Abstract
BACKGROUND
Retention of patients in buprenorphine medication treatment for opioid use disorder (B-MOUD) reduces harms associated with opioid use disorder (OUD). We sought to characterize the patients receiving B-MOUD and courses of B-MOUD in a large healthcare system.
METHODS
We conducted a retrospective, open cohort study of patients with OUD who either did or did not receive B-MOUD courses within the Veterans Health Administration (VHA) from January 2006 through July 2019, using VHA clinical data. We compared patients receiving or not receiving B-MOUD, characterized B-MOUD courses (e.g., length and doses), and examined persistence, across patient characteristics, over time. We used analyses for normally or non-normally distributed continuous variables, categorical data, and persistence over time (Kaplan-Meier persistence curves).
RESULTS
We identified 255,726 Veterans with OUD; 40,431 (15.8%) had received 63,929 B-MOUD courses. Compared to patients with OUD without B-MOUD, patients with B-MOUD were younger, more often of white race, and had more co-morbidities. The frequency of new B-MOUD starts and prevalent B-MOUD patients ranged from 1,550 and 1,989 in 2007 to 8,146 and 16,505 in 2018, respectively. The median duration of B-MOUD was 157 (IQR: 37–537) days for all courses and 44% patients had more than one course. The average proportion days covered was 90% (SD: 0.15), and the average prescribed daily dose was 13.44 (SD: 6.5).
CONCLUSIONS
Within a VHA B-MOUD cohort, courses increased more than 10-fold from 2006 to 2016 with nearly half of patients experiencing multiple courses. Patient demographics seem to dictate the length of courses.
Keywords: Opioid-Related Disorders, Buprenorphine, Humans, Veterans
1.1. INTRODUCTION
In 2021 over 80,000 persons died of opioid-related events in the US, a 15% increase since 2020 and a 62% increase since 2019 (Centers for Disease Control and Prevention, 2022; Centers for Disease Control and Prevention National Center for Health Statistics, 2022). Both the incidence of opioid use disorder (OUD) and the risks of use contribute to mortality (Blanco and Volkow, 2019; Keyes et al., 2022; Substance Abuse and Mental Health Services Administration, 2020; Volkow, N. et al., 2017; Volkow, 2020; Volkow and Collins, 2017; Volkow, N.D. et al., 2017). Medications for opioid use disorder (MOUD)—including formulations of buprenorphine, naltrexone, and methadone—are the standard of care and are associated with reduced illicit opioid use, mortality, criminal activity, healthcare costs, and high-risk behaviors (Clausen et al., 2008; Gowing et al., 2011; Mattick et al., 2014; Thomas et al., 2014; Tkacz et al., 2014; Volkow and Collins, 2017; Volkow et al., 2014). In addition, MOUD improves patients’ quality of life (Giacomuzzi et al., 2005; Giacomuzzi et al., 2003; Ponizovsky and Grinshpoon, 2007; Ponizovsky et al., 2010). Outcomes improve with longer treatment duration; conversely, relapse to illicit use and mortality rise when MOUD ceases (Bentzley et al., 2015a; Bentzley et al., 2015b; Clausen et al., 2008; Degenhardt et al., 2009; Dunn et al., 2011; Gordon et al., 2015; Kakko et al., 2003; Lo-Ciganic et al., 2016). Thus, retention in treatment is an important clinical goal.
The US Department of Veterans Affairs’ Veterans Health Administration (VHA) is the largest direct provider of addiction care in the US (Hagedorn et al., 2018; Wyse et al., 2018). With the rise in opioid use disorder (OUD) among Veterans in the last two decades, the VHA has taken steps to improve access to MOUD, and VHA mandates that clinicians offer MOUD to all Veterans with OUD (Wyse et al., 2018). In 2022, more than 40% of patients seen in the VHA with OUD receive an MOUD formulation at any given time, compared to less than a third outside the VHA (Becker et al., 2020; Gordon et al., 2011; Gordon et al., 2007; Mauro et al., 2022; Oliva et al., 2012; Oliva et al., 2013; Radmall et al., 2022; Substance Abuse and Mental Health Services Administration, 2021).
Within the VHA, expansion of MOUD care has been primarily driven by increased access to buprenorphine (B-MOUD), similar to trends outside the VHA (Chen et al., 2022; Oliva et al., 2013; Stein et al., 2018). In the VHA, buprenorphine was a non-formulary medication—providers had to get an authorization to prescribe—until 2005. Buprenorphine then gained formulary status, making it available as a pharmacy benefit, without prior approval (Gordon et al., 2007). Since then, B-MOUD access has increased over time. In a 12-month period between 2016 and 2017, approximately 14,500 Veterans were treated with B-MOUD by 1,150 VHA prescribers (Wyse et al., 2018). As access to B-MOUD improved, clinical priorities shifted to retention of patients in care. Among a small sample of 3,151 Veterans initiated on B-MOUD in the VHA from October 2012 to March 31, 2013, Manhapra et. al. found the mean duration of treatment was 1.68 years; 61.6% were in treatment for more than one year and 31.8% for more than three years, figures comparable to non-VHA populations (Manhapra et al., 2018; Manhapra et al., 2017).
Despite this literature, there is minimal information about the historical characteristics of B-MOUD courses (or B-MOUD treatment episodes) within the VHA over time. Because the VHA is an integrated system serving as both insurer and provider, evidence derived from VHA may provide a more straightforward profile of patient-level and provider-level influences in the absence of payor restrictions commonly evident in traditional private insurance settings. VHA also provides comprehensive, generally well-resourced and team-based care, all factors that enhance access and retention in care in non-VHA environments (Bentzley et al., 2015a; Bentzley et al., 2015b; Hui et al., 2017; Oliva et al., 2011; Weinstein et al., 2017a; Weinstein et al., 2018; Weinstein et al., 2017b). Thus, the VHA may mitigate considerations regarding not only access to, but retention in care (e.g., insurance, geography) and highlight importance of patient-level and provider factors. Considering that the duration of OUD treatment correlates with clinical outcomes, we sought to examine B-MOUD medication retention within VHA, with careful consideration of potentially influential patient characteristics. Secondarily, we sought to describe characteristics of patients with OUD who received B-MOUD to those who did not receive B-OUD, and to profile their courses of care (e.g., number of courses per patient, typical prescription length, common doses).
1.2. MATERIALS and METHODS
1.2.1. General Study Design and Timeframe
We report a National Institute on Drug Abuse (NIDA) Clinical Trials Network Study—NIDA CTN-0087—a retrospective, open cohort study of Veteran patients with OUD who either did, or did not, obtain a B-MOUD course within the VHA from January 1, 2006, through July 30, 2019 (the designated study period). The units of observations were either (a) patients with OUD or (b) B-MOUD courses, depending on the specific analysis. Patients qualified for analysis by either having a documented OUD diagnosis (OUD patient cohort) or documentation of B-MOUD dispensation (B-MOUD patient cohort) in the electronic medical record. B-MOUD courses were followed until the end of the treatment episode (defined as the last day of the prescribed B-MOUD), until the end of the study period, or patient death. We disseminate the results of this study consistent with the STROBE reporting guidelines for observational studies in epidemiology (von Elm et al., 2007). The University of Utah and the VA Salt Lake City Institutional Review Board approved this study.
1.2.2. Data Source
Data were obtained from the VHA’s Corporate Data Warehouse (CDW) within the VA’s Veterans Informatics and Computing Infrastructure (VINCI) framework. In brief, the CDW contains all clinical, laboratory, pharmacy, and other administrative data for care provided by VHA. The CDW data domains for this study included patient (e.g., demographics) characteristics, inpatient and outpatient encounters, and inpatient and outpatient pharmacy dispensing. We only used data on VHA services, as non-VHA care was not consistently recorded in the CDW over the study period. We also used the Compensation and Pension Records Interchange (CAPRI) system, which provides national “read-only” access to Veteran electronic health records and medical notes available in CDW for data quality review and to inform algorithm development and confirm correct classification of clinical concepts.
1.2.3. Study Population and Inclusion and Exclusion Criteria
Among patients in CDW, we defined a B-MOUD patient cohort of Veterans with OUD and who had a documented dispensation of buprenorphine formulations for OUD from VHA pharmacies, or the consolidated mail order pharmacy. Veterans were identified as having an OUD if they had an inpatient or outpatient International Classification of Disease 9th or 10th revision (ICD-9 or ICD-10) coded diagnosis of opioid abuse or opioid dependence. Buprenorphine formulations indicated for B-MOUD by the US Food and Drug Administration were used to define the B-MOUD patient cohort; patients who received courses of buprenorphine formulations indicated for pain were excluded from the B-MOUD cohort. Sublingual proprietary and generic buprenorphine or buprenorphine/naloxone tablets and films were formulary medications and readily available during the study period. The year 2006 was chosen as the first full year of the study period; for that complete year, buprenorphine formulations were formulary medications in the VHA. Extended-release buprenorphine or implantable buprenorphine products were non-formulary during the study period. VHA RxNorm was used to identify a list of brands and generic products containing buprenorphine with indications for OUD (Cimino and Zhu, 2006).
We also defined an OUD patient cohort of those with a documented diagnosis of OUD, but who did not receive B-MOUD within the study period. Patients with OUD who received buprenorphine prescriptions indicated for pain (formulations not approved for OUD treatment) were included in this OUD patient cohort. Patients were excluded if they were not Veterans or had inconsistent or invalid patient identifiers. Within this OUD patient cohort, we defined a cohort who received methadone treatment. These patients were identified by receiving at least four VHA opioid treatment program visits in any calendar year, consistent with how methadone treatment for OUD has been defined in previous studies (Wyse et al., 2021). If a patient received courses of buprenorphine and also methadone, they were included in the B-MOUD cohort.
Patient integrated control number (ICN) is the national Veteran identifier that should have a one-to-one relationship with each patient social security number. In both mutually exclusive cohorts, patients were removed when multiple social security numbers were mapped to one patient ICN or multiple patient ICNs mapped to one social security number. The baseline periods were defined in each cohort, as the year prior to either (a) the day of OUD diagnosis or (b) the day of B-MOUD initiation (index dates); baseline periods were used to describe patient characteristics (e.g., age, demographics, co-morbidities).
1.2.4. Measures
Within the CDW, patient demographic characteristics such as age, gender, race, and ethnicity were obtained in the baseline period. Race was obtained from the CDW’s Patient Race table, which uses self-reported race when available. The most frequent race was chosen when patients listed more than one race category; a race was selected randomly when patients listed more than one race at an equal frequency. We applied the Healthcare Cost and Utilization Project (HCUP) Clinical Classifications System Revised (CCSR) and an in-house developed crosswalk with ICD-9/ICD-10 codes, to outpatient and inpatient discharge data to classify physical health, mental health, and SUD comorbidities (see Supplementary Material 1 for codes).
1.2.5. Outcomes
The primary study outcome was buprenorphine medication persistence among the B-MOUD courses. The CDW had data regarding prescription metrics including formulation, dose, and the days of medication supplied. We defined a B-MOUD course that began with the first observed buprenorphine dispensing event or the first dispensing event after a 30-day gap in treatment. A B-MOUD course end date was defined as the last day of the dispensing event before a 30-day gap in BUP dispensing events. A gap of 30 days was chosen as a conservative criterion to indicate a period without B-MOUD, where the Veteran or provider intentionally ended the B-MOUD course; this approach is typical for a course of medication treatment calculated in in the general pharmacoepidemiologic literature (Stein et al., 2022).
If the patient had B-MOUD and refills of this medication, we examined the length of the prescription (e.g., 7, 14, 28, 30 days) and examined the date of the next dispensation of B-MOUD. If the patient had dispensation of less than 30 days from the last date that would have been covered from the prescription to the date of the dispensation, then the patient was deemed to have continued their treatment course. For example, if the patient received an initial dispensation on January 1, 2010, for 30 days, then received a second dispensation at 45 days after January 1, 2010, for 30 days, this was considered a continuous episode of care or “course”. If the patient did not receive the second dispensation 61 days after January 1 (i.e., 30 days after January 30, 2010), then there was a gap of treatment of at least 30 days, and this treatment episode was considered discontinued.
Medication persistence refers to continuing treatment for a prescribed duration and course duration (Cramer et al., 2008). Our primary measures of adherence are the proportion of days covered (PDC) and the Medication Possession Ratio (MPR) (Hess et al., 2006; Peterson et al., 2007). The PDC reflects the proportion of available days that the patient has B-MOUD (with a maximum of 100%), while MPR may in some ways indicate how medication is used. Notably, the MPR will be greater than 100% if the medication is consistently refilled early.
We also evaluated the number of courses of B-MOUD received per patient. Additionally, during the courses of B-MOUD, we examined the characteristics of the course (e.g., mean, mode, median, range) of (a) the length (days) of the prescriptions and (b) the average daily doses (in mg). Dispensing events aggregated multiple doses dispensed on the same day into a single dose and days-supply. For example, if the patient received an 8mg and 4mg buprenorphine product on the same day with the same days-supply, and instructions were to take one per day, then the daily dose was recorded as 12mg per day. The algorithms also accounted for situations where multiple doses of one product strength occurred alone or in combination with different unit strengths. For example, the algorithms accounted for specifications for the patient to take two 8mg and one 4mg buprenorphine dose per day or other complex dosing schedules that included initiation and titration. We calculated an average prescribed dose as the average of prescribed daily dose for each dispensing event during a course (total mg dispensed during course/sum of days supplied during a course). The average prescribed dose generally represents the dose the prescriber intended. We also calculated an average observed dose or the average daily dose when gaps in dispensing events are included in the denominator (total mg dispensed during course/days during course).
1.2.6. Data Quality
Effort was made to identify records in pharmacy dispensing data that indicated a B-MOUD was received by the patient. Dispensing records in pharmacy data occur for many reasons and in some VHA facilities, a record is prepopulated for the next available dispense date. Pharmacy database workflows were established to remove records that did not appear to represent treatment received by the patient but rather reflected return to stock, dispense date but no fill or release date. Duplicates, where the same product and strength were released on the same day were removed. Partial fills with duplicate dates were combined. Daily doses of <1mg and >40mg were flagged and updated from the medication schedule and days-supply.
1.2.7. Analyses
We described population characteristics and B-MOUD courses of care in Veteran patients with an OUD diagnosis using means and 95% Confidence Intervals (CI) for normally distributed continuous variables, median and interquartile range (IQR) for non-normally distributed continuous variables, and proportions and 95% CI based on binomial probability for categorical data. Persistence with B-MOUD was described using the Kaplan-Meier method to estimate days to discontinuation during the study period and the first year of a B-MOUD course. B-MOUD courses were right-censored if the patient died or remained persistent on buprenorphine at the end of the follow-up period. We examined and compared courses of those who persisted on B-MOUD at the end of the study period against those who discontinued all courses prior to the end of the study period. The Kaplan-Meier method provided visual comparisons of days to B-MOUD discontinuation by age group, and race.
1.3. RESULTS
Within the period of January 1, 2006 through July 30, 2019, 255,726 Veterans had an OUD diagnosis, and 40,431 (15.8%) received B-MOUD over 63,929 courses (B-MOUD patient Cohort), while 215,295 did not (“OUD patient cohort”). When comparing Veterans receiving any B-MOUD to the OUD patient cohort, similarities and differences were noted (TABLE 1). Large majorities of both cohorts were male. The average age of Veterans in the B-MOUD and OUD patient cohorts were 43.6 years versus 52.8 years, respectively. Those receiving B-MOUD were more likely to be White (79.9%) and less likely to be Black (13.7%) compared to receipt of methadone (47.4% White, 44.9% Black); 68.6% of whites and 23.0% of Blacks did not receive either buprenorphine or methadone during the analytic period.” At baseline, 72.3% of the B-MOUD cohort and 75.7% of the OUD cohort had a mental health condition, which increased to 95.1% for the B-MOUD cohort and 89.8% for OUD cohort when ascertained during the study period.
TABLE 1:
Demographics of the BUP, Methadone, and OUD Cohorts
| B-MOUD Patient Cohort (n=40,431) | M-MOUD Patient Cohort (OUD without receipt of B-OUD) (n=10,281) | OUD Patient Cohort (OUD without receipt of B-OUD or M-OUD) (n=205,014) | |||||
|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| Age | Mean, std and (95% CI) | 43.6 (13.5) | 43.5–43.8 | 52.5 (9.9) | 52.3–52.7 | 52.8 (13.1) | 52.8–52.9 |
| 18–29 | 9031 | 22.3 (21.922.7) | 514 | 5.0 (4.6–5.4) | 14859 | 7.3 (7.1–7.4) | |
| 30–39 | 8828 | 21.8 (21.422.2) | 650 | 6.3 (5.9–6.8) | 22981 | 11.2 (11.1–11.3) | |
| 40–49 | 7162 | 17.7 (17.318.1) | 1806 | 17.6 (16.8–18.3) | 33315 | 16.3 (16.1–16.4) | |
| 50–59 | 10470 | 25.9 (25.526.3) | 5522 | 53.7 (52.7–54.7) | 71325 | 34.8 (34.6–35.0) | |
| 60+ | 4939 | 12.2 (11.912.5) | 1789 | 17.4 (16.7–18.1) | 62529 | 30.5 (30.3–30.7) | |
| Unknown | 1 | 0 (0.0–0.0) | 0 | 0 (0.0–0.0) | 5 | 0 (0.0–0.0) | |
| Gender | Male | 37503 | 92.8 (92.5–93.0) | 9953 | 96.8 (96.4–97.1) | 191586 | 93.5 (93.3–3.6) |
| Race | White | 32293 | 79.9 (79.5–80.3) | 4868 | 47.4 (46.4–48.3) | 140629 | 68.6 (68.4–68.8) |
| Black | 5523 | 13.7 (13.3–14.0) | 4618 | 44.9 (44.0–45.9) | 47118 | 23.0 (22.8–23.2) | |
| Other | 2615 | 6.5 (6.2–6.7) | 795 | 7.7 (7.2–8.3) | 17267 | 8.4 (8.3–8.5) | |
| Ethnicity | Not Hispanic/ Latino | 37047 | 91.6 (91.491.9) | 9261 | 90.1 (89.5 90.6) | 186344 | 90.9 (90.891.0) |
| Hispanic/Latino | 2281 | 5.6 (5.4–5.9) | 740 | 7.2 (6.7–7.7) | 10982 | 5.4 (5.3–5.5) | |
| Unknown | 1103 | 2.7 (2.6–2.9) | 280 | 2.7 (2.4–3.1) | 7688 | 3.8 (3.7–3.8) | |
| MENTAL HEALTH CONDITIONS | |||||||
| Any Mental Health Condition | 29241 | 72.3 (71.972.8) | 5808 | 56.5 (55.5–57.4) | 157133 | 76.7 (76.5–76.8) | |
| Depressive disorders | 18772 | 46.4 (45.946.9) | 3514 | 34.2 (33.3–35.1) | 107122 | 52.3 (52.0–52.5) | |
| Trauma- and stressor-related disorders | 15035 | 37.2 (36.737.7) | 2601 | 25.3 (24.5–26.1) | 79546 | 38.8 (38.6–39.0) | |
| Anxiety and fear-related disorders | 11775 | 29.1 (28.729.6) | 1536 | 14.9 (14.3–15.6) | 58529 | 28.6 (28.4–28.7) | |
| Suicidal ideation/attempt/intentional self-harm | 4411 | 10.9 (10.611.2) | 519 | 5.1 (4.6–5.5) | 30077 | 14.7 (14.5–14.8) | |
| Bipolar and related disorders | 3775 | 9.3 (9.1–9.6) | 597 | 5.8 (5.4–6.3) | 24171 | 11.8 (11.7–11.9) | |
| Other specified and unspecified mood disorders | 2943 | 7.3 (7.0–7.5) | 373 | 3.6 (3.3–4.0) | 19826 | 9.7 (9.5–9.8) | |
| Personality disorders | 2521 | 6.2 (6.0–6.5) | 549 | 5.3 (4.9–5.8) | 18616 | 9.1 (9.0–9.2) | |
| Schizophrenia spectrum and other psychotic disorders | 2488 | 6.2 (5.9–6.4) | 856 | 8.3 (7.8–8.9) | 25196 | 12.3 (12.1–12.4) | |
| Disruptive, impulse-control and conduct disorders | 990 | 2.5 (2.3–2.6) | 245 | 2.4 (2.1–2.7) | 7471 | 3.6 (3.6–3.7) | |
| Somatic symptom disorders | 862 | 2.1 (2.0–2.3) | 186 | 1.8 (1.6–2.1) | 5533 | 2.7 (2.6–2.8) | |
| Obsessive-compulsive and related disorders | 386 | 1.0 (0.9–1.1) | 55 | 0.5 (0.1–1.1) | 2326 | 1.1 (1.1–1.2) | |
| Miscellaneous mental and behavioral disorders/conditions | 3194 | 7.9 (7.6–8.2) | 878 | 8.5 (8.0–9.1) | 23308 | 11.4 (11.2–11.5) | |
| SUBSTANCE USE CONDITIONS | |||||||
| Tobacco-related disorders | 14933 | 36.9 (36.537.4) | 3943 | 38.4 (37.4–39.3) | 84363 | 41.2 (40.9–41.4) | |
| Alcohol-related disorders | 11670 | 28.9 (28.429.3) | 3010 | 29.3 (28.4–30.2) | 83854 | 40.9 (40.7–41.1) | |
| Stimulant-related disorders | 7737 | 19.1 (18.819.5) | 2964 | 28.8 (28.0–29.7) | 52362 | 25.5 (25.4–25.7) | |
| Inhalant-related disorders | 7183 | 17.8 (17.418.1) | 1738 | 16.9 (16.2–17.6) | 31662 | 15.4 (15.3–15.6) | |
| Amphetamine-related disorders | 3578 | 8.9 (8.6–9.1) | 532 | 5.2 (4.8–5.6) | 11271 | 5.5 (5.4–5.6) | |
| Cannabis-related disorders | 5443 | 13.5 (13.113.8) | 994 | 9.7 (9.1–10.3) | 40750 | 19.9 (19.7–20.1) | |
| Sedative-related disorders | 2830 | 7.0 (6.8–7.3) | 628 | 6.1 (5.7–6.6) | 14388 | 7.0 (6.9–7.1) | |
| Hallucinogen-related disorders | 430 | 1.1 (1.0–1.2) | 85 | 0.8 (0.6–1.0) | 2720 | 1.3 (1.3–1.4) | |
| Other specified substance-related disorders | 14924 | 36.9 (36.437.4) | 4063 | 39.5 (38.6–40.5) | 67138 | 32.8 (32.5–33.0) | |
| Mental and substance use disorders in remission | 7726 | 19.1 (18.719.5) | 2490 | 24.2 (23.4–25.1) | 59375 | 29.0 (28.8–29.2) | |
TABLE 2 characterizes buprenorphine courses by course duration, PDC and MPR, average prescribed daily dose, and average observed daily dose. Three groups are presented: all B-MOUD courses observed in the study, courses active at the end of the study period, and courses that met criteria for B-MOUD discontinuation before the end of the study period. The median durations of B-MOUD courses, after censoring patients who died, were: 157 (IQR: 37–537) days for all courses; 618 (IQR: 202–1,495) days for the 15,132 courses that were active at the end of the study period; and 105 (IQR: 27–328) days for the 48,797 courses that were discontinued. For patients (n=26,781) who had only one B-MOUD course, the median durations of B-MOUD courses, after censoring patients who died, were: 213 (IQR: 45–796) days for all courses; 741 (IQR: 252–1750) days for the 9,623 courses that were active at the end of the study period; and 100 (IQR: 24–354) days for the 17,158 courses that were discontinued. Measures of adherence (PDC and MPR) indicated patients did not experience many delays in fills or gaps <30 days during their buprenorphine treatment courses. Patients with an active B-MOUD course at the end of the study period had higher daily doses (median 15.54, IQR: 10–19.21) compared to discontinued courses (median 12.77, IQR: 8–16).
TABLE 2:
Buprenorphine course descriptions
| All Buprenorphine Courses | Course Active 7/31/2019 | Discontinued Courses | ||||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % (row) | Frequency | % (row) | |
| Number of Courses | 63929 | 100 | 15132 | 24 | 48797 | 76 |
| Number of patients | 40431 | 15132 | 30817 | |||
| Number of Deaths (by the end of study) | 5572 | 55 | 5517* | |||
| Total Follow up time (to the last VA visit or death) | ||||||
| Mean (std) | Median (IQR) | Mean (std) | Median (IQR) | Mean (std) | Median (IQR) | |
| Drug Course Duration (days) | 461 (729) | 158 (38–538) | 1009 (1055) | 619 (203–1496) | 291 (478) | 106 (28–329) |
| Drug Course Duration (days) ** | 460 (729) | 157 (37–537) | 1008 (1055) | 618 (202–1495) | 290 (478) | 105 (27–328) |
| Proportion of Days Covered (PDC) | 0.90 (0.15) | 0.96 (0.88–1) | 0.93 (0.11) | 0.97 (0.91–0.99) | 0.89 (0.16) | 0.96 (0.86–1) |
| Medication Possession Ratio (MPR) | 0.96 (0.21) | 1 (0.92–1.01) | 0.98 (0.14) | 1 (0.96–1.02) | 0.95 (0.22) | 1 (0.90–1.01) |
| Average Prescribed Dose (mg per day) | 13.44 (6.50) | 13.66 (8–16.51) | 14.88 (6.42) | 15.54 (10–19.21) | 13.00 (6.46) | 12.77 (8–16) |
| Average Observed Dose (mg per day) | 12.93 (7.07) | 12.35 (7.81–16.58) | 14.61 (6.80) | 14.93 (9.32–19.02) | 12.41 (7.08) | 11.79 (7.33–16) |
PDC=Proportion of days covered; MPR=medication possession ratio; mg=milligrams.
Among them, 627 patients died before the drug course end.
censored for patients who died during the drug course
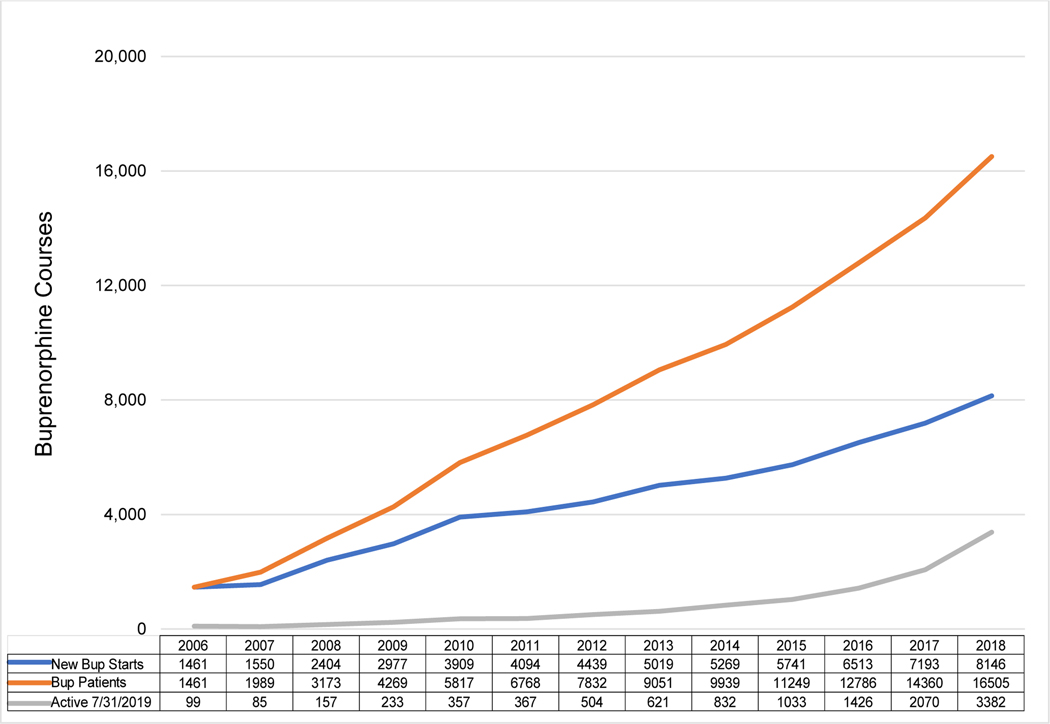
FIGURE 1 displays the annual trends for the three B-MOUD groups over time: new B-MOUD courses, any B-MOUD course during the calendar year, and the number of B-MOUD courses started in the calendar year that are active at the end of the study period. New B-MOUD courses increased annually and ranged from 1,461 in 2006 to 8,146 in 2018, the last full calendar year of the study. The number of B-MOUD courses during each calendar year ranged from 1,461 in 2006 to 16,505 in 2018. Ninety-nine patients who initiated their B-MOUD course in 2006 remained persistent until the end of the study period. The number of Veterans who remained persistent on B-MOUD at the end of the study period increased from 157 in 2008 to 15,132 the end of the study period.
FIGURE 1. ANNUAL TRENDS IN BUPRENORPHINE USE FOR NEW BUP COURSES, VETERANS ON BUPRENORPHINE, AND BUPRENORPHINE COURSES ACTIVE AT THE END OF 2018.
BUP = Buprenorphine. BUP Patients indicate count of unique Veterans receiving buprenorphine.
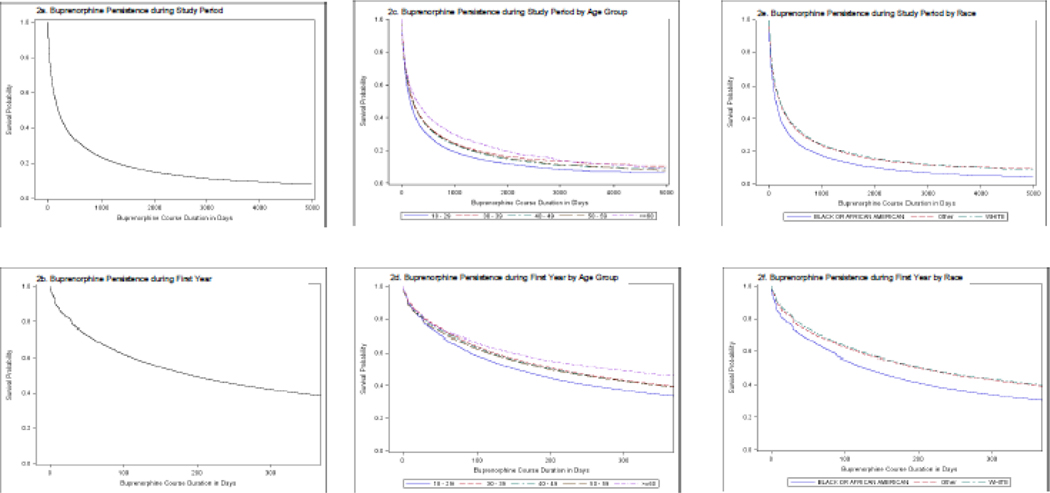
Persistence curves for B-OUD are shown for the entire study period (FIGURE 2a-c) and restricted to the first year of follow-up after initiating a B-MOUD course (FIGURE 2d-f). Notably, Figure 2b shows that half of all buprenorphine courses were discontinued by 180 days. The persistency curves by age (FIGURES 2c & 2d) show that the youngest age group (18–29) discontinued B-MOUD sooner than the others, with less than 40% persisting by the end of the first year. Those 60 and older had the highest persistence, with 50% remaining on their buprenorphine course at the end of the first year. The other age groups (30–39, 40–49, 50–59) displayed similar persistence at the end of the first year.
FIGURE 2. BUPRENORPHINE PERSISTENCE CURVES DURING FULL STUDY PERIOD AND RESTRICTED TO THE FIRST YEAR.
The number of B-MOUD courses per Veteran ranged from 1 to 14 (Supplemental Material 2); 66% patients had one B-MOUD course, 20% had two courses, 8% had three courses, and 6% had four or more courses. Within the B-MOUD course, there were 2,048 unique daily doses (mean 15.6, SD 7.3, range 1 to 64 mg) (Supplemental Material 3). The most common dose (mode) was 16mg, followed by 24mg, 8mg, 12mg, 20mg, 4mg, and 32mg daily doses. There were 94 distinct days of prescribed doses of buprenorphine in the B-MOUD patient cohort. The mode days’ supply was 28 days (mean 18.6%, 10.8 SD), with the minimum days dispensed one day and the maximum 90 days. The next most frequent dispensed intervals were 8, 30,14, 2, and 21 days. (Supplemental Material 4)
1.4. DISCUSSION
We examined the history of B-MOUD in the VHA by examining the characteristics of B-MOUD courses and the patients who received them. We found that from 2006 to 2019—a time when B-MOUD was on formulary—over 40,000 Veterans (representing >15% of unique Veterans with OUD) experienced over 63,000 courses of B-MOUD. A vast majority of patients had only one course of B-MOUD. Course duration increased over time, and courses were characterized by relatively high doses of buprenorphine daily doses (mode 16mg, mean 15.6mg) with common prescription fills of 28 days. Compared to those who had OUD without receiving B-MOUD, patients with a B-MOUD treatment episode were younger and more likely to not be of a minority race or ethnicity.
The number of new B-MOUD courses and the length of B-MOUD courses increased over time. Over this period, the VHA exerted itself to increase access to B-MOUD in both specialty SUD environments and also non-SUD clinical environments (Becker et al., 2020; Wyse et al., 2018). In 2005, the buprenorphine formulations for B-MOUD became available for all Veterans, where indicated, without prior approval. Later, Criteria for Use documents of VHA’s Pharmacy Benefits Management Service were established, then iteratively liberalized as more supporting evidence became available, allowing for increased prescribing (e.g., allowing refills, encouraging B-MOUD in non-specialty environments, mandating continuation in residential addiction settings). By 2022, the VHA had eliminated the Criteria for Use documents entirely, and B-MOUD had become a standard of care treatment without restrictions. The 2008 Department of Veterans Affairs/Department of Defense Clinical Practice Guidelines for SUD and two subsequent iterations recommended B-MOUD as first-line therapy (Perry et al., 2022; United States Department of Veterans Affairs and Department of Defense, 2015).
A plethora of studies have examined patient-, provider-, and system-level barriers to the provision of B-MOUD within the VHA (Gordon et al., 2020; Gordon et al., 2011; Gordon et al., 2022; Gordon et al., 2008; Hawkins et al., 2021; Manhapra et al., 2017; Manhapra et al., 2016; Manhapra et al., 2020; Oliva et al., 2012; Wyse et al., 2018). During our study timeline, the VHA provided resources to improve access to quality B-MOUD care. For example, by 2008, the VHA created the Buprenorphine in the VA (later renamed to the Medication Addiction Treatment in the VA Initiative). With this Initiative’s monthly national webinars, ad-hoc consultations, and education resources, it sought to disseminate knowledge and skills to provide B-MOUD. Recognizing that nearly all B-MOUD was provided in SUD specialty settings, in 2018, the VHA created the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) Initiative with a mission to improve access to B-MOUD in primary care, general mental health, and pain clinic settings (Gordon et al., 2020; Hawkins et al., 2021). As of 2022, at any given time, in the VHA, over 40% of patients with OUD are on either methadone, buprenorphine, or naltrexone for MOUD (Wyse et al., 2022). These data confirm that even with a rise of the number of Veterans with OUD over time, B-MOUD access and retention accelerated (Oliva et al., 2013).
“Patients in the VHA who initiated B-MOUD differed from those who did not. Racial and ethnic disparities are known to exist to access MOUD and even the type of MOUD patients receive both in VHA and non-VHA environments (Goedel et al., 2020; Manhapra et al., 2020). Our retention results also were consistent with the literature; minority patients tend to have shorter MOUD (and B-MOUD) treatment courses than non-minority patients (Manhapra et al., 2018). While our current results are unable to specifically determine the reasons for the inequity we identified in access to B-MOUD, the opioid prescribing literature provides some putative reasons for the disparity (Burgess et al., 2016; Burgess et al., 2013; Burgess et al., 2014). The reasons for the inequity may be multifactorial and could include how OUD itself originated (more often in pain care, for Whites), tacit bias among clinicians, patient preference, or variations in treatment-seeking among White and Black patients. These factors merit future primary data collection and analytic efforts. In addition, research is needed to compare how patient factors like race and ethnicity may influence retention, daily doses, and settings of care.”
Looking at the characteristics of B-MOUD treatment courses, we found typical doses of single or combination of buprenorphine tablet strengths. The VHA has almost exclusively used brand name or generic tablets in buprenorphine doses of 8mg or 2mg. Thus, it is not surprising that the modal dose was 16mg/d. The next most common dose, 24mg/d, was the maximum recommended dose in the package insert for buprenorphine products from Food and Drug Administration and many of the VHA’s Criteria for Use documents. Prior work has found that up to 32mg/d have been prescribed in the VHA (Fareed et al., 2012). Further evaluation should examine whether typical daily doses of B-MOUD in the VHA changed over time. These studies should assess whether rising doses of B-MOUD contributed to B-MOUD retention, given rising concern that low B-MOUD daily doses may not sufficiently treat patients exposed to opioids of increasingly high potency. Higher pill counts per prescription may encourage diversion. However, our persistence results hint at a high degree of adherence to B-MOUD prescriptions. New formulations of buprenorphine (e.g., extended-release buprenorphine) are being studied and increasingly used in the VHA, and these could further alleviate diversion concerns (Petrakis et al., 2022).
There are limitations of these findings. We did not assess the environment, setting, or other context of B-MOUD courses, as we opted to concentrate on patient and B-MOUD course characteristics at the outset. For example, it is well known that access to B-MOUD varies greatly by VHA facility (Gordon et al., 2011; Manhapra et al., 2017; Oliva et al., 2012; Wyse et al., 2018). As our study period ended in 2019, we did not assess the impact of COVID on access and retention of B-MOUD within the VHA. We chose not to examine data regarding B-MOUD offered in implantable buprenorphine or extended-release formulations, as implantable buprenorphine was not available in the VHA and extended-release buprenorphine medication only recently (2022) became a VHA formulary medication. Our administrative data cannot assess the reasons for B-MOUD discontinuation and specifically whether discontinuation reflected primarily patient, provider, or system factors. Subsequent investigations should examine reasons for discontinuation in the VHA as typical barriers to retention (e.g., loss of insurance, costs of treatment, geography) may be mitigated in the VHA system of care.
Despite these limitations, our results provide a snapshot of the history of B-MOUD in the VHA. We examined select characteristics of not only who initiates B-MOUD care, but characteristics of B-MOUD treatment courses. This work highlights some of the major policy and prescription frequency changes of B-MOUD in the VHA over time and its potential effect on B-MOUD utilization by veterans with OUD. In this large system of care, we found that patients are increasingly accessing this care, and are increasingly being retained for longer courses of care, over time. Patients tend to be adherent to their treatment courses. The results do suggest an equity shortfall in regard to who receives this care, most notably in regard to lower receipt among Blacks in comparison to Whites. Acknowledging the need to uncover and remediate this matter in particular, rising B-MOUD access and length of treatment in the VHA may be instructive to other health systems as the US continues to confront the increasing harms of OUD.
Supplementary Material
HIGHLIGHTS.
We describe courses of patients who received buprenorphine for opioid use disorder
Over 15% of Veterans with opioid use disorder received 63,929 buprenorphine courses
Veterans who had buprenorphine courses were different than those who did not
Buprenorphine courses were characterized
ACKNOWLEDGEMENTS
We thank the staff of the NIDA Clinical Trials Network for their participation in the study design and execution. NIDA staff’s (Udi E. Ghitza’s) participation in this publication arises from his role as a project scientist on a cooperative agreement (3UG1DA040316-04S3 and 1UG1DA049444-01), but Dr. Ghitza has not had and will not have any programmatic involvement with the non-cooperative agreement grants cited. We also thank the staff of the Greater Intermountain Node of the NIDA Clinical Trials Network.
ROLE OF FUNDING SOURCE
Research reported in this publication was supported by Helping to End Addiction Long-term® Initiative or NIH HEAL Initiative® under award numbers 3UG1DA040316-04S3 and 1UG1DA049444-01. The work was also supported by the National Institute on Drug Abuse (NIDA) Clinical Trials Network. Institutional support for this work occurred from the VA Salt Lake City Health Care System’s Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation (CIN 13-414); Program for Addiction Research, Clinical Care, Knowledge, and Advocacy (PARCKA) at the University of Utah; and the Vulnerable Veteran Innovative PACT (VIP) Initiative at the VA Salt Lake City Health Care System.
Staff of the Center for the Clinical Trials Network in the National Institute on Drug Abuse Clinical Trials Network had an advisory role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, or review of the manuscript; and decision to submit the manuscript for publication. Supporting organizations including the NIH, VHA, and academic institutions, had no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors are solely responsible for the content of this article. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Federal Government, including the Veterans Health Administration, the National Institute of Health, National Institute on Drug Abuse, or any of the authors’ academic affiliates.
CONFLICTS OF INTEREST
AJG receives an honorarium for an online chapter on alcohol management in the perioperative period from Wolters-Kluwer; is on the board of directors (not-for profit; not remunerated) for the American Society of Addiction Medicine (ASAM), the Association for Multidisciplinary Education and Research in Substance use and Addiction (AMERSA), and the International Society of Addiction Journal Editor (ISAJE), all non-for profit organizations; and receives current grant support from the Veterans Health Administration (VHA) and NIH. SGK receives an honorarium for an online chapter on homeless health care from Wolters-Kluwer, and research grant funding from the Veterans Health Administration (VHA). He serves on the board of scientific advisors (not-for-profit; not remunerated) of both the National Pain Advocacy Center and the Albert Schweitzer Fellowship, Inc. He reports current ownership of stock in medical product companies unrelated to this topic, Zimmer Biomet, Dow and Thermo Fisher. He reports stock ownership in CVS/Caremark in 2020, only.
ABBREVIATIONS
- B-MOUD
Buprenorphine Medication treatment for Opioid Use Disorder
- CAPRI
Compensation and Pension Records Interchange
- CCSR
Clinical Classifications System Revised
- CDW
Corporate Data Warehouse
- DoD
US Department of Defense
- HCUP
Healthcare Cost and Utilization Project
- ICD-9/ICD-10
International Classification of Disease, ninth or tenth edition
- ICN
Integrated Control Number
- MG
Milligram
- MG/D
Milligram/Day
- MOUD
Medication treatment for Opioid Use Disorder
- MPR
Medication Possession Ratio
- OUD
Opioid Use Disorder
- PDC
Proportion Days Covered
- SUD
Substance Use Disorder
- VHA
Veterans Health Administration
- VA
US Department of Veterans Affairs
- VINCI
Veterans Informatics and Computing Infrastructure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
2.0 REFERENCES
- Becker WC, Krebs EE, Edmond SN, Lin LA, Sullivan MD, Weiss RD, Gordon AJ, 2020. A Research Agenda for Advancing Strategies to Improve Opioid Safety: Findings from a VHA State of the Art Conference. J Gen Intern Med 35(Suppl 3), 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, Aronson G, Book SW, 2015a. Patient Perspectives Associated with Intended Duration of Buprenorphine Maintenance Therapy. J Subst Abuse Treat 56, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, Book SW, 2015b. Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat 52, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Volkow ND, 2019. Management of opioid use disorder in the USA: present status and future directions. Lancet 393(10182), 1760–1772. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, Gravely AA, Nelson DB, Bair MJ, Kerns RD, Higgins DM, Farmer MM, Partin MR, 2016. Association between pain outcomes and race and opioid treatment: Retrospective cohort study of Veterans. J Rehabil Res Dev 53(1), 13–24. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, Gravely AA, Nelson DB, van Ryn M, Bair MJ, Kerns RD, Higgins DM, Partin MR, 2013. A national study of racial differences in pain screening rates in the VA health care system. Clin J Pain 29(2), 118–123. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, Nelson DB, Gravely AA, Bair MJ, Kerns RD, Higgins DM, van Ryn M, Farmer M, Partin MR, 2014. Racial differences in prescription of opioid analgesics for chronic noncancer pain in a national sample of veterans. J Pain 15(4), 447–455. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2022. Provisional Drug Overdose Data. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- Centers for Disease Control and Prevention National Center for Health Statistics, 2022. U.S. Overdose Deaths In 2021 Increased Half as Much as in 2020 – But Are Still Up 15%. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm. (Accessed July 12 2022).
- Chen AY, Powell D, Stein BD, 2022. Changes in Buprenorphine and Methadone Supplies in the US During the COVID-19 Pandemic. JAMA Netw Open 5(7), e2223708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino JJ, Zhu X, 2006. The practical impact of ontologies on biomedical informatics. Yearb Med Inform, 124–135. [PubMed]
- Clausen T, Anchersen K, Waal H, 2008. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend 94(1–3), 151–157. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK, 2008. Medication compliance and persistence: terminology and definitions. Value Health 11(1), 44–47. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L, 2009. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 105(1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST, 2011. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend 119(1–2), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Casarella J, Drexler K, 2012. Treatment outcome for flexible dosing buprenorphine maintenance treatment. Am J Drug Alcohol Abuse 38(2), 155–160. [DOI] [PubMed] [Google Scholar]
- Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A, 2005. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. ScientificWorldJournal 5, 452–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomuzzi SM, Riemer Y, Ertl M, Kemmler G, Rossler H, Hinterhuber H, Kurz M, 2003. Buprenorphine versus methadone maintenance treatment in an ambulant setting: a health-related quality of life assessment. Addiction 98(5), 693–702. [DOI] [PubMed] [Google Scholar]
- Goedel WC, Shapiro A, Cerda M, Tsai JW, Hadland SE, Marshall BDL, 2020. Association of Racial/Ethnic Segregation With Treatment Capacity for Opioid Use Disorder in Counties in the United States. JAMA Netw Open 3(4), e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Drexler K, Hawkins EJ, Burden J, Codell NK, Mhatre-Owens A, Dungan MT, Hagedorn H, 2020. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus 41(3), 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Kavanagh G, Krumm M, Ramgopal R, Paidisetty S, Aghevli M, Goodman F, Trafton J, Liberto J, 2011. Facilitators and barriers in implementing buprenorphine in the Veterans Health Administration. Psychol Addict Behav 25(2), 215–224. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Kenny M, Dungan M, Gustavson AM, Kelley AT, Jones AL, Hawkins E, Frank JW, Danner A, Liberto J, Hagedorn H, 2022. Are x-waiver trainings enough? Facilitators and barriers to buprenorphine prescribing after x-waiver trainings. Am J Addict 31(2), 152–158. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Liberto J, Granda S, Salmon-Cox S, Andree T, McNicholas L, 2008. Outcomes of DATA 2000 certification trainings for the provision of buprenorphine treatment in the Veterans Health Administration. Am J Addict 17(6), 459–462. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Lo-Ciganic WH, Cochran G, Gellad WF, Cathers T, Kelley D, Donohue JM, 2015. Patterns and Quality of Buprenorphine Opioid Agonist Treatment in a Large Medicaid Program. J Addict Med 9(6), 470–477. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Trafton JA, Saxon AJ, Gifford AL, Goodman F, Calabrese VS, McNicholas L, Liberto J, Buprenorphine Work Group of the Substance Use Disorders Quality Enhancement Research, I., 2007. Implementation of buprenorphine in the Veterans Health Administration: results of the first 3 years. Drug Alcohol Depend 90(2–3), 292–296. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R, 2011. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev(8), CD004145. [DOI] [PubMed]
- Hagedorn H, Kenny M, Gordon AJ, Ackland PE, Noorbaloochi S, Yu W, Harris AHS, 2018. Advancing pharmacological treatments for opioid use disorder (ADaPT-OUD): protocol for testing a novel strategy to improve implementation of medication-assisted treatment for veterans with opioid use disorders in low-performing facilities. Addict Sci Clin Pract 13(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins EJ, Malte CA, Gordon AJ, Williams EC, Hagedorn HJ, Drexler K, Blanchard BE, Burden JL, Knoeppel J, Danner AN, Lott A, Liberto JG, Saxon AJ, 2021. Accessibility to Medication for Opioid Use Disorder After Interventions to Improve Prescribing Among Nonaddiction Clinics in the US Veterans Health Care System. JAMA Netw Open 4(12), e2137238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess LM, Raebel MA, Conner DA, Malone DC, 2006. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 40(7–8), 1280–1288. [DOI] [PubMed] [Google Scholar]
- Hui D, Weinstein ZM, Cheng DM, Quinn E, Kim H, Labelle C, Samet JH, 2017. Very early disengagement and subsequent re-engagement in primary care Office Based Opioid Treatment (OBOT) with buprenorphine. J Subst Abuse Treat 79, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M, 2003. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 361(9358), 662–668. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Rutherford C, Hamilton A, Barocas JA, Gelberg KH, Mueller PP, Feaster DJ, El-Bassel N, Cerdá M, 2022. What is the prevalence of and trend in opioid use disorder in the United States from 2010 to 2019? Using multiplier approaches to estimate prevalence for an unknown population size. Drug and Alcohol Dependence Reports. [DOI] [PMC free article] [PubMed]
- Lo-Ciganic WH, Gellad WF, Gordon AJ, Cochran G, Zemaitis MA, Cathers T, Kelley D, Donohue JM, 2016. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction 111(5), 892–902. [DOI] [PubMed] [Google Scholar]
- Manhapra A, Agbese E, Leslie DL, Rosenheck RA, 2018. Three-Year Retention in Buprenorphine Treatment for Opioid Use Disorder Among Privately Insured Adults. Psychiatr Serv 69(7), 768–776. [DOI] [PubMed] [Google Scholar]
- Manhapra A, Petrakis I, Rosenheck R, 2017. Three-year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. Am J Addict 26(6), 572–580. [DOI] [PubMed] [Google Scholar]
- Manhapra A, Quinones L, Rosenheck R, 2016. Characteristics of veterans receiving buprenorphine vs. methadone for opioid use disorder nationally in the Veterans Health Administration. Drug Alcohol Depend 160, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhapra A, Stefanovics E, Rosenheck R, 2020. Initiating opioid agonist treatment for opioid use disorder nationally in the Veterans Health Administration: Who gets what? Subst Abus 41(1), 110–120. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev(2), CD002207. [DOI] [PubMed]
- Mauro PM, Gutkind S, Annunziato EM, Samples H, 2022. Use of Medication for Opioid Use Disorder Among US Adolescents and Adults With Need for Opioid Treatment, 2019. JAMA Netw Open 5(3), e223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, Harris AH, Trafton JA, Gordon AJ, 2012. Receipt of opioid agonist treatment in the Veterans Health Administration: facility and patient factors. Drug Alcohol Depend 122(3), 241–246. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Maisel NC, Gordon AJ, Harris AH, 2011. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep 13(5), 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, Trafton JA, Harris AH, Gordon AJ, 2013. Trends in opioid agonist therapy in the Veterans Health Administration: is supply keeping up with demand? Am J Drug Alcohol Abuse 39(2), 103–107. [DOI] [PubMed] [Google Scholar]
- Perry C, Liberto J, Milliken C, Burden J, Hagedorn H, Atkinson T, McKay JR, Mooney L, Sall J, Sasson C, Saxon A, Spevak C, Gordon AJ, Group*, V.A.D.G.D., 2022. The Management of Substance Use Disorders: Synopsis of the 2021 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med. [DOI] [PubMed]
- Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M, 2007. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 10(1), 3–12. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Springer SA, Davis C, Ralevski E, Gu L, Lew R, Hermos J, Nuite M, Gordon AJ, Kosten TR, Nunes EV, Rosenheck R, Saxon AJ, Swift R, Goldberg A, Ringer R, Ferguson R, 2022. Rationale, design and methods of VA-BRAVE: a randomized comparative effectiveness trial of two formulations of buprenorphine for treatment of opioid use disorder in veterans. Addict Sci Clin Pract 17(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponizovsky AM, Grinshpoon A, 2007. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse 33(5), 631–642. [DOI] [PubMed] [Google Scholar]
- Ponizovsky AM, Margolis A, Heled L, Rosca P, Radomislensky I, Grinshpoon A, 2010. Improved quality of life, clinical, and psychosocial outcomes among heroin-dependent patients on ambulatory buprenorphine maintenance. Subst Use Misuse 45(1–2), 288–313. [DOI] [PubMed] [Google Scholar]
- Radmall AO, Calder S, Codell N, Kelley AT, Hawkins E, Jones AL, Hagedorn HJ, Reynolds MA, Gordon AJ, 2022. Roles and Perceptions of Nurses During Implementation of a Medication Treatment for Opioid Use Disorder National Initiative. J Addict Nurs 33(2), 70–79. [DOI] [PubMed] [Google Scholar]
- Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, Pacula RL, 2018. A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Subst Abus 39(4), 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Saloner B, Kerber R, Sorbero M, Gordon AJ, 2022. Subsequent Buprenorphine Treatment Following Emergency Physician Buprenorphine Prescription Fills: A National Assessment 2019 to 2020. Ann Emerg Med 79(5), 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2021. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21–07-01–003, NSDUH Series H-56). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME, 2014. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv 65(2), 158–170. [DOI] [PubMed] [Google Scholar]
- Tkacz J, Volpicelli J, Un H, Ruetsch C, 2014. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat 46(4), 456–462. [DOI] [PubMed] [Google Scholar]
- United States Department of Veterans Affairs and Department of Defense, 2015. VA/DoD clinical practice guideline for the management of substance use disorders (Version 3.0). https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
- Volkow N, Benveniste H, McLellan AT, 2017. Use and Misuse of Opioids in Chronic Pain. Annu Rev Med. [DOI] [PubMed]
- Volkow ND, 2020. Personalizing the Treatment of Substance Use Disorders. Am J Psychiatry 177(2), 113–116. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS, 2017. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377(4), 391–394. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS, 2014. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med 370(22), 2063–2066. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Poznyak V, Saxena S, Gerra G, Network U-WIIS, 2017. Drug use disorders: impact of a public health rather than a criminal justice approach. World Psychiatry 16(2), 213–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S, 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147(8), 573–577. [DOI] [PubMed] [Google Scholar]
- Weinstein ZM, Cheng DM, Quinn E, Hui D, Kim H, Gryczynski G, Samet JH, 2017a. Psychoactive medications and disengagement from office based opioid treatment (obot) with buprenorphine. Drug Alcohol Depend 170, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ZM, Gryczynski G, Cheng DM, Quinn E, Hui D, Kim HW, Labelle C, Samet JH, 2018. Tapering off and returning to buprenorphine maintenance in a primary care Office Based Addiction Treatment (OBAT) program. Drug Alcohol Depend 189, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ZM, Kim HW, Cheng DM, Quinn E, Hui D, Labelle CT, Drainoni ML, Bachman SS, Samet JH, 2017b. Long-term retention in Office Based Opioid Treatment with buprenorphine. J Subst Abuse Treat 74, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse JJ, Gordon AJ, Dobscha SK, Morasco BJ, Tiffany E, Drexler K, Sandbrink F, Lovejoy TI, 2018. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus 39(2), 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse JJ, Mackey K, Lovejoy TI, Kansagara D, Tuepker A, Gordon AJ, Todd Korthuis P, Herreid-O’Neill A, Williams B, Morasco BJ, 2022. Expanding access to medications for opioid use disorder through locally-initiated implementation. Addict Sci Clin Pract 17(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse JJ, McGinnis KA, Edelman EJ, Gordon AJ, Manhapra A, Fiellin DA, Moore BA, Korthuis PT, Kennedy AJ, Oldfield BJ, Gaither JR, Gordon KS, Skanderson M, Barry DT, Bryant K, Crystal S, Justice AC, Kraemer KL, 2021. Twelve-Month Retention in Opioid Agonist Treatment for Opioid Use Disorder Among Patients With and Without HIV. AIDS Behav. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.