Abstract
Murine cerebral malaria (CM) induced by Plasmodium berghei ANKA kills susceptible mice within 24 to 48 h of onset of symptoms and is characterized by the production of inflammatory cytokines in the brain. C57BL/6J mice are sensitive to lethal CM, while A/J mice are resistant. These strains of mice were immunized with an adjuvant vaccine of killed whole-blood-stage parasites. The immunization protected C57BL/6 mice from lethal CM following virulent challenge. The same immunization increased the incidence of lethal CM in A/J mice challenged similarly. Histopathologic examination of the brains of mice from these studies revealed two distinct types of lesions. Type I CM is acute in onset; usually lethal; and characterized by widespread microglial activation, endothelial cell damage, and microvascular disruption in the brain. Type II CM is characterized by intense, but focal, mononuclear cell inflammation without endothelial cell damage or microvascular destruction. Animals with type II lesions were clinically normal and protected from type I lesions. Available clinical, epidemiological, and biochemical evidence suggests that type I and type II lesions might exist in human CM as well.
Cerebral malaria (CM) is induced in susceptible strains of mice by injection of virulent Plasmodium berghei ANKA blood-stage parasites. The mice die after paralysis and coma between 6 and 9 days after infectious challenge, with percentages of parasitized erythrocytes averaging 10%. In contrast, resistant strains of mice suffer similar parasite growth but no cerebral disease. They die 3 weeks after infection, with anemia and percentages of erythrocytes with parasites of greater than 60% (20).
It is well established that fatal murine CM is an immunopathological process mediated by proinflammatory cytokines. Depletion of CD4+ or CD8+ T cells, tumor necrosis factor (TNF), or gamma interferon (IFN-γ) prevents the disease (8, 9, 19, 23, 24). Using bioluminescent quantitative reverse transcriptase PCR, we demonstrated simultaneous increases in messages for TNF-α, IFN-γ, and parasites in the brains of mice coincident with the onset of lethal CM. The timing, magnitude, and pattern of the production of messages for these proinflammatory cytokines in the brain were qualitatively and quantitatively different from changes observed in the spleen or liver. This finding demonstrated that murine CM is an encephalitis or inflammation of the brain, not simply a local manifestation in the brain of a systemic process.
Histopathologically, murine CM has been most frequently characterized by inflammation in the central nervous system (CNS), with monocyte adherence to the endothelium of the microvasculature (16). However, activation of microglial cells, swelling of endothelial cell nuclei, microvasculature damage, and breakdown of the blood-brain barrier with cerebral edema have also been reported (16, 29). Morphologic evidence of activation of microglial cells was observed 2 to 3 days after infection and 3 or more days prior to the onset of cerebral symptoms (18). Microglia are pluripotent members of the macrophage/monocyte lineage that react to many pathological changes in the CNS. Using immunohistochemistry and in situ reverse transcriptase PCR, Medana et al. (19) further demonstrated that both TNF-α message and protein are induced in microglial cells and astrocytes before the onset of mononuclear cell inflammation. This result suggested that intrinsic brain cells rather than extrinsic inflammatory cells are of primary importance in the pathogenesis of lethal murine CM.
In an earlier study, we observed that certain vaccines designed to induce cell-mediated immunity (CMI) to malaria antigens increased the susceptibility of mice to CM (22). A/J mice are normally resistant to CM. However, immunized A/J mice developed typical lethal CM following challenge with virulent parasites. In another study, Curfs et al. reported that a different kind of vaccine was able to prevent the development of CM in susceptible mice (5). Mice immunized with this vaccine developed no signs of CM even though the multiplication of parasites was unaffected, and the mice eventually died of anemia due to overwhelming parasitemia. In our experience, this type of vaccine is likely to induce predominantly an antibody response (10).
The present experiments were conducted to evaluate the effects of vaccines designed to induce predominantly antibody- or cell-mediated responses on the development of lethal disease in CM-susceptible and CM-resistant strains of mice. We were able to reproduce both the enhancement and the suppression of CM. However, two unexpected observations were made. First, the same vaccines that protected susceptible mice from lethal CM enhanced the disease in resistant mice, causing them to die rapidly of CM. Second, histopathologic examination of the brains of these mice demonstrated that lethal disease in both normal and immunized mice was associated with widespread microvascular destruction with minimal inflammation. Mononuclear cell inflammation, in contrast, was associated with reversible disease and protection from lethal malaria. While all of the histopathologic changes that we observed had been reported previously, the patterns of change induced by immunization facilitated identification of two distinct pathologic mechanisms of CM that had not been recognized previously.
(This research was conducted in partial fulfillment of the requirement for a Ph.D. from Emory University, Atlanta, Ga., 1998.)
MATERIALS AND METHODS
Mouse strains and dose of parasite.
Preliminary experiments with five inbred strains of mice (Jackson Laboratory, Bar Harbor, Maine) identified C57BL/6J and BALB/c mice as being susceptible to CM, DBA and CBA mice as being intermediately susceptibile to CM, and A/J mice as being resistant to CM (12a). Therefore, 12-week-old pathogen-free female A/J and C57BL/6J mice (Jackson Laboratory) were selected as prototype CM-resistant and CM-susceptible strains, respectively. They were infected intravenously with 104 P. berghei ANKA-parasitized erythrocytes from homologous donors that had been infected with frozen stocks maintained in Glycerolyte 57 solution (Baxter Health Corp., Deerfield, Ill.) at −70°C in 200-μl aliquots (3).
Preparation of whole-blood-stage antigen with and without fatty acid tails.
Whole-blood-stage plasmodial antigen was prepared from blood of ICR mice infected with P. berghei ANKA, with percentages of parasitized erythrocytes of approximately 10 to 15%, as described by Hunter et al. (11). Approximately one-half of the total antigen obtained was chemically conjugated with fatty acid tails as described previously (10). The antigen was incubated with an excess of dodecanoic anhydride (0.5 M) buffer (pH 9) for 24 h at 4°C. The pH was lowered to 7.0 to hydrolyze remaining anhydride. Excess fatty acid was removed by dialysis against phosphate-buffered saline.
Immunization and challenge.
Groups of A/J and C57BL/6J mice (8 to 10 per group) were immunized subcutaneously with the preparations of whole-blood-stage antigen on days 0, 14, and 28. The first immunization on day 0 contained 100 μg of antigen with or without fatty acid tails, 50 μg of detoxified lipopolysaccharide (LPS), and 1 mg of P1005 adjuvant in saline. Nonionic block copolymer (P1005) was obtained from Vaxcel Inc. (Norcross, Ga.), and LPS detoxified by deacylation (LPS) from Escherichia coli EH-100 was obtained from Kuni Takayama (Veteran’s Administration Hospital, Madison, Wis.). The preparation and characterization of these materials have been described previously (27). Boosters on days 14 and 28 were prepared similarly but contained 50 μg of antigen. Both immunized and unimmunized control mice were challenged intravenously on day 42 with 104 P. berghei ANKA-parasitized erythrocytes. Survival time was monitored, and parasitemia was assessed from Giemsa-stained thin smears of tail blood prepared every other day for most infections. Smears were used to determine percentages of parasitized erythrocytes.
Histology.
Brain samples were formalin fixed and paraffin embedded for preparation of histologic sections. Five-micrometer-thick sections were cut and stained with hematoxylin and eosin (H&E) or reticulum stain. Similar unstained sections were immunohistochemically analyzed with monoclonal antibodies for HAM-56 (ENZO Diagnostics, Farmington, N.Y.).
RESULTS
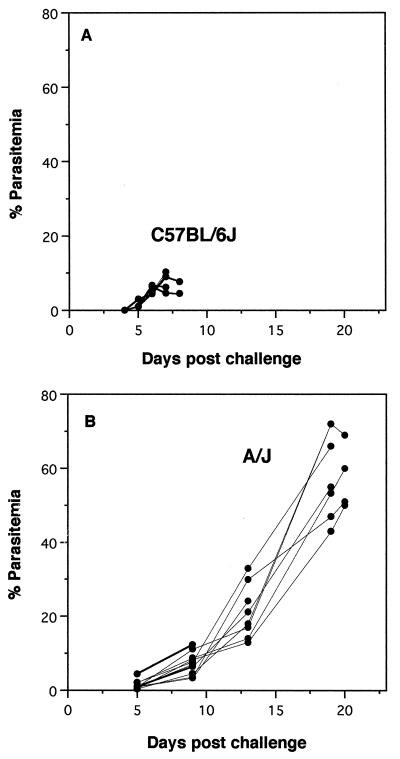
Groups of unimmunized CM-susceptible (C57BL/6J) and CM-resistant (A/J) mice were infected intravenously with 104 blood-stage P. berghei ANKA-parasitized erythrocytes. Clinical signs and parasitemia were monitored until the animals became moribund (Fig. 1A). All of the C57BL/6J mice developed CM between days 6 and 8, with percentages of erythrocytes that were parasitized of less than 12%. They were euthanized when neurologic signs of CM (posterior paralysis, coma, and seizures) were observed. Around 20% of the A/J mice also died early with signs of CM. The remaining 80% survived nearly 3 weeks with no evidence of neurologic disease. The levels of parasitemia in these mice rose steadily throughout the course of the infection, with percentages of parasitized erythrocytes reaching a peak of 50 to 70% at the time of death (Fig. 1B). These mice were euthanized when they became moribund with anemia and hyperparasitemia. This biphasic pattern of survival is typical for A/J mice and other CM-resistant strains of mice. Some die early with typical signs of CM, while others die late of anemia. Very few deaths occur at intermediate times.
FIG. 1.
Parasitemia of unimmunized infected mice. Groups of C57BL/6J and A/J mice were challenged intravenously with 104 P. berghei ANKA-parasitized erythrocytes. Percentages of erythrocytes with parasites were determined by examination of thin blood smears from individual mice taken at 2- to 4-day intervals until death. The groups are C57BL/6J mice (n = 10), which are susceptible to CM (A), and A/J mice (n = 10), which are resistant to CM (B).
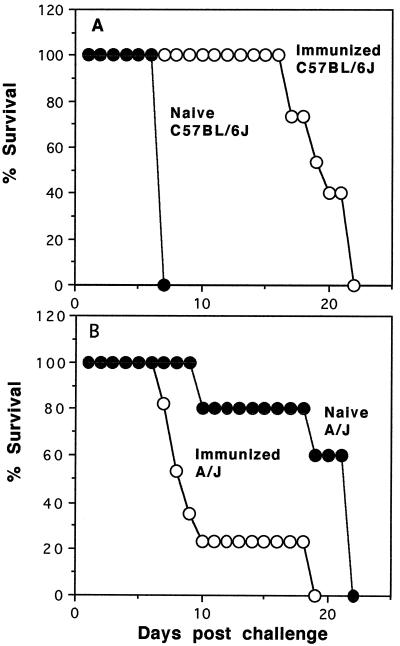
Immunization with a whole-blood-stage P. berghei vaccine prior to challenge with virulent blood-stage parasites had dramatic effects on the pattern of disease and survival of both CM-susceptible and CM-resistant mice. Parasitemia became detectable by day 4 or 5 after infection, and percentages of erythrocytes that were parasitized rose to approximately 10% by day 8 in both strains of mice. All of the unimmunized control C57BL/6J mice died by day 8 with typical CM. All of the immunized C57BL/6J mice, in contrast, survived to at least day 17 postinfection (Fig. 2A). These mice appeared completely healthy through the time when all the unimmunized controls developed CM. They died later (day 22 postinfection) with high levels of parasitemia and anemia but without signs of CM.
FIG. 2.
Effect of immunization on survival following infection. Groups of C57BL/6J and A/J mice were immunized three times subcutaneously with a whole-blood-stage P. berghei vaccine. Percentages of survival of naive control (filled circles) and immunized (open circles) groups of C57BL/6J (n = 15) (A) and A/J (n = 17) (B) mice following challenge infection with 104 parasitized erythrocytes are shown.
The opposite effect of immunization was observed in CM-resistant A/J mice. The same immunizations that protected the C57BL/6J mice from CM enhanced the disease and shortened survival of A/J mice (Fig. 2B). Most (78%) of the unimmunized A/J mice died with anemia between days 19 and 22 postinfection, whereas the remaining 22% died with CM by day 10. These percentages were reversed by immunization. Most (80%) of the immunized mice died before day 10 postinfection with signs of CM, while only 20% survived until day 18 postinfection, after which they died of anemia without signs of CM.
The growth rate of parasites in A/J mice that had been immunized with unconjugated antigen was indistinguishable from that of unimmunized controls except that a higher portion of mice died early with low levels of parasitemia. Fatty acid conjugation of the antigen used for immunization, in contrast, was associated with a lower rate of growth of parasites (less than 20%) in mice that did not die of CM. The observation that immunization with lipid-conjugated antigen slowed the growth of parasites was reproducible for both the A/J and the C57BL/6J mice in this and a previous study (22). Slowed growth of parasites was never observed in control mice or those immunized with unconjugated antigen.
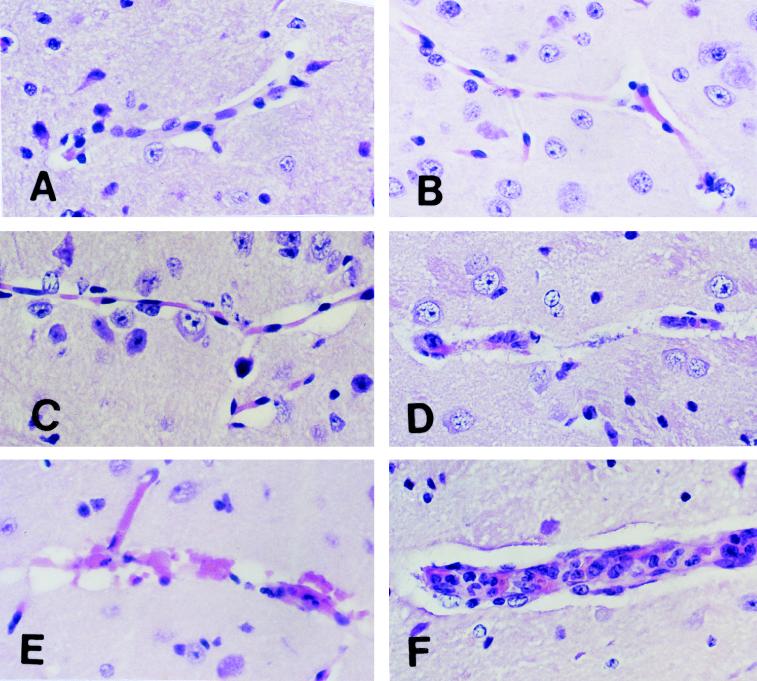
Samples of brains from each of these groups of mice were processed for histologic examination. Figure 3A is a section of a normal C57BL/6J mouse brain. The first sign of disease in C57BL/6J mice infected with P. berghei was activation of microglial cells on day 3 after infection (Fig. 3B). This activation progressed, and by day 5 a redistribution of activated microglia towards the involved vessels was apparent (Fig. 3C). Retraction of ramified processes, nuclear enlargement, intensification of staining, an increasingly amoeboid appearance, and vacuolation were also apparent. Enlargement of spaces around the blood vessels suggested increasing edema. No margination or infiltration of mononuclear inflammatory cells was observed. These changes progressed, so that on day 7, when mice were moribund, there was widespread destruction of the microvasculature of the brain (Fig. 3D and E). The walls of the blood vessels were disrupted with the death of endothelial cells, severe edema, focal hemorrhage, and occasional thrombosis. Sequestered parasitized erythrocytes were evident adhering to the walls of some blood vessels. However, most parasites in the sections were contained in extravascular erythrocytes (not shown).
FIG. 3.
Morphology of CM lesions in the brains of C57BL/6J mice. Normal C57BL/6J mice were challenged intravenously with 104 parasitized erythrocytes. Brains were removed for histologic examination at different times. (A) Section of normal mouse brain showing a healthy unaffected blood vessel. (B) Section of mouse brain on day 3 after challenge showing microglial cells in the perivascular space. (C) Section of mouse brain on day 5 after challenge showing increased numbers of activated microglial cells in perivascular spaces. (D and E) Sections of mouse brain on day 7 after challenge showing destruction of endothelial cells and disruption of vessel walls. Endothelial and other cell nuclei were occasionally condensed and/or fragmented in a fashion consistent with apoptosis. (F) Section of mouse brain on day 7 after challenge showing mononuclear cell accumulation and endothelial cell activation in a blood vessel from the brain of a mouse moribund with CM. These were focal lesions. Larger areas of the brains of the same mice demonstrated the changes shown in panels D and E. All sections were stained with H&E. Magnification, ×500.
Occasionally, a second type of vascular lesion was observed focally in the brains of C57BL/6J mice that were moribund with CM (Fig. 3F). Monocytes were adherent to the endothelial cells and occasionally occluded the lumens of vessels. The endothelial cells of these vessels had enlarged nuclei that appeared to be activated, but there was little sign of activation of microglial cells or astrocytes and the vessel walls remained intact. These findings suggested that mononuclear cell adhesion to endothelial cells was not associated with microvascular destruction.
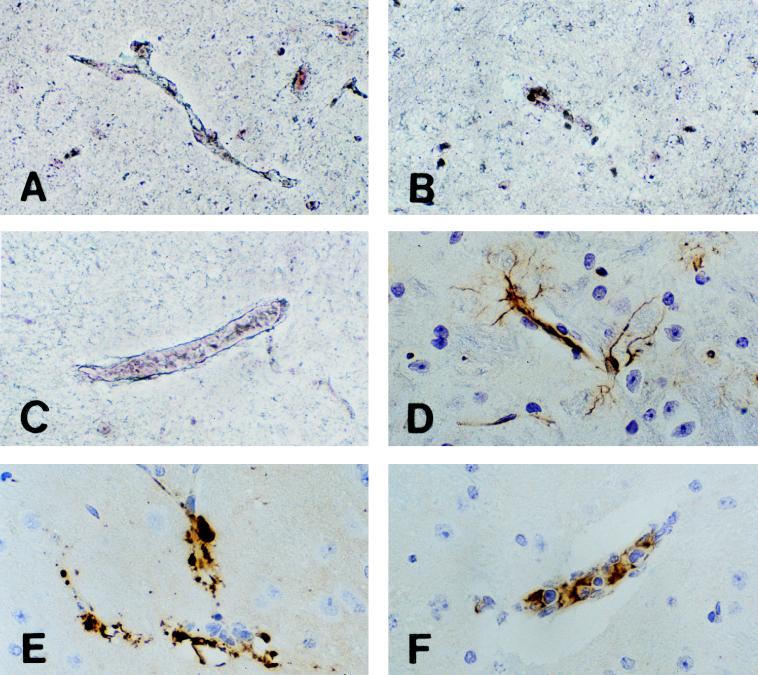
The connective tissue framework of affected vessel walls appeared to be severely disrupted, which was confirmed by examination of reticulum stains. These stains demonstrate an uninterrupted framework of blood vessels of normal mice (Fig. 4A). The reticulum fibers associated with the blood vessels in the brains of mice moribund with CM were severely disrupted (Fig. 4B). This disruption was observed only around damaged vessels. The reticulum was preserved around vessels with mononuclear cell inflammation (Fig. 4C).
FIG. 4.
Reticulum and HAM-56 stainings of CM lesions in the brains of C57BL/6J mice. (A) Section of normal mouse brain showing a healthy uninterrupted blood vessel reticulum. (B) Section of mouse brain on day 7 after challenge showing destruction of reticulum fibers of a vessel. This change was characteristic of vessels with the lesions shown in Fig. 3D and E. (C) Section of mouse brain on day 7 after challenge showing preservation of reticulum fibers around a blood vessel with monocyte inflammation. This pattern of reticulum staining was characteristic of vessels with the lesions shown in Fig. 3F. Magnification, ×200. (D) Section of normal mouse brain showing a HAM-56 monoclonal antibody staining of endothelial cells and glial processes. (E) Section of mouse brain on day 7 after challenge showing a HAM-56 staining of aggregates of material associated with disrupted blood vessels. This pattern of staining was characteristic of vessels with the lesions shown in Fig. 3D and E. (F) Section of mouse brain on day 7 after challenge showing a HAM-56 staining of monocytes in an intact blood vessel with monocyte inflammation. This pattern of staining was characteristic of vessels with the lesions shown in Fig. 3F. All sections were stained with H&E. Magnification, ×200.
HAM-56 is a monoclonal antibody that is widely used as a marker for macrophages/monocytes. It also reacts with endothelial cells and some glial processes in the brain (1, 7). Immunohistochemical staining with this antibody demonstrated striking changes in C57BL/6J mice dying of CM. In normal brains, HAM-56 stained endothelial cells and processes of glial cells in a delicate ramified pattern (Fig. 4D). The microvascular destructive lesions in mice with lethal CM showed intense staining in the most severely affected vessels (Fig. 4E). The staining of recognizable endothelial cells and glial processes had been replaced by intense staining of amorphous aggregates of material in the dead vessels.
The vessels with focal monocyte inflammation showed a different pattern of staining with HAM-56. Monocytes and endothelial cells of these vessels demonstrated moderate cytoplasmic staining with the monoclonal antibody (Fig.4 F). The staining was much less intense than in the destructive lesions. Staining of astrocytes with glial fibrillar acidic protein demonstrated relatively less morphologic change than staining with either the reticulum or HAM-56 stain. There was retraction and thickening of ramified processes in some areas with severe lesions (not shown).
Histologic sections of the brains of C57BL/6J mice that had been immunized and protected from CM showed different histopathologic changes. Some mice were sacrificed on day 10 after infection to evaluate the histopathologic changes at the end of the period in which unimmunized control mice died of CM. These immunized mice did not display any signs of CM and appeared clinically normal. Nevertheless, their brains demonstrated conspicuous inflammatory hemorrhagic lesion (Fig. 5A). These lesions consisted of vessels largely occluded by monocytes, with infiltration of inflammatory cells into the brain parenchyma together with hemorrhage. Parasitized erythrocytes were especially prominent in the hemorrhagic foci. These lesions were observed in focal areas throughout the cortex and meninges (Fig. 5B). The widespread destruction of the microvasculature characteristic of the unimmunized mice with lethal CM was not present in immunized mice protected from lethal CM. In these mice, blood vessels away from the focal inflammatory lesions showed only mild changes consisting of swelling of endothelial cells and evidence of activation of glial cells. These changes were mild, and the blood vessels maintained their normal architecture and integrity (not shown).
FIG. 5.
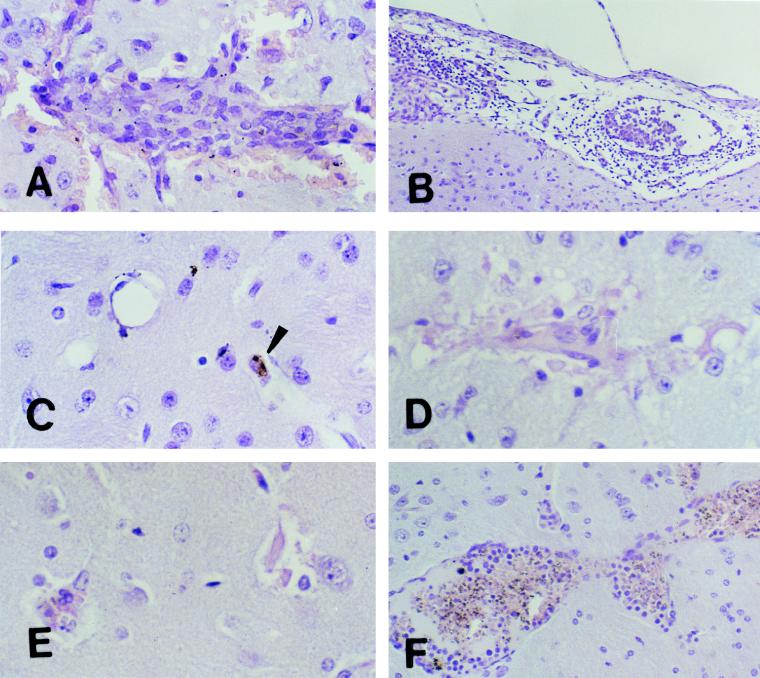
Morphologies of lesions in the brains of immunized C57BL/6J (A to C) and A/J (D to F) mice. Immunized C57BL/6J and A/J mice were challenged intravenously with 104 parasitized erythrocytes. C57BL/6J mice were clinically normal, with no signs of CM. In contrast, some A/J mice developed CM while others remained clinically normal, with no signs of CM. Brains were removed from both groups for histologic examination at different times. (A) Section of mouse brain on day 10 after challenge showing an inflammatory hemorrhagic vascular lesion in the cortex. Magnification, ×500. (B) Section of mouse brain and meninges on day 10 after challenge showing meningitis. The monocyte was the predominant inflammatory cell within both vessels and surrounding tissue. Magnification, ×200. (C) Section of mouse brain 21 days after challenge showing resolution and reversibility of the inflammatory and hemorrhagic lesions. Small hemosiderin deposits (arrowhead) are the only remnants of previous lesions. Magnification, ×500. (D and E) Sections of the brains of mice moribund with CM on day 8 after challenge. Changes in microglial cells, destruction of endothelial cells, and disruption of the vessel walls similar to that observed in C57BL/6J mice with CM are evident. The sections appear pale because of loss of cellular integrity. Magnification, ×500. (F) Section of the brain of a mouse protected from lethal CM on day 18 after challenge showing an intense inflammatory infiltrate within blood vessels made up primarily of activated lymphocytes, macrophages, and malarial pigment but in which the endothelium remains intact. These were focal lesions. Areas of the brains of these mice away from these lesions appeared normal. Magnification, ×200. All sections were stained with H&E.
The inflammatory lesions in immunized mice proved to be rapidly reversible. Groups of C57BL/6J mice that had been protected from lethal CM by immunization were sacrificed 21 days after challenge when they became clinically ill with progressive anemia and parasitemia. Sections of the brains of these mice were nearly normal (Fig. 5C). The inflammatory lesions had completely resolved in both the cortex and meninges. Small deposits of hemosiderin in the cortex were the only clearly identifiable remnants of the intense inflammatory reactions present earlier in the course of the disease. In addition, the endothelia of some vessels appeared to be regenerating in that the endothelial cell nuclei stained more darkly and more irregularly than normal nuclei in brain.
Histopathologic examination of the brains of normal and immunized A/J mice also produced surprising findings. Normal unimmunized A/J mice challenged with virulent parasites did not develop CM. They had essentially normal brains histologically throughout the course of the infection (not shown). A/J mice that had been immunized presented more complex histopathologic patterns. Those that developed clinical CM had lesions similar to those of naive infected C57BL/6J mice dying of CM. Widespread microglial activation, disruption of the microvasculature, death of endothelial cells, and hemorrhage with little or no inflammatory reaction occurred (Fig. 5D and E).
Focal mononuclear cell inflammatory lesions similar to those observed in immunized C57BL/6J mice were observed in some mice. In the brains of these A/J mice, the microvascular destructive lesions associated with lethal CM occurred only at a distance from the inflammatory lesions. The small vessels near the inflammatory lesions remained viable. This pattern was similar to that observed in C57BL/6J mice.
The largest inflammatory lesions found in A/J mice occurred in immunized mice that survived to day 19 without signs of CM. Large numbers of mononuclear cells and a few parasitized erythrocytes were adherent to the walls of dilated venules, with endothelial cells that appeared activated (Fig. 5F). Malarial or blood pigment was present in cells throughout the lesions. Vessels with these changes were present both in the deep cortex and in the meninges of the brain. These blood vessels had intact endothelial cells, and there was no evidence of hemorrhage. The surrounding microvasculature was normal, with no evidence of microglial or endothelial cell activation. Mice with these persistent inflammatory lesions showed no clinical signs of CM.
DISCUSSION
Previous investigators assumed that monocytes acted in concert with microglia and other CNS cells to produce cytokines in the pathogenesis of lethal murine CM (24). The key departure of results of the present study from earlier findings was the observation that florid mononuclear cell inflammation is not part of the pathogenesis of lethal CM but is part of a second pathological process that may actually contribute to protection. This observation was made possible because of the modulation of disease afforded by prior immunization of mice.
Immunization of CM-susceptible C57BL/6J mice with either plain or lipid-conjugated P. berghei antigen prevented CM in 100% of mice in several experiments. These mice survived to 3 weeks postinfection with no evidence of CM and died of overwhelming parasitemia. Surprisingly, the same immunizations that protected susceptible mice from CM enhanced it in resistant A/J mice. Over 80% of the immunized A/J mice died of typical CM between 7 and 10 days following challenge. These experiments confirmed previous reports that murine CM can be either suppressed or enhanced by immunization but failed to support our prior hypothesis that lipid conjugation of antigen changes the antigen’s ability to modulate CM. Histopathologic studies demonstrated a correlation of mononuclear cell inflammation with protection rather than with death from lethal CM. Collectively, these results demonstrate the existence of two distinct types of CM in mice. Many aspects of these experiments require discussion.
Two distinct histopathologic patterns were observed in the brains of C57BL/6J mice infected with P. berghei ANKA. The changes in unimmunized mice that developed lethal CM were identical to those that we and others have described previously (13). There was widespread microvascular destruction and focal mononuclear cell inflammation in some mice. The brains of C57BL/6J mice that had been protected from CM by immunization showed intense, but focal, mononuclear cell inflammation. Many vessels appeared occluded by monocytes. The inflammation extended into the brain substance and was accompanied by hemorrhage. However, these changes were reversible in spite of a progressive increase in parasitemia. Tiny deposits of hemosiderin pigment and irregular staining of endothelial cell nuclei were the only evidence of these lesions that remained at 3 weeks, when the mice became moribund.
Three distinct patterns of change were observed in the brains of A/J mice infected with P. berghei ANKA. First, the brains of unimmunized A/J that failed to develop CM upon challenge did not show either microvascular destruction or mononuclear cell infiltration. They appeared normal throughout the course. Second, the sections of brains of immunized mice that developed lethal CM were indistinguishable from those of C57BL/6J mice with the disease. They showed widespread microvascular destruction with little mononuclear cell inflammation. Finally, sections of brains of A/J mice that had been immunized and did not develop lethal CM showed especially prominent mononuclear cell inflammation of the vessels in the brain, which persisted through the course of the disease. These mice never showed signs of CM in spite of persistent focal inflammation in their brains and meninges.
These patterns of histopathologic lesions suggest the existence of two distinct pathologic processes of CM (Table 1). The first, resulting in type I lesions, consisted of widespread destruction of the microvasculature in the cortex of the brain with, at best, inconspicuous evidence of inflammation. Glial cells appeared activated beginning at day 3 after infection. This progressed to total destruction of vessel walls when the mice became moribund on day 7 or 8. Type I lesions were observed consistently in CM-susceptible C57BL/6J mice and in A/J mice made susceptible by immunization.
TABLE 1.
Characteristics of the two types of histopathologic lesions caused by murine CM
| Lesion type | Disease characteristics |
|---|---|
| 1 | Death within 18 to 24 h of CNS signs |
| Microglial and endothelial cell activation | |
| Production of TNF-α and IFN-γ within CNS | |
| Paucity of mononuclear inflammatory cells in CNS | |
| Widespread CNS microvascular destruction, including disruption of microvascular reticulum and conglomeration of material that reacts with HAM-56 staining | |
| Lesions observed in all naive C57BL/6J mice and 80% of immunized A/J mice | |
| 2 | Nonlethal, associated with protection |
| Reversible | |
| Focal mononuclear cell inflammation within brain and meninges | |
| Focal hemorrhage | |
| Minimal abnormalities visible in reticulum or with HAM-56 staining | |
| Lesions observed in all immunized C57BL/6J mice and 20% of immunized A/J mice |
The second, resulting in type II lesions, consisted of mononuclear cell inflammation centered around veins in the cortices and meninges of the mice brains. Type II lesions occurred in both C57BL/6J and A/J mice that had been immunized and protected from CM. Type II lesions were much more prominent on cursory examination than type I lesions, but they were focal. Most of the microvasculatures of the brains of mice with these lesions were intact and viable. Type I and type II changes were observed together in the brains of a few mice. In these instances, the microvascular breakdown and destruction were located at a distance from the foci of mononuclear inflammation. The vessels adjacent to the mononuclear cell foci appeared viable and intact, suggesting a local anatomic degree of protection.
In our murine infections with malaria parasites, C57BL/6J mice were highly sensitive to P. berghei-induced CM, which was associated with production of proinflammatory cytokines in the brain (13). Furthermore, we demonstrated that immunization of CM-resistant A/J mice increased susceptibility to type I CM. We hypothesized that this increased susceptibility involved induction of a TH1-type response which worked in conjunction with malarial products, such as malaria toxin or glycosylphosphatidylinositol. This response augmented synthesis of IFN-γ by microglial cells and astrocytes to produce TNF-α, IFN-γ, and interleukin 1B. TNF-α and IFN-γ, when they are produced simultaneously, are known to cause endothelial cell damage. Also, it has been shown that TNF-α may regulate CM induction via tumor necrosis factor receptors (15). Therefore, the widespread endothelial cell damage, which we have documented histologically, is most likely due to the action of these proinflammatory cytokines and can account for the severity of this syndrome. Resistance to CM in A/J mice appeared to be associated with two mechanisms. First, most naive A/J mice appear to be hyporesponders which fail to make a sufficient response to the parasites. Second, a fraction of the immunized A/J mice were protected from lethal CM. The protective immune response was associated with marked and persistent mononuclear cell inflammation in the brains in response to infection. In these mice and in the immunized C57BL/6J mice, immunization appears to have induced a response that suppressed lethal CM.
The responses of A/J and C57BL/6J mice to P. chabaudi malaria support this interpretation. A/J mice are sensitive to P. chabaudi and they fail to make a CMI response, while C57BL/6J mice are resistant because they make a strong CMI response (25, 26). Sensitivity in this disease means that the numbers of parasites grow to high levels and that the mice die at 3 weeks of anemia, while resistance means that they clear the parasites. A/J mice fail to make strong CMI responses. This fact makes them resistant to P. berghei CM but sensitive to uncontrolled parasitemia from P. chabaudi. C57BL/6J mice, in contrast, make stronger CMI responses that protect them from P. chabaudi and augment CM from P. berghei ANKA.
Our limited observations of the effect of immunization with lipid-conjugated antigen on the rate of growth of parasites add further support to this interpretation. Lipid conjugation of antigens increases their ability to stimulate CMI (11). In the present study and in that published by Curfs et al. (5), immunizations with conventional antigen prevented CM but failed to reduce the rate of increase of parasitemia. In contrast, parasites grew at a lower rate in mice immunized with lipid-conjugated antigens in this and our previous studies (22).
Finally, the relationship of these findings to human CM requires comment. The often repeated argument that P. berghei ANKA CM is not a good model of Plasmodium falciparum CM because it is characterized by mononuclear cell inflammation rather than sequestration of parasitized erythrocytes has been challenged on multiple grounds. Human CM, like the murine model, has been associated with the production of proinflammatory cytokines, especially TNF. Patients with CM typically have high levels of TNF in their circulation and tend to have a genetic phenotype of high-level expression of the TNF promoter (2, 4, 6, 8, 17).
The murine model has been criticized because it reflects mononuclear inflammation that is not characteristic of the human disease. The present study demonstrated that such inflammation is associated with protection from lethal CM and may not be part of CM’s pathogenesis. Such changes, if they occurred in humans, would be nearly impossible to demonstrate because biopsies are not made during recovery from CM.
Clinical and epidemiological experiments also provided evidence that type I and type II CM may occur in humans. Lethal CM in mice was associated with widespread microvascular destruction with minimal infiltration of inflammatory cells. The time from onset of signs of disease to death was less than 2 days. If the mice survived, the lesions cleared completely by 3 weeks. Patients with lethal human CM present similarly. Three-quarters of the deaths occur within 24 h of admission, and recovery is typically complete in survivors (12). The clinical sequellae of widespread endothelial cell damage, namely, papilloedema and sonographic features of progressive intracranial hypertension, are common in children with CM who die (14, 21). This time course and these clinical findings are consistent with type I diseases.
Type II murine CM occurred in immunized mice. It was focal, nonlethal, and associated with mononuclear cell inflammation and hemorrhage. One might expect such focal lesions to produce seizures and a spectrum of neurologic deficits that resolve in parallel with the inflammatory processes. Our methods were unable to detect such signs in mice. However, they do occur in humans (28). Many patients recovering from CM have focal neurologic signs such as paresis that resolve over a period of months. On the basis of clinical and laboratory features associated with ophthalmologic findings, Lewallen et al. suggested that there may be at least two different pathogenic processes in patients with CM (14). In a prospective study of 624 children with CM in The Gambia, multiple logistic regression analysis suggested that fatal outcome and neurologic sequellae arise from separate pathologic processes (28). It appears that both the human and the murine CM diseases are more complex processes than most of us appreciated.
Finally, the present study demonstrated that vaccines can modulate susceptibility to CM without affecting parasitemia. Most vaccines for malaria have attempted to prevent infection. However, many of our most valuable vaccines do not prevent infection. They prevent only disease or the worst manifestations of disease. Since people become resistant to CM with repeated exposure to the parasites, it seems clear that immune mechanisms can prevent human CM. Further studies aimed at understanding the potential of vaccines for modulating the various manifestations of CM and other forms of severe malaria seem a worthwhile goal.
ACKNOWLEDGMENT
This work was supported by grant AI31064 from the National Institutes of Health.
REFERENCES
- 1.Adams C W, Poston R N. Macrophage histology in paraffin-embedded multiple sclerosis plaques is demonstrated by the monoclonal panmacrophage marker HAM-56: correlation with chronicity of the lesion. Acta Neuropathol. 1990;80:208–211. doi: 10.1007/BF00308926. [DOI] [PubMed] [Google Scholar]
- 2.Allen S, Genton B, Alexander N, Gibson N, Raiko A. Malaria vaccine in children under 12 months of age. Lancet. 1995;346:1555–1556. [PubMed] [Google Scholar]
- 3.Barnwell J W, Howard R J, Coon H G, Miller L H. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun. 1983;40:985–994. doi: 10.1128/iai.40.3.985-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark I A, Ilschner S, MacMicking J D, Cowden W B. TNF and Plasmodium berghei ANKA-induced cerebral malaria. Immunol Lett. 1990;25:195–198. doi: 10.1016/0165-2478(90)90114-6. [DOI] [PubMed] [Google Scholar]
- 5.Curfs J H, Hermsen C C, Meuwissen J H, Eling W M. Immunization against cerebral pathology in Plasmodium berghei-infected mice. Parasitology. 1992;105:7–14. doi: 10.1017/s0031182000073625. [DOI] [PubMed] [Google Scholar]
- 6.de Kossodo S, Grau G E. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 7.Gown A, Tsokada T, Ross R. Human artherosclerosis II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986;125:191–207. [PMC free article] [PubMed] [Google Scholar]
- 8.Grau G E, Lou J N. Experimental cerebral malaria: possible new mechanisms in the TNF-induced microvascular pathology. Soz- Praevmed. 1995;40:50–57. doi: 10.1007/BF01615662. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology. 1997;114:7–12. doi: 10.1017/s0031182096008293. [DOI] [PubMed] [Google Scholar]
- 10.Hunter R L, Kidd M R, Olsen M R, Patterson P S, Lal A A. Induction of long-lasting immunity to Plasmodium yoelii malaria with whole blood-stage antigens and copolymer adjuvants. J Immunol. 1995;154:1762–1769. [PubMed] [Google Scholar]
- 11.Hunter R L, Stricklan F. Immunization with a lipid-conjugated membrane antigen to suppress growth of a fibrosarcoma induced by simian virus 40. J Natl Cancer Inst. 1975;54:1157–1163. doi: 10.1093/jnci/54.5.1157. [DOI] [PubMed] [Google Scholar]
- 12.Jaffar S, Van H M, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. Am J Trop Med Hyg. 1997;57:20–24. doi: 10.4269/ajtmh.1997.57.20. [DOI] [PubMed] [Google Scholar]
- 12a.Jennings V M. Ph.D. thesis. Atlanta, Ga: Emory University; 1998. [Google Scholar]
- 13.Jennings V M, Actor K J, Lal A A, Hunter R L. Cytokine profile suggesting that murine cerebral malaria is an encephalitis. Infect Immun. 1997;65:4883–4887. doi: 10.1128/iai.65.11.4883-4887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewallen S, Bakker H, Taylor T E, Wills B A, Courtright P, Molyneux M E. Retinal findings predictive of outcome in cerebral malaria. Trans R Soc Trop Med Hyg. 1996;90:144–156. doi: 10.1016/s0035-9203(96)90116-9. [DOI] [PubMed] [Google Scholar]
- 15.Lucas R, Juillard P, Decoster E, et al. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- 16.Ma N, Madigan M C, Chan L T, Hunt N H. Compromised blood-nerve barrier, astrogliosis, and myelin disruption in optic nerves during fatal murine cerebral malaria. Glia. 1997;19:135–151. doi: 10.1002/(sici)1098-1136(199702)19:2<135::aid-glia5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.McGuire W, Hill A V, Allsopp C E, Greenwood B M, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 18.Medana I M, Hunt N H, Chan L T. Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia. 1997;19:91–103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Medana I M, Hunt N H, Chaudhri G. Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes. Am J Pathol. 1997;150:1473–1486. [PMC free article] [PubMed] [Google Scholar]
- 20.Neill A L, Chan L T, Hunt N H. Comparisons between microvascular changes in cerebral and non-cerebral malaria in mice, using the retinal whole-mount technique. Parasitology. 1993;107:477–487. doi: 10.1017/s0031182000068050. [DOI] [PubMed] [Google Scholar]
- 21.Newton C R, Marsh K, Peshu N, Kirkham F J. Pertubations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol. 1996;15:41–49. doi: 10.1016/0887-8994(96)00115-4. [DOI] [PubMed] [Google Scholar]
- 22.Reed R C, Verhuel A F, Hunter R L, Udhayakumar V, Louis-Wileman V, Jennings V M, Jue D L, Wohlhueter R M, Lal A A. Rapid onset of malaria-induced mortality by immunizations with lipo-peptides: an experimental model to study deleterious immune responses and immunopathology in malaria. Vaccine. 1997;15:65–70. doi: 10.1016/s0264-410x(96)00103-x. [DOI] [PubMed] [Google Scholar]
- 23.Rudin W, Eugster H P, Bordmann G, Bonato J, Muller M, Yamage M, Ryffel B. Resistance to cerebral malaria in tumor necrosis factor-alpha/beta-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol. 1997;150:257–266. [PMC free article] [PubMed] [Google Scholar]
- 24.Rudin W, Favre N, Bordmann G, Ryffel B. Interferon-gamma is essential for the development of cerebral malaria. Eur J Immunol. 1997;27:810–815. doi: 10.1002/eji.1830270403. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson M M, Tam M F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi as infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 27.Takayama K, Olsen M, Dattam P, Hunter R L. Adjuvant activity of nonionic block copolymers. V. Modulation of antibody isotype by lipopolysaccharides, lipid A and precursors. Vaccine. 1991;9:257–265. doi: 10.1016/0264-410x(91)90109-j. [DOI] [PubMed] [Google Scholar]
- 28.van Hensbroek M B, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997;131:125–129. doi: 10.1016/s0022-3476(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 29.Yoeli M. Chadwick lecture. Cerebral malaria—the quest for suitable experimental models in parasitic diseases of man. Trans R Soc Trop Med Hyg. 1976;70:24–35. doi: 10.1016/0035-9203(76)90003-1. [DOI] [PubMed] [Google Scholar]