Abstract
We found that the culture supernatant of the periodontopathic bacterium Actinobacillus actinomycetemcomitans had a cytotoxic effect on several cell lines. In this study, we purified the toxin from the culture supernatant of A. actinomycetemcomitans Y4 by a four-step procedure: ammonium sulfate precipitation, POROS HQ/M column chromatography, polymyxin B matrix column chromatography, and Mono-Q column chromatography. The purified toxin gave two major bands of protein with molecular masses of 80 and 85 kDa upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The mechanism of cell death of the B-cell hybridoma cell line HS-72 was examined by observing changes in nuclear morphology, an increase in the proportion of fragmented DNA, and the typical ladder pattern of degraded chromosomal DNA, indicating the induction of apoptosis. Overexpression of human Bcl-2 suppressed apoptosis in HS-72 cells, indicating that the toxin from A. actinomycetemcomitans induces apoptosis by a Bcl-2-inhibitable mechanism. Flow cytometric analysis revealed that the toxin caused cell cycle arrest in the G2/M phase and apoptosis in HS-72 cells. In addition, aurintricarboxylic acid, a DNA endonuclease inhibitor, markedly decreased the percentage of apoptotic cells but had no effect on cell cycle arrest in the G2/M phase. Taken together, these findings suggest that the toxin from A. actinomycetemcomitans could mediate the development of periodontal diseases through cell cycle arrest in the G2/M phase and apoptosis in B lymphocytes of periodontal tissue.
Actinobacillus actinomycetemcomitans, a gram-negative, nonmotile, capnophilic, fermentative coccobacillus, has been recovered from periodontally diseased gingival tissues (3) and implicated in the pathogenesis of severe juvenile and adult periodontitis (30, 31). In addition, A. actinomycetemcomitans has been reported to be a causative agent of various infectious diseases, such as endocarditis, pericarditis, meningitis, osteomyelitis, empyema, and subcutaneous abscess (9). A. actinomycetemcomitans elaborates a multiplicity of virulence factors and tissue-damaging products, such as a leukotoxin (33, 34), collagenase (28), lipopolysaccharide (LPS), alkaline and acid phosphatases, an epitheliotoxin, a fibroblast-inhibitory factor (6), and a bone resorption-inducing toxin (29). Several investigators have been reported that the leukotoxin is continuously released into gingival tissue during chronic infection and induces tissue destruction (3). We reported that LPS and capsular polysaccharide from A. actinomycetemcomitans were potent mediators of bone resorption (7, 21, 37).
Apoptosis has been shown to play important roles in the control of various biological systems, such as immune responses, hematopoiesis, and embryonic development (26). This process is an active cell death and can be triggered by a variety of pharmacological and physical agents. It is sometimes referred to as programmed cell death, being fundamentally distinct from accidental cell death or necrosis. Cells undergoing apoptosis show morphological changes with loss of the plasma membrane of microvilli, chromatin condensation, nuclear fragmentation, and shrinkage. Its hallmark biochemical feature is an endonuclease-mediated cleavage of internucleosomal DNA linker sections (4). We have previously reported evidence for apoptosis in macrophages infected with A. actinomycetemcomitans, suggesting that the ability of A. actinomycetemcomitans to promote apoptosis in macrophages may be important for the development of periodontitis (10).
Bcl-2 and related proteins are important regulators of cell death in mammalian cells. The first member of the family, Bcl-2, is a 26-kDa protein localized in outer mitochondria, the nuclear envelope, and endoplasmic reticula. This molecule has been shown to protect cells from apoptotic cell death in a variety of cellular systems (35). Bcl-2 and related proteins are well known to be products of a family of genes including bcl-X, bax, bak, mcl-1, A1, and bad. bcl-2 and bcl-X exhibit striking patterns of regulation during B-cell maturation and inhibit several forms of apoptosis (22). The function of these genes appears to be critical in regulating cell number in complex eukaryotes during embryonic and postembryonic development (12). Recently, it has been demonstrated that Bcl-2 functions as an ion channel and as an adapter/docking protein to protect cells from apoptosis (27).
Exposure of mammalian cells to several DNA-damaging agents evokes a complicated cellular response, such as a reversible block in cell cycle progression at G1 and G2/M phases, and the induction of programmed cell death (5). The cell cycle arrest at G1 and G2/M checkpoints reflects the necessity of mammalian cells to gain time to repair the damaged DNA (39). It has been reported that culture supernatant from A. actinomycetemcomitans blocks human gingival fibroblast cell division in the G2/M phase (6). However, we are not aware of any reports concerning cell cycle arrest and apoptosis in mammalian cells induced by the toxin from A. actinomycetemcomitans. We report in this study that the toxin induces cell cycle arrest in the G2/M phase before the appearance of apoptosis.
MATERIALS AND METHODS
Purification of the toxin from A. actinomycetemcomitans.
A. actinomycetemcomitans Y4 was grown in dialysates of Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 1% (wt/vol) yeast extract at 37°C for 4 days in an atmosphere of 5% CO2 in air. The cell-free culture supernatant was collected by centrifugation, and the crude toxin was precipitated from the culture supernatant by adding solid ammonium sulfate to 40% saturation. The precipitate was collected by centrifugation, dissolved in phosphate-buffered saline (PBS; pH 7.2), and dialyzed extensively against PBS (18). The protein content of sample was determined using a Bio-Rad protein assay reagent (Bio-Rad Laboratories, Richmond, Calif.).
To purify the toxin from the culture supernatant, A. actinomycetemcomitans Y4 was grown in RPMI 1640 medium (GIBCO BRL, Grand Island, N.Y.) at 37°C for 4 days in an atmosphere of 5% CO2 in air. After ammonium sulfate was added to 40% saturation as described above, the precipitate was collected and dialyzed against 10 mM ammonium bicarbonate buffer. The solution (60 mg of protein) was applied to a preparative POROS HQ/M column (4.6 by 100 mm; PerSeptive Biosystems, Framingham, Mass.) equilibrated with 10 mM ammonium bicarbonate buffer and eluted with same buffer extensively at 5 ml/min, followed by a linear gradient of 10 to 500 mM ammonium bicarbonate buffer for 30 min. Fractions that showed growth-inhibitory activity were pooled, dialyzed against PBS, and used as a partially purified toxin. The sample was applied to a column (18 by 200 mm) of polymyxin B matrix (Affi-Prep polymyxin matrix; Bio-Rad) according to the manufacturer’s instructions. The unbound fraction was biologically active and then dialyzed against 10 mM ammonium bicarbonate buffer. The dialysate was applied to a Mono-Q HR5/5 column (5 by 50 mm; Pharmacia, Uppsala, Sweden) equilibrated with 10 mM ammonium bicarbonate buffer for 30 min and eluted with same buffer extensively at 0.5 ml/min, followed by a linear gradient of 10 to 500 mM ammonium bicarbonate buffer for 30 min (20). The biologically active fractions from the Mono-Q column were pooled and lyophilized. The lyophilized sample was dissolved in PBS and used as a purified toxin.
Cell lines and culture conditions.
J774.1 (mouse macrophage cell line) and EL-4 (mouse lymphoma) were maintained in RPMI 1640 medium (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). WEHI-231 (mouse B-cell lymphoma) was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 50 μM 2-mercaptoethanol, and antibiotics (17). Mouse hybridoma cell line HS-72 was maintained in Iscove’s modified Dulbecco’s medium (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (19). KB (human oral epidermoid carcinoma) was propagated in Dulbecco’s modified Eagle medium (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (17). HS-72 cells were transfected by electroporation with pCΔJ-SV-2 and pCΔJ-Bcl-2 as described previously (13). HS-72 transfectants were selected by growth in the presence of G418 (450 μg/ml; GIBCO BRL), and individual clones were isolated by limiting dilution. Human bcl-2-transfected clone HS-72 B-16 and control plasmid-transfected clone HS-72 S-4 were grown in medium containing 10% heat-inactivated fetal bovine serum and 450 μg of G418 per ml (13).
Cell viability assay.
The cells were washed extensively to remove the growth factors and suspended to a density of 4 × 105/ml in medium containing 5% heat-inactivated fetal bovine serum and antibiotics. The cells (2 × 104/well of a 96-well plate) were cultured with appropriate amounts of the toxin in an atmosphere of 5% CO2 in air. After the cells were cultured with the toxin for 44 h, stock MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; 2.5 mg/ml; Sigma Chemical Co., St. Louis, Mo.) solution (20 μl/well) was added to the wells, and the plate was incubated at 37°C for 4 h. After acid-isopropanol (100 μl of 0.04 N HCl in isopropanol) was added and mixed thoroughly, the plate was read on a Multiskan bichromatic microplate reader (Labsystems, Helsinki, Finland), using a test wavelength of 570 nm and a reference wavelength of 620 nm (MTT viability assay). Percent cytotoxicity was calculated as 100 × (1 − optical density at 570 to 620 nm with the toxin/optical density at 570 to 620 nm without the toxin) (10).
Detection of apoptotic cells.
HS-72 cells were cultured with appropriate amounts of the toxin in an atmosphere of 5% CO2 in air. In some experiments, HS-72 cells were cultured with the toxin in the presence of a DNA endonuclease inhibitor, aurintricarboxylic acid (ATA; 200 μM; Sigma). For the Hoechst staining, HS-72 cells were fixed with 1% glutaraldehyde for 1 h and washed with PBS. The samples were stained with 56 μg of Hoechst dye 33342 per ml and mounted on a slide glass. Nuclei were visualized by fluorescence microscopy with excitation wavelength 355 nm and emission wavelength 465 nm, and the number of cells with apoptotic nuclei was determined. To detect apoptotic nuclei and analyze the cell cycle of HS-72 cells, the cells (106) were suspended in hypotonic solution (3.4 mM sodium citrate, 0.1% Triton X-100, 0.1 mM EDTA, 1 mM Tris-HCl [pH 8.0]), stained with 5 μg of propidium iodide per ml, and analyzed by a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The population of cells in each cell cycle phase was determined by CellFIT software (Becton Dickinson). In the DNA fragmentation assay, the DNA was extracted from the cells (5 × 106) by the method of Moore and Matlashewski (16). In brief, the cells were lysed with 10 mM Tris-HCl (pH 7.4)–5 mM EDTA–1% Triton X-100. The lysates were centrifuged to remove integral nuclei. The supernatants were digested with RNase (0.5 mg/ml) for 1 h at 37°C, incubated with proteinase K (10 mg/ml) for 1 h at 50°C, and extracted with phenol-chloroform (1:1, vol/vol) before precipitation with ethanol. The precipitates were dried and solubilized in 10 mM Tris-HCl (pH 8.0)–1 mM EDTA. Electrophoresis was performed with a 2% agarose gel, which was stained with ethidium bromide.
SDS-PAGE analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in a 10 to 20% polyacrylamide gradient gel (PAGEL, NPG-1020L; ATTO Co., Tokyo, Japan) under reducing conditions. The gel was stained with 2D-Silver Stain II DAIICHI (Daiichi Pure Chemicals Co., Tokyo, Japan).
Immunoblot analysis.
The lyophilized A. actinomycetemcomitans Y4 whole cells were suspended in PBS to a final concentration of 1 mg/ml. The bacterial cell suspension (10 μg of protein) was mixed with equal volume of SDS-containing sample buffer, boiled for 5 min, and used as a whole-cell lysate. The toxin was mixed with SDS-containing sample buffer and boiled for 5 min. The samples were separated by SDS-PAGE as described above and transferred electrophoretically to a polyvinylidene fluoride membrane. The membrane was treated with a rabbit polyclonal antiserum against the purified leukotoxin from A. actinomycetemcomitans 301-b (23). Immunodetection was performed with an enhanced chemiluminescence Western blotting detection system (Amersham International, Little Chalfont, England) according to the manufacturer’s instructions. Proteins were stained with Coomassie brilliant blue to confirm the amount of protein in each lane.
RESULTS
Purification of the toxin from A. actinomycetemcomitans Y4 culture supernatant.
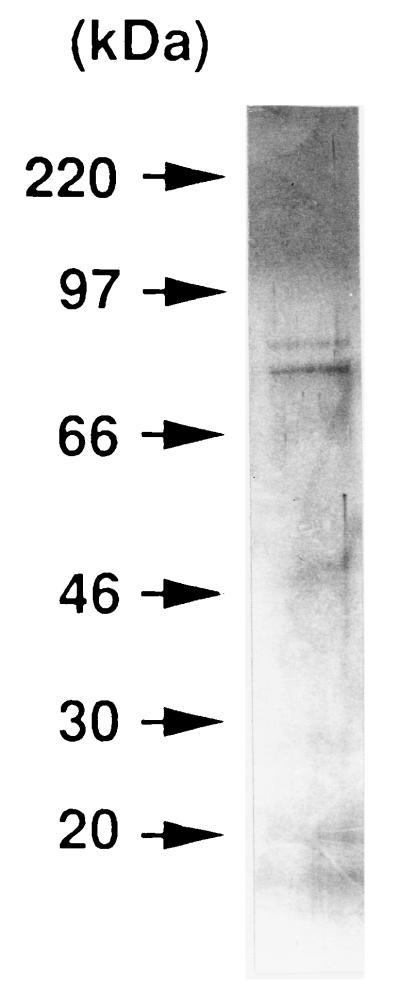
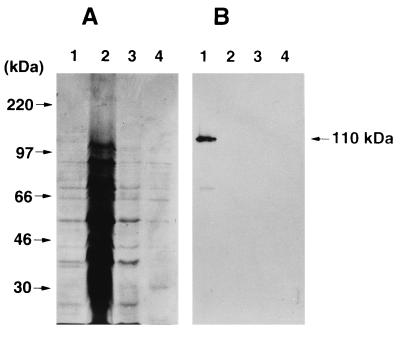
We purified the toxin from the culture supernatant of A. actinomycetemcomitans Y4 by a four-step procedure. Biologic activity was monitored by the MTT viability assay, using HS-72 cells as indicator cells. The crude toxin was precipitated from the cell-free culture supernatant by addition of solid ammonium sulfate to 40% saturation and then applied to a preparative POROS column. The biologically active fractions eluted at 200 to 350 mM ammonium bicarbonate were pooled, dialyzed against PBS, and used as the partially purified toxin. To remove LPS from the partially purified toxin, the sample was applied to a column of polymyxin B matrix. The unbound fraction was found to be biologically active. The active fraction was dialyzed against 10 mM ammonium bicarbonate buffer and applied to a Mono-Q ion-exchange column. A peak that exhibited cytotoxic activity by the MTT viability assay was eluted at 250 to 300 mM ammonium bicarbonate. The active fractions were pooled, lyophilized, and used as a purified toxin from A. actinomycetemcomitans Y4. The purification procedure (Table 1) resulted in 432-fold purification with 43% yield. SDS-PAGE of the purified toxin yielded two major bands with molecular masses of 80 and 85 kDa by the silver staining method (Fig. 1). Next, we examined the immunologic reactivity to the leukotoxin by immunoblot analysis. As shown in Fig. 2, a polyclonal antibody against the leukotoxin purified from A. actinomycetemcomitans 301-b recognized the leukotoxin derived from A. actinomycetemcomitans Y4 whole-cell lysate. However, no protein bands with a molecular mass of 110 kDa were detected in the crude (10 and 100 μg) and partially purified (5 μg) toxin derived from A. actinomycetemcomitans Y4.
TABLE 1.
Scheme for purification of the toxin from A. actinomycetemcomitans Y4a
| Purification step | Protein (mg) | Total activity (U) | Sp act (U/mg) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Culture supernatant | 330.01 | 17,161 | 52 | 1 | 100 |
| Ammonium sulfate precipitation | 60.00 | 15,600 | 260 | 5 | 91 |
| POROS HQ/M column | 17.27 | 15,267 | 884 | 17 | 89 |
| Polymyxin B matrix | 15.35 | 13,892 | 905 | 17 | 81 |
| Mono-Q HR5/5 column | 0.33 | 7,413 | 22,464 | 432 | 43 |
Cytotoxicity (in units per milliliter) was expressed as the reciprocal of the highest dilution producing 50% inhibition by the MTT viability assay. Culture supernatant (5 liters) was used as the starting material.
FIG. 1.
Silver-stained gel after SDS-PAGE of the purified toxin from A. actinomycetemcomitans Y4 under reducing conditions.
FIG. 2.
Western blot analysis of the toxin purified from A. actinomycetemcomitans Y4 culture supernatant with a rabbit antileukotoxin serum. The whole-cell lysate (10 μg [lane 1]), crude toxin (100 μg [lane 2] and 10 μg [lane 3]), and partially purified toxin (5 μg [lane 4]) derived from A. actinomycetemcomitans Y4 were reacted with a rabbit antileukotoxin serum (A). A gel was stained with Coomassie brilliant blue to confirm the amount of protein on each lane (B).
Cytotoxic effect of the toxin from A. actinomycetemcomitans Y4.
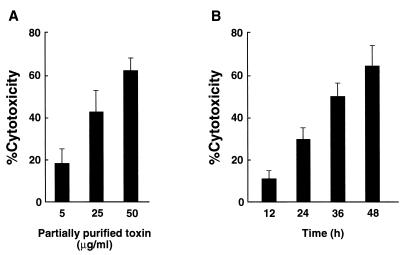
We first examined the effect of the crude toxin from A. actinomycetemcomitans Y4 on cell viability of several cell lines by the MTT viability assay. The crude toxin from A. actinomycetemcomitans Y4 was found to be dose dependently toxic to HS-72, J774.1, WEHI-231, KB, and EL-4 cells after 48 h of culture (data not shown). We incubated HS-72 cells with the partially purified toxin from A. actinomycetemcomitans Y4 and examined the cytotoxicity by the MTT viability assay. As shown in Fig. 3, the partially purified toxin from A. actinomycetemcomitans Y4 showed toxic effect dose and time dependently. HS-72 cells exhibited 65% cytotoxicity when cultured with the partially purified toxin (50 μg/ml) for 48 h (Fig. 3B).
FIG. 3.
Cell death of HS-72 cells induced by the toxin from A. actinomycetemcomitans Y4. HS-72 cells (2 × 104) were cultured with partially purified toxin (5, 25, and 50 μg/ml) for 48 h (A); HS-72 cells were cultured with the partially purified toxin (50 μg/ml) for 12, 24, 36, and 48 h (B). Percent cytotoxicity was determined by the MTT viability assay as described in Materials and Methods. Data are expressed as means ± standard deviations of triplicate determinations. The experiment was performed three times, with similar results.
Detection of apoptosis in HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4.
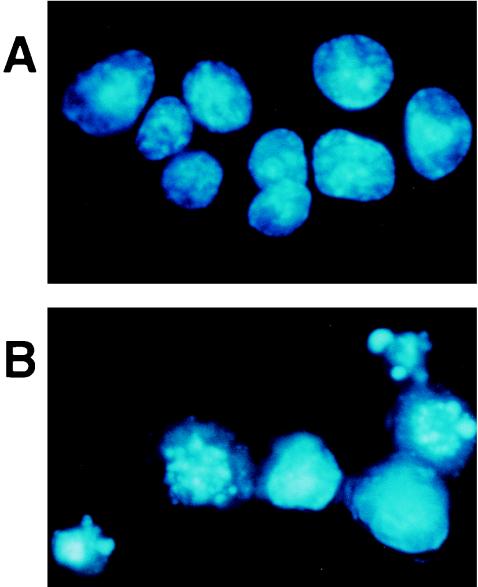
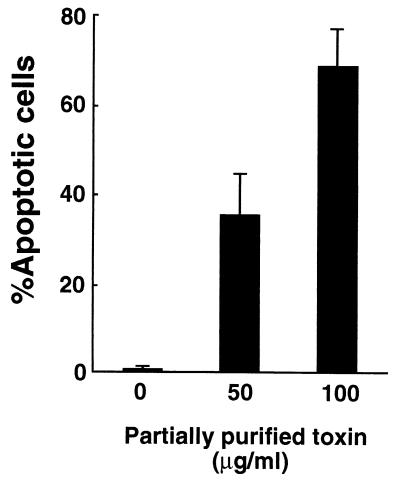
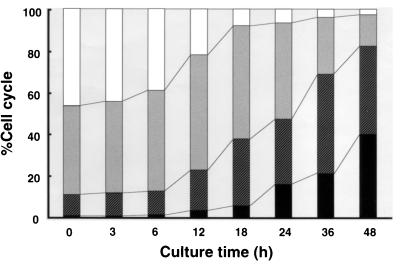
After HS-72 cells were incubated with the partially purified toxin from A. actinomycetemcomitans Y4, the cells were stained with Hoechst dye and visualized by fluorescence microscopy. As shown in Fig. 4, the apoptotic cells were identified according to characteristic cell morphology such as condensation and degradation of the nuclei. Hoechst staining revealed that the partially purified toxin increased the number of apoptotic HS-72 cells in a dose-dependent manner (Fig. 5). The partially purified toxin (50 μg/ml) exhibited 70% apoptosis in HS-72 cells. These results were verified by flow cytometric analysis. The propidium iodide-stained histogram clearly distinguished nuclei with normal diploid DNA from apoptotic nuclei with hypodiploid DNA. When HS-72 cells were cultured without the partially purified toxin, less than 2% of the cells had hypodiploid DNA (Fig. 6). After HS-72 cells were cultured with the partially purified toxin (50 μg/ml) for 48 h, the population of HS-72 cells with hypodiploid DNA increased time dependently (Fig. 6).
FIG. 4.
Representative morphology of HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4. HS-72 cells were cultured without (A) or with (B) partially purified toxin (50 μg/ml) for 48 h and stained with the DNA-specific fluorochrome Hoechst dye 33342. Apoptotic cells exhibiting the characteristic chromatin condensation were observed by fluorescence microscopy (magnification, ×250).
FIG. 5.
Apoptotic cell death of HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4. HS-72 cells were cultured with appropriate amounts of the partially purified toxin for 48 h and stained with Hoechst dye 33342. One hundred cells were observed by fluorescence microscopy, and apoptotic cells exhibiting the characteristic chromatin condensation were counted.
FIG. 6.
Cell cycle analysis of HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4 by flow cytometry. HS-72 cells (106) were cultured with the partially purified toxin (50 μg/ml) for 48 h and then stained with propidium iodide. DNA content was analyzed at the times indicated. □, G1 phase;  , S phase; ▨, G2/M phase; ■, apoptosis.
, S phase; ▨, G2/M phase; ■, apoptosis.
Cell cycle arrest in the G2/M phase and apoptosis induced by the toxin from A. actinomycetemcomitans Y4.
HS-72 cells were incubated with the toxin from A. actinomycetemcomitans Y4 and analyzed for cell cycle distribution by flow cytometry. Cultivation with the partially purified toxin (50 μg/ml) time dependently increased the population of HS-72 cells in the G2/M phase but decreased the population in the G1 phase. A time course study indicated that the population in the S phase decreased gradually after being cultured with the partially purified toxin for 24 h (Fig. 6). As shown in Table 2, the purified toxin (1 μg/ml) remarkably increased the population of HS-72 cells in the G2/M phase and apoptosis but decreased the population in the G1 phase and S phase.
TABLE 2.
Effect of ATA on cell cycle arrest in the G2/M phase and apoptosis in HS-72 cells cultured with the purified toxin from A. actinomycetemcomitans Y4a
| Condition | % of cells at indicated stage of cell cycle
|
|||
|---|---|---|---|---|
| G1 | S | G2/M | Apoptosis | |
| No toxin | 48.3 | 40.2 | 8.8 | 2.7 |
| Purified toxin | ||||
| Alone | 2.4 | 16.5 | 31.2 | 49.9 |
| With ATA | 5.2 | 26.3 | 62.3 | 6.2 |
HS-72 cells (106) were cultured without or with purified toxin (1 μg/ml) in the absence or presence of ATA (200 μM) for 48 h and stained with propidium iodide. DNA content was analyzed by flow cytometry.
Effects of ATA and bcl-2 on apoptosis induced by the toxin from A. actinomycetemcomitans Y4.
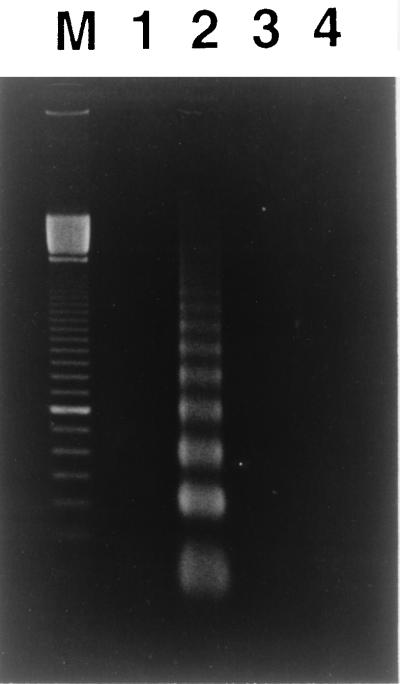
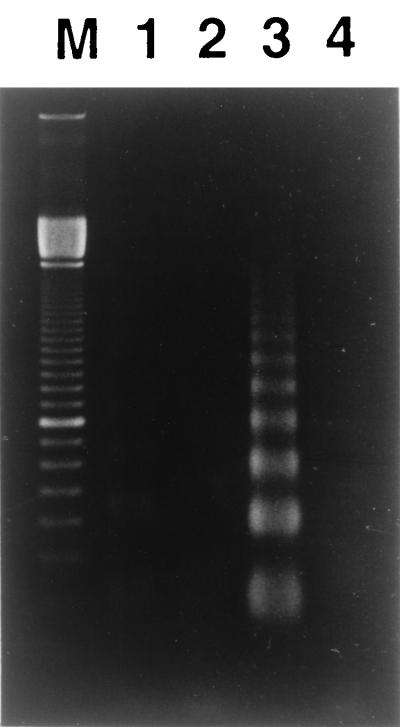
We determined whether ATA, a DNA endonuclease inhibitor, rescues HS-72 cells from apoptotic cell death induced by the partially purified toxin from A. actinomycetemcomitans Y4. As shown in Fig. 7, a nucleosomal ladder pattern of DNA degradation was observed in HS-72 cells cultured with the partially purified toxin (50 μg/ml). However, HS-72 cells cultured with the same concentrations of the toxin in the presence of ATA (200 μM) had an undetectable level of low-molecular-weight DNA, indicating a lack of DNA fragmentation that is characteristic of apoptosis. We next assessed the ability of bcl-2 to protect HS-72 cells from the partially purified toxin-induced apoptosis, using HS-72 cells that overexpress human Bcl-2. Gel electrophoresis analysis of cellular DNA revealed that the fragmented DNA content was less in HS-72 cells with constitutive expression of Bcl-2 (HS-72 B16) than in the plasmid control (HS-72 S4) when cells were cultured with the partially purified toxin (50 μg/ml). Incubation of HS-72 S4 and HS-72 B16 cells without the partially purified toxin resulted in an undetectable level of low-molecular-weight DNA (Fig. 8). Next, we examined the effects of ATA and bcl-2 on apoptosis in HS-72 cells cultured with the purified toxin from A. actinomycetemcomitans Y4 by flow cytometry. ATA (200 μM) was found to inhibit completely apoptosis in HS-72 cells cultured with the purified toxin (1 μg/ml) from A. actinomycetemcomitans Y4 but not cell cycle arrest in the G2/M phase (Table 2). Flow cytometric analysis revealed that an overexpression of Bcl-2 remarkably inhibited the purified toxin-induced apoptosis in HS-72 cells (Table 3). When HS-72 B16 cells were cultured with the purified toxin (1 μg/ml) for 48 h, the population of the cells in the G1 phase decreased from 48.6 to 8.8%, but those in the G2/M phase increased from 11.3 to 52.4% (Table 3).
FIG. 7.
Effect of ATA on the DNA cleavage induced by the toxin from A. actinomycetemcomitans Y4. HS-72 cells were cultured with the partially purified toxin (50 μg/ml) in the absence or presence of ATA (200 μM) for 48 h, and cellular DNA was detected as described in Materials and Methods. Lane M, 100-bp ladder (Pharmacia Biotech, Piscataway, N.J.); lane 1, untreated; lane 2, cultured with partially purified toxin; lane 3, cultured with partially purified toxin and ATA; lane 4, cultured with ATA.
FIG. 8.
bcl-2 protects HS-72 cells from apoptosis induced by the toxin from A. actinomycetemcomitans Y4. Cells were cultured with the partially purified toxin (50 μg/ml) for 48 h, and then cellular DNA was detected as described in Materials and Methods. Lane M, 100-bp ladder; lane 1, HS-72 S4 cultured without partially purified toxin; lane 2, HS-72 B16 cultured without partially purified toxin; lane 3, HS-72 S4 cultured with partially purified toxin; lane 4, HS-72 B16 cultured with partially purified toxin.
TABLE 3.
Effect of Bcl-2 on cell cycle arrest in the G2/M phase and apoptosis in HS-72 cells cultured with the purified toxin from A. actinomycetemcomitans Y4a
| Cells | % at indicated stage of cell cycle
|
|||
|---|---|---|---|---|
| G1 | S | G2/M | Apoptosis | |
| HS-72 S4 | ||||
| −Purified toxin | 47.3 | 39.3 | 10.8 | 2.6 |
| +Purified toxin | 4.8 | 17.0 | 30.3 | 47.9 |
| HS-72 B16 | ||||
| −Purified toxin | 48.6 | 37.5 | 11.3 | 2.6 |
| +Purified toxin | 8.8 | 29.6 | 52.4 | 9.2 |
The cells (106) were cultured with or without purified toxin (1 μg/ml) for 48 h and stained with propidium iodide. DNA content was analyzed by flow cytometry.
DISCUSSION
In this study, we purified the toxin from A. actinomycetemcomitans Y4 through POROS column chromatography, polymyxin B matrix column chromatography, and Mono-Q column chromatography. SDS-PAGE of the purified toxin showed two major bands corresponding to molecular masses of approximately 80 and 85 kDa (Fig. 1). To remove LPS contamination, the toxin was applied to a column of polymyxin B matrix. The silver-staining profile of SDS-PAGE revealed no ladder bands indicative of A. actinomycetemcomitans Y4 LPS (18), suggesting no contamination with LPS in the purified toxin from A. actinomycetemcomitans Y4 (Fig. 1). Furthermore, the toxin purified from A. actinomycetemcomitans Y4 did not exhibit Limulus amebocyte lysate clotting activity (data not shown). These findings indicate that contamination with LPS would not explain the ability of the toxin from A. actinomycetemcomitans Y4 to induce a cytotoxic effect on HS-72 cells. However, the possibility that a substance other than 80- and 85-kDa proteins may be responsible for the cytotoxic activity has not been excluded in this study. We are continuing efforts to prepare a sufficient quantity of the highly purified toxin for definitive identification.
This study reports the presence of a new toxin in culture supernatant from the periodontopathic bacterium A. actinomycetemcomitans. This is the first evidence that the toxin from A. actinomycetemcomitans induces cell cycle arrest in the G2/M phase and subsequently apoptosis in mammalian cells. Periodontitis is associated with an infectious disease process. Bacteria and their products interact with the junctional epithelium and penetrate the underlying connective tissue. The host immune system is activated by periodontopathic bacterial products (8), and the lesion with periodontitis becomes dominated by B cells (11). Finally, a large number of plasma cells accumulate in local tissue with chronic inflammation. In this study, the toxin from the periodontopathic bacterium A. actinomycetemcomitans was found to induce the growth inhibition of several mammalian cell lines derived from B cells, T cells, macrophages, and oral epidermoid cells (data not shown). In addition, the toxin contributed to the induction of cell cycle arrest in the G2/M phase and subsequent apoptosis in B lymphocytes, suggesting that the toxin purified from A. actinomycetemcomitans Y4 may play an important role in the progression of periodontitis.
Several studies have shown that the products of A. actinomycetemcomitans kill mammalian cells. Among these products, the leukotoxin is well known to be a potent virulence factor of A. actinomycetemcomitans. It has been reported that 55% of A. actinomycetemcomitans clinical isolates from localized juvenile periodontitis patients produce the leukotoxin able to lyse human neutrophils and monocytes (43). The leukotoxin, a 110-kDa, pore-forming, basic protein which destroys human neutrophils and monocytes by osmotic lysis (40), is not released into culture supernatants but remains associated with the outer membrane. Mangan et al. (14) have reported that the leukotoxin kills up to 70% of human lymphocytes by the pathway resembling necrosis and apoptosis. Taichman et al. (33) have demonstrated that the leukotoxin destroys human polymorphonuclear leukocytes and monocytes but not cells derived from rabbits, rats, mice, and guinea pigs. In this study, the crude toxin from A. actinomycetemcomitans Y4 killed mouse B cells and human oral epidermoid cells (data not shown). In addition, a 110-kDa protein band of leukotoxin was not detected in the toxin purified from A. actinomycetemcomitans Y4 culture supernatant by immunoblot analysis (Fig. 2). A leukotoxin was extracted with polymyxin B sulfate from A. actinomycetemcomitans whole cells and purified with ion exchange chromatography and gel filtration chromatography, resulting in the recovery of 48% with a 99-fold increase in specific activity (34). Ohta et al. (23) have also reported that the leukotoxin is not secreted extracellularly and remains associated with the bacterial cells and that the extracellular secretion of toxin occurs with increased ionic strength of medium. These findings suggest that contamination with the leukotoxin would not explain the ability of the toxin to induce apoptosis in mouse B-cell hybridoma HS-72 cells. However, the possibility remains that the toxin purified from culture supernatant of A. actinomycetemcomitans Y4 is released from the outer membrane into the culture media by proteolytic cleavage. To elucidate whether the toxin is a leukotoxin-derived substance or some other protein, work is in progress to determine the amino acid sequence of the highly purified toxin.
During apoptosis, or programmed cell death, endogenous endonucleases cut DNA in the nucleosomal linker regions. Selective activation of endonucleases appears to be responsible not only for chromatin cleavage but also for nuclear morphologic change. In this study, we demonstrated that murine B-cell hybridoma HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4 exhibited the typical patterns of DNA fragmentation and the nuclear morphology of apoptotic cells. ATA, a DNA endonuclease inhibitor, was found to efficiently inhibit apoptosis in purified toxin-treated HS-72 cells (Table 2). Furthermore, Hoechst staining revealed the typical morphologic change of apoptotic nuclei of HS-72 cells cultured with the toxin. These results indicate that death of HS-72 cells cultured with the toxin from A. actinomycetemcomitans Y4 occurs through apoptosis mediated by an endonuclease. We previously demonstrated that A. actinomycetemcomitans infection induces apoptosis in the macrophage cell line J774.1 and that the invasion of macrophages by A. actinomycetemcomitans is essential for induction of apoptosis (10). In addition, ATA was found to suppress apoptosis in J774.1 cells infected with live A. actinomycetemcomitans (10). In this study, the toxin from A. actinomycetemcomitans induced death of J774.1 cells (data not shown). Taken together, these findings suggest that A. actinomycetemcomitans inside J774.1 cells might produce the apoptosis-inducing toxin within the cytoplasm, resulting in the induction of apoptosis in J774.1 cells infected with A. actinomycetemcomitans.
The bcl-2 gene was identified as the breakpoint site of the t(14; 18) chromosomal translocation that is associated with follicular lymphoma (2, 36). The role of Bcl-2-related molecules, such as Bcl-2, Bcl-X, and Bax, in regulating apoptosis has been characterized over several years. In particular, a variety of studies have demonstrated that overexpression of Bcl-2 can prevent or delay many forms of apoptosis (22). We have previously demonstrated that Bcl-2-overexpressing HS-72 cells are resistant to activin A-induced apoptosis (13). In the present study, we examined the response of HS-72 cells transfected with bcl-2 to the toxin from A. actinomycetemcomitans Y4 to determine whether Bcl-2 is involved in modulating the cell cycle arrest in the G2/M phase and apoptosis. As shown in Fig. 8 and Table 2, overexpression of Bcl-2 completely protected HS-72 cells from the toxin-induced apoptosis. However, exposure of the purified toxin to bcl-2-transfected HS-72 cells for 48 h had no effect on cell cycle arrest in the G2/M phase (Table 3). These findings are in accord with the results showing that Bcl-2 appears to function by suppressing apoptosis at a point after cell cycle arrest (38). We have previously reported that induction of a high level of Bcl-2 blocks activin A-induced apoptosis but not cell cycle arrest in the G1 phase in HS-72 cells (13, 42). These findings raise the question as to precisely how Bcl-2 functions in cell cycle arrest and apoptosis. We have no ready exact explanation for this phenomenon but think that Bcl-2 operates at a signaling point downstream of cell cycle stasis.
DNA damage in proliferating cells induces a complex intracellular response comprising perturbation of the cell cycle and apoptotic cell death (41). It is well known that loss of cell cycle control and the inability of the cells to repair DNA at cell cycle checkpoints results in the propagation of genetic lesions which ultimately leads to cancer (24). Irradiation of mammalian cells was shown to cause delays in completion of the S phase followed by an extended G2/M arrest and apoptosis (1, 15). It has been reported that human immunodeficiency virus type 1 viral protein R induces apoptosis following cell cycle arrest in the G2/M phase (32) and that a new cytolethal distending toxin from Escherichia coli blocks HeLa cell division in the G2/M phase (25). These studies help us to elucidate the role of the viral protein and bacterial toxin family in serious infectious diseases.
We demonstrate here that after the arrest of cells in the G2/M phase, the toxin from A. actinomycetemcomitans induces apoptosis in mouse lymphocytes. Our results are contrary to a report by Helgeland and Nordby (6), who suggested that the crude toxin from A. actinomycetemcomitans is cytostatic but not cytotoxic. They reported that the crude toxin isolated from the growth medium of A. actinomycetemcomitans inhibits cell growth of human gingival fibroblasts in an irreversible manner, although without killing the cells (6). It is likely that A. actinomycetemcomitans produces several types of toxin which have cytostatic and cytotoxic effects on mammalian cells. Another possible explanation regarding the discrepancy relates to the difference in cells used in the experiments.
In conclusion, the present results indicate a novel cytopathic effect of the toxin from A. actinomycetemcomitans characterized by a cell cycle block in the G2/M phase and subsequent induction of apoptosis. Although A. actinomycetemcomitans is implicated in the pathogenesis of juvenile and severe adult periodontitis, this microorganism has also been associated with systemic infections in humans, such as endocarditis, pericarditis, meningitis, osteomyelitis, empyema, and subcutaneous abscess. These findings may provide insight into the important pathological roles of the toxin from A. actinomycetemcomitans in the initiation and progression of not only periodontitis but also severe systemic infectious diseases.
ACKNOWLEDGMENTS
We thank H. Ohta and K. Fukui for generously providing the antileukotoxin antibody.
This work was supported partially by grants-in-aid from the Ministry of Education, Science, and Culture of Japan and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Aloni-Grinstein R, Schwartz D, Rotter V. Accumulation of wild-type p53 protein upon γ-irradiation induces a G2 arrest-dependent immunoglobulin κ light chain gene expression. EMBO J. 1995;14:1392–1401. doi: 10.1002/j.1460-2075.1995.tb07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakhshi A, Jensen J P, Goldman P, Wright J J, McBride O W, Epstein A L, Korsmeyer S J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985;41:899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- 3.Christersson L A, Wikesjo U M, Albini B, Zambon J J, Genco R J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J Periodontol. 1987;58:540–545. doi: 10.1902/jop.1987.58.8.540. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J J. Apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 6.Helgeland K, Nordby O. Cell cycle-specific growth inhibitory effect on human gingival fibroblasts of a toxin isolated from the culture medium of Actinobacillus actinomycetemcomitans. J Periodontal Res. 1993;28:161–165. doi: 10.1111/j.1600-0765.1993.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara Y, Nishihara T, Maki E, Noguchi T, Koga T. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J Periodontal Res. 1991;26:155–160. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa I, Nakashima K, Koseki T, Arakawa S, Nitta H, Nishihara T. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997;14:79–111. doi: 10.1111/j.1600-0757.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan A H, Weber D J, Oddone E Z, Perfect J R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev Infect Dis. 1989;11:46–63. doi: 10.1093/clinids/11.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Muro M, Akifusa S, Hanada N, Semba I, Fujii T, Kowashi Y, Nishihara T. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infect Immun. 1995;63:3914–3919. doi: 10.1128/iai.63.10.3914-3919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornman K S, Page R C, Tonetti M S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 12.Korsmeyer S J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- 13.Koseki T, Yamato K, Krajewski S, Reed J C, Tsujimoto Y, Nishihara T. Activin A-induced apoptosis is suppressed by BCL-2. FEBS Lett. 1995;376:247–250. doi: 10.1016/0014-5793(95)01290-7. [DOI] [PubMed] [Google Scholar]
- 14.Mangan D F, Taichman N S, Lally E T, Wahl S M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna W G, Bernhard E J, Markiewicz D A, Rudoltz M S, Maity A, Muschel R J. Regulation of radiation-induced apoptosis in oncogene-transfected fibroblasts: influence of H-ras on the G2 delay. Oncogene. 1996;12:237–245. [PubMed] [Google Scholar]
- 16.Moore K J, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 17.Nishihara T, Akifusa S, Koseki T, Kato S, Muro M, Hanada N. Specific inhibitors of vacuolar type H+-ATPases induce apoptotic cell death. Biochem Biophys Res Commun. 1995;212:255–262. doi: 10.1006/bbrc.1995.1964. [DOI] [PubMed] [Google Scholar]
- 18.Nishihara T, Koga T, Hamada S. Extracellular proteinaceous substances from Haemophilus actinomycetemcomitans induce mitogenic responses in murine lymphocytes. Oral Microbiol Immunol. 1987;2:48–52. doi: 10.1111/j.1399-302x.1987.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara T, Ohsaki Y, Ueda N, Koseki T, Eto Y. Induction of apoptosis in B lineage cells by activin A derived from macrophages. J Interferon Cytokine Res. 1995;15:509–516. doi: 10.1089/jir.1995.15.509. [DOI] [PubMed] [Google Scholar]
- 20.Nishihara T, Ohsaki Y, Ueda N, Saito N, Mundy G R. Mouse interleukin-1 receptor antagonist induced by Actinobacillus actinomycetemcomitans lipopolysaccharide blocks the effects of interleukin-1 on bone resorption and osteoclast-like cell formation. Infect Immun. 1994;62:390–397. doi: 10.1128/iai.62.2.390-397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishihara T, Ueda N, Amano K, Ishihara Y, Hayakawa H, Kuroyanagi T, Ohsaki Y, Nagata K, Noguchi T. Actinobacillus actinomycetemcomitans Y4 capsular-polysaccharide-like polysaccharide promotes osteoclast-like cell formation by interleukin-1α production in mouse marrow cultures. Infect Immun. 1995;63:1893–1898. doi: 10.1128/iai.63.5.1893-1898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Núñez G, Merino R, Grillot D, González-Garciá M. Bcl-2 and Bcl-x: regulatory switches for lymphoid death and survival. Immunol Today. 1994;15:582–588. doi: 10.1016/0167-5699(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 23.Ohta H, Hara H, Fukui K, Kurihata H, Murayama Y, Kato K. Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infect Immun. 1993;61:4878–4884. doi: 10.1128/iai.61.11.4878-4884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orren D K, Petersen L N, Bohr V A. Persistent DNA damage inhibits S-phase and G2 progression, and results in apoptosis. Mol Biol Cell. 1997;8:1129–1142. doi: 10.1091/mbc.8.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 26.Raff M C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 27.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 28.Robertson P B, Lantz M, Marucha P T, Kornman K S, Trummel C L, Holt S C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982;17:275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 29.Slots J, Genco R J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 30.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 31.Slots J, Reynolds H S, Genco R J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980;29:1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taichman N S, Dean R T, Sanderson C J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai C C, Shenker B J, DiRienzo J M, Malamud D, Taichman N S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984;43:700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci USA. 1989;86:1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujimoto Y, Cossman J, Jaffe E, Croce C M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 37.Ueda N, Nishihara T, Ishihara Y, Amano K, Kuroyanagi T, Noguchi T. Role of prostaglandin in the formation of osteoclasts induced by capsular-like polysaccharide antigen of Actinobacillus actinomycetemcomitans strain Y4. Oral Microbiol Immunol. 1995;10:69–75. doi: 10.1111/j.1399-302x.1995.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 38.Walton M I, Whysong D, O’Connor P M, Hockenbery D, Korsmeyer S J, Kohn K W. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993;53:1853–1861. [PubMed] [Google Scholar]
- 39.Weinert T A, Hartwell L H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 40.Wilson M, Henderson B. Virulence factors of Actinobacillus actinomycetemcomitans relevant to the pathogenesis of inflammatory periodontal diseases. FEMS Microbiol Rev. 1995;17:365–379. doi: 10.1111/j.1574-6976.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 41.Woloschak M, Yu A, Xiao J. Molecular and cellular responses to DNA damage in a murine pituitary adenoma cell line. Mol Cell Endocrinol. 1996;119:61–68. doi: 10.1016/0303-7207(96)03795-1. [DOI] [PubMed] [Google Scholar]
- 42.Yamato K, Koseki T, Ohguchi M, Kizaki M, Ikeda Y, Nishihara T. Activin A induction of cell-cycle arrest involves modulation of cyclin D2 and p21CIP1/WAF1 in plasmacytic cells. Mol Endocrinol. 1997;11:1044–1052. doi: 10.1210/mend.11.8.9953. [DOI] [PubMed] [Google Scholar]
- 43.Zambon J J, DeLuca C, Slots J, Genco R J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect Immun. 1983;40:205–212. doi: 10.1128/iai.40.1.205-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]