Abstract
Rationale
Racial disparities in sepsis outcomes have been previously reported. However, recently, there have been inconsistencies in identifying which socioeconomic variables, such as race, account for these disparities. The objective of this study was to perform a systematic review in order to examine the impact of race on sepsis-attributable mortality.
Methods
Systematic searches for English-language articles identified through MEDLINE, EBSCOhost, PubMed, ERIC, and Cochrane Library databases from 1960 to 1 February 2017. Included studies examined sepsis outcomes in the context of sepsis incidence and/or mortality. Two investigators independently extracted data and assessed study quality. The meta-analysis was performed in accordance with the Cochrane Collaboration guidelines.
Results
Twenty-one studies adhered to the predefined selection criteria and were included in the review. Of the 21 studies, we pooled data from 6 studies comparing African American/Black race as a risk factor for sepsis-related mortality disparities (reference group being Caucasian/White). From the meta-analysis on these six studies, African American/Black race was found to have no statistical significant relationship with sepsis-related mortality (odds ratio 1.20, 95% CI, 0.81 to 1.77). Similar results were found for other races (Native Americans, Asians) and ethnicities (Hispanic/Latinos).
Conclusion
On the basis of available evidence from a limited number of observation retrospective studies, race alone cannot fully explain sepsis-related disparities, especially sepsis-attributable mortality.
Keywords: Sepsis, Race, Mortality
Background
Health inequities by race and/or socioeconomic status are prevalent in the most common morbidities and mortalities in the USA [1]. Eliminating these health inequities has become a significant national priority in all fields of medicine, especially in critical care medicine [2–4]. For instance, race-specific mortality disparities have been reported in cardiac arrests [5], trauma [6], and venous thrombo-embolisms [7]. Understanding disease-specific factors along with socioeconomic variables, such as race, may result in identifying areas for quality improvement, reduction in healthcare costs, and allocation of equitable and efficient resources.
Sepsis is one condition specific to critical care medicine that has been identified as having socioeconomic disparities [3]. Of all the socioeconomic variables, race has been the most explored in regard to sepsis; however, there are inconsistent findings by race on the impact of sepsis-related outcomes, specifically on mortality [2, 8, 9]. Further, it is unclear if this racial inconsistency is due to the impact of other socioeconomic variables (e.g., insurance status, median income), as race is often used as a proxy for these factors [10]. Given that human genetic variation does not naturally aggregate into subgroups that match racial categories [11], insight into race’s influence on sepsis-related mortality is needed.
The objective of this study was to perform a systematic review of literature in order to synthesize evidence with the goal of examining the impact of race on sepsis-attributable mortality.
Methodology
Data Sources
We searched for English-language articles identified through MEDLINE, EBSCOhost, PubMed, ERIC, and Cochrane Library databases (from 1960 to 1 February 2017). A potential source of bias may be how race is defined in studies outside of the USA, and if race is considered a proxy for other socioeconomic variables. Therefore, to limit this bias, we only consider studies conducted within the USA and in English.
Eligibility Criteria
Eligibility criteria included studies that explicitly examined sepsis outcomes in the context in disparities in sepsis incidence and/or mortality. We excluded studies that did not provide demographic and/or socioeconomic variables. We also excluded animal studies and studies that provided insufficient information on population characteristics (e.g., did not provide information on the population’s racial identities).
Study Identification
Two persons (JW and PG) worked on finalizing the research criteria, and one person (PG) worked on reviewing all titles and abstracts identified form the search strategy. The full texts were then reviewed by PG and sent to third person (AS) to confirm inclusion or exclusion of a study.
Data Extraction
PG independently reviewed each eligible paper. The final list of studies was prepared by PG and reviewed by AS. Data collected from each paper included number of patients, how sepsis was defined (by billing code or definition), time frame the study spanned, what database the paper used, race, English-speaking status, insurance, HIV status, median income (self-reported or pulled from national-level data), poverty level of patient community, education, urbanicity of patient community, behaviors (tobacco use, alcohol use). Race and ethnicity were operationalized asAfrican American/Black versus Caucasian/White, Asian American versus Caucasian/White, and Hispanic/Latino versus Caucasian/White.
Outcome Measures
In regard to outcomes, we focused on the relationship of race with sepsis-related mortality. Reasons for not focusing on other outcomes include change in the clinical definition of sepsis over time [12–14] (which would impact incidence) and time to antibiotics, and completion of sepsis bundles have more recently gained attention [15] (resulting in fewer studies focusing on management of sepsis [16, 17]).
Data Synthesis and Analysis
We examined the following comparisons in regard to sepsis-related mortality: minority race (African American/Black, Asian, or Native American) versus Caucasian/White and ethnicity (Hispanic/Latino) versus Caucasian/White.
Qualitative Analysis
We used a narrative summary approach to describe study characteristics and variation in quality indicators among studies. Further, we evaluated how these factors affect our understanding of sepsis-related mortality outcomes of the studies included in this review.
Quantitative Analysis
The meta-analysis was performed in accordance with the Cochrane Collaboration guidelines [18]. All statistical analyses were performed with R (version 3.4.3) [19] and package meta (version 4.9.1) [20]. The pooled effect estimates for binary variables were summarized as odds ratios with 95% confidence intervals (CI). We examined heterogeneity by using the Cochran Q and the I2 test [21]. All meta-analysis were performed using random-effect models. We considered two-sided p value less than 0.05 to be significant.
Results
Study Selection
The search identified 123 studies of possible relevance. Of these, 21 studies adhered to the predefined selection criteria and were included in the review. Figure 1 shows the results of the search strategy. Forty-five manuscripts were removed after being assigned as “not primary focus,” indicating that sepsis incidence and/or mortality were discussed in more of an editorial rather than reviewed through epidemiological statistical evaluations. We evaluated the concern for publication bias within our studies. Studies were reviewed by AS who completely agreed with the selection of the included studies.
Fig. 1.
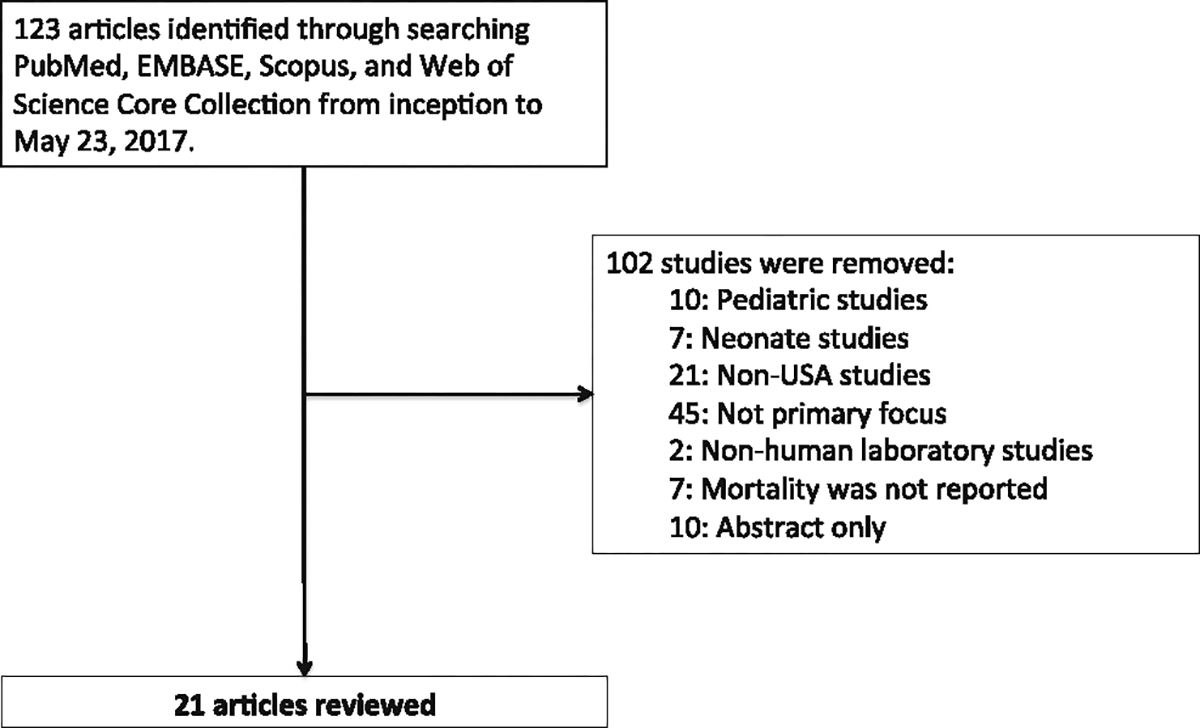
Flow of identified studies
Study Description
Table 1 summarizes the 21 studies, none of which are randomized-controlled trials [2, 3, 8, 16, 22–38].A total of 21,868,054 patients were included in this review. Seven studies evaluated only sepsis mortality [16, 22, 29, 30, 32, 34, 36], while the remaining 14 studies evaluated both incidence and mortality. Sepsis was identified by either coding system or clinical chart review. Seventeen studies used ICD-9 coding to identify sepsis: five using admission ICD-9 coding [16, 23, 27, 34, 37], five using discharge ICD-9 coding [2, 8, 24–26, 31], and 7 used ICD-9 coding but did not specify admission or discharge [3, 28, 30, 32, 35, 36, 38]. The remaining studies used clinical chart review to determine if the diagnosis was sepsis [9, 23, 33, 37]. The studies’ investigational period ranged from 1 year to as many as 25 years (mean of 9.5 ± 7.1 years). The earliest year of investigational tracking began in 1979. All of the 21 studies concluded in 2000 or later, with the latest completion being in 2013. Qualitative analysis of key study characteristics, as well as quality indicators, revealed the following differences.
Table 1.
Characteristics of included studies
| Author (year) | Patient characteristics | Sample size | Study years | Sepsis-related disparity outcome finding |
|---|---|---|---|---|
|
| ||||
| Bamato et al. [2] (2008) | Patients pulled from hospital discharge data sets (both state and federal agencies) | 282,292 | 2001 | African Americans/Blacks had higher incidence and mortality for sepsis, while Hispanics/Latinos had lower rates of incidence for sepsis. Adjusted for race, gender, poverty categories, and urbanization versus rural |
| Bedri et al. [29] (2017) | Evaluated patients with burns and their outcomes | 172,640 | 2002–2011 | No difference in sepsis-related mortality between races; only race was evaluated for sepsis incidence. Adjusted for insurance status and other co-morbidities |
| Bime et al. [22] (2016) | Evaluated patients with acute respiratory distress syndrome [39] and their outcomes | 821,902 | 2008–2012 | Blacks, Hispanics, and other minorities were observed to exhibit significantly higher sepsis-related respiratory failure-associated mortality compared to non-Hispanic whites. Adjusted for race, sex, age, disease severity, type of hospital, and median household income |
| Cheek et al. [30] (2014) | Described mortality due to infections in American Indians/Alaskan Natives | 53,520 | 1999–2009 | American Indians/Alaskan Natives had higher rates of sepsis-related mortality than Whites. No adjustment models were performed. |
| Cribbs et al. [23] (2015) | Outcomes of HIV-infected patients with severe sepsis | 1095 | 2006–2010 | HIV-infected patients had worse sepsis-related mortality than those without HIV. No adjustment to evaluate racial influence. |
| Danai et al. [24] (2006) | Evaluated sepsis incidence and mortality in cancer patients | 1,781,445 | 1979–2001 | African Americans/Blacks had higher rates of sepsis-related mortality, as did patients with HIV. Race was adjusted for gender, severity of illness, source of infection, and other chronic comorbid medical condi- |
| Dombrovskiy et al. [25] (2007) | Patients pulled from HCUP New Jersey database | 24,839 | 2002 | African Americans/Blacks had higher rates of hospitalization for sepsis, but no racial case fatality differences for sepsis. Adjusted only for age (African Americans/Blacks with sepsis were younger) |
| Esper et al. [26] (2006) | Patients pulled from National Hospital Discharge Survey to evaluate sepsis-related disparities | 2,505,082 | 1979–2003 | African Americans/Blacks had higher rates of sepsis-related incidence; however, African Americans/Blacks had higher rates of immunomodulating diseases (HIV, alcohol use). No difference in mortality |
| Firempong etal. [31] (2014) | Sepsis-related outcomes of patients undergoing neurosurgical procedures | 206,902 | 2001–2009 | African Americans/Blacks had higher rates of sepsis-related incidence and mortality. Adjusted for age, gender, comorbidities, length of stay, insurance, hospital volume, and site of the procedure |
| Goodwin et al. [27] (2016) | Patients were pulled from non-federal South Carolina hospitals. | 24,395 | 2010 | Patients from medically underserved areas had higher rates of sepsis incidence and sepsis-attributable mortality. Such community variables were adjusted for race. |
| Kumar et al. [28] (2014) | Using HCUP and National Inpatient Sample database to sepsis outcomes | 1,600,269 | 2000–2008 | Patients without insurance had higher rates of sepsis-attributable mortality. Adjusted for race, gender, age, neighborhood median income, hospital characteristics, number of organ dysfunction, and individual comorbidities. |
| Madsen et al. [16] (2016) | Evaluated variables associated with delay in antibiotic administration in patients with sepsis | 768 | 2005–2012 | No impact of race on time to antibiotics. Adjusted for sex, age, sequential organ failure assessment |
| Martin et al. [3] (2003) | Analyzed occurrence of sepsis from the National Center for Health Statistics | 10,319,418 | 1979–2000 | Higher incidence of sepsis and sepsis-attributable mortality in African Americans/Blacks. No adjusted models |
| Mayr et al. [8] (2010) | Pulled patients from the NHAMCS and US Hospital Discharge Data to evaluate disparities in sepsis | 381,787 | 2003–2007 | African Americans/Blacks had higher rates of incidence and mortality related to sepsis versus Caucasian/Whites. Adjusted for race, age, and sex |
| Melamed et al. [32] (2009) | Analyzed burden of sepsis using population-based estimates from National Center for Health Statistics | 1,017,616 | 1999–2005 | African Americans/Blacks had an increase in mortality as compared to Caucasian/Whites, while Asians had a lower sepsis-attributable mortality. Adjusted only for age |
| Moore et al. [36] (2016) | Data pulled from the National Center for Health Statistics to identify regions high in sepsis-attributable mortality | Not applicable | 2003–2012 | While sepsis-attributable mortality clustered in geographic regions, this remained the case after adjusting for race and community-level variables. |
| Moore et al. [37] (2017) | Explored community variables on incidence of sepsis and sepsis mortality | 29,680 | 2003–2012 | Found that both incidence and deaths due to sepsis clustered around communities, often of high poverty. Adjusted for age, sex, race, education, income, and alcohol use |
| Plurad et al. [33] (2010) | Sepsis incidence and sepsis-attributable mortality in post-trauma patients | 3998 | 2000–2007 | African American/Black had a lower rate of sepsis-attributable mortality as compared to Caucasian/White. Adjusted for age, emergency room hypotension, and presenting neurological status and trauma severity |
| Sammon et al. [38] (2015) | Sepsis incidence and mortality in patients with cancer | 2,502,710 | 1999–2009 | African Americans/Blacks more likely to develop sepsis, but no impact on sepsis-related mortality. Medicare patients more likely to die from sepsis. Adjusted for race, comorbidities, surgery, and cancer type |
| Sandoval et al. [34] (2016) | Patients hospitalized for sepsis, pulled from HCUP database | 131,831 | 2011 | Minorities had less sepsis-related fatalities as compared to Caucasian/White. Adjusted for race, urbanicity, insurance, and neighborhood median income |
| Vogel et al. [35] (2009) | Surgical patients pulled from HCUP New Jersey database | 5865 | 1990–2006 | Higher rates of sepsis-related incidence and mortality in African Americans/Blacks versus Caucasian/Whites. No adjusted models |
HCUP Healthcare Cost and Utilization Project, NHAMCS National Hospital Ambulatory Medical Care Survey
Use of Databases
Databases ranged from hospital-specific database, to medical registries, to national-level data. In regard to national-level registries, they included US Census, American Community Survey [36], Healthcare Cost and Utilization Project (HCUP) [22, 25, 28, 34, 35], Indian Health Services [30], National Center for Health Statistics [3, 32, 36], Nationwide Inpatient Sample [28, 38], and National Hospital Discharge Survey [24, 26]. Three studies pulled data from the 2000 US Census [2, 8, 27]. One study pulled data from state-level database (California) [31]. Medical registries were used by two studies: National Burn Registry [29] and REGARDS [37]. Three single-center studies used only their hospital’s available data [16, 23, 33], while two studies supplemented their hospital pulled data with the US Census [2, 8].
Defining Race
All of the 21 studies evaluated race as a variable for identifying sepsis-related disparities in regard to mortality. Of the 21, only 3 reported that race was self-identified in their methodology [31, 33, 38]. All the studies used Caucasian/White as the comparator/reference group. Thirteen studies evaluated more than two races in their analysis [2, 3, 22–24, 26, 28, 31–36]. From these studies, only one operationalized Hispanic/Latino as an ethnicity category [32] (creating unique categories such as non-Hispanic black versus Hispanic black), while the remainder categorized these individuals under race. Of the studies that assessed only two races, one study evaluated Alaskan Natives/American Indians exclusively, comparing their outcomes to Caucasian/White [30]. The remaining eight studies compared African American/Black to Caucasian/White.
Defining Socioeconomic Individual-Level Variables and Behaviors
There were several socioeconomic individual-level variables that were explored in the 21 studies. The most frequent variable examined after race was insurance status (11 studies [16, 22, 25, 27–29, 31, 34, 36–38]). Five studies explored behaviors to explain sepsis-related disparities: two studies evaluated tobacco use [29, 37] and three studies assessed alcohol use [26, 29, 37]. Other variables included education [27, 36, 37], weight (as defined by body mass index) [28, 29, 37], income [37], and non-English speaking patients [16]. A complete list of variables are provided in Table 2.
Table 2.
List of demographic and socioeconomic variables, both at the individual and community level, from the 21 studies
| Author | Patient-level data |
Community-level data |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race* | Language§ | HIV Status | BMI | Employment | Income° | Tobacco use | Alcohol use | Insurance | Education | Neighbor-hood median income | Urbanicity | Poverty | |
|
| |||||||||||||
| Bamato et al. [2] | √ | √ | √ | ||||||||||
| Bedri et al. [29] | √ | √ | √ | √ | |||||||||
| Binre et al. [22] | √ | √ | √ | ||||||||||
| Cheek et al. [30] | |||||||||||||
| Cribbs et al. [23] | √ | √ | |||||||||||
| Danai et al. [24] | √ | √ | |||||||||||
| Donrbrovskiy et al. [25] | √ | √ | |||||||||||
| Esper et al. [26] | √ | √ | √ | ||||||||||
| Firenrpong et al. [31] | √ | √ | √ | ||||||||||
| Goodwin et al. [27] | √ | √ | √ | √ | |||||||||
| Kumar et al. [28] | √ | √ | √ | √ | |||||||||
| Madsen et al. [16] | √ | √ | |||||||||||
| Martin et al. [3] | √ | ||||||||||||
| Mayr et al. [8] | √ | ||||||||||||
| Melamed et al. [32] | √ | √ | √ | ||||||||||
| Moore et al. [36] | √ | √ | √ | √ | √ | √ | √ | ||||||
| Moore et al. [37] | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Plurad et al. [33] | √ | ||||||||||||
| Samnron et al. [38] | √ | ||||||||||||
| Sandoval et al. [34] | √ | √ | √ | √ | |||||||||
| Vogel et al. [35] | √ | ||||||||||||
| Total studies | 13 | 1 | 6 | 3 | 2 | 1 | 2 | 3 | 11 | 3 | 5 | 3 | 5 |
BMI body mass index
More than two races explored
English versus non-English speaking
Income from patient-level data
Defining Socioeconomic Community-Level Variables
Three variables evaluated were neighborhood median income, poverty, and urbanicity (Table 2). Five studies explored neighborhood median income, where data was pulled from the 2000 US Census, 2010 American Community Survey, or HCUP [22, 27, 28, 34, 36]. Five studies evaluated poverty, pulling data from the 2000 US Census, 2010 American Community Survey, HCUP, or the REGARDS database [2, 8, 27, 37]. Three studies explored the effect of urbanicity versus rural on sepsis-related disparities [2, 34, 36].
Evidence Synthesis
Of the 21 studies reviewed, we narrowed our meta-analysis to trials that compared (a) minority races versus Caucasian/White (b) in adjusted regression models with provided odds ratios or hazard ratios in order to (c) estimate the impact of race on sepsis-related mortality. Seven studies were used for this meta-analysis out of the 21 studies included in the systematic review. Pooling data from six studies [8, 27, 31, 32, 34, 38] (with a sample size of 4,265,241) showed significant heterogeneity (I2 = 100%) in studies comparing the African American/Black race as a risk factor for sepsis-related mortality disparities. Further, African American/Black race had no statistical significant relationship with sepsis-related mortality (odds ratio 1.20, 95% CI, 0.81 to 1.77) (Fig. 2). Figure 3 displays our funnel plot evaluating for bias. It should be noted that these six studies in their adjusted models did not adjust for similar variables. Goodwin, for example, adjusted for age, income, and poverty [27], while Sandoval explored income and urbanicity on the community level and insurance, gender, and age at the patient level [34], and Sammon only explored individual-level variables (gender and insurance)[38].A complete list of variables used in the adjusted models for the other three studies can be found in Table 2.
Fig. 2.
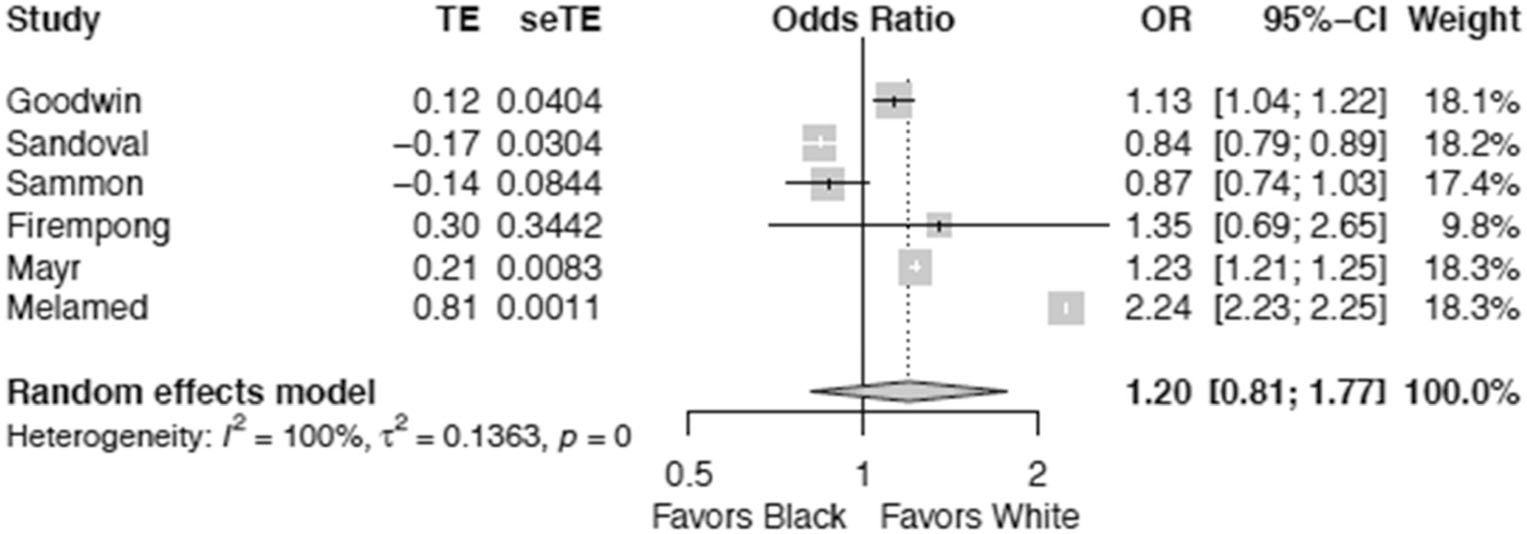
Race as associated with risk of sepsis-related mortality between African American/Black and Caucasian/White
Fig. 3.
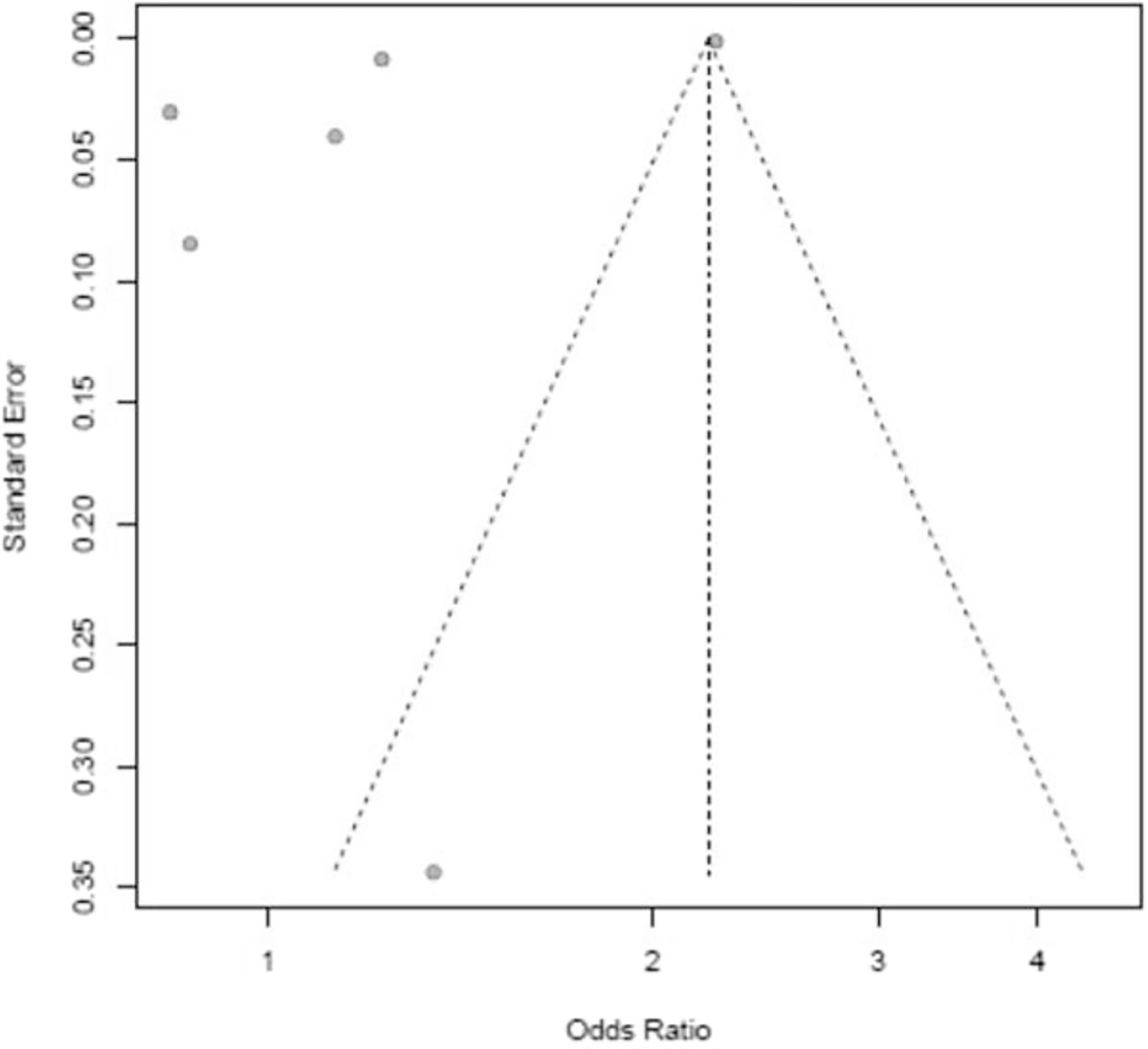
Funnel plot evaluating for bias in meta-analysis of race and sepsis-related mortality
Similar findings in regard to significant heterogeneity were seen with other races: with regard to Native American, explored by two studies [30, 32], I2 = 98% and odds ratio were 1.77 (95% CI, 0.02 to 64.33); for Asians, I2 = 97% and odds ratio were 0.85 (95% CI, 0.28 to 2.60); for Hispanic/Latino, I2 = 98% and odds ratio were 1.06 (95% CI, 0.72 to 1.56).
Discussion
Our results reveal that there is inconsistency in current evidence to support whether race has any significant impact on sepsis-related mortality and that there was significant heterogeneity among the reviewed and analyzed studies. The inconsistency may have to do with the lack of consistency of inclusion of the potential relevant covariates that were used in the multivariable models. Further, there was significant heterogeneity in regard to the source of patient-specific variables (e.g., from hospital-level records to national databases) and to what socioeconomic variables the authors of the respective studies chose to explore.
Race is both an evolving term and one that is difficult to pinpoint definitive individual characteristics [10]. Further, there are fallacies when using race as a proxy for more complex issues to explain certain health disparities. For instance, believing race is a proxy for common genetic stock has been shown to be invalid, warranting caution over believing there are genetic issues that predispose one race towards a disease versus another [39–41]. In addition, using race as a proxy for socioeconomic status has too been shown to be imprecise, as the two are independent of each other and carry different consequences in regard to healthcare outcomes [42]. Therefore, our findings of race being an inconsistent variable in explaining sepsis-related disparities are in accordance with these concerns and evident in studies that took into account multiple individual-level and community-level socioeconomic factors when evaluating sepsis-related disparities [29, 37].
Exploring race’s impact on sepsis mortalities is important as it may reveal gaps in clinical care or identify other social and environmental factors that impact sepsis-related disparities. For example, Becker et al. identified that African Americans/Blacks had a higher incidence of cardiac arrest and lower rates of survival as compared to Caucasian/Whites [43]. The authors discuss that such disparities could be explained by environment, genetics, or healthcare access. However, they do raise the concern that socioeconomic status could very well explain these disparities, but they were unable to assess such variables due to, at the time, unreliable data [43]. Over time, as socioeconomic data became accessible and reliable, the foresight of Becker et al. came to fruition for this population, with race no longer explaining the disparities in cardiac arrest incidence and survival. An alternative explanation was access to healthcare, quality of healthcare, and socioeconomic status were the major contributors to this critical care disparity of cardiac arrest incidence and survival [44–46]. Such a parallel may be relevant for sepsis-related disparities, as our review reveals that when racial inequities are not considered in isolation, as was performed by Martin et al. [3], race’s association with sepsis-related mortality is often lost when accounting for other socioeconomic variables, both at the individual level [38] and community level.
What may further cloud race-related disparities in sepsis is how racial and ethnic data is collected itself. In our review, only four studies discussed in their methodology how race was identified [31, 33, 38]; otherwise, there was no mention to how race was captured (self-identified or provider-identified) by the study teams. This lack of consistently defining race is significant in critical care research as critically ill patients are often unable to speak, engage in conversations, or have a next-of-kin present; thus, demographic data entry will be what the healthcare provider is able to identify. An example that parallels this clinical dilemma is in the inconsistencies in race identification between self-selected race and observer-selected race in death certificates [47]. Comparisons between next-of-kin racial identifications and death certificates have shown a significant proportion of death certificates that are misidentified, leading to an overestimation of life expectancy for race-specific mortality rates [47]. Therefore, without providing insight into how race is captured in these studies on sepsis-related disparities, caution must be raised in order to prevent inaccurate estimations of race’s overall affect.
We conducted an extensive literature search to retrieve all relevant eligible studies, as well as to minimize publication bias. However, the high level of heterogeneity observed has many reasons; one being that much of the databases varied between studies (from the use of national databases to medical registries to hospital-specific data). Further, we cannot assure that there was no overlap of data, whereby the few hospital-level studies may have also been used in national-level data cohorts and result in biasing the results of our analysis. In regard to outcomes, while several sepsis-related disparities exist (mortality, incidence, time to antibiotics, completion of sepsis bundle), we conducted a meta-analysis only on mortality. Reasons to focus on mortality include the change in the clinical definition of sepsis over time [12–14] (which would impact incidence), and time to antibiotics and completion of sepsis bundles have more recently gained attention [15] (resulting in fewer studies evaluating them in regard to the presence of disparities [16, 17]). Finally, how poverty is defined was not addressed by all studies. Given poverty has several definitions [48], the inability to assure that all the studies used the same definition may be one explanation as to the inconsistencies surrounding poverty’s influence on sepsis-related outcomes.
In conclusion, on the basis of available evidence from a limited number of observation retrospective studies in addition to significant heterogeneity among the analyzed studies, race alone cannot fully explain sepsis-related mortality between African Americans/Blacks and Caucasians/Whites. The lack of a clear relationship between race and sepsis-related mortality warrants further investigations. Other sociodemographic and socioeconomic variables may prove to be clinically meaningful than race; however, we cannot conclude this from our study. Creating clear and feasible requirements on how race and other sociodemographic and socioeconomic variables are captured should be a priority in order to assure precise and consistent data is being reported for sepsis-related disparities. An increase understanding of where and what type of socioeconomic variable impacts these outcomes is vital prior to embarking on any clinical intervention aimed at reducing this inequity.
Acknowledgments
Disclosure The Intramural Research Programs of National Institutes of Health (Clinical Center, Critical Care Medicine Department) supported this work. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the US Department of Health and Human Services.
Footnotes
Competing Interests The authors declare that they have no conflicts of interest.
References
- 1.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–92. [DOI] [PubMed] [Google Scholar]
- 2.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. [DOI] [PubMed] [Google Scholar]
- 4.Cooke CR, Erickson SE, Eisner MD, Martin GS. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PS, Nallamothu BK, Krumholz HM, Spertus JA, Li Y, Hammill BG, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368(11):1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider AH, Chang DC, Efron DT, Haut ER, Crandall M, Cornwell EE 3rd. Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008;143(10):945–9. [DOI] [PubMed] [Google Scholar]
- 7.Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. J Natl Med Assoc. 2006;98(12):1967–72. [PMC free article] [PubMed] [Google Scholar]
- 8.Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore JX, Donnelly JP, Griffin R, Safford MM, Howard G, Baddley J, et al. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Crit Care. 2015;19:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winker MA. Measuring race and ethnicity: why and how? JAMA. 2004;292(13):1612–4. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348(12):1166–70. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–3. [DOI] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. [DOI] [PubMed] [Google Scholar]
- 14.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama. 2016;315(8): 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen TE, Napoli AM. Analysis of race and time to antibiotics among patients with severe sepsis or septic shock. J Racial Ethn Health Disparities. 2017;4(4):680–686. [DOI] [PubMed] [Google Scholar]
- 17.Madsen TE, Simmons J, Choo EK, Portelli D, McGregor AJ, Napoli AM. The DISPARITY study: do gender differences exist in Surviving Sepsis Campaign resuscitation bundle completion, completion of individual bundle elements, or sepsis mortality? J Crit Care. 2014;29(3):473.e477–11. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Green S. Cochrane Handbook for systematic reviews of interventions. Version 4.2.6. The Cochrane Collaboration. 2006. https://training.cochrane.org/handbook. Accessed 1 May 2018. [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 20.Schwarzer G Meta: an R package for meta-analysis. R News. 2007;7(3):40–5. [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 22.Bime C, Poongkunran C, Borgstrom M, Natt B, Desai H, Parthasarathy S, et al. Racial differences in mortality from severe acute respiratory failure in the United States, 2008–2012. Ann Am Thorac Soc. 2016;13(12):2184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cribbs SK, Tse C, Andrews J, Shenvi N, Martin GS. Characteristics and outcomes of HIV-infected patients with severe sepsis: continued risk in the post-highly active antiretroviral therapy era. Crit Care Med. 2015;43(8):1638–45. [DOI] [PubMed] [Google Scholar]
- 24.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129(6):1432–40. [DOI] [PubMed] [Google Scholar]
- 25.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35(3):763–8. [DOI] [PubMed] [Google Scholar]
- 26.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW. Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest. 2016;150(4):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar G, Taneja A, Majumdar T, Jacobs ER, Whittle J, Nanchal R. The association of lacking insurance with outcomes of severe sepsis: retrospective analysis of an administrative database*. Crit Care Med. 2014;42(3):583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedri H, Romanowski KS, Al-Ramahi G, et al. A national study of the effect of race, socioeconomic status, and gender on burn outcomes. J Burn Care Res. 2017;38(3):161–8. [DOI] [PubMed] [Google Scholar]
- 30.Cheek JE, Holman RC, Redd JT, Haberling D, Hennessy TW. Infectious disease mortality among American Indians and Alaska Natives, 1999–2009. Am J Public Health. 2014;104(Suppl 3): S446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firempong AO, Shaheen MA, Pan D, Drazin D. Racial and ethnic disparities in the incidence and mortality from septic shock and respiratory failure among elective neurosurgery patients. Neurol Res. 2014;36(10):857–65. [DOI] [PubMed] [Google Scholar]
- 32.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care. 2009;13(1):R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plurad DS, Lustenberger T, Kilday P, et al. The association of race and survival from sepsis after injury. Am Surg. 2010;76(1):43–7. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval E, Chang DW. Association between race and case fatality rate in hospitalizations for sepsis. J Racial Ethn Health Disparities. 2016;3(4):625–34. [DOI] [PubMed] [Google Scholar]
- 35.Vogel TR, Dombrovskiy VY, Lowry SF. Trends in postoperative sepsis: are we improving outcomes? Surg Infect. 2009;10(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore JX, Donnelly JP, Griffin R, Howard G, Safford MM, Wang HE. Defining sepsis mortality clusters in the United States. Crit Care Med. 2016;44(7):1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xavier Moore J, Donnelly JP, Griffin R, et al. Community characteristics and regional variations in sepsis. Int J Epidemiol. 2017;46(5):1607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sammon JD, Klett DE, Sood A,Olugbade K Jr, Schmid M, Kim SP, et al. Sepsis after major cancer surgery. J Surg Res. 2015;193(2):788–94. [DOI] [PubMed] [Google Scholar]
- 39.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. [DOI] [PubMed] [Google Scholar]
- 40.Wilson JF, Weale ME, Smith AC, Gratrix F, Fletcher B, Thomas MG, et al. Population genetic structure of variable drug response. Nat Genet. 2001;29(3):265–9. [DOI] [PubMed] [Google Scholar]
- 41.Sankar P, Cho MK. Genetics. Toward a new vocabulary of human genetic variation. Science. 2002;298(5597):1337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaacs SL, Schroeder SA. Class - the ignored determinant of the nation’s health. N Engl J Med. 2004;351(11):1137–42. [DOI] [PubMed] [Google Scholar]
- 43.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329(9):600–6. [DOI] [PubMed] [Google Scholar]
- 44.Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167(11):1177–82. [DOI] [PubMed] [Google Scholar]
- 45.Werner RM, Goldman LE, Dudley RA. Comparison of change in quality of care between safety-net and non-safety-net hospitals. Jama. 2008;299(18):2180–7. [DOI] [PubMed] [Google Scholar]
- 46.Virnig BA, Lurie N, Huang Z, Musgrave D, McBean AM, Dowd B. Racial variation in quality of care among Medicare+Choice enrollees. Health Aff (Millwood). 2002;21(6):224–30. [DOI] [PubMed] [Google Scholar]
- 47.National Research Council. 2004. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press. 10.17226/11086. Accessed 30 March 2018. [DOI] [PubMed] [Google Scholar]
- 48.Wagle UR. Multidimensional poverty: an alternative measurement approach for the United States? Soc Sci Res. 2008;37(2):559–80. [DOI] [PubMed] [Google Scholar]


