed infants. Infants receiving SF showed a somewhat different pattern of Bifidobacterium species, containing relatively high levels of B. adolescentis and B. catenulatum and lower levels of B. breve.
Species-specific quantitative real-time PCR.
The high sensitivity and specificity of 5′ nuclease assays makes it possible to accurately quantify small amounts of bacterial species in fecal samples. These assays with specific primers and probes are therefore more suitable for the study of the intestinal microbiota than the SYBR green methods. The 5′ nuclease assays were developed using the 16S-23S rRNA gene intergenic spacer region sequences instead of the 16S rRNA gene sequences, which are often used for the phylogenetic analyses and specific detection of bacteria. Due to the high similarities of the bifidobacterial 16S rRNA gene sequences, it is not feasible to develop highly specific primer and probe sets for the different species based on these genes (26). The intergenic spacer of the 16S-23S rRNA gene can be used for a more detailed analysis of Bifidobacterium species because sequences are less conserved than the 16S rRNA gene sequence (36).
The choice for the intergenic spacer was also determined by the fact that contamination and sensitivity issues were described for quantitative real-time PCR when the 16S rRNA gene was used (10). The presence of 16S rRNA gene contamination of Escherichia coli in the recombinant Taq DNA polymerase, as reported by Corless et al. (10), can lead to false-positive results. The eubacterial 5′ nuclease assay described by Nadkarni et al. (35) and used in this study is also targeted at the 16S rRNA gene, but when determining the total bacterial load, the target DNA is highly abundant and the contaminating DNA does not interfere with the assays.
The determined CV values (0.02 to 0.21) for the different species-specific duplex 5′ nuclease assays lie within the range of CV values (0.09 to 0.28) obtained with the FISH technique (12, 17) for determinations at the genus level. Data regarding CV values for determination of bacterium levels in the feces with quantitative real-time PCR have not been reported so far, and a comparison can therefore not be made. The two independent techniques, FISH and real-time PCR, gave very similar results for the levels of fecal bifidobacteria. For FISH, fluorescent-labeled whole bacterial cells are counted in a microscopic field, whereas the 5′ nuclease assay is based on a PCR targeted to isolated chromosomal DNA. The correspondence of the results obtained with both methods demonstrates the power of these techniques for the enumeration of bacteria at the genus level.
A multicolor FISH method for the analysis of seven Bifidobacterium species has been published recently (43). With this method, it is possible to determine semiquantitatively the presence of highly abundant Bifidobacterium species, which are present in fecal samples, with the help of relative fluorescent intensities. However, quantitative real-time PCR is more sensitive then FISH and allows the quantification of less-abundant groups of bacteria.
Bifidobacteria in fecal samples from the intervention study.
The relative quantification of the total percentage of bifidobacteria with real-time PCR showed an increase in fecal samples from infants receiving a formula supplemented with GOS/FOS in contrast to infants receiving a standard formula. The data obtained with this newly developed method support earlier studies in which GOS/FOS were shown to stimulate bifidobacteria (5, 24, 33, 42).
Fecal samples from infants receiving a standard formula supplemented with GOS/FOS showed a large variety of Bifidobacterium species, with a profile very similar to that of breast-fed infants. The levels of the different species show that this specific mixture of GOS/FOS does not selectively stimulate one particular species to dominate the intestinal microbiota. However, in each feeding group, there is still a significant proportion of bifidobacteria that cannot be detected with the probes and primers used (∼45 to 50%), indicating that there are probably still other Bifidobacterium species present in these samples.
During the whole study period, B. infantis, B. breve, and B. longum were detected as the predominant species in the GOS/FOS and breast-fed groups. These same species were also reported by Matsuki et al. (32), Malinen et al. (29), and Kleessen et al. (23) to be dominant in the feces of breast-fed infants. At the end of the intervention period, the Bifidobacterium species profile of infants receiving a standard formula supplemented with GOS/FOS closely resembled the profile of breast-fed infants. The infants receiving a standard formula have lower levels of B. breve and higher levels of B. catenulatum and B. adolescentis. The latter two are more common in adult feces, indicating that a standard infant formula gives a more adult-like microbiota at the level of Bifidobacterium species (19, 32, 37).
At the start of the intervention period, the levels of B. adolescentis are relatively high in all groups but decrease significantly during the study in the breast-fed infants and in infants receiving the GOS/FOS formula. In infants receiving a standard formula, these levels remained constant. It has been reported before by Kalliomaki and Isolauri (21), He et al. (19), and Ouwehand et al. (37) that B. adolescentis is not a dominant species in breast-fed infants. All infants are probably colonized with B. adolescentis from the mother, but the prebiotic substances change the levels of Bifidobacterium species to give a pattern that is common for breast-fed infants.
Bifidobacteria are considered to be important for a well-balanced intestinal microbiota (2), and it has been postulated that bifidobacteria can have several health-promoting effects. This includes the prevention of diarrhea and intestinal infections (41) but also maturation of the immune system. Specific bifidobacteria, for example, were shown to have effects on the symptoms of atopic eczema and allergy (19, 21, 22, 37, 39). Several papers have reported differences in the levels of Bifidobacterium species between allergic and nonallergic infants, with a more adult-like microbiota in allergic infants (19, 21, 37). This might indicate that the specific prebiotic GOS/FOS mixture used in this study can have implications for the immune development of infants by changing the intestinal microbiota at the Bifidobacterium species level. To test whether these prebiotics can have a preventive effect on the incidence of allergy and atopy, larger, well-designed, clinical trails need to be performed.
Acknowledgments
We thank the pediatric practitioners Sabine Groβ, Klaus Helm, Malte Klarczyk, and Helmut Schöpfer for collaboration and collection of the stool specimens. We thank Petra Scholtens for help with the statistical analysis and Esmeralda van der Linde for excellent technical support on the FISH analysis. We thank John Wells and Günther Boehm for critically reading the manuscript and for valuable advice throughout the study.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ballongue, J. 1998. Bifidobacteria and probiotic action, p. 519-587. In S. Salminen and A. von Wright (ed.), Lactic acid bacteria, microbiology and functional aspects, 2nd ed. Marcel Dekker, New York, N.Y.
- 3.Balmer, S. E., and B. A. Wharton. 1989. Diet and faecal flora in the newborn: breast milk and infant formula. Arch. Dis. Child. 64:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm, G., S. Fanaro, J. Jelinek, B. Stahl, and A. Marini. 2003. Prebiotic concept for infant nutrition. Acta Paediatr. Suppl. 91:64-67. [DOI] [PubMed] [Google Scholar]
- 5.Boehm, G., M. Lidestri, P. Casetta, J. Jelinek, F. Negretti, B. Stahl, and A. Marini. 2002. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 86:F178-F181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm, G., and B. Stahl. 2003. Oligosaccharides, p. 203-243. In T. Mattila-Sandholm and M. Saarela (ed.), Functional dairy products. Woodhead Publishing, Cambridge, United Kingdom.
- 7.Brigidi, P., B. Vitali, E. Swennen, L. Altomare, M. Rossi, and D. Matteuzzi. 2000. Specific detection of Bifidobacterium strains in a pharmaceutical probiotic product and in human feces by polymerase chain reaction. Syst. Appl. Microbiol. 23:391-399. [DOI] [PubMed] [Google Scholar]
- 8.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 9.Collins, M. D., and G. R. Gibson. 1999. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 69:1052S-1057S. [DOI] [PubMed] [Google Scholar]
- 10.Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, E. B. Kaczmarski, and A. J. Fox. 2000. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J. Clin. Microbiol. 38:1747-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, X., G. Cheng, and W. Jian. 2000. Simultaneous identification of five Bifidobacterium species isolated from human beings using multiple PCR primers. Syst. Appl. Microbiol. 23:386-390. [DOI] [PubMed] [Google Scholar]
- 12.Franks, A. H., H. J. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller, R. 1991. Factors affecting the composition of the intestinal microflora of the human infant, p. 121-130. In W. C. Heird (ed.), Nutritional needs of the 6-12 month infant. Raven Press, New York, N.Y.
- 14.Fuller, R., and G. R. Gibson. 1997. Modification of the intestinal microflora using probiotics and prebiotics. Scand. J. Gastroenterol. Suppl. 222:28-31. [DOI] [PubMed] [Google Scholar]
- 15.Germond, J. E., O. Mamin, and B. Mollet. 2002. Species specific identification of nine human Bifidobacterium spp. in feces. Syst. Appl. Microbiol. 25:536-543. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 17.Harmsen, H. J., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmsen, H. J., A. C. Wildeboer-Veloo, G. C. Raangs, A. A. Wagendorp, N. Klijn, J. G. Bindels, and G. W. Welling. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61-67. [DOI] [PubMed] [Google Scholar]
- 19.He, F., A. C. Ouwehand, E. Isolauri, H. Hashimoto, Y. Benno, and S. Salminen. 2001. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol. Med. Microbiol. 30:43-47. [DOI] [PubMed] [Google Scholar]
- 20.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real-time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 21.Kalliomaki, M., and E. Isolauri. 2003. Role of intestinal flora in the development of allergy. Curr. Opin. Allergy Clin. Immunol. 3:15-20. [DOI] [PubMed] [Google Scholar]
- 22.Kirjavainen, P. V., T. Arvola, S. J. Salminen, and E. Isolauri. 2002. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut 51:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleessen, B., H. Bunke, K. Tovar, J. Noack, and G. Sawatzki. 1995. Influence of two infant formulas and human milk on the development of the faecal flora in newborn infants. Acta Paediatr. 84:1347-1356. [DOI] [PubMed] [Google Scholar]
- 24.Knol, J., P. A. M. J. Scholtens, G. M. A. Steenbakkers, S. Gross, K. Helm, M. Klarczyk, H. Schopfer, C. Kafka, and J. Wells. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 40:36-42. [DOI] [PubMed] [Google Scholar]
- 25.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leblond-Bourget, N., H. Philippe, I. Mangin, and B. Decaris. 1996. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. Int. J. Syst. Bacteriol. 46:102-111. [DOI] [PubMed] [Google Scholar]
- 27.Liu, W., and D. A. Saint. 2002. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal. Biochem. 302:52-59. [DOI] [PubMed] [Google Scholar]
- 28.Liu, W., and D. A. Saint. 2002. Validation of a quantitative method for real time PCR kinetics. Biochem. Biophys. Res. Commun. 294:347-353. [DOI] [PubMed] [Google Scholar]
- 29.Malinen, E., J. Matto, M. Salmitie, M. Alander, M. Saarela, and A. Palva. 2002. PCR-ELISA II: analysis of Bifidobacterium populations in human faecal samples from a consumption trial with Bifidobacterium lactis Bb-12 and a galacto-oligosaccharide preparation. Syst. Appl. Microbiol. 25:249-258. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu-Daude, F., J. Welsh, T. Vogt, and M. McClelland. 1996. DNA rehybridization during PCR: the ‘Cot effect’ and its consequences. Nucleic Acids Res. 24:2080-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Kado, T. Takada, K. Matsumoto, and R. Tanaka. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro, G., I. Minoli, M. Mosca, S. Fanaro, J. Jelinek, B. Stahl, and G. Boehm. 2002. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 34:291-295. [DOI] [PubMed] [Google Scholar]
- 34.Morrison, C., and F. Gannon. 1994. The impact of the PCR plateau phase on quantitative PCR. Biochim. Biophys. Acta 1219:493-498. [DOI] [PubMed] [Google Scholar]
- 35.Nadkarni, M. A., F. E. Martin, N. A. Jacques, and N. Hunter. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257-266. [DOI] [PubMed] [Google Scholar]
- 36.O'Sullivan, D. J. 2001. Screening of intestinal microflora for effective probiotic bacteria. J. Agric. Food Chem. 49:1751-1760. [DOI] [PubMed] [Google Scholar]
- 37.Ouwehand, A. C., E. Isolauri, F. He, H. Hashimoto, Y. Benno, and S. Salminen. 2001. Differences in Bifidobacterium flora composition in allergic and healthy infants. J. Allergy Clin. Immunol. 108:144-145. [DOI] [PubMed] [Google Scholar]
- 38.Raeymaekers, L. 2000. Basic principles of quantitative PCR. Mol. Biotechnol. 15:115-122. [DOI] [PubMed] [Google Scholar]
- 39.Rautava, S., and E. Isolauri. 2002. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr. Issues Intest. Microbiol. 3:15-22. [PubMed] [Google Scholar]
- 40.Requena, T., J. Burton, T. Matsuki, K. Munro, M. A. Simon, R. Tanaka, K. Watanabe, and G. W. Tannock. 2002. Identification, detection, and enumeration of human bifidobacterium species by PCR targeting the transaldolase gene. Appl. Environ. Microbiol. 68:2420-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saavedra, J. M., N. A. Bauman, I. Oung, J. A. Perman, and R. H. Yolken. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046-1049. [DOI] [PubMed] [Google Scholar]
- 42.Schmelzle, H., S. Wirth, H. Skopnik, M. Radke, J. Knol, H. M. Bockler, A. Bronstrup, J. Wells, and C. Fusch. 2003. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J. Pediatr. Gastroenterol. Nutr. 36:343-351. [DOI] [PubMed] [Google Scholar]
- 43.Takada, T., K. Matsumoto, and K. Nomoto. 2004. Development of multi-color FISH method for analysis of seven Bifidobacterium species in human feces. J. Microbiol. Methods 58:413-421. [DOI] [PubMed] [Google Scholar]
- 44.Ventura, M., and R. Zink. 2002. Rapid identification, differentiation, and proposed new taxonomic classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 68:6429-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitali, B., M. Candela, D. Matteuzzi, and P. Brigidi. 2003. Quantitative detection of probiotic Bifidobacterium strains in bacterial mixtures by using real-time PCR. Syst. Appl. Microbiol. 26:269-276. [DOI] [PubMed] [Google Scholar]
- 46.Walter, J., G. W. Tannock, A. Tilsala-Timisjarvi, S. Rodtong, D. M. Loach, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver, L. T. 2003. Improving infant milk formulas: near the end of the trail for the holy grail? J. Pediatr. Gastroenterol. Nutr. 36:307-310. [DOI] [PubMed] [Google Scholar]