Abstract
Salmonella enterica forms biofilms that are relatively resistant to chemical sanitizing treatments. Ionizing radiation has been used to inactivate Salmonella on a variety of foods and contact surfaces, but the relative efficacy of the process against biofilm-associated cells versus free-living planktonic cells is not well documented. The radiation sensitivity of planktonic or biofilm-associated cells was determined for three food-borne-illness-associated isolates of Salmonella. Biofilms were formed on sterile glass slides in a coincubation apparatus, using inoculated tryptic soy broth, incubated at 37°C for 48 h. Resulting biofilms were 18 to 24 μm in height as determined by confocal scanning laser microscopy. The planktonic and biofilm cultures were gamma irradiated to doses of 0.0 (control), 0.5, 1.0, 1.5, 2.0 and 2.5 kGy. The D10 value (the dose of radiation required to reduce a population by 1 log10, or 90%) was calculated for each isolate-culture based on surviving populations at each radiation dose. The D10 values of S. enterica serovar Anatum were not significantly (P < 0.05) different for biofilm-associated (0.645 kGy) and planktonic (0.677 kGy) cells. In contrast, the biofilm-associated cells of S. enterica serovar Stanley were significantly more sensitive to ionizing radiation than the respective planktonic cells, with D10 values of 0.531 and 0.591 kGy, respectively. D10 values of S. enterica serovar Enteritidis were similarly reduced for biofilm-associated (0.436 kGy) versus planktonic (0.535 kGy) cells. The antimicrobial efficacy of ionizing radiation is therefore preserved or enhanced in treatment of biofilm-associated bacteria.
Salmonella spp. are important food-borne pathogens, responsible for food-borne illness (FBI) associated with a variety of food products (16, 18, 19, 24). Numerous studies have documented the ability of Salmonella spp. to adhere and form biofilms on surfaces such as plastic, cement, glass, and stainless steel (4, 10). The presence of these organisms in food processing environments can serve as a persistent source of contamination.
A biofilm has been defined as a “community of bacteria living in organized structures at a liquid interface” (2). Microscopic investigations of biofilm structure have revealed that bacteria exist in microcolonies that are encapsulated in a matrix of extracellular polymeric material (23). Due to the protection afforded to cells enclosed within this matrix, chemical sanitizers are generally unable to eliminate most biofilm-associated bacteria. Several reports have documented the increased resistance of biofilm-associated (attached) bacteria to various antimicrobial agents compared to their planktonic (suspended) counterparts. For example, Luppens et al. (12) demonstrated that a 600-fold increase in chlorine concentration was required to kill biofilm-associated cells of Staphylococcus aureus compared with their planktonic counterparts. Biofilm-associated Salmonella bacteria were reduced by less than 1 log10 unit after treatment for 10 min using 10 ppm of chlorine whereas 106 CFU/ml of planktonic cells were completely killed using the same treatment (10). Other chemical sanitizers such as trisodium phosphate (21) and iodophor (10) have also been shown to have similarly reduced efficacy. Biofilm-associated bacteria have also demonstrated enhanced resistance to heat treatment (5).
Ionizing radiation (X ray, gamma ray, and electron beam) has long been recognized as a method for destroying spoilage and pathogenic microorganisms in foods (16, 22). Numerous reports document the radiation D10 values (the dose of radiation require to reduce population by 1 log10, or 90%) of various Salmonella spp. Values ranging from 0.13 to 1.1 kGy have been reported, with great dependence on the suspending menstrum (15, 16, 22). When treated on radish sprouts, Salmonella isolates were shown to possess a D10 value of 0.46 to 0.54 kGy, depending on the source of the isolates (18).
The primary mode of action of ionizing radiation is via oxygen and hydroxyl radicals, created when the high-energy photons (X ray and gamma) or electrons (electron beam) split water molecules within the product and the resident bacteria (13). These radicals damage cell membranes, protein structures, and nucleic acid strands. Irradiation involves a directed transfer of radiant energy into products and inhabitant bacteria, rather than the necessary transfer and/or migration of an antimicrobial chemical compound. Direct contact with the target organism is required for aqueous sanitizers to be effective, and this is hindered in cells encased within a biofilm.
To our knowledge, the effect of ionizing radiation on biofilm-associated cells of Salmonella spp. and the differences in radiation sensitivity between biofilm and planktonic cells have not been reported. The goal of our study was to investigate the relative susceptibilities of planktonic versus biofilm (surface-associated) cells of Salmonella spp. to ionizing radiation and to compare the variation, if any, with that of chemical sanitizers.
MATERIALS AND METHODS
Bacteria.
All isolates utilized in this study were from the U.S. Department of Agriculture-Agricultural Research Service-Eastern Regional Research Center culture collection. Stock cultures of Salmonella enterica serovar Anatum F4317 (isolated from sprouts implicated in FBI outbreak), S. enterica serovar Stanley H0558 (clinical strain from a sprout-related FBI outbreak), and S. enterica serovar Enteritidis 15159 (orange juice-related outbreak strain) were stored in tryptic soy broth (TSB; Difco, Detroit, MI) containing 30% glycerol at −80°C. Working cultures were maintained on tryptic soy agar (TSA; Difco, Detroit, MI) slants at 4°C.
Preparation of biofilms.
Various methodologies have been proposed for the production of biofilms. Depending on the intent of the study, these methods vary in incubation times and temperatures, medium, medium changes, and dynamic conditions (13). Planktonic and biofilm-associated cells were prepared in sterile 50-ml centrifuge tubes as follows. For each individual isolate, sterile TSB (5 ml) was inoculated with 200 μl of a stock culture and incubated overnight at 37°C to make a fresh culture (approximately 109 CFU/ml) used to inoculate the biofilm coincubation apparatus. Precleaned glass microscope slides, 7.62 cm by 2.54 cm, were wrapped in foil and sterilized by autoclaving (121°C, 15 min) using a gravity cycle. Slides were aseptically placed into 50-ml tubes containing 25 ml of sterile TSB and inoculated with 200 μl of the fresh culture. Tubes were held upright in a rack and incubated at 35°C for 48 h under static conditions. This configuration resulted in approximately 3.5 cm of the slide being submerged in culture medium; the upper part of the slide in the headspace of the tube remained dry. The studies were performed in duplicate, with separate replications prepared on the same day. For each replication, separate stock cultures were grown, and separate coincubation tubes were inoculated, irradiated concurrently, and sampled individually (described below).
CSLM.
To confirm and characterize the extent of biofilm production of these isolates, 3 ml of sterile TSB in glass-bottomed culture dishes (part no. P35G-1.5-14C; MatTek Corp., Ashland, MA) was inoculated with 100 μl of fresh culture and incubated at 35°C for 48 h under static conditions. This style of culture dish is suited to confocal imaging of the resulting biofilms, due to the optical quality of the glass insert in the bottom of the dish. The glass bottom of the culture dish also presents the same type of surface as the glass slides used in the 50-ml centrifuge tube coincubation apparatus, described above, making this type of culture plate appropriate for comparisons of resulting biofilms. The TSB in each microwell dish was removed using a sterile pipette, and samples were stained by the method of Hope et al. (9). Briefly, 1 ml of 0.1 M phosphate-buffered saline (pH 7.2) containing 1 μl each of component A (SYTO 9) and component B (propidium iodide) of the BacLight bacterial viability kit (Molecular Probes, Inc., Eugene, OR) was added to the well within each microplate. Plates were incubated in the dark at room temperature for 10 min, and then 1 ml of fresh phosphate-buffered saline was added to ensure that samples remained hydrated. Stained samples were examined using a Leica TCS-SP confocal scanner connected to an inverted microscope equipped with a 20× objective (model IRBE; Leica Microsystems, Heidelberg, Germany). Fluorochromes were excited using an argon laser source at 488 nm. Images were collected in two channels, 490 to 515 nm and 620 to 640 nm, corresponding to the emission maxima for SYTO 9 and propidium iodide, respectively. Optical sections approximately 1 μm in height were collected starting from below the focal plane containing the glass bottom of each microplate upward through the entire biofilm. These conditions were sufficient to produce a biofilm approximately 18 to 24 μm in height. The confocal scanning laser microscopy (CSLM) studies were performed in duplicate, on separate days.
Irradiation.
Samples were irradiated using a Lockheed-Georgia (Marietta, GA) cesium-137 temperature-controlled gamma radiation source, with a dose rate of 5.88 kGy/h, according to the method of Niemira et al. (15). The sample tubes of the separate, duplicate replicates were irradiated concurrently. The sample tubes were given doses ranging from 0.0 to 2.5 kGy. The temperature was held constant at 22°C during irradiation by the injection of gas-phase liquid nitrogen into the top of the chamber. Alanine pellets (Bruker, Inc., Billerica, MA) were used for dosimetry. The pellets were read on a Bruker EMS 104 electron paramagnetic resonance analyzer and compared with a previously determined standard curve. The delivered dose, as determined by electron paramagnetic resonance dosimetry, was typically within 5% of the nominal dose.
Enumeration of survivors.
Tubes were opened, and surviving planktonic cells were enumerated by withdrawal of a 1-ml aliquot of the liquid culture, serial dilution with Butterfield's phosphate buffer (BPB), and pour plating using TSA. In withdrawing the sample of liquid culture containing planktonic cells, extreme care was taken to avoid contacting either the glass slide or the sides of the culture tube. Biofilm-associated cells were enumerated by removing the microscope slide using sterile forceps to grip the clean, dry upper portion of the slide, rinsing for 10 seconds under a stream of sterile distilled water to remove unattached cells, and then vigorously shaking the slide in 25 ml of BPB in a fresh, sterile 50-ml centrifuge tube. Aliquots were then pour plated using TSA. Plates were incubated overnight at 35°C.
In order to assess the efficiency of bacterial removal of the shaking process, the glass slides from control (0.0 kGy) and irradiated (2.0 kGy) samples were removed from the BPB and stained approximately 1 h with 0.1% ruthenium red to identify extracellular polysaccharide (EPS) adhering to the glass substrate. The slides were rinsed with sterile distilled water and examined in a Zeiss HB50 compound light microscope. Aliquots (100 μl) of the BPB used in the shaking procedure were mounted on clean slides, allowed to air dry, and stained with the ruthenium red as described above. Disrupted fragments of EPS were extensively visible in the BPB aliquot used for sampling, but only trace EPS was detected on the slides themselves. The shaking procedure was therefore determined to have effectively removed essentially all of the biofilm-associated cells. Preliminary experiments in our laboratory with alternative methods of removing biofilm-associated cells indicated that shaking in BPB recovered levels of biofilm-associated cells similar to those of other methods such as swabbing with sterile cotton swabs.
Statistical analysis.
Data were normalized against the control and plotted as the log10 reduction to produce a survivor ratio. The data were pooled, and the slopes of the individual survivor curves were calculated with linear regression (SigmaPlot 5.0; SPSS Inc., Chicago, IL) and compared using analysis of covariance (Excel 97; Microsoft Corp., Redmond, WA). The ionizing radiation D10 value was calculated by taking the negative reciprocal of the survivor curve slope.
RESULTS AND DISCUSSION
Biofilm formation.
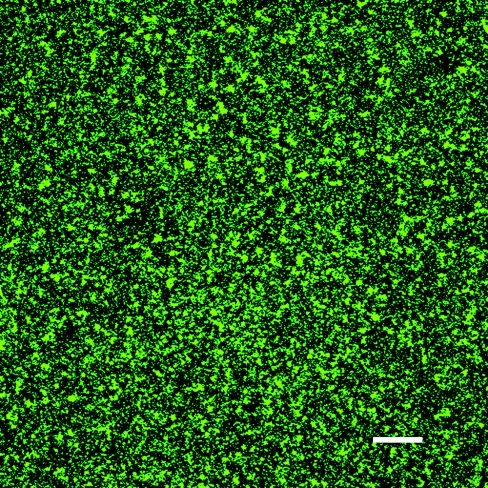
All three isolates produced significant biofilms under the conditions utilized in our experiments. Optical series captured using CSLM demonstrated the presence of a biofilm between 18 and 24 μm in height. The majority of the cells within the biofilm were located in the region between 3 and 12 μm above the glass substrate. This spatial localization of cells within the biofilm is in general agreement with previously published reports (2, 14). A section of S. enterica serovar Anatum 7 μm above the glass slide is shown in Fig. 1. Comparable biofilms were produced and microscopy results were obtained for S. enterica serovar Stanley and serovar Enteritidis (not shown). Numerous authors have investigated biofilm formation by salmonellae and other Enterobacteriaceae (24, 25, 26). The coincubation apparatus used in the present work both provided a simple means of investigating the survival of planktonic and biofilm-associated cells and resulted in reproducible levels of biofilm formation throughout our experiments.
FIG. 1.
Confocal laser scanning micrograph of Salmonella serovar Anatum stained with SYTO 9 and propidium iodide. Bar, 50 μm.
Planktonic cells.
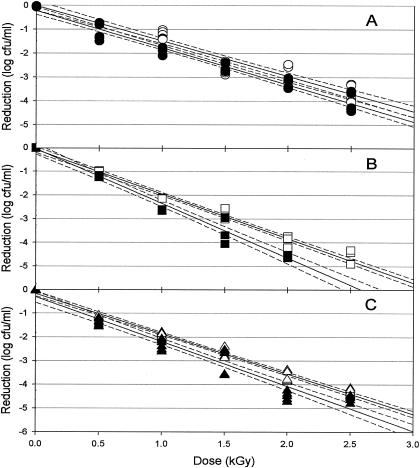
Ionizing radiation effectively reduced the populations of planktonic cells of the three isolates examined (Fig. 2). Radiation D10 values for planktonic cells ranged from 0.535 to 0.677 kGy (Table 1). Resistance of the planktonic cells to irradiation is in agreement with previously published D10 values for Salmonella in/on a variety of food products (16, 22). The three strains of Salmonella used in this study showed significantly different resistance to irradiation. This strain-dependent response has been previously observed with Salmonella irradiated on a variety of food and nonfood substrates (15, 16, 18). Four sprout-related outbreak strains exhibited D10 values ranging from 0.35 to 0.71 kGy when suspended in orange juices of various turbidities (15). Sherry et al. (20) reported D10 values between 0.36 and 0.65 kGy for four strains suspended in spent tryptone soy broth supplemented with 0.6% yeast extract. Four isolates of animal origin exhibited D10 values of 0.39 to 0.41 kGy in liquid whole eggs (19). Kim and Thayer (11) reported D10 values of between 0.394 and 0.561 for S. enterica serovar Typhimurium suspended in phosphate buffer, depending on the presence or absence of air. A conceptual understanding of the mechanism of isolate-associated variability in radiation resistance has yet to be developed.
FIG. 2.
Radiation sensitivity of planktonic (white) and biofilm-associated (black) Salmonella serovar Anatum (circles, graph A), serovar Enteritidis (squares, graph B), and serovar Stanley (triangles, graph C). The unbroken line indicates linear regression (with 95% confidence intervals [dashed]). Within each isolate-growth condition, n equals 6 at each dose.
TABLE 1.
Radiation sensitivity of three Salmonella isolates in planktonic and biofilm-associated forms
| Isolate (serovar) | Culture | CFU/ml (log10)a | Radiation D10 (kGy)b | R2 | 5-log10 dose (kGy)c |
|---|---|---|---|---|---|
| Anatum | Planktonic | 8.98 | 0.677 ab | 0.94 | 3.385 |
| Biofilm | 5.98 | 0.645 a | 0.96 | 3.225 | |
| Enteritidis | Planktonic | 9.15 | 0.535 c | 0.98 | 2.675 |
| Biofilm | 6.25 | 0.436 d | 0.97 | 2.180 | |
| Stanley | Planktonic | 9.08 | 0.591 b | 0.99 | 2.955 |
| Biofilm | 6.30 | 0.531 c | 0.95 | 2.655 |
Cell density in the nonirradiated control samples, log10 CFU/ml.
D10 values among the six isolate-culture combinations followed by the same letter are not significantly different (analysis of covariance, P < 0.05).
The dose required to achieve a 5-log10 (99.999%) reduction in population.
Biofilm cells.
Ionizing radiation was similarly effective in reducing the populations of biofilm-associated cells of the three isolates examined (Fig. 2). D10 values for biofilm-associated cells ranged from 0.436 to 0.645 for the three strains (Table 1). These values are similar to previously reported radiation sensitivities of Salmonella attached to surfaces or foods. Grant and Patterson (7) reported D10 values ranging from 0.371 to 0.697 kGy for S. enterica serovar Typhimurium in components of a roast beef ready-to-eat meal. D10 values of between 0.374 and 0.773 were reported for six strains of Salmonella when irradiated in mechanically deboned chicken meat (27). Clavero et al. (1) found D10 values ranging from 0.618 to 0.800 kGy for salmonellae in raw ground beef, depending on irradiation temperatures and fat content.
Relative efficacy.
In a comparison of the relative irradiation sensitivities of the three isolates tested, S. enterica serovar Anatum was the most radiation-resistant isolate, followed by S. enterica serovar Stanley, with S. enterica serovar Enteritidis being the most sensitive (Table 1). D10 values for biofilm-associated cells of S. enterica serovar Anatum were similar to those found for planktonic cells, while biofilm-associated S. enterica serovar Stanley and serovar Enteritidis were significantly (P < 0.05) more sensitive than planktonic cells (Table 1). The D10 values of S. enterica serovar Stanley and serovar Enteritidis were reduced by 10.2% and 18.5%, respectively.
The implications of these results are readily apparent when contrasted with the relative efficacy of chemical sanitizers on planktonic versus biofilm-associated bacteria. The differences between the radiation D10 values obtained, while statistically significant in the cases of S. enterica serovar Stanley and serovar Enteritidis, do not invalidate the use of irradiation as an effective antimicrobial intervention against biofilm-associated bacteria.
The primary mode of action of ionizing radiation is via oxygen and hydroxyl radicals (16). In suspensions with a high antioxidant capacity, these radicals can be neutralized before doing damage to bacterial cell membranes, protein structures, and nucleic acid strands, thereby protecting the bacteria and reducing the efficacy of the process (22). It had been suggested that the microstructural polysaccharide elements of a biofilm may serve a similar protective role for biofilm-associated pathogens (16). While the maximal thickness of the biofilms observed in this study was ∼24 μm, gamma radiation has a very high penetrability, approximately 22 cm in material of unit (1 g/cm3) density (16, 22). The observed antimicrobial efficacy of the process against biofilm-associated pathogens indicates that it is probable that neither the polysaccharide elements nor other associated elements such as DNA, proteins, etc., inhibit the generation of radical molecules throughout the biofilm, including within the resident bacteria.
The microbial ecology of biofilm-associated bacteria which have been damaged, but not killed, by the penetrating irradiation process is poorly understood. The possibility exists of regrowth of the pathogen and recolonization of the surface. However, in the complex, dynamic, multispecies ecologies of foods and food contact surfaces, competition, predation, and other interactions between pathogenic and nonpathogenic members of the community make predictions and even qualitative risk assessments problematic. Studies of postintervention population dynamics are ongoing to define the behavior of irradiated biofilms.
In contrast to the unchanged or increased efficacy of irradiation on biofilm-associated versus planktonic cells shown herein, the efficacy of aqueous sanitizers, biocides, or antibiotics is commonly reduced by orders of magnitude when applied to biofilm-associated bacteria. This is most probably due to the limited penetration of sanitizers into biofilms (2). de Beer et al. (3) demonstrated the lack of penetration of chlorine into biofilms of Klebsiella pneumoniae and Pseudomonas aeruginosa. Typically, chlorine concentrations measured within the biofilm were 20% or less than the concentration in the bulk fluid, with increased contact time resulting in no significant increase in penetration. Listeria monocytogenes within a multispecies biofilm was reduced by less than 2 log cycles after treatment with 1,000 ppm sodium hypochlorite for 20 minutes (17). In contrast, 30 seconds of treatment with 10 ppm reduced planktonic cells by 8 log cycles. Similar results have been reported for alkaline hypochlorite, chlorsulfamates, and trisodium phosphate (21, 26).
Chemical treatments which may be effective against planktonic cells, therefore, are typically not effective against biofilm-associated cells. The structural organization of biofilms effectively diminishes or prevents the introduction of active chemical species with antimicrobial efficacy. Processes which instead rely on the direct transfer of energy, such as ionizing radiation, UV irradiation (8), radio frequency treatments (6), etc., may have greater potential when directed against biofilm-associated pathogens. The combination of chemical and energy transfer antimicrobial treatments, particularly with regard to the potential for synergistic interactions, is a subject of ongoing research.
Summary.
This study has demonstrated that ionizing radiation effectively reduces the populations of both planktonic and biofilm-associated salmonellae. The radiation sensitivity of Salmonella is isolate specific. Similarly, the influence on radiation sensitivity of the cultured state of the organism, i.e., planktonic versus biofilm associated, is also isolate specific. In general, these results show that, in contrast to chemical antimicrobial treatments, the antimicrobial efficacy of ionizing radiation is preserved or enhanced when treating biofilm-associated bacteria.
Acknowledgments
We thank B. Annous, N. Gunther, and W. Fett for their valuable discussion; P. Cooke for assistance with confocal microscopy; and K. Lonczynski for technical assistance.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Clavero, M. R. S., J. D. Monk, L. R. Beuchat, M. P. Doyle, and R. E. Brackett. 1994. Inactivation of Escherichia coli O157:H7, salmonellae, and Campylobacter jejuni in raw ground beef by gamma irradiation. Appl. Environ. Microbiol. 60:2069-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 3.de Beer, D., R. Srinivasan, and P. S. Stewart. 1994. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ Microbiol. 60:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhir, V. K., and C. E. R. Todd. 1995. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 61:1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank, J., and R. Koffi. 1990. Surface-adherence growth of Listeria monocytogenes is associated with increased resistance to surface sanitizers and heat. J. Food Prot. 53:550-554. [DOI] [PubMed] [Google Scholar]
- 6.Geveke, D. J., M. F. Kozempel, O. J. Scullen, and C. Brunkhorst. 2002. Radio frequency energy effects on microorganisms in foods. Innovative Food Sci. Emerg. Technol. 3:133-138. [Google Scholar]
- 7.Grant, I. R., and M. F. Patterson. 1992. Sensitivity of foodborne pathogens to irradiation in the components of a chilled ready meal. Food Microbiol. 9:95-103. [Google Scholar]
- 8.Guerrero-Beltran, J. A., and G. V. Barbossa-Canovas. 2004. Review: advantages and limitations on processing foods by UV light. Food Sci. Technol. Int. 10:137-147. [Google Scholar]
- 9.Hope, C. K., D. Clements, and M. Wilson. 2002. Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. J. Appl. Microbiol. 93:448-455. [DOI] [PubMed] [Google Scholar]
- 10.Joseph, B., S. K. Otta, I. Karunasagar, and I. Karunasagar. 2001. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 64:367-372. [DOI] [PubMed] [Google Scholar]
- 11.Kim, A. Y., and D. W. Thayer. 1996. Mechanism by which gamma irradiation increases the sensitivity of Salmonella typhimurium ATCC 14028 to heat. Appl. Environ. Microbiol. 62:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luppens, S. B. I., M. W. Reij, R. W. L. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphyloccoccus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean, R. J. C., C. L. Bates, M. B. Barnes, C. L. McGowin, and G. M. Aron. 2004. Methods of studying biofilms, p. 379-413. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms. ASM Press, Washington, D.C.
- 14.Morris, C. E., J.-M. Monier, and M.-A. Jaques. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemira, B. A., C. H. Sommers, and G. Boyd. 2001. Irradiation inactivation of four Salmonella serotypes in orange juices with various turbidities. J. Food Prot. 64:614-617. [DOI] [PubMed] [Google Scholar]
- 16.Niemira, B. A. 2003. Irradiation of fresh and minimally processed fruits, vegetables and juices, p. 279-300. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), The microbial safety of minimally processed foods. CRC Press, Boca Raton, Fla.
- 17.Norwood, D. E., and A. Gilmour. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520. [DOI] [PubMed] [Google Scholar]
- 18.Rajkowski, K. T., and D. W. Thayer. 2000. Reduction of Salmonella spp. and strains of Escherichia coli O157:H7 by gamma radiation of inoculated sprouts. J. Food Prot. 63:871-875. [DOI] [PubMed] [Google Scholar]
- 19.Serrano, L. E., E. A. Murano, K. Shenoy, and D. G. Olson. 1997. D values of Salmonella enteritidis isolates and quality attributes of shell eggs and liquid whole eggs treated with irradiation. Poult. Sci. 76:202-206. [DOI] [PubMed] [Google Scholar]
- 20.Sherry, A. E., M. F. Patterson, and R. H. Madden. 2004. Comparison of 40 Salmonella enterica serovars injured by thermal, high-pressure and irradiation stress. J. Appl. Microbiol. 96:887-893. [DOI] [PubMed] [Google Scholar]
- 21.Somers, E. B., J. L. Schoeni, and A. C. L. Wong. 1994. Effect of trisodium phosphate on biofilm and planktonic cells of Campylobacter jejuni, Escherichia coli O157:H7, Listeria monocytogenes and Salmonella typhimurium. Int. J. Food Microbiol. 22:269-276. [DOI] [PubMed] [Google Scholar]
- 22.Sommers, C. H. 2003. Irradiation of minimally processed meats, p. 301-318. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), The microbial safety of minimally processed foods. CRC Press, Boca Raton, Fla.
- 23.Starkey, M., K. A. Gray, S. I. Chang, and M. R. Parsek. 2004. A sticky business: the extracellular polymeric substance matrix of bacterial biofilms, p. 174-191. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms. ASM Press, Washington, D.C.
- 24.Stepanovic, S., I. Cirkovic, M. Vijac, and M. Svabic-Vlahovic. 2003. Influence of the incubation temperature, atmosphere and dynamic conditions on biofilm formation by Salmonella spp. Food Microbiol. 20:339-343. [Google Scholar]
- 25.Stepanovic, S., I. Cirkovic, L. Ranin, and M. Svabic-Vlahovic. 2004. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 38:428-432. [DOI] [PubMed] [Google Scholar]
- 26.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorsulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 27.Thayer, D. W., G. Boyd, W. S. Muller, C. A. Lipson, W. C. Hayne, and S. H. Baer. 1990. Radiation resistance of Salmonella. J. Ind. Microbiol. 5:387-390. [Google Scholar]