Abstract
The host immune response to Helicobacter pylori infection might be of importance with regard to the outcome of infection by this organism, e.g., to explain why only a proportion of infected subjects develop peptic ulcers. In this study we have analyzed the local response of different cytokines—i.e., the proinflammatory interleukin-1β, (IL-1β), IL-6, tumor necrosis factor alpha, and IL-8; the immunoregulatory gamma interferon (IFN-γ); and IL-4; and the anti-inflammatory transforming growth factor beta (TGF-β)—in antral biopsy specimens from H. pylori-infected duodenal ulcer (DU) patients and asymptomatic (AS) carriers (i.e., with chronic gastritis only). For comparison, biopsy specimens from uninfected healthy individuals were also analyzed. An immunohistochemical technique was used to allow quantification of the cytokine responses as well as identification of the cell types associated with the cytokine expression. We found that the levels of all of the studied cytokines except IL-4 were increased in the H. pylori-infected subjects compared to the levels in the healthy individuals. Our results indicate that the antral cytokine response is of the Th1 type since IFN-γ, but not IL-4, was up-regulated both in H. pylori-infected DU patients and in AS carriers. However, there were no significant differences in either proinflammatory or immunoregulatory cytokine levels when H. pylori-infected subjects with and without peptic ulcers were compared. Some of the cytokines, particularly IL-1β and TGF-β, were also found in the gastric mucosae of healthy, uninfected subjects. We also showed that the gastric epithelium contributes substantially to the antral cytokine response of the proinflammatory cytokines IL-1β and IL-6 in addition to IL-8.
Helicobacter pylori causes chronic antral gastritis and peptic ulcers and is associated with gastric adenocarcinoma and primary gastric lymphoma (30, 32, 34). H. pylori infection almost invariably causes chronic gastritis, but only a proportion of the infected subjects develop peptic ulcers. In addition to the possibility that some H. pylori strains might be more ulcerogenic than others, the nature of the host immune response might explain these different outcomes of infection by this organism. The local inflammation in H. pylori infection is characterized by infiltration of neutrophils and specific lymphocytes into the gastric mucosa as well as by increased production of several cytokines (10, 12, 16, 22, 43). Sometimes the infiltrating B and T cells form lymphoid follicles (15).
The immunoregulatory and proinflammatory cytokines induced by H. pylori may influence the nature of the local T-cell response. It is thought that helper T (Th) cells can be divided into two subsets, Th1 and Th2. The Th1 subset promotes cell-mediated immunity by producing mainly interleukin-2 (IL-2) and gamma interferon (IFN-γ), and the Th2 subset, which is important for antibody responses and also for down-regulation of chronic inflammatory reactions, produces IL-4, IL-5, IL-6, and IL-13 (37). The local Th cell response in H. pylori infection is generally held to be of the Th1 type since the levels of IFN-γ, but not IL-4 and IL-5, have been shown to be increased in H. pylori-induced gastritis (20, 24). Furthermore, gastric H. pylori-specific T-cell clones isolated from peptic ulcer patients are more often of the Th1 type than clones isolated from subjects with chronic gastritis only (12).
The gastric mucosal levels of the proinflammatory cytokines IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) have been reported to be increased in H. pylori-infected subjects (26, 29, 43). In particular, the mucosal production of the neutrophil chemotactic and activating factor IL-8 has been suggested to play an important role in H. pylori-associated diseases (3, 8–10). H. pylori strains carrying the pathogenicity island, including the gene encoding the cytotoxin-associated protein (CagA), are more commonly isolated from duodenal ulcer (DU) patients than from infected subjects with chronic gastritis only, and infection with such strains has been found to be correlated with increased IL-8 production both in vivo and in vitro (10, 33). In addition, TNF-α and IL-1β can cause epithelial cell damage and induce epithelial IL-8 expression and therefore might also be of importance for the H. pylori-induced pathology (14). However, the inflammatory effects induced by the proinflammatory cytokines might be counteracted not only by IL-4 but also by locally produced transforming growth factor beta (TGF-β), which has been shown to have anti-inflammatory effects, e.g., inhibition of T cells, endothelial transmigration of neutrophils, and IL-8 production (11, 31, 39). The TGF-β response to H. pylori infection has not been extensively studied, however.
Most previous studies of H. pylori-induced cytokines have focused on detection of cytokine mRNA or quantification of protein in supernatants from in vitro cultures of gastric biopsy specimens, isolated gastric lymphocytes, or gastric epithelial cell lines (5, 19, 26, 29, 43). However, these approaches do not allow determination of either the localization or the nature of the cytokine-producing cells. In particular, the possible contribution of the antral epithelial cells to the cytokine response in H. pylori infection has hitherto not been extensively studied, except for that of IL-8 (8). In this study, we have therefore used immunohistochemical methods allowing identification as well as enumeration of the gastric cells associated with the expression of some of the cytokines which may be of importance for pathological mechanisms of H. pylori infection, i.e., IL-1β, IL-4, IL-6, IL-8, IFN-γ, TNF-α, and TGF-β. This has included a comparison of the expression of these cytokines in antral biopsy specimens from H. pylori-infected DU patients and subjects with chronic gastritis only (i.e., asymptomatic [AS] carriers), as well as from healthy, uninfected subjects, to evaluate whether there is any association between increased levels of one or more cytokines and peptic ulcer disease.
MATERIALS AND METHODS
Subjects and specimen collection.
The study was approved by the Human Research Ethics Committee of the Medical Faculty, Göteborg University, Göteborg, Sweden, and comprised 30 subjects. Informed consent was obtained from all participants. Ten H. pylori-infected DU patients (mean age, 49.5 years; range, 25 to 75 years; 6 men and 4 women) and 10 H. pylori-infected subjects with chronic gastritis only (AS carriers) (mean age, 51.5 years; range, 39 to 59 years; 9 men and 1 woman) were recruited for the study. The AS carriers were recruited among Swedish blood donors by serological screening for H. pylori-specific antibodies as described below. For comparison, 10 healthy, uninfected (H. pylori-negative) Swedish volunteers (mean age, 45.5 years; range 24 to 60 years; 4 men and 6 women) were included in the study. The AS carriers as well as the uninfected healthy subjects were thoroughly interviewed with regard to gastrointestinal symptoms (e.g., dyspepsia), and none of them had any history of gastrointestinal or any other relevant disease. The DU patients had chronic relapsing duodenal disease, confirmed by endoscopy, but none of the subjects had active ulcers at the time of the study. Five of the DU patients were on antisecretory therapy (i.e., omeprazol), which was terminated 1 to 2 weeks before the onset of the study. The remaining DU patients had not taken any antisecretory drug or antibiotics for at least 3 months. All participants were specifically asked to refrain from taking nonsteroidal anti-inflammatory drugs for at least 1 week prior to the endoscopy.
Six antral biopsy specimens were collected from each subject during endoscopy. Four of the six biopsy specimens were embedded in O.C.T. compound (Tissue-Tek; Miles Inc., Elkhart, Ind.) and immediately snap frozen in liquid nitrogen and processed for immunohistochemistry as described below. One biopsy specimen was fixed in formalin, embedded in paraffin, stained with hematoxylin, and examined by an experienced histopathologist who was unaware of the subject’s clinical symptoms. Gastritis and the presence of H. pylori-like organisms were graded from 0 to 3 (for none, mild, moderate, and severe, respectively) in accordance with the Sydney system (35). The sixth biopsy specimen was used for culturing of H. pylori.
Diagnosis of H. pylori infection.
One biopsy specimen was homogenized in phosphate-buffered saline (PBS) and then inoculated on Skirrow blood agar plates containing 10% horse blood. After 3 days of incubation under microaerobic conditions (10% CO2, 5% O2, and 85% N2), the plates were examined for H. pylori-like colonies. A rapid urease test and a dot blot assay using the H. pylori-specific monoclonal antibody (MAb) HP30-1:1.6 (6) were used to identify H. pylori strains. Only subjects with positive cultures were considered to be H. pylori infected. Sera collected from all subjects were screened for H. pylori-specific antibodies (17), and these analyses showed that all culture-positive subjects also had positive serological results. The healthy control subjects were all H. pylori negative in both culture and serology.
PCR amplification of cagA.
The bacterial strains were analyzed for the presence of cagA by PCR essentially as previously described (7). Briefly, DNA was isolated by the use of a Puregene kit (Gentra Systems, Minneapolis, Minn.), and cagA was demonstrated by using the specific primers R008 (5′-TTAGAATAATCAACAAACATCACGCCAT-3′) and D008 (5′-ATAATGCTAAATTAGACAACTTGAGCGA-3′). The PCR master mix contained 0.3 μM each primer, 200 μM each deoxynucleoside triphosphate, 2.5 mM MgCl2, and 1.25 U of Taq-2000 DNA polymerase (Stratagene, La Jolla, Calif.) in Taq PCR (Stratagene) buffer. The samples were amplified in a thermocycler PTC-200 DNA engine (MJ Research Inc., Watertown, Mass.). Strain CCUG 17874 was used as a positive control. The PCR products were visualized by ethidium bromide staining of 2% agarose gels.
Cytokine-specific MAbs.
The cytokine-specific MAbs used, all mouse anti-human antibodies, were anti-IL-1β (2-D-8; a kind gift from H. Towbin, Ciba-Geigy, Basel, Switzerland), anti-IL-4 (8F12; ImmunoKontact, Bioggio, Switzerland), IL-6 (Genzyme Diagnostics, Cambridge, Mass.), anti-IL-8 (NAP I; Skafte-Claesson, Mölndal, Sweden), anti-TNF-α (MAb 1; PharMingen, San Diego, Calif.), anti-IFN-γ (1-D1K; MABTECH AB, Nacka, Sweden), and anti-TGF-β (Genzyme). All MAbs were of the immunoglobulin G1 (IgG1) isotype. The optimal MAb concentrations were determined in preliminary experiments using lipopolysaccharide or staphylococcal enterotoxin B-stimulated peripheral blood mononuclear cells (MNCs) in addition to gastric tissue sections. The MAbs were used at 2 to 5 μg of Ig/ml except for the MAbs from Genzyme, which were used at a concentration of 40 μg of Ig/ml. An irrelevant, isotype-matched MAb (Dako, Glostrup, Denmark) was used as a control for nonspecific staining in each experiment. The specificities of the MAbs were ascertained by preabsorption with recombinant cytokines.
Immunohistochemistry.
Immunohistochemical staining of cryopreserved antral biopsy specimens was performed as previously described (2). Briefly, 8-μm-thick sections mounted on glass slides (SuperFrost/Plus; Menzel-Gläser, Braunschweig, Germany) were fixed with 4% paraformaldehyde, washed with PBS, and permeabilized with 0.1% saponin (Sigma, St. Louis, Mo.) in PBS. Endogenous peroxidase activity was blocked with PBS–0.1% saponin containing 1% H2O2 and 0.02% NaN3, and endogenous biotin was blocked by using an avidin-biotin blocking kit (Vector Laboratories, Burlingame, Calif.) according to the manufacturer’s instructions. The tissue sections were then incubated with the cytokine-specific MAbs at 4°C overnight, washed with PBS–0.1% saponin, and blocked with 1% normal goat serum before incubation with biotinylated goat anti-mouse IgG1 (1:300; Caltag Laboratories, South San Francisco, Calif.) at room temperature for 30 min. The sections were washed and treated with avidin-biotin-horseradish peroxidase complex (Vectastain ABC-HP kit; Vector Laboratories) for 30 min, washed twice with PBS–0.1% Saponin, and then overlaid with the substrate chromogen 3,3-diaminobenzidine (DAB; Vector Laboratories). The sections were then washed with distilled water, counterstained with Mayer’s hematoxylin, dehydrated, and mounted with Mountex (Histolab, Göteborg, Sweden).
Determination of cytokine-specific immunostaining.
Entire tissue sections were examined. The tissue area was determined by applying an eyepiece grid, consisting of 100 squares covering 1.21 mm2 of tissue, on a Zeiss microscope at 100× magnification and counting the number of squares covering the tissue section. The numbers of cytokine-stained cells were counted under 400× magnification, and the numbers of positively stained MNCs and polymorphonuclear (PMN) cells per square millimeter of tissue were calculated. Only cells with a distinct intracellular (cytoplasmic) staining were counted. The proportion of superficial epithelial cells stained was determined by dividing the stained surface epithelial area by the total surface epithelial area in the same tissue section. The proportion of stained gastric neck and pit epithelial cells was determined by dividing the number of cross-sectioned gastric necks and pits with immunoreactivity for the cytokine by the total number of cross-sectioned gastric necks and pits in the section.
In initial studies, the possibility of using a single tissue section to represent an entire biopsy sample was evaluated by cryosectioning the entire biopsy specimen and then immunostaining the sections from different parts of the specimen. Since the numbers of cytokine-producing cells as well as the proportions of epithelial cytokine staining were very similar in all sections obtained from the same biopsy specimen, one section per biopsy sample was analyzed for each cytokine in subsequent studies.
Furthermore, to test the representativity of each biopsy specimen with regard to cytokine expression in the antrum, cryosections of all four biopsy samples obtained from the same subject were prepared (n = 6; 2 DU, 2 AS, and 2 H. pylori negative) and then immunohistochemically stained. The numbers of cytokine-producing cells as well as the proportions of positively stained gastric epithelium were very similar in all four biopsy specimens from the six subjects studied for all cytokines except IL-8 and TGF-β, which differed substantially between the samples. Therefore, sections from all four biopsy specimens were examined for the presence of IL-8- or TGF-β-expressing cells in all subsequent experiments.
Statistical evaluation.
The Mann-Whitney test was used for statistical evaluation of comparisons between groups. P values of ≤0.05 were considered to indicate statistically significant differences. Spearman’s rank correlation coefficients were calculated to evaluate correlations.
RESULTS
Histopathological evaluation.
One antral biopsy specimen from each subject was used for a histopathological evaluation. All biopsy samples obtained from the 20 H. pylori-infected subjects showed moderate active chronic gastritis (score of 2) except for one specimen from a DU patient and one from an AS carrier who had severe gastritis (scores of 3) and two (one from a DU patient and one from an AS carrier) who were scored as having mild gastritis (scores of 1). In three of the H. pylori-infected subjects (2 DU patients and 1 AS carrier), Helicobacter-like organisms could not be detected histopathologically despite the positive cultures and serologies of these subjects. The majority of the remaining subjects had numerous Helicobacter-like organisms (score of 3) in the antral sections. All uninfected individuals had normal antral mucosae without Helicobacter-like organisms. No differences in scores for inflammation or Helicobacter-like organisms were seen in the five DU patients who had been on antisecretory therapy recently and the untreated DU patients.
Frequencies of subjects with immunostaining for different cytokines.
Immunohistochemical staining for IL-β, IL-4, IL-6, IL-8, TNF-α, IFN-γ, and TGF-β was performed on cryosections of antral biopsy samples obtained from H. pylori-infected DU patients and AS carriers and from healthy controls. The intracellular cytokine staining was predominantly localized to the cytoplasm of the cells. In some instances, the staining showed a juxtanuclear position, suggesting an accumulation of the cytokines in the Golgi complex and indicating that the stained cells were actually producing the cytokine.
The cytokines under study could be detected in biopsy specimens from most of the infected DU patients and AS carriers (Table 1). Concomitant expression of at least six of the seven cytokines was seen in 9 of the 10 DU patients and in all of the AS carriers, compared to only 1 of the 10 H. pylori-negative subjects. However, all of the uninfected individuals showed positive staining for at least one of the cytokines studied; in particular, IL-1β, IL-4, and TGF-β were frequently observed in biopsy samples from the healthy subjects (Table 1).
TABLE 1.
Frequencies of positively stained biopsy specimens from H. pylori-infected DU patients, AS H. pylori carriers, and uninfected subjects
| Cytokine | Frequency of cytokine positivity amonga:
|
||
|---|---|---|---|
| H. pylori-infected DU patients | H. pylori-infected AS carriers | H. pylori-negative individuals | |
| IL-1β | 7/10 | 10/10 | 8/10 |
| IL-4 | 9/10 | 9/10 | 8/10 |
| IL-6 | 9/10 | 10/10 | 5/10 |
| IL-8 | 9/10 | 10/10 | 4/10 |
| IFN-γ | 9/10 | 9/10 | 1/10 |
| TNF-α | 8/10 | 10/10 | 1/10 |
| TGF-β | 10/10 | 10/10 | 10/10 |
Number of biopsy samples with positively stained epithelium and/or MNCs per total number studied.
Comparison of cytokine-specific staining in different antral biopsy specimens obtained from the same subject.
In initial studies, immunohistochemical stainings for IL-1β, IL-4, IL-6, IL-8, TNF-α, IFN-γ, and TGF-β was performed with sections made from all four antral biopsy samples obtained from the same subject (n = 6; 2 DU, 2 AS, and 2 H. pylori negative) in order to determine how well the staining of a single biopsy specimen represented the cytokine response of the antral mucosa. These analyses showed that all of the cytokines except IL-8 and TGF-β were evenly distributed in the different biopsy samples from the same individual. In contrast, the number of IL-8-producing MNCs and PMN cells varied considerably; there was a mean variation of 10 cells/mm2 of tissue (range, 0 to 29 cells/mm2) when sections of the four different biopsy samples obtained from the same subject were compared. The epithelial IL-8 staining of the different biopsy specimens varied from no staining at all to up to 100% IL-8-positive epithelium (mean variation, 20%). In those subjects in whom no mucosal IL-8 could be detected, i.e., in the majority of the uninfected subjects, the sections from the four biopsy samples were all negative for IL-8. An even larger degree of variability between the antral biopsy specimens was seen when the number of TGF-β-specific cells was analyzed (mean variation, 19 cells/mm2 of tissue; range, 2.8 to 57 cells/mm2). Also, the epithelial staining for TGF-β differed considerably. The mean variation between biopsy specimens was 30% (range, 0 to 100%) when the four different biopsy samples obtained from the same subject were compared.
Cytokine staining of lamina propria and intraepithelial cells in antral mucosa.
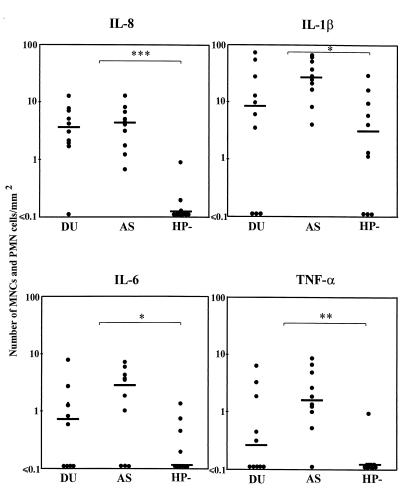
When the cytokine-specific lamina propria cells and intraepithelial lymphocytes (IELs) were enumerated, it was found that the numbers of cells staining for the proinflammatory cytokines—i.e., IL1-β, IL-6, IL-8, and TNF-α—were significantly larger in the H. pylori-infected subjects than in the uninfected volunteers (Fig. 1). In contrast, the frequencies of positive cells in the H. pylori-infected DU patients and AS carriers did not differ significantly. Neither did the cytokine responses in the five DU patients who had recently been on antisecretory therapy differ from the responses observed in the untreated DU patients or the AS carriers (with gastritis only). However, there was a tendency for a higher frequency of IL-1β-producing cells in the AS carriers (P = 0.058). The largest numbers of positively stained MNCs and PMN cells located in the lamina propria and intraepithelially were observed when using MAbs specific for IL-1β and TGF-β (Fig. 1 and 2, respectively). As shown in Fig. 3C and D, MNCs staining specifically for IL-1β were found to be evenly distributed throughout the entire lamina propria, whereas the TGF-β-specific cells often were located in clusters in the lamina propria surrounding the neck region of the antral glands. IL-8- or TNF-α-specific cells were almost always found only in the biopsy samples from infected subjects (Fig. 1). The IL-8-positive cells were located in clusters around the neck of the antral glands, whereas the less frequently encountered TNF-α-producing cells were scattered throughout the lamina propria. IL-6-positive MNCs were located both in the lamina propria and intraepithelially. PMN cells in the lamina propria occasionally also showed immunoreactivity with the anti-IL-6 MAb. In some antral sections, the vascular endothelial cells were positively stained, particularly for TGF-β and IL-1β and, in some instances, also for IL-6 and IL-8.
FIG. 1.
Comparison of the proinflammatory cytokine staining of antral biopsy specimens from H. pylori-infected DU patients and AS carriers (i.e., with gastritis only) and uninfected subjects (HP−). Numbers of cytokine-specific stained intraepithelial and lamina propria MNCs and PMN cells/mm2 of tissue are given. Bars represent median values. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (Mann-Whitney test, DU and AS versus HP−).
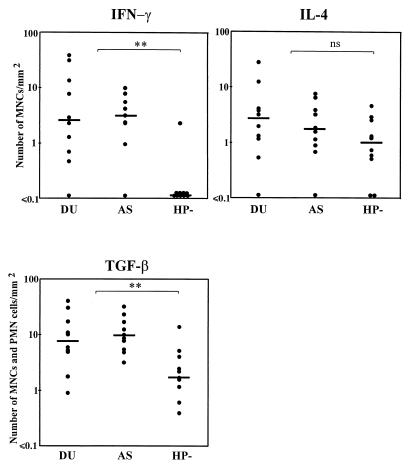
FIG. 2.
Comparison of numbers of leukocytes specifically stained for IFN-γ, IL-4, or TGF-β in antral biopsy samples from H. pylori-infected DU patients and AS carriers (i.e., with gastritis only) and uninfected subjects (HP−). Numbers of cytokine-specific stained intraepithelial and lamina propria MNCs/mm2 of tissue are given (for TGF-β, values are MNCs plus PMN cells/mm2 of tissue). Bars represent median values. ∗∗, P < 0.01 (Mann-Whitney test, DU and AS versus HP−).
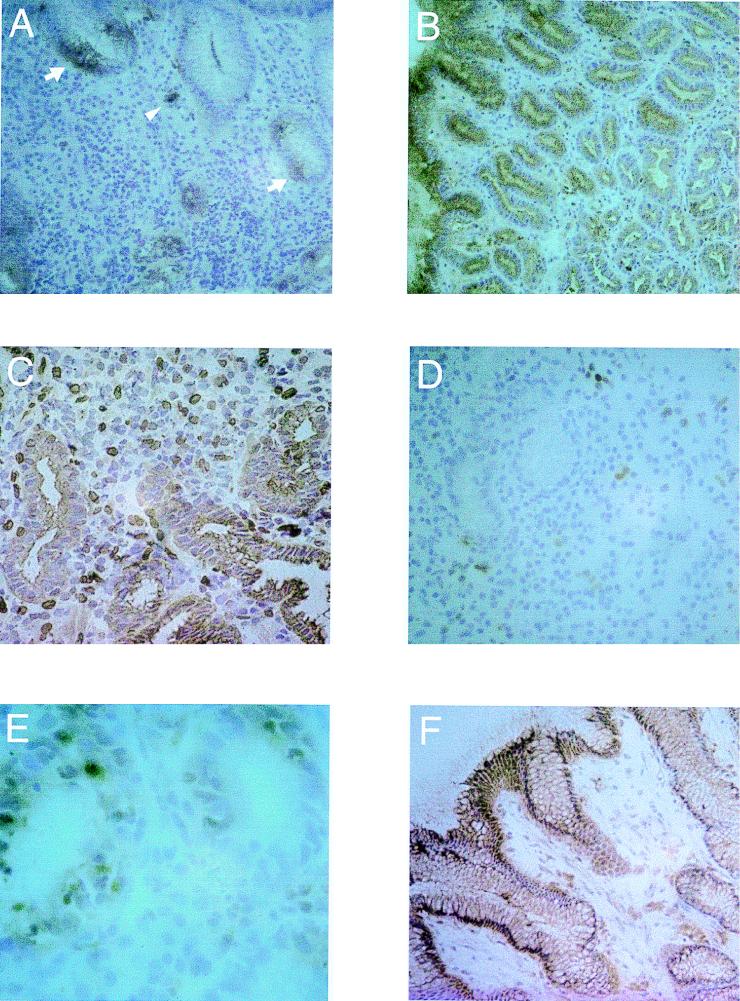
FIG. 3.
Microphotographs showing cytokine-specific immunoperoxidase staining of cells in cryopreserved antral tissue obtained from H. pylori-infected subjects and uninfected volunteers. The sections were counterstained with hematoxylin. (A) IL-8-specific staining in the antrum of an H. pylori-infected AS carrier (i.e. with gastritis only), showing the typical patchy distribution of epithelial IL-8 reactivity (arrows) and cytoplasmic localization of IL-8 in a monocyte (arrowhead). Original magnification, ×125. (B) Epithelial staining for IL-6 localized to the superficial as well as to the neck and pit epithelium in the antrum of an AS carrier. Original magnification, ×100. (C) Biopsy specimen from an AS carrier showing IL-1β staining of the epithelium as well as of MNCs in the lamina propria. Original magnification, ×200. (D) IL-1β-specific staining of MNCs in the antrum of an uninfected individual. Original magnification, ×160. (E) Intraepithelial IFN-γ-producing lymphocytes in the neck region of a cross-sectioned antral gland in a biopsy sample obtained from an H. pylori-infected DU patient. Original magnification, ×400. (F) Epithelial staining for TGF-β in a biopsy specimen from an uninfected subject. Original magnification, ×125.
As for the proinflammatory cytokines, the frequencies of TGF-β-specific cells were also higher in the H. pylori-infected subjects than in the uninfected subjects, and again no differences were seen when comparing DU patients and AS carriers (Fig. 2). The numbers of lamina propria lymphocytes and IELs producing IFN-γ (Fig. 3E) were significantly higher in the H. pylori-infected subjects than in the controls; however, no significant difference between DU patients and AS carriers was observed (Fig. 2). In contrast, similar frequencies of IL-4-containing cells could be detected in biopsy specimens of both H. pylori-infected DU patients and AS carriers as well as uninfected subjects.
The lymphoid follicles seen in many of the biopsy samples from the H. pylori-infected subjects usually did not show immunoreactivity for the studied cytokines, with the exception of IL-4, which often was detected extracellularly between the cells of the follicle. In some instances, cells with cytoplasmic staining for IL-4 could also be detected. In a few biopsy samples, IL-6- or TGF-β-specific cells as well as some positive extracellular staining for these cytokines could be seen in the lymphoid follicles.
Cytokine staining of gastric epithelial cells.
In addition to the cytokine-specific staining of lymphocytes and PMN cells, a substantial proportion of the cytokine staining was localized to the gastric epithelial cells (Table 2). Positive epithelial staining was especially seen for IL-6, IL-1β, and TGF-β, localized to the superficial epithelial cells as well as to the epithelial cells of the neck and pit region of the antral glands (Fig. 3B, C, and F, respectively).
TABLE 2.
Cytokine staining of epithelial cells in the antrum
| Cytokine | Proportion of positively stained epithelial cells (%) amonga:
|
DU and AS vs H. pylori negativeb
|
||||||
|---|---|---|---|---|---|---|---|---|
|
H. pylori-positive DU patients
|
H. pylori-positive AS carriers
|
H. pylori-negative individuals
|
||||||
| Superficial epithelium | Neck and pit epithelium | Superficial epithelium | Neck and pit epithelium | Superficial epithelium | Neck and pit epithelium | Superficial epithelium | Neck and pit epithelium | |
| IL-1β | 0 (0–64) | 9 (0.2–22) | 25 (0.1–74) | 29 (9–83) | 2.9 (0–9.5) | 0 (0–0) | NS | ** |
| IL-4 | 42 (10–100) | 38 (21–52) | 88 (50–94) | 24 (19–42) | 40 (17–87) | 9.6 (2–44) | NS | NS |
| IL-6 | 8.3 (0–62) | 14 (3–15) | 65 (6–98) | 18 (8–36) | 0 (0–13) | 0 (0–4) | NS | * |
| IL-8 | 1.4 (0–4.1) | 3.5 (0.4–12) | 8.4 (0–12) | 4.9 (0.3–6.3) | 0 (0–0) | 0 (0–0) | NS | ** |
| IFN-γ | 0 (0–21) | 5.4 (0–28) | 0 (0–15) | 5.5 (1–9.4) | 0 (0–0) | 0 (0–0) | NS | * |
| TNF-α | 0 (0–1.9) | 5.8 (0–8.7) | 0 (0–1.9) | 2.4 (0.4–12) | 0 (0–0) | 0 (0–0) | NS | ** |
| TGF-β | 17 (1.2–25) | 7.6 (2.7–19) | 10 (1.2–21) | 6.1 (3.5–10) | 14 (5.3–22) | 5.1 (1.1–17) | NS | NS |
Given as a percentage of antral superficial surface and neck-and-pit epithelial cells with positive cytokine staining. Data given are medians; interquartile ranges are shown in parentheses.
H. pylori positive (DU and AS) versus H. pylori negative, determined by Mann-Whitney test. *, P < 0.05; **, P < 0.01; NS, not significant.
Quantification of specific epithelial staining revealed that the proinflammatory cytokines—i.e., IL-1β, IL-6, IL-8, and TNF-α—were all significantly more abundant in the H. pylori-infected DU patients and AS carriers than in the uninfected subjects (Table 2). Epithelial staining for IL-6 and IL-8 was seen in specimens from most of the infected subjects. IL-8 could not be detected in any of the healthy controls, whereas positive epithelial staining for IL-6 was seen in four of the controls. However, the staining intensity for IL-6 in these subjects was much weaker than the immunoreaction seen in the H. pylori-infected subjects. Furthermore, the epithelial staining for IL-8 showed a typical patchy distribution (Fig. 3A). TNF-α was nearly always found only in the pit epithelium of the antral gland and was never observed in any of the biopsy specimens obtained from uninfected subjects.
The proportions of epithelium staining for the anti-inflammatory TGF-β in H. pylori-infected and uninfected subjects did not differ.
Surprisingly, IFN-γ and IL-4 could also be detected in the gastric epithelial cells in a majority of the biopsy samples from H. pylori-infected subjects. IFN-γ was mostly seen in the epithelial cells of the pits, whereas IL-4 was located in the superficial epithelial cells as well. All of the biopsy specimens with positive epithelial IFN-γ or IL-4 staining also had MNCs stained positively for the respective cytokines located in the lamina propria and/or intraepithelially. There were weak positive correlations between the number of IFN-γ-producing IELs and the proportion of positively stained gastric epithelium (rs = 0.50, P < 0.05) and also between lymphocytes stained for IL-4 and epithelial IL-4 staining (rs = 0.53, P < 0.02), suggesting that the epithelial staining may represent receptor-bound IFN-γ and IL-4, respectively.
The cytokine-specific staining of epithelial cells showed a large variability between subjects for all of the studied cytokines, particularly the staining of the superficial epithelium for IL-1β and IL-6, but the mean proportion of the epithelial cytokine-specific staining did not differ significantly between DU patients and AS carriers for any of the cytokines studied. However, the proportion of IL-1β staining of gastric epithelial cells was somewhat higher in the AS carriers than in the DU patients.
cagA status.
Fifteen of the bacterial strains isolated from the antral biopsy samples were analyzed for the presence of the cagA gene. Five of seven strains isolated from the DU patients and six of eight strains isolated from the AS carriers were cagA positive.
DISCUSSION
We have characterized the local IL-4, IFN-γ, IL-1β, IL-6, IL-8, TNF-α, and TGF-β responses in antral biopsy specimens obtained from H. pylori-infected subjects with and without peptic ulcers and from uninfected healthy subjects by an immunohistochemical technique. Earlier studies have reported increased levels of IL-1β, IL-2, IL6, IL-7, IL-8, IL-10, TNF-α, IFN-γ, and TGF-β (20, 23, 29, 33, 43) in H. pylori-infected subjects, but the local distribution of the cytokine-associated cells has hitherto only been described for IL-8 (3, 8).
All of the presently studied cytokines except IL-4 were found to be increased in H. pylori-infected subjects compared to their levels in the uninfected subjects. In addition, our study suggests that the gastric epithelial cells contribute substantially to the proinflammatory cytokine response to H. pylori infection, either by active cytokine production or by uptake of cytokines produced by the lamina propria or intraepithelial leukocytes. Our finding of an up-regulation of IFN-γ, but not of IL-4, in H. pylori-infected subjects compared to the uninfected subjects is in agreement with previous studies suggesting a predominant Th1 response in H. pylori-infected mucosa (4, 12, 20). However, it is not known if this Th1 cell-mediated immune response is protective or if it contributes to the pathogenesis of H. pylori-associated diseases, e.g., gastritis and peptic ulcers. Data from recent murine studies suggest that the Th1 response is associated with gastric pathology but not with protection against Helicobacter infections. Thus, in vivo neutralization of IFN-γ reduced gastritis in H. felis-infected mice, and adoptive transfer of specific spleen cells from infected IL-4 gene-targeted mice exacerbated gastric inflammation in infected recipients (24, 25). Furthermore, it has been shown that H. pylori-specific Th cell clones isolated from the gastric mucosae of peptic ulcer patients are of the Th1 type more frequently than are clones isolated from subjects with gastritis only, as reported by D’Elios et al. (12). However, in our study, no differences in IFN-γ levels were seen when biopsy specimens from H. pylori-infected subjects with and without DU were compared, but we studied the total IFN-γ response, from both CD8+ and CD4+ T cells, whereas the differences in IFN-γ responses found by D’Elios et al. (12) concerned CD4+ cells only.
The increased levels of IFN-γ might contribute to gastric inflammation not only by activating mononuclear phagocytes and neutrophils but also by up-regulating the expression of major histocompatibility complex type II molecules on epithelial cells, which has been observed in H. pylori-infected subjects (13). Furthermore, the epithelial barrier function is decreased by IFN-γ (1). In the present study, we found IFN-γ-specific staining not only of IELs but also of the gastric epithelium. The epithelial IFN-γ staining probably reflects receptor-bound IFN-γ, since this cytokine is produced only by T cells and NK cells. Furthermore, it was found only in biopsy samples with positive MNCs, suggesting a local entrapment of the cytokine in H. pylori-infected mucosa. Also, our recent studies have shown high-level production of IFN-γ by cells, primarily CD8+, isolated from H. pylori-infected subjects after in vitro stimulation with H. pylori antigens, suggesting that IFN-γ may, at least in part, be produced by gastric CD8+ cells (36). Additionally, it has been shown that IELs in the gastric epithelium are mainly of the CD8+ phenotype (18).
We could also detect increased levels of the proinflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α, as previously reported, in the H. pylori-infected subjects (29, 33, 43). However, it is still unclear whether the proinflammatory responses in H. pylori-infected subjects with DU are different from those in infected individuals without DU. Noach et al. (29) found similar levels of IL-1β, IL-8, and TNF-α when analyzing supernatants of biopsy specimens from H. pylori-infected patients with and without DU, whereas Peek et al. (33) found expression of IL-1α and IL-8, as analyzed by PCR, significantly more often in DU patients than in patients with gastritis only. These conflicting results are probably due to methodological limitations; i.e., in vitro culture of antral biopsies induces an IL-8 response in both infected and uninfected biopsy specimens (21a) and does not reflect the actual mucosal cytokine response in vivo. In addition, PCR techniques are not the optimal methods for quantitative analyses. Furthermore, single biopsies might not be representative of the overall mucosal cytokine response, as shown for IL-8 and TGF-β in this study; these cytokines varied considerably among the biopsy samples taken from different parts of the antral mucosa. However, the immunohistochemical method used in our study allows enumeration and identification of the cytokine-specific stained cells in the antral mucosa. Using this approach, no differences were seen when the numbers of MNCs and PMN cells staining specifically for IL-1β, IL-6, IL-8, or TNF-α in biopsy specimens from H. pylori-infected patients with and without DU were compared. Similarly, the epithelial levels of IL-1β, IL-6, IL-8, and TNF-α did not differ between DU patients and subjects with gastritis only but both cases were significantly higher than in the uninfected subjects. Indeed, positive epithelial staining for IL-8 or TNF-α was seen only in biopsy samples from infected subjects and never in those from any of the healthy subjects. This finding is in contrast to that of Crabtree et al. (8), who could detect IL-8 in the surface epithelium of normal gastric mucosa. This difference might be explained by the use of different MAbs and/or tissue fixation techniques. The positive epithelial staining for IL-1β, IL-6, and TNF-α might represent the uptake of locally produced cytokines rather than cytokine production by the epithelial cells themselves, but this must be further investigated by techniques other than immunohistochemistry (e.g., in situ hybridization). Nevertheless, the presence of IL-1β, IL-6, and TNF-α in the gastric epithelial cells, as demonstrated in the present study, suggests that these cytokines might have effects on the gastric epithelium. The presence of IL-6 in normal gastric mucosa has previously been reported (38). Our finding of IL-1β and IL-6 in the uninfected gastric epithelium indicates that these cytokines might also have effects on normal epithelial cell functions.
The potential role of the proinflammatory cytokines in DU disease is still unclear, and data from animal studies are contradictory. Several investigations have shown that IL-1β can inhibit gastric acid secretion both in vivo and in vitro (27, 41), suggesting that a gastric IL-1β response might protect against DU disease. On the other hand, IL-1β and TNF-α have been shown to stimulate gastrin secretion by rabbit antral gastrin-producing G cells in vitro (42), suggesting a role for these cytokines in H. pylori-induced hypergastrinemia (21), which is linked to increased gastric acid secretion. However, our data suggest a protective role of IL-1β, since both the number of MNCs and PMN cells and the proportion of epithelium being stained for IL-1β were higher, although not significantly, in the H. pylori-infected subjects with gastritis only than in the DU patients.
TGF-β may have a local effect, e.g., counteracting the effects of the proinflammatory cytokines or the Th1 type of cellular immune response (31, 39, 40). However, we did not find any difference in TGF-β responses in biopsy specimens from DU patients and those from infected subjects with gastritis only. We found a substantial epithelial TGF-β-specific staining also in normal antral mucosa, which is in agreement with the finding of this cytokine in normal fundic mucosa (28), suggesting a role for this cytokine in maintaining mucosal homeostasis under physiological conditions.
In summary, we have shown that the local cytokine response to H. pylori infection exhibits the Th1 profile; i.e., the IFN-γ, but not the IL-4, levels are increased in H. pylori-infected subjects. However, no differences in the levels of IFN-γ were seen when infected DU patients and AS carriers (i.e., with gastritis only) were compared. Neither did the levels of proinflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α, which all were increased in H. pylori-infected subjects, differ between DU patients and AS carriers. The contribution of the gastric epithelium to the antral cytokine responses may be considerable, which may be of importance with regard to the pathogenic mechanisms of H. pylori-associated diseases.
ACKNOWLEDGMENTS
Ulf and Jan Andersson are gratefully acknowledged for their kind and invaluable help in establishing the immunohistochemical technique in our laboratory. We thank Ingela Ahlstedt for excellent technical assistance and the staff at the Gastroenterological Endoscopy Unit, Sahlgrenska University Hospital, for skillful assistance with the biopsy samplings.
This study was financially supported by a grant from Astra Research Center, Boston, Mass.
REFERENCES
- 1.Adams R B, Planchon S M, Roche J K. IFN-γ modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 2.Andersson J, Abrams J, Björk L, Funa K, Litton M, Ågren K, Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–1156. [PubMed] [Google Scholar]
- 4.Bamford K B, Fan X, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 5.Basso D, Scrigner M, Toma A, Navaglia F, Dimario F, Rugge M, Plebani M. Helicobacter pylori infection enhances mucosal interleukin 1β, interleukin 6, and the soluble receptor of interleukin 2. Int J Clin Lab Res. 1996;26:207–210. doi: 10.1007/BF02592984. [DOI] [PubMed] [Google Scholar]
- 6.Bölin I, Lönroth H, Svennerholm A-M. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J Clin Microbiol. 1995;33:381–384. doi: 10.1128/jcm.33.2.381-384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci A, Rappuoli R. PCR amplification of gene sequences from Helicobacter pylori strains. In: Lee A, Mégraud F, editors. Helicobacter pylori: techniques for clinical diagnosis and basic research. W. B. London, United Kingdom: Saunders; 1996. pp. 94–112. [Google Scholar]
- 8.Crabtree J E, Wyatt J I, Tredjosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J D. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crabtree J E, Xiang Z, Lindley I J, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree J E. Immune and inflammatory responses to Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1996;215:3–10. [PubMed] [Google Scholar]
- 11.Czarniecki C W, Chiu H H, Wong G H, McCabe S M, Palladino M A. Transforming growth factor beta 1 modulates the expression of class II histocompatability antigens on human cells. J Immunol. 1988;140:4217–4223. [PubMed] [Google Scholar]
- 12.D’Elios M M, Manghetti M, Almerigogna F, Amedei A, Costa F, Burroni D, Baldari C T, Romagagni S, Telford J L, Del Prete G. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 13.Engstrand L, Scheynius A, Påhlson C, Grimelius L, Schwan A, Gustavsson S. Association of Campylobacter pylori with induced expression of class II transplantation antigens on gastric epithelial cells. Infect Immun. 1989;57:827–832. doi: 10.1128/iai.57.3.827-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst, P. B., S. E. Crowe, and V. E. Reyes. 1997. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology 113(Suppl. 6):S35–S42. [DOI] [PubMed]
- 15.Genta R M, Gurer I E, Graham D Y. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577–583. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 16.Genta R M. The immunobiology of Helicobacter pylori gastritis. Semin Gastrointest Dis. 1997;8:2–11. [PubMed] [Google Scholar]
- 17.Hamlet A K, Erlandsson K I, Olbe L, Svennerholm A-M, Backman V E, Pettersson A B. A simple, rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1058–1063. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 18.Hatz R A, Meimarakis E, Bayerdörffer E, Stolte M, Kirchner T, Enders G. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scan J Gastroenterol. 1996;31:222–228. doi: 10.3109/00365529609004870. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karttunen R, Karttunen T, Ekre H E, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989;i:1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- 21a.Lindholm, C., et al. Unpublished data.
- 22.Mattsson A, Quiding-Järbrink M, Lönroth H, Hamlet A, Ahlstedt I, Svennerholm A-M. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705–2712. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messa C, DiLeo A, Greco B, Caradona L, Amati L, Linsalata M, Giorgio I, Jirillo E. Successful eradicating treatment of Helicobacter pylori in patients with chronic gastritis: gastric levels of cytokines, epidermal growth factor and polyamines before and after therapy. Immunopharmacol Immunotoxicol. 1996;18:1–13. doi: 10.3109/08923979609007106. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter pylori specific cell mediated immune responses display a predominant TH1 phenotype and promote a delayed type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 25.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1998;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 26.Moss S F, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, M. J., and R. H. Hunt. 1993. Cytokines in peptic ulcer disease. Eur. J. Gastroenterol. Hepatol. 5(Suppl. 3):S69–S73.
- 28.Naef M, Ishiwata T, Friess H, Buchler M W, Gold L I, Korc M. Differential localization of transforming growth factor-β isoforms in human gastric mucosa and overexpression in gastric carcinoma. Int J Cancer. 1997;71:131–137. doi: 10.1002/(sici)1097-0215(19970410)71:2<131::aid-ijc1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Noach L A, Bosma N B, Jansen J, Hoek F J, van Deventer S J, Tytgat G N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 30.Nomura A, Stemmermann G N, Chyou P-H, Kato I, Pérez-Pérez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 31.Palladino M A, Morris R E, Starnes H F, Levinson A D. The transforming growth factor-betas. A new family of immunoregulatory molecules. Ann N Y Acad Sci. 1990;593:181–187. doi: 10.1111/j.1749-6632.1990.tb16110.x. [DOI] [PubMed] [Google Scholar]
- 32.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 33.Peek R M, Jr, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+Helicobacter pylori strains. Lab Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- 34.Peek R M, Jr, Blaser M J. Pathophysiology of Helicobacter pylori-induced gastritis and peptic ulcer disease. Am J Med. 1997;102:200–207. doi: 10.1016/s0002-9343(96)00273-2. [DOI] [PubMed] [Google Scholar]
- 35.Price A B. The Sydney system: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 36.Quiding-Järbrink, M., H. Lönroth, and A.-M. Svennerholm. CD4+ and CD8+ T cell responses in Helicobacter pylori infected individuals. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 37.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 38.Shirota K, LeDuy L, Yuan S, Jothy S. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58:303–308. doi: 10.1007/BF02890085. [DOI] [PubMed] [Google Scholar]
- 39.Smith W B, Noach L, Khew-Goodall Y, Isenmann S, Vadas M A, Gamble J R. Transforming growth factor-β1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996;157:360–368. [PubMed] [Google Scholar]
- 40.Strober W, Kesall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 41.Uehara A, Okumura T, Sekiya C, Okamura K, Takasugi Y, Namiki M. Interleukin-1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun. 1989;162:1578–1584. doi: 10.1016/0006-291x(89)90855-3. [DOI] [PubMed] [Google Scholar]
- 42.Weigert N, Schaffer K, Schusdziarra V, Classen M, Schepp W. Gastrin secretion from primary cultures of rabbit antral G cells: stimulation by inflammatory cytokines. Gastroenterology. 1996;110:742–745. doi: 10.1053/gast.1996.v110.pm8536851. [DOI] [PubMed] [Google Scholar]
- 43.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]