Abstract
In Australia, the cotton bollworm, Helicoverpa armigera, has a long history of resistance to conventional insecticides. Transgenic cotton (expressing the Bacillus thuringiensis toxin Cry1Ac) has been grown for H. armigera control since 1996. It is demonstrated here that a population of Australian H. armigera has developed resistance to Cry1Ac toxin (275-fold). Some 70% of resistant H. armigera larvae were able to survive on Cry1Ac transgenic cotton (Ingard) The resistance phenotype is inherited as an autosomal semidominant trait. Resistance was associated with elevated esterase levels, which cosegregated with resistance. In vitro studies employing surface plasmon resonance technology and other biochemical techniques demonstrated that resistant strain esterase could bind to Cry1Ac protoxin and activated toxin. In vivo studies showed that Cry1Ac-resistant larvae fed Cy1Ac transgenic cotton or Cry1Ac-treated artificial diet had lower esterase activity than non-Cry1Ac-fed larvae. A resistance mechanism in which esterase sequesters Cry1Ac is proposed.
During the last 50 years, worldwide use of synthetic insecticides to control insect pests has led to both insecticide resistance and environmental problems (10). A more recent and environmentally friendly strategy for pest management has incorporated the use of insecticidal proteins of Bacillus thuringiensis both in sprays and in genetically engineered crops. Transgenic crops, such as cotton, are now grown globally to help control insect pests. Initially when B. thuringiensis was used as a pesticide, it was hoped that its coevolution with insects would preclude resistance. However, a number of insects have developed resistance to B. thuringiensis Cry toxins (12). In Australia, the People's Republic of China, the Indian subcontinent, and Africa, the cotton bollworm, Helicoverpa armigera, is arguably the most important agricultural pest and has a long history of insecticide resistance (7). The possibility that this cosmopolitan pest could develop resistance to B. thuringiensis Cry toxins in transgenic cotton is thus of great concern.
B. thuringiensis toxins are ingested by the insect as protoxins, which are then activated by midgut proteases to form a crystal capable of disrupting the midgut membrane wall, leading to insect death. Transgenic cotton varieties coding for expression of the B. thuringiensis delta-endotoxin Cry1Ac (Ingard) have been grown in Australia to control H. armigera since 1996, and a two-gene variety, Bollgard II, coding for both Cry1Ac and Cry2Ab toxins, was commercially released in late 2003. The continuous expression of B. thuringiensis toxins in transgenic cotton presents an enduring threat of resistance selection, a risk increased in Australian cotton by variable Cry1Ac expression (9). Resistance monitoring of H. armigera field populations from Australia and the People's Republic of China has suggested that there has been some decline in the susceptibility to Cry1Ac in the field (4, 11). In 2001, an H. armigera strain (silver strain) was formed from field survivors of the Cry1Ac resistance monitoring program (4) from New South Wales and Queensland cotton areas. Due to inadequate bioassay methods, there has been some disagreement among Australian researchers as to the resistance status of the silver strain. In this work we optimized the bioassay protocol and increased the availability of the Cry1Ac toxin to H. armigera larvae, and we obtained unequivocal evidence that this H. armigera strain, bred from field survivors of a Cry1Ac resistance screening procedure, is resistant to Cry1Ac. A class of serine hydrolases, called nonspecific esterases, which are found in the insect gut, have been implicated as an insecticide resistance mechanism in numerous insect pests due to their ability to hydrolyze insecticidal esters and their ability to sequester xenobiotics. In Australia, H. armigera resistance to insecticide groups as diverse as pyrethroids and chlorfenapyr has been linked these enzymes (5, 6). In this work, we investigated the possibility that such an esterase mechanism confers resistance to B. thuringiensis toxins, and innovative surface plasmon resonance (SPR) using BIAcore and other biochemical techniques were employed to study H. armigera esterase interactions with Cry1Ac.
MATERIALS AND METHODS
Insect strains.
The Australian H. armigera strains used in this work were a Cry1Ac-susceptible laboratory strain, the silver strain, the silver selected strain, and a reciprocal backcross of the susceptible and “silver selected” strains. The silver strain originated from Cry1Ac resistance screening of survivors from New South Wales and Queensland cotton (Emerald, Darling Downs, and St. George, Queensland; Macintrye Valley, Queensland and New South Wales; Namoi Valley, Macquarie Valley, and Hillston, New South Wales).
Five Cry1Ac-susceptible H. armigera field strains collected from conventional cotton and maize crops in 2002, which been exposed to a wide variety of conventional insecticides, were also used for comparison of esterase activity in this study (strain 1, Warren, New South Wales; strain 2, Narromine, New South Wales; strain 3, Gunnedah; strain 4, Griffith, New South Wales; and strain 5, Willow Tree, New South Wales).
Diet incorporation bioassay.
Feeding bioassays with third-instar larvae were used, in which Cry1Ac was incorporated into an artificial diet. Formulated Cry1Ac (MVP) was serially diluted with distilled water containing 0.1% Triton X-100 and pipetted onto the diet surface. Larvae were confined to the B. thuringiensis-treated diet at 25°C for 4 days before they were transferred to a fresh, non-B. thuringiensis-treated diet. Mortality was assessed 14 days after Cry1Ac dosage. Dosage mortality data were analyzed by probit analysis (3). We bioassayed the silver strain and a lab susceptible strain, but since the silver strain had been maintained unselected for some time, to better gauge the magnitude of resistance, the silver strain was selected once with MVP at the 50% lethal concentration (LC50), and the F1 progeny (silver selected strain) were bioassayed. The “silver selected” strain was also reciprocally backcrossed with the susceptible strain (silver selected females × susceptible males; silver selected males × susceptible females), and progeny were bioassayed with MVP.
Feeding bioassays with cotton leaves.
First-instar larvae of the susceptible and “silver selected” strains were placed on cotton leaf disks of non-B. thuringiensis (Sicot 189), Ingard (Sicot 189i), and Bollgard II (Sicot 289b) cotton varieties. The Ingard and Bollgard II cotton varieties expressed Cry1Ac toxin at a rate of 1.2 to 1.4 ppm. The larvae were sealed in vented, air-tight, polystyrene, 5-cm-diameter petri dishes (five larvae per petri dish). The larvae were allowed to feed on the cotton leaf disks at 25°C. Each experiment was replicated eight times. Additional leaf material was provided if required, and mortality was assessed at 10 days. Surviving larvae were kept on the artificial diet until pupation and then until eclosion to verify normal development, fertility, and fecundity into the next generation.
Electrophoresis.
The polyacrylamide gel electrophoresis preparation methods were similar to those used by Devonshire and Moores (2). Larvae (3 to 4 mg) were homogenized in microtiter plates with 200 μl of 1.6% Triton X-100 in distilled water containing 10% sucrose and a few grains of bromocresol purple. Aliquots (10 μl) were loaded directly onto a polyacrylamide gel. Gels were run at 250 V and maximum current until the solvent front had run off the gel. The gels were stained for esterase activity in a solution of 0.1 mM 1-naphthyl acetate-0.2% Fast Blue RR salt in phosphate buffer (pH 6.0) for 30 min in the dark at 25°C and fixed in 5% acetic acid.
Total-esterase determinations.
Small (3- to 4-mg) larvae were used for total-esterase determinations. Fifty larvae of each strain were homogenized in 2.5 ml of 0.02 M phosphate buffer (pH 7.0) containing 0.05% Triton X-100. Replicate aliquots (10 μl) were transferred to a clean microplate containing 240 μl of 0.2 M phosphate buffer (pH 6.0) with 0.6% Fast Blue RR salt and 1.86% 1-naphthyl acetate. Kinetic assays were immediately performed with a Bio-Rad microplate reader utilizing kinetic software, and absorbance readings (450 nm) were taken automatically at 14-s intervals. The kinetic velocity was calculated by the online computer as the slope of the fitted regression line using an absorbance limit of 2,000 mOD/(optical density × 10−3).
In vivo esterase determinations.
The in vivo esterase determination method (7) was as follows. First-instar susceptible and “silver selected” larvae were fed a Cry1Ac-treated diet (0.0012 mg Cry1Ac/0.5 g diet) or Cry1Ac-expressing Ingard cotton leaves at 25°C. At times from 30 min to 70 h later, samples of larvae were taken and stored at −15°C until the conclusion of the experiments. Control larvae were fed non-B. thuringiensis cotton or diet. Larval tissue (20 mg) from each sampling time was assayed for esterase activity.
Inhibition of esterase by Cry1Ac toxin.
Cry1Ac protoxin (crystals and spores) and activated Cry1Ac (crystals) were dissolved in 50 mM carbonate buffer (pH 9.5). Small (3- to 4-mg) larvae were used for these experiments. Fifty larvae of each strain were homogenized in 1.0 ml of 0.02 M phosphate buffer (pH 7.0) containing 0.05% Triton X-100. Aliquots (50 μl), were pipetted into Eppendorf tubes. Cry1Ac was added to the aliquots, and the volume was adjusted to 150 μl with carbonate buffer. The tubes were incubated for 60 min at 25°C. Esterase activity was determined for replicate 10-μl aliquots. The final concentrations of Cry1Ac protoxin and activated toxin ranged from 2.0 × 10−8 to 2.0 × 10−5 μg/assay and from 5.0 × 10−8 to 5.0 × 10−6 μg/assay, respectively.
Surface plasmon resonance.
SPR techniques using BIAcore were used to study H. armigera esterase/Cry1Ac interactions. Esterase isoenzymes from the resistant “silver selected” strain were purified by anion-exchange chromatography. Approximately 1,800 response units of activated Cry1Ac toxin in 10 mM sodium acetate (pH 4.0) was bound to a CM5 carboxymethyl surface using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide-N-hydroxysuccinimide chemistry. Purified H. armigera esterases from the Cry1Ac-resistant silver selected strain were then passed across this surface at concentrations ranging from 1.74 μM to 27.8 μM in 10 mM disodium tetraborate-1 M NaCl (pH 8.5) (to approximate midgut conditions). The Cry1Ac surface was regenerated between each injection with two 10-s injections of glycine (pH 1.5).
RESULTS
Bioassay.
Bioassay data are shown in Table 1. There was an excellent relationship between Cry1Ac dose and H. armigera mortality for the susceptible strain (as shown by the slope value). Compared to the susceptible strain, the silver strain was 14- and 50-fold more resistant at the LC50 and LC99.9, respectively. A lower slope value of 1.9 for the silver strain compared to 3.1 for the susceptible strain showed the heterogeneity of the response to Cry1Ac in the population, which is indicative of some resistance. Proof of Cry1Ac resistance in the silver strain was provided by the “silver selected” strain. The results showed that the single selection with Cry1Ac considerably increased both the resistance factors and the slope value of the dose-response curve, giving resistance factors of 150- and 275-fold at the LC50 and LC99.9, respectively, and a slope value of 2.5. Further evidence of genetic resistance to Cry1Ac was provided by the reciprocal backcrosses of the “silver selected” and susceptible strains. Both backcrosses produced levels of resistance intermediate between those of the susceptible and resistant parents, showing that resistance was semidominant. The resistance factors for the “silver selected” strain male × susceptible strain female cross were 39 and 67 for the LC50 and LC99.9, respectively, with a slope of 2.6. The silver strain female × susceptible male cross had resistance factors of 37 and 51 at the LC50 and LC99.9, respectively, and a slope of 2.8. Data from the reciprocal backcrosses were not significantly different, indicating that resistance was not sex linked.
TABLE 1.
Responses of Cry1Ac-resistant -and susceptible Australian H. armigera strains to formulated Cry1Aca
| Strainb | Slope | χ2 | LC50 (95% fiducial limits) (mg Cry1Ac/0.5 g diet) | Resistance factorc | LC99.9 (95% fiducial limits) (mg Cry1Ac/0.5 g diet) | Resistance factorc |
|---|---|---|---|---|---|---|
| Susceptible | 3.1 | 2.0 | 0.000125 (0.00010-0.00015) | 1 | 0.0012 (0.00069-0.0023) | 1 |
| Silver strain 2001/2 | 1.9 | 5.9 | 0.0018 (0.001-0.003) | 14 | 0.063 (0.0075-0.57) | 50 |
| “Silver selected” | 2.5 | 0.7 | 0.019 (0.010-0.024) | 150 | 0.33 (0.15-0.69) | 275 |
| “Silver selected” (m) × susceptible (f) F1 | 2.6 | 1.4 | 0.0049 (0.0041-0.0061) | 39 | 0.08 (0.036-0.17) | 67 |
| “Silver selected” (f) × susceptible (m) F1 | 2.8 | 0.7 | 0.0046 (0.003-0.006) | 37 | 0.061 (0.022-0.16) | 51 |
Dosage mortality data were calculated by probit analysis.
m, males; f, females.
The resistance factor was calculated from the ratio of resistant LC50 or LC99.9 to susceptible LC50 or LC99.9.
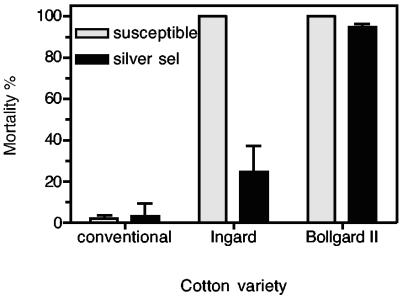
Feeding bioassays with conventional and transgenic cotton varieties and the lab susceptible and “silver selected” strains (Fig. 1) showed that while both strains survived on non-B. thuringiensis cotton, only the “silver selected” strain survived on Ingard cotton. Some 70% of “silver selected” strain larvae survived on Ingard cotton, compared to 100% mortality for the susceptible strain. A small but significant percentage of “silver selected” larvae also survived on Bollgard II cotton. Surviving larvae developed to pupation and eclosed into normal, fertile moths.
FIG. 1.
Survival of larvae of Cry1Ac-susceptible and “silver selected” (silver sel) strains of H. armigera on leaves of conventional non-B. thuringiensis (Sicot 189), Ingard (Sicot 189i), and Bollgard II (Sicot 289b) cotton varieties. The errors bars indicate 95% confidence intervals.
Esterases.
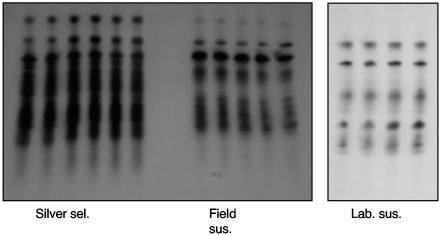
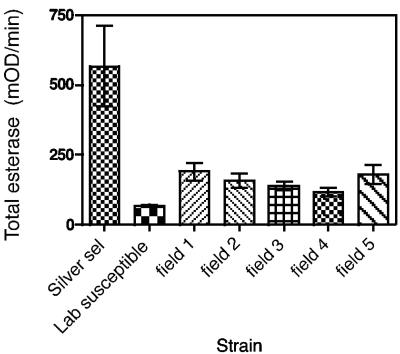
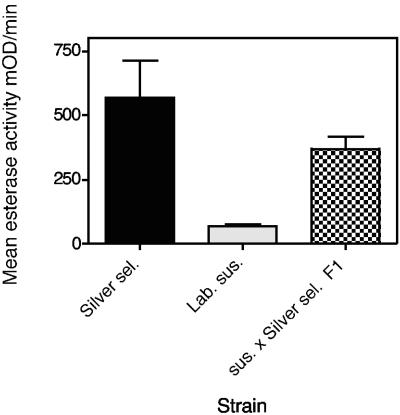
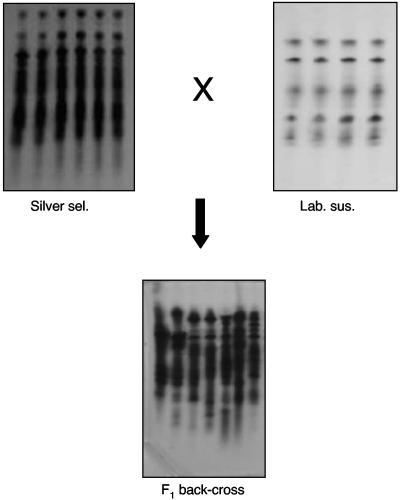
Electrophoretic data (Fig. 2) showed that the “silver selected” H. armigera strain had greatly increased esterase levels, as indicated by heavily staining bands compared to the bands for the lab susceptible strain and a typical field strain. Total-esterase studies showed that the “silver selected” strain of H. armigera had 3- to 12-fold-greater esterase activity than the lab susceptible strain and other H. armigera field strains (which had faced strong chemical selection in the field) (Fig. 3). The extra enzyme activity in the “silver selected” strain was inherited along with resistance and was not induced by Cry1Ac exposure. Electrophoresis and total-esterase determinations for the “silver selected” and susceptible strains and the F1 backcross proved that the extra esterase was inherited. The F1 backcross had a level of esterase that was intermediate between the levels for the susceptible and resistant parents (Fig. 4 and 5), proving the link between resistance and increased levels of esterase.
FIG. 2.
Polyacrylamide gels showing esterase activities in larvae of lab susceptible (Lab. sus.), field susceptible (Field sus.), and silver selected (Silver sel.) strains of H. armigera. Each track contained the equivalent of 5% of a 3- to 4-mg larva.
FIG. 3.
Total esterase activities in 3- to 4-mg larvae of strains of H. armigera, including the “silver selected” strain, the Cry1Ac-susceptible lab strain, and Cry1Ac-susceptible field strains. The error bars indicate 95% confidence intervals. mOD, optical density × 10−3.
FIG. 4.
Total esterase activities in 3- to 4-mg larvae of strains of H. armigera, including the “silver selected” strain (Silver sel.), the Cry1Ac-susceptible lab strain (Lab st), and F1 backcrosses. The error bars indicate 95% confidence intervals. sus., susceptible; mOD, optical density × 10−3.
FIG. 5.
Polyacrylamide gels showing esterase activities in larvae of the lab susceptible (Lab. sus.), “silver selected” (Silver sel.), and F1 backcross strains of H. armigera. Each track contained the equivalent of 5% of a 3- to 4-mg larva.
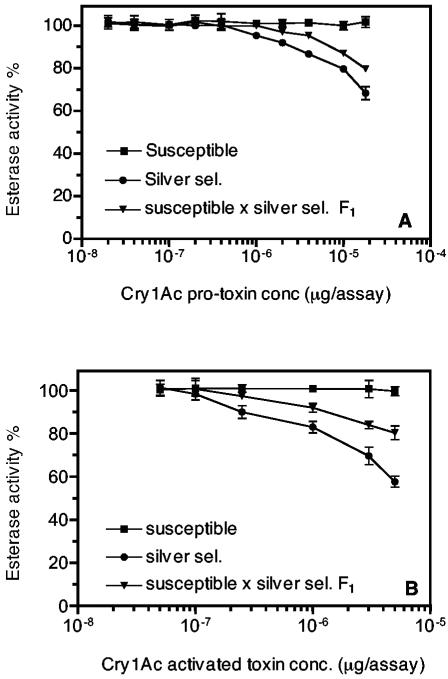
Using the artificial substrate to measure esterase activity, in vitro esterase inhibition experiments were performed by incubating esterase with Cry1Ac. The results demonstrated that the “silver selected” strain esterase bound to both Cry1Ac protoxin and activated toxin (Fig. 6). Esterase from susceptible H. armigera did not bind to Cry1Ac (Fig. 6). Esterase binding data for the F1 backcross revealed an intermediate level of binding to both Cry1Ac protoxin and activated toxin (Fig. 6); thus, the ability of esterase to bind to Cry1Ac toxin was inherited in a semidominant fashion.
FIG. 6.
In vitro esterase inhibition by Cry1Ac protoxin (A) and activated toxin (B) in the Cry1Ac-susceptible, resistant “silver selected” (Silver sel.), and F1 backcross strains of H. armigera. The error bars indicate 95% confidence intervals.
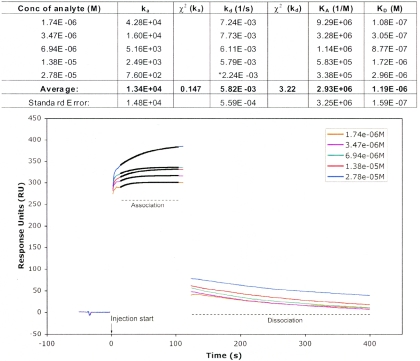
To further study the resistance mechanism, we used novel SPR techniques (BIAcore) to directly investigate H. armigera esterase binding to Cry1Ac toxin. BIAcore data were captured in real time, and the inline blank surface response was subtracted automatically from the binding curves. The data were then plotted, and association and dissociation rates were fitted globally across the concentration range using the Langmuir model. The resulting sensogram and kinetic data (Fig. 7) confirmed that there was binding between analyte and ligand. The resultant equilibrium dissociation constants (KD) are in the μM range (average KD = 1.2 μM), demonstrating that the esterase has a strong affinity for the Cry1Ac toxin.
FIG. 7.
Sensogram plot of association and dissociation curves for purified esterase from the Cry1Ac-resistant “silver selected” H. armigera strain to activated Cry1Ac toxin and kinetic data from the surface plasmon resonance analysis of binding.
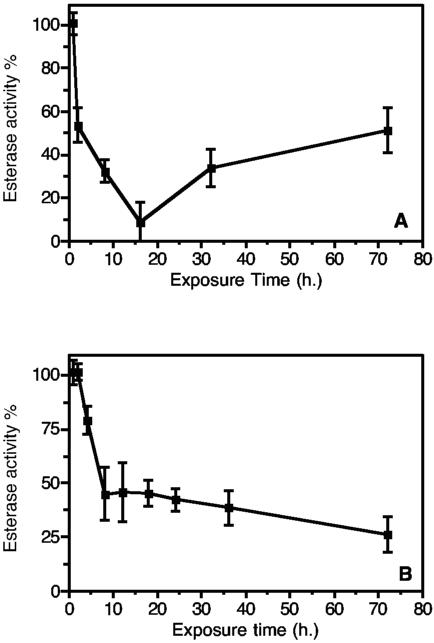
While the in vitro data clearly showed that the “silver selected” strain esterase bound to Cry1Ac toxin, the resistance significance for B. thuringiensis cotton requires the ability of esterases in neonate larvae to sequester Cry1Ac toxin in vivo. In the field, H. armigera is exposed as first-instar larvae to Cry1Ac toxin, and the resistance mechanism would have little ecological significance for resistance selection unless it was expressed in live, first-instar larvae. Esterase inhibition by Cry1Ac was studied in vivo with first-instar “silver selected” strain larvae either by using formulated Cry1Ac in the diet or by feeding the larvae leaves of Cry1Ac-expressing Ingard cotton plants. Larvae were sampled at regular time intervals for esterase analysis. In vivo inhibition data are shown in Fig. 8. Esterase inhibition, compared to non-Cry1Ac diet controls, was detected 2 h after feeding on the formulated Cry1Ac diet and continued to increase for 16 h (Fig. 8A). The esterase activity remained significantly inhibited while the actively feeding larvae had access to the Cry1Ac-treated diet. Susceptible strain larvae did not survive the course of the experiment. With Ingard cotton (Fig. 8B), esterase inhibition was detected after 4 to 5 h of active feeding, and esterase activity continued to decline for the duration of the experiment. The esterase activity remained inhibited while the feeding larvae had access to Ingard cotton. Susceptible strain larvae did not survive the course of the experiment with Ingard cotton.
FIG. 8.
In vivo esterase inhibition (with respect to non-Cry1Ac-treated controls) in “silver selected” strain larvae fed formulated Cry1Ac (0.0012 mg Cry1Ac/0.5 g diet) (A) or fed actively Cry1Ac-expressing Ingard cotton leaves (B). The error bars indicate 95% confidence intervals.
DISCUSSION
While in all insect species there is some naturally occurring variation in susceptibility to insecticides, mere vigor tolerance can be discounted for the silver strain because of the ease of selection for higher levels of resistance. Had the observed resistance level (14-fold at the LC50) been the result of mere variation in susceptibility within susceptible strains, one mild selection with Cry1Ac would not have resulted in any resistance. The increase in resistance observed in the “silver selected” strain indicated that genetic resistance was already present in the silver strain, and this was proven by the backcross data. Our conclusion, therefore, was that the H. armigera silver strain, bred from field survivors of Cry1Ac resistance monitoring, was clearly resistant to Cry1Ac toxin. The transgenic cotton feeding bioassay data confirmed Cry1Ac resistance to Ingard cotton and further emphasized the potential field significance of the resistance.
Findings concerning esterase binding to Cry1Ac are very significant because sequestration is recognized as a potential B. thuringiensis delta-endotoxin resistance mechanism. Given the greatly increased esterase titer in the “silver selected” strain, esterase could have the ability to bind to and thus detoxify considerable quantities of Cry1Ac. F1 backcross data proved that extra esterase and the ability of this esterase to bind to Cry1Ac were undoubtedly linked to resistance. Although no appreciable hydrolysis of the protein by resistant esterase may take place, the very large molar amounts of esterase in resistant H. armigera could be sufficient to sequester quantities of the toxin, thus rendering it harmless before it reaches the target site.
In vivo data (Fig. 8) showed that esterase in live first-instar larvae of the H. armigera “silver selected” strain binds to Cry1Ac and thus could be significant in the field. Esterase sequestration, a potential resistance mechanism, has great ecological significance because of the capacity for selection by transgenic cotton crops. The finding that resistant larvae apparently sequester and detoxify Cry1Ac toxin while they are feeding on transgenic cotton is highly significant because it also provides a direct field mechanism for the observed resistance to Cry1Ac in the silver strain of H. armigera.
Mechanisms previously reported to confer resistance to B. thuringiensis toxins are based on modifications to the receptor binding site (8) or alterations in the proteases that cleave the protoxin, processing it into a smaller active toxin (1). The potential for H. armigera esterase to bind to and detoxify B. thuringiensis toxins is of great concern because esterase-based resistance mechanisms in insects are not uncommon. The vast overproduction of esterase in the “silver selected” strain may be unique to Cry1Ac resistance. However, given that Australian H. armigera has developed esterase-based resistance mechanisms in the past, it is unclear whether this esterase-mediated Cry1Ac resistance mechanism is in any way connected with the widespread use of conventional insecticides. Cross-resistance studies and further experiments with other B. thuringiensis resistant species with widespread resistance to both chemical and B. thuringiensis insecticides may help clarify the situation.
Confirmation of Cry1Ac resistance in a strain of H. armigera derived from survivors of a field Cry1Ac resistance monitoring program in Australia and the finding that there is inherited increased esterase activity, which genetically segregates with resistance and can sequester Cry1Ac, are important to the future of B. thuringiensis crops. Of further concern is the semidominant status of the resistance mechanism, which makes management of H. armigera resistance with Bollgard II cotton more difficult. Survival on transgenic cotton further emphasizes the field significance of resistance to Cry1Ac. Cry1Ac resistance places additional selection pressure on the Cry2Ab toxin component of Bollgard II cotton. Finally, given that H. armigera is a cosmopolitan pest of cotton and other crops, the existence of an esterase-mediated resistance mechanism may pose a considerable threat to the future efficacy of B. thuringiensis transgenic crops worldwide.
Acknowledgments
This work was partially funded by the Cotton Research and Development Corporation, Australia. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
We thank Ray Ackhurst (CSIRO Entomology) for providing the laboratory susceptible strain.
REFERENCES
- 1.Candas, M., O. Loseva, B. Oppert, P. Kosaraju, and L. A. Bulla. 2003. Insect resistance to Bacillus thuringeniesis—alterations in the Indianmeal moth larval gut proteome. Mol. Cell. Proteomics 2:19-28. [DOI] [PubMed] [Google Scholar]
- 2.Devonshire, A. L., and G. D. Moores. 1982. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate, and pyrethroid resistance in peach-potato aphids (Myzus persicae). Pestic. Biochem. Physiol. 18:235. [Google Scholar]
- 3.Finney, D. J. 1971. Probit analysis, 3rd ed. Cambridge University Press, London, United Kingdom.
- 4.Gunning, R., H. Dang, and I. Christiansen. 2001. Play your part in resistance testing. Aust. Cotton Grower 22(6):18. [Google Scholar]
- 5.Gunning, R. V., and G. D. Moores. 2002. Chlorfenapyr resistance in Helicoverpa armigera in Australia, p. 793-798. In Pests and diseases 2002. Proceedings of the British Crop Protection Council Conference. British Crop Protection Council, United Kingdom.
- 6.Gunning, R. V., G. D. Moores, and A. L. Devonshire. 1996. Esterases and fenvalerate resistance in Australian Helicoverpa armigera. Pestic. Biochem. Physiol. 54:12-23. [DOI] [PubMed] [Google Scholar]
- 7.Gunning, R. V., G. D. Moores, and A. L. Devonshire. 1999. Esterase inhibitors synergise the toxicity of pyrethroids in Australian Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 63:50-62. [DOI] [PubMed] [Google Scholar]
- 8.Jurat-Fuentes, J. I., F. L. Gould, and M. J. Adang. 2002. Altered glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. Appl. Environ. Microbiol. 68:5711-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon, R., J. Finnegen, K. Olsen, and L. Laurence. 2002. Environmental stress and the efficacy of BT cotton. Aust. Cotton Grower 23(2):18-21. [Google Scholar]
- 10.Roush, R. T., and B. E. Tabashnik (ed.). 1990. Pesticide resistance in arthropods. Chapman and Hall, New York, N.Y.
- 11.Shen, J., W. Zhou, Y. Wu, X. Lin, and X. Zhu. 1998. Early resistance of Helicoverpa armigera (Hubner) to Bacillus thuringiensis and its relation to the effect of transgenic cotton lines expressing Bt toxin on the insect. Acta Entomol. Sin. 41:8-14. [Google Scholar]
- 12.Zhuang, M., and S. S. Gill. 2003. Mode of action of Bacillus thuringiensis toxins, p. 213-234. In G. Voss and G. Ramos (ed.), Chemistry of crop protection. Wiley VCH, Weinheim, Germany.