Abstract
Background
Tranexamic acid (TXA) has been hypothesized to mitigate coagulopathy in patients after traumatic injury. Despite previous prehospital clinical trials demonstrating a TXA survival benefit, none have demonstrated correlated changes in thromboelastography (TEG) parameters. We sought to analyze if missing TEG data contributed to this paucity of findings.
Methods
We performed a secondary analysis of the Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport Trial. We compared patients that received TEG (YES-TEG) and patients unable to be sampled (NO-TEG) to analyze subgroups in which to investigate TEG differences. TEG parameter differences across TXA intervention arms were assessed within subgroups disproportionately present in the NO-TEG relative to the YES-TEG cohort. Generalized linear models controlling for potential confounders were applied to findings with p<0.10 on univariate analysis.
Results
NO-TEG patients had lower prehospital systolic blood pressure (SBP) (100 (78, 140) vs 125 (88, 147), p<0.01), lower prehospital Glascow Coma Score (14 (3, 15) vs 15 (12, 15), p<0.01), greater rates of prehospital intubation (39.4% vs 24.4%, p<0.01) and greater mortality at 30 days (36.4% vs 6.8%, p<0.01). NO-TEG patients had a greater international normalized ratio relative to the YES-TEG subgroup (1.2 (1.1, 1.5) vs 1.1 (1.0, 1.2), p=0.04). Within a severe prehospital shock cohort (SBP<70), TXA was associated with a significant decrease in clot lysis at 30 min on multivariate analysis (β=−27.6, 95% CI (−51.3 to –3.9), p=0.02).
Conclusions
Missing data, due to the logistical challenges of sampling certain severely injured patients, may be associated with a lack of TEG parameter changes on TXA administration in the primary analysis. Previous demonstration of TXA’s survival benefit in patients with severe prehospital shock in tandem with the current findings supports the notion that TXA acts at least partially by improving clot integrity.
Level of evidence
Level II.
Keywords: thromboelastography, Multiple Trauma, tranexamic acid, blood transfusion
WHAT IS ALREADY KNOWN ON THIS TOPIC
Tranexamic acid (TXA) is hypothesized to mitigate hyperfibrinolysis and reduce bleeding.
However, previous prehospital clinical trials have not concomitantly demonstrated a TXA survival benefit and reduction in clot lysis at 30 min (LY30).
WHAT THIS STUDY ADDS
In a secondary analysis of a prehospital clinical trial, we demonstrate that prehospital TXA administration is associated with a significant reduction of LY30 in the same cohort (severe prehospital shock patients) in which a survival benefit was found in the primary trial analysis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Importantly, we analyzed that missing TEG measurements may play a role in the paucity of previous findings.
Introduction
Resuscitation after severe traumatic injury has transformed during the past decade, with the use of early blood products and adjunctive strategies to prevent coagulopathy, improve hemostasis and modulate the downstream immune response that complicates traumatic injury.1–5 Concomitantly, there has been an increasing number of randomized clinical trials characterizing early resuscitation adjuncts to reduce coagulopathy and mortality attributable to traumatic injury.6–9
Tranexamic acid (TXA) improves survival in patients with traumatic injury7 9–12 and is hypothesized to mitigate hyperfibrinolysis and the ensuing coagulopathy induced by shock and traumatic hemorrhage.13 Despite previous clinical trials demonstrating TXA is associated with a survival benefit and an improvement in endothelial cell damage markers,9 12 none have demonstrated improvement in thromboelastography (TEG) parameters.7 11 The underlying mechanisms responsible for the benefits of prehospital TXA must be better characterized.
Abnormal TEG parameters are clinically relevant and predict coagulopathy and mortality in patients with traumatic injury.14 15 A significant proportion of patients enrolled in TXA clinical trials could not be sampled for TEG analysis resulting in substantial missing data.7 11 Little is known regarding the characteristics of patients with missing TEG measurements and how this missingness relates to the lack of TEG parameter differences found across TXA intervention arms.
Our objectives were to identify specific subgroups of patients unable to be sampled for TEG measurements and analyze whether TXA is associated with TEG parameter changes within subgroups highly correlated with missing TEG in the Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport (STAAMP) Trial. These objectives may provide insight into TXA and its mechanism of action. We hypothesized that injury characteristics attributable to missingness may be responsible for the absence of TEG differences found across TXA intervention arms.
Methods
Trial design and study population
We performed a secondary analysis of the STAAMP Trial.7 The STAAMP Trial (NCT02086500) was a prospective prehospital phase III multicenter double-blind randomized placebo-controlled trial. The study included patients from the scene or transferred from an outside emergency department to one of four participating trauma centers within 2 hours of injury with either hypotension (systolic blood pressure (SBP) <90 mm Hg) or tachycardia (heart rate >110 beats per minute). Patients were randomized to receive TXA (1 gram bolus during 10 min en route to hospital) or placebo in the prehospital phase. This was followed by an in-hospital phase in which patients were randomized to an abbreviated (1 g total TXA), standard (2 g total TXA), or repeat bolus (3 g total TXA) dose at trauma center arrival. All in-hospital doses of TXA were administered within the first 8 hours of hospital admission. TEG was a principal secondary outcome and a required measurement specified in the protocol.
The STAAMP Trial enrolled subjects through the Emergency Exception From Informed Consent protocol, after a period of community consultation and public notification. Informed consent for continuing participation was obtained from all subjects enrolled in the trial. All study methods were performed in accordance with relevant guidelines and regulations.
Sample collection and measurement
Patients who met all the inclusion and no exclusion criteria of STAAMP en route via emergency medical transport received point of care rapid TEG performed for coagulation parameter measurements within 6 hours of arrival at definitive trauma care. TEG analysis was performed on a TEG 5000 Thromboelastograph Hemostasis Analyzer. Activated clotting time (ACT; sec), kinetic time (K; min), alpha angle (degrees), maximum amplitude (MA; mm) and clot lysis at 30 min (LY30; %) were analyzed. Standard international normalized ratio (INR) was drawn and measured at each institution within 6 hours of arrival.
Statistical analysis
We first compared patients that received TEG analysis (YES-TEG) and patients who did not (NO-TEG) to characterize injury-related differences and identify subgroups with a significant amount of missing TEG data. Kaplan Meier analysis was also performed to assess 24-hour survival differences between the NO-TEG and YES-TEG groups to further investigate the relative injury severity of NO-TEG patients. In an attempt to assess whether TXA administration was associated with TEG parameter differences within subgroups correlated with missing TEG data, TEG measurement differences were compared between the TXA and placebo arms in subgroups disproportionately present in the NO-TEG cohort relative to the YES-TEG cohort.
Finally, for associations approaching significance (p<0.10) we employed a generalized linear model to analyze the independent effect of prehospital TXA administration on TEG parameters. We modeled the relationship between intervention and TEG parameters using regression analysis controlling for Injury Severity Score (ISS), a known confounder in this trauma population. We analyzed ACT, K, alpha angle, MA and LY30 at admission as a function of prehospital TXA controlling for arrival Glascow Coma Score (GCS) and ISS. We evaluated variance inflation factors to ensure that the variance of our regression coefficients was not due to multicollinearity.
Descriptive statistics characterized the demographics and injuries of the patients and outcomes of interest. A Shapiro-Wilk test was conducted on all continuous variables to test for normality. Categorical variables were presented as frequencies and percentages and tested using the χ2 test. Continuous variables were expressed as medians with IQRs and were tested using Wilcoxon rank-sum. Statistical significance was analyzed at the p<0.05 level (two-sided). All data were analyzed using STATA V.17.0 (College Station, Texas, USA).
Results
The STAAMP Trial assessed 30-day mortality in 903 patients who were randomized to prehospital TXA (n=447) or placebo (n=456). This cohort was moderately injured with a median (IQR) ISS of 12 (5, 22) and 30-day mortality of 9.0%. There were no significant differences in TEG parameters between patients that received TXA and those that received placebo in the overall study cohort. In the overall study cohort, there were 66 (7.3%) NO-TEG patients and this missingness was similar across the TXA and placebo arms (9.0% vs 5.7%, p=0.06).
The subset of YES-TEG patients (n=837) was similar to those patients included in the primary STAAMP Trial analysis regarding injury severity and demographics. In the YES-TEG cohort patients were predominantly male (75%) patients with median ages of 38 (26, 55) years who sustained blunt injuries (84%) with a median ISS of 12 (5, 22). This subgroup of patients had a 30-day mortality rate of 6.8%, which was lower than that of the overall STAAMP Trial cohort. There were no significant differences between the TXA and placebo arms within the YES-TEG subgroup (table 1).
Table 1.
Demographic and clinical characteristics of the STAAMP cohort with TEG samples stratified by TXA
| Variable | Overall (n=837) | Placebo (n=430) | TXA (n=407) | P value |
| Age | 38.0 (26.0–55.0) | 39.0 (26.0–56.0) | 38.0 (26.0–51.0) | 0.20 |
| Male | 624 (74.6%) | 322 (74.9%) | 302 (74.2%) | 0.82 |
| Blunt Injury | 702 (83.9%) | 366 (85.1%) | 336 (82.6%) | 0.31 |
| TBI | 196 (23.4%) | 100 (23.3%) | 96 (23.6%) | 0.92 |
| ISS | 12.0 (5.0–22.0) | 11.0 (4.0–22.0) | 12.0 (5.0–22.0) | 0.39 |
| Prehospital GCS | 15.0 (12.0–15.0) | 14.0 (11.0–15.0) | 15.0 (12.0–15.0) | 0.25 |
| Transfer | 113 (13.7%) | 57 (13.4%) | 56 (14.0%) | 0.78 |
| Prehospital Intubation | 204 (24.4%) | 109 (25.3%) | 95 (23.3%) | 0.50 |
| Prehospital SBP | 125.0 (88.0–147.0) | 126.0 (88.0–148.0) | 124.0 (88.0–144.0) | 0.89 |
| 24-hour whole blood | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.64 |
| 24-hour PRBC | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.50 |
| 24-hour plasma | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.11 |
| 24-hour platelets | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.85 |
| INR | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.77 |
| Lactate | 2.7 (1.8–3.9) | 2.6 (1.8–4.1) | 2.8 (1.8–3.8) | 0.97 |
| 30-day mortality | 57 (6.8%) | 35 (8.1%) | 22 (5.4%) | 0.12 |
| MOF | 69 (8.2%) | 37 (8.6%) | 32 (7.9%) | 0.70 |
| VTE | 35 (4.2%) | 13 (3.0%) | 22 (5.4%) | 0.09 |
Values are represented by numbers (percentages) and means (IQR). TXA refers to patients who received prehospital tranexamic acid (TXA).
GCS, Glasgow Coma Scale; INR, international normalized ratio; ISS, Injury Severity Score; MOF, multiple organ failure; PRBC, packed red blood cells; SBP, systolic blood pressure; STAAMP, Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport; TBI, traumatic brain injury; TEG, thromboelastography; transfer, transferred from another hospital; VTE, venous thromboembolism.
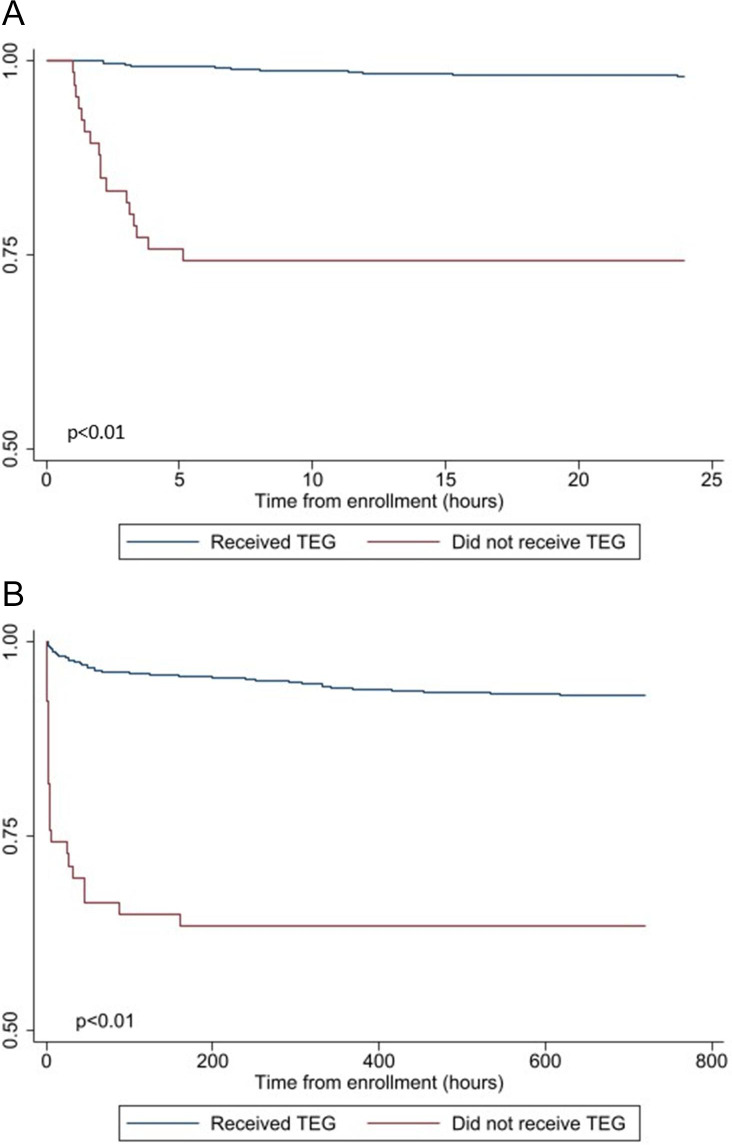
In a comparison of YES-TEG (n=837) and NO-TEG (n=66) patients, NO-TEG patients were similar in terms of demographics but were more severely injured. Specifically, NO-TEG patients had lower prehospital SBP (100 (78, 140) vs 125 (88, 147), p<0.01), lower prehospital GCS (14 (3, 15) vs 15 (12, 15), p<0.01), higher rates of prehospital intubation (39.4% vs 24.4%, p<0.01) and significantly greater mortality at 30 days (36.4% vs 6.8%, p<0.01; table 2). Importantly, NO-TEG patients had a significantly higher INR relative to the YES-TEG subgroup. However, 32 (48.5%) of the NO-TEG patients were also missing INR analysis. Kaplan-Meier survival analysis demonstrated a significant higher mortality for the NO-TEG subgroup with early separation of the survival curves that persisted out to 30 days (log rank p<001; figure 1).
Table 2.
Demographic and clinical characteristics of the STAAMP cohort stratified by TEG sampling
| Variable | Overall (n=903) | YES-TEG (n=837) | NO-TEG (n=66) | P value |
| Age | 39.0 (26.0–55.0) | 38.0 (26.0–55.0) | 39.5 (25.0–61.0) | 0.57 |
| Male | 668 (74.0%) | 624 (74.6%) | 44 (66.7%) | 0.16 |
| Blunt Injury | 755 (83.6%) | 702 (83.9%) | 53 (80.3%) | 0.45 |
| TBI | 212 (23.6%) | 196 (23.4%) | 16 (25.4%) | 0.72 |
| ISS | 12.0 (5.0–22.0) | 12.0 (5.0–22.0) | 14.0 (5.0–29.0) | 0.13 |
| Prehospital GCS | 15.0 (11.0–15.0) | 15.0 (12.0–15.0) | 14.0 (3.0–15.0) | <0.01 |
| Transfer | 127 (14.3%) | 113 (13.7%) | 14 (21.5%) | 0.08 |
| Prehospital Intubation | 230 (25.5%) | 204 (24.4%) | 26 (39.4%) | <0.01 |
| Prehospital SBP | 124 (88–146) | 125 (88–147) | 100 (78–140) | <0.01 |
| 24-hour whole blood | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.05 |
| 24-hour PRBC | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–4.0) | <0.01 |
| 24-hour plasma | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | <0.01 |
| 24-hour platelets | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.03 |
| INR | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.2 (1.1–1.5) | 0.04 |
| Lactate | 2.8 (1.8–4.0) | 2.7 (1.8–3.9) | 3.9 (2.3–5.8) | <0.01 |
| 30-day mortality | 81 (9.0%) | 57 (6.8%) | 24 (36.4%) | <0.01 |
| MOF | 72 (8.0%) | 69 (8.2%) | 3 (4.5%) | 0.29 |
| VTE | 36 (4.0%) | 35 (4.2%) | 1 (1.5%) | 0.29 |
Values are represented by numbers (percentages) and means (IQR).
GCS, Glascow Coma Score; INR, international normalized ratio; ISS, Injury Severity Score; MOF, multiple organ failure; NO-TEG, patients unable to be sampled; SBP, systolic blood pressure; STAAMP, Study of Tranexamic Acid During Air Medical and Ground Prehospital Transport; TBI, traumatic brain injury; TEG, thromboelastography; VTE, venous thromboembolism; YES-TEG, patients that received TEG.
Figure 1.
(A) Kaplan-Meier survival analysis comparing patients that received thromboelastography (TEG) measurements and those unable to be sampled at 24 hours. (B) Kaplan-Meier survival analysis comparing patients that received TEG measurements and those unable to be sampled at 30 days.
Based on these missingness related injury characteristics we further analyzed the severe shock subgroup (SBP <70 mm Hg, n=58), low prehospital GCS (GCS<8, n=196), and those patients that required prehospital intubation (n=230). We next compared TEG measurements ACT, K, alpha, MA and LY30 at hospital admission stratified by prehospital TXA administration using univariate analysis within each missingness informed subgroup. On univariate analysis, there were no significant differences in TEG parameters between patients that received TXA and those that received placebo in the prehospital GCS <8 subgroup, patients that required prehospital intubation subgroup and in the severe prehospital shock subgroup (table 3). However, within the severe prehospital shock cohort, TXA trended towards an association with lower LY30 relative to placebo (2.7% vs 40.0%, p=0.07). After controlling for ISS, within the severe prehospital shock cohort, TXA was associated with a significant decrease in LY30 (β=−27.6, 95% CI −51.3 to –3.9, p=0.02; table 4) without significant associations for other TEG parameters.
Table 3.
TEG measurements stratified by TXA administration in specified subgroups
| Variable | Placebo (n=96) | TXA (n=76) | P value |
| Prehospital GCS <8 subgroup | |||
| ACT | 121.0 (105.0–136.0) | 113.0 (105.0–128.0) | 0.40 |
| K | 1.8 (1.4–2.6) | 1.8 (1.4–2.5) | 0.83 |
| Alpha | 69.8 (64.8–74.5) | 70.5 (66.1–74.2) | 0.63 |
| MA | 59.8 (53.5–64.9) | 60.1 (55.2–64.1) | 1.00 |
| LY30 | 20.0 (1.8–70.0) | 12.3 (1.8–60.0) | 0.99 |
| Prehospital intubation subgroup | |||
| CT | 121.0 (105.0–136.0) | 113.0 (105.0–128.0) | 0.46 |
| K | 1.8 (1.2–2.6) | 1.8 (1.4–2.6) | 0.57 |
| Alpha | 70.8 (65.1–74.9) | 70.1 (65.5–73.9) | 0.80 |
| MA | 59.6 (53.6–65.2) | 58.7 (53.4–63.2) | 0.18 |
| LY30 | 20.0 (1.8–70.0) | 10.0 (1.75–60.0) | 0.57 |
| Severe prehospital shock subgroup | |||
| ACT | 121.0 (101.0–132.0) | 121.0 (105.0–136.0) | 0.65 |
| K | 1.7 (1.2–3.1) | 1.6 (1.1–2.3) | 0.27 |
| Alpha | 69.8 (61.9–74.3) | 72.2 (64.5–75.6) | 0.58 |
| MA | 60.7 (51.4–66.2) | 60.2 (55.7–67.5) | 0.62 |
| LY30 | 40.0 (2.2–60.0) | 2.7 (1.6–10.0) | 0.07 |
ACT, activated clotting time; GCS, Glascow Coma Score; LY30, lysis at 30 min; MA, maximum amplitude; TEG, thromboelastography; TXA, tranexamic acid.
Table 4.
Adjusted coefficients of TEG measurements by TXA administration in severe prehospital shock cohort
| Variable | Adjusted coefficient (95% CI) | P value |
| ACT | 93.0 (−52.9 to 238.9) | 0.21 |
| K | −7.5 (−19.2 to 4.0) | 0.20 |
| Alpha | −1.8 (−10.1 to 6.6) | 0.68 |
| MA | −0.5 (−9.0 to 8.0) | 0.90 |
| LY30 | −27.6 (−51.3 to 3.9) | 0.02 |
ACT, activated clotting time; LY30, lysis at 30 min; MA, maximum amplitude; TEG, thromboelastography; TXA, tranexamic acid.
Discussion
In prior randomized prehospital clinical trials after injury, TXA administration has not been associated with changes in TEG parameters,7 11 despite the proposed antifibrinolytic mechanism of action of TXA treatment.13 Missing data may contribute to a lack of observed difference in TEG parameters due to the logistics of sampling severely injured patients and the time sensitive nature of trauma treatment.16 17 The results from the current secondary analysis demonstrate that TXA administration in patients with severe shock is independently associated with a lower LY30. Importantly, the reduction in LY30 found is consistent with TXA’s hypothesized mechanism of action.
TXA prevents the conversion of plasminogen to plasmin. As a result, inhibition of plasminogen activation stabilizes the preformed fibrin meshwork produced by secondary hemostasis and ultimately reducing bleeding.13 18 19 Alternate hypotheses for TXA’s mechanism of action have been explored, particularly as a pro-endothelial therapeutic agent to reduce glycocalyx shedding.20 Our results add to the existing literature and our TEG findings correspond to the historic TXA mechanism of action.
NO-TEG patients were significantly more injured than YES-TEG patients supporting the likelihood that these patients were unable to undergo sampling due to the logistical difficulties associated with the management of the severely injured patient. In addition, approximately half of these patients had INR values calculated, indicating that this challenge may be unique to TEG. It has been previously demonstrated that severely injured patients at higher risk of mortality consistently demonstrate TEG parameter abnormalities relative to those less severely injured.14 15 Importantly, it is in the severe prehospital shock subgroup where TEG improvements were demonstrated and TXA treatment was associated with a significant reduction in 30-day mortality in the primary STAAMP analysis.7 The concomitant demonstration of a TXA survival benefit and a reduction of LY30 is novel in the setting of traumatic injury.
However, it is critical to note that TXA administration, in the other subgroups disproportionately present in NO-TEG patients, was not associated with TEG parameter differences relative to placebo. In addition, the primary STAAMP analysis7 did not demonstrate a survival benefit in these subgroups. This finding suggests that the administration of TXA and its impact on TEG parameters and survival is dependent on specific characteristics of traumatic injury. It’s plausible that missing TEG data from the severe prehospital shock cohort, as opposed to severely injured patients in general, may underlie the importance of missing TEG data.
Limitations
There are limitations to this analysis. First, this is a post hoc secondary analysis with the potential for confounding and is hypothesis generating in nature. Second, although our models were statistically robust, the small sample size limits our ability to include additional variables for adjustment and there may be unknown and unaccounted confounding factors missing in our models. Third, this analysis is limited by the times at which we were able to sample blood from injured trauma patients. Specifically, TEG was only sampled at hospital admission, which may mask delayed effects of prehospital TXA on TEG parameters. Of equal importance, the early sampling that was accomplished may vary significantly from the time of injury. Fourth, the number of patients with severe prehospital shock was relatively low in this cohort which may limit the generalizability of our findings. Lastly, patients in the missingness defined subgroups were not randomized relative to the overall trial cohort. We attempted to minimize the impact of this by using a generalized linear model accounting for potential confounding variables.
Conclusions
In conclusion, the logistical difficulties of sampling patients with severe shock and the attributable missingness may be responsible for the paucity of TEG differences found in previous prehospital TXA trials. TXA is associated with TEG parameter improvement in patients with severe prehospital shock, a subgroup disproportionately present in patients unable to be sampled for TEG. Importantly, TXA’s associated decrease in LY30 corresponds to its historical mechanism of action and the subgroup in which this decrease was found is the same subgroup in which TXA was associated with a survival benefit. Further translational and basic science research is crucial to investigating the proposed mechanism of TXA in traumatic injury.
Footnotes
Contributors: JBB, FG, BE, RN, GV, TO, BJ, MN, JLS designed and executed the trials. FG, JBB, JLS collected the trial data. JKD designed the study, harmonized data sets, analyzed data and drafted the article. JLS designed the study, analyzed data and drafted the article. NI and JML interpreted data. All authors performed critical revision and approved the final article for submission. JLS is the guarantor for this manuscript.
Funding: The STAAMP Trial was funded by the US Army Medical Research and Materiel Command (W81XWH 13-2-0080). MN was supported by the National Institute of General Medical Sciences (R35GM119526).
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
Data are available upon reasonable request. After publication of the primary and all secondary analyses detailed in study protocols, individual de-identified data will be available upon request and approval of the proposed use of the data after 3 years of the close of the trial. The trial protocol, statistical analysis plan embedded in the protocol, and the trial publications are available online. Requests should be sent to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the University of Pittsburgh Institutional Review Board (STUDY19060072) and at all other study sites. Participants gave informed consent to participate in the study before taking part.
References
- 1.Harris T, Davenport R, Mak M, Brohi K. The evolving science of trauma resuscitation. Emerg Med Clin North Am 2018;36:85–106. 10.1016/j.emc.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 2.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011;254:598–605. 10.1097/SLA.0b013e318230089e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013;148:127–36. 10.1001/2013.jamasurg.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82. 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meizoso JP, Barrett CD, Moore EE, Moore HB. Advances in the management of coagulopathy in trauma: the role of viscoelastic hemostatic assays across all phases of trauma care. Semin Thromb Hemost 2022;48:796–807. 10.1055/s-0042-1756305 [DOI] [PubMed] [Google Scholar]
- 6.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med 2018;379:315–26. 10.1056/NEJMoa1802345 [DOI] [PubMed] [Google Scholar]
- 7.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial. JAMA Surg 2020;156:11–20. 10.1001/jamasurg.2020.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyette FX, Zenati M, Triulzi DJ, Yazer MH, Skroczky H, Early BJ, Adams PW, Brown JB, Alarcon L, Neal MD, et al. Prehospital low titer group O whole blood is feasible and safe: results of a prospective randomized pilot trial. J Trauma Acute Care Surg 2022;92:839–47. 10.1097/TA.0000000000003551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruen RL, Mitra B, Bernard SA, McArthur CJ, Burns B, Gantner DC, Maegele M, Cameron PA, Dicker B, Forbes AB, et al. Prehospital tranexamic acid for severe trauma. N Engl J Med 2023;389:127–36. 10.1056/NEJMoa2215457 [DOI] [PubMed] [Google Scholar]
- 10.Li SR, Guyette F, Brown J, Zenati M, Reitz KM, Eastridge B, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, et al. Early prehospital tranexamic acid following injury is associated with a 30-day survival benefit: a secondary analysis of a randomized clinical trial. Ann Surg 2021;274:419–26. 10.1097/SLA.0000000000005002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, Bulger EM, Idris AH, Christenson J, Morrison LJ, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA 2020;324:961–74. 10.1001/jama.2020.8958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1–79. 10.3310/hta17100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J, Ribkoff J, Olson S, Raghunathan V, Al-Samkari H, DeLoughery TG, Shatzel JJ. The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol 2020;104:79–87. 10.1111/ejh.13348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane I, Ong A, Orozco FR, Post ZD, Austin LS, Radcliff KE. Thromboelastography predictive of death in trauma patients. Orthop Surg 2015;7:26–30. 10.1111/os.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg 2012;215:496–502. 10.1016/j.jamcollsurg.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Coughenour J. Initial evaluation and management of the injured patient. Mo Med 2018;115:429–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Guyette FX, Peitzman AB, Billiar TR, Sperry JL, Brown JB. Identifying patients with time-sensitive injuries: association of mortality with increasing prehospital time. J Trauma Acute Care Surg 2019;86:1015–22. 10.1097/TA.0000000000002251 [DOI] [PubMed] [Google Scholar]
- 18.Ockerman A, Vanassche T, Garip M, Vandenbriele C, Engelen MM, Martens J, Politis C, Jacobs R, Verhamme P. Tranexamic acid for the prevention and treatment of bleeding in surgery, trauma and bleeding disorders: a narrative review. Thromb J 2021;19:54. 10.1186/s12959-021-00303-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts I, Prieto-Merino D, Manno D. Mechanism of action of tranexamic acid in bleeding trauma patients: an exploratory analysis of data from the CRASH-2 trial. Crit Care 2014;18:685. 10.1186/s13054-014-0685-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruen DS, Brown JB, Guyette FX, Johansson PI, Stensballe J, Li SR, Leeper CM, Eastridge BJ, Nirula R, Vercruysse GA, et al. Prehospital tranexamic acid is associated with a dose-dependent decrease in Syndecan-1 after trauma: a secondary analysis of a prospective randomized trial. J Trauma Acute Care Surg 2023;95:642–8. 10.1097/TA.0000000000003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. After publication of the primary and all secondary analyses detailed in study protocols, individual de-identified data will be available upon request and approval of the proposed use of the data after 3 years of the close of the trial. The trial protocol, statistical analysis plan embedded in the protocol, and the trial publications are available online. Requests should be sent to the corresponding author.