Abstract
Several human pathogens and fecal-pollution indicators may persist as viable organisms in natural environments, owing to their ability to activate different types of survival strategies. These strategies include adhesion on both abiotic and biotic surfaces and the entrance to the so-called viable but nonculturable (VBNC) state. In an 18-month survey for the detection of enterococci in both lake water and seawater, C. Signoretto et al. (Appl. Environ. Microbiol. 70:6892-6896, 2004) have shown that Enterococcus faecalis was detected mostly bound to plankton and in the VBNC state. In the present study, we show that in vitro adhesion of E. faecalis to copepods accelerated the entry of cells into the VBNC state relative to that of planktonic bacteria. VBNC E. faecalis cells maintained their adhesive properties to copepods and chitin (the main component of the copepod carapace), though to a reduced extent in comparison with growing cells. Sugar competition experiments showed interference with adhesion to both copepods and chitin by GlcNAc and only to copepods by d-mannose. Four enterococcal cell wall proteins present in both growing and VBNC cells and lipoteichoic acid were shown to be capable of binding chitin. The results indicate that copepods may represent an additional environmental reservoir of enterococci, thus suggesting the advisability of redesigning the protocols currently used for microbial detection during the evaluation of the microbiological quality of environmental samples.
Several human pathogens and fecal-pollution indicators may persist as viable organisms in natural environments because of their ability to activate different types of survival strategies. These strategies include adhesion, possibly the formation of biofilm on both abiotic and biotic surfaces (5, 13-15, 23, 34), and the entry into the so-called viable but nonculturable (VBNC) state. Bacteria in the VBNC state are characterized by loss of culturability on conventional growth media, but cells maintain viability, pathogenicity factors, and the potential ability to reinfect humans on resuscitation to the culturable state (2, 8-10, 17, 20, 21, 26).
Among human pathogens, the role of the adhesion of Vibrio cholerae, the etiological agent of a severe waterborne diarrheal disease (cholera), as well as of other vibrios, was extensively investigated, and it has been suggested that altered forms of V. cholerae in specific association with plankton organisms are the most plausible reservoirs of fully virulent strains during interepidemic periods (9). Bacterial binding to various surfaces involves several forces, ranging from hydrophobic and ionic bonds to the lectin-like interactions between the bacterial ligand(s) and complementary receptor(s) displayed by the substrate. A few examples of specific interactions between human-pathogenic bacteria and chitin-containing surfaces are known, but they are entirely restricted to the Vibrio genera. Lectins with specificity for N-acetylglucosamine (GlcNAc), the sugar component of chitin, have been demonstrated to occur in V. cholerae, Vibrio harveyi, Vibrio damsela, and Vibrio furnissii (24, 29, 36). In addition, specific chitin-binding proteins (CBPs) are displayed on the surfaces of V. cholerae (29, 35), Vibrio alginolyticus (5, 27), and V. harveyi (24) and have been shown to be directly correlated to the binding ability of the microorganisms to various substrates.
In an 18-month survey for the detection of enterococci in both lake water and seawater, Signoretto et al. (32) have shown that Enterococcus faecalis was bound mostly to plankton when present. In addition, E. faecalis, either in bound or in free form, was most frequently detected in the VBNC state. We believe that this unexpected result deserves particular attention because it constitutes new evidence which, together with the vibrio model, indicates that this may be the main mode of persistence of medically important bacteria in surface waters. The creation of an unexpected pathogen reservoir might possibly invalidate the culture methods currently used to assess the microbiological quality of surface waters in that zooplankton, by removing bacteria from water, may concentrate and move bacteria with currents and tides.
In this work, we analyze the ability of enterococci to bind lake zooplankton in vitro in both the growing and VBNC states and try to identify the possible enterococcal ligand(s) involved. The data presented are the means of results of three distinct experiments. The standard deviation (SD) is indicated in each table or figure. Data were analyzed for significance using Student's t test. Differences were considered significant at a P of ≤0.05.
Bacterial strains and copepods.
E. faecalis 56R (33) and E. faecalis JH2-2 (16) were used. Strain 56R is a clinical isolate and is a producer of the enzyme chitinase, while JH2-2 is a laboratory strain and a nonproducer of chitinase as evaluated by the hydrolysis of ethylene glycol chitin, as described by Connell et al. (11; C. Pruzzo, unpublished observation). Enterococcal strains were grown in brain heart infusion (BHI) broth or BHI agar (BHIA) (Difco) at 37°C. Cell growth in liquid media was monitored by reading optical density at a 640-nm wavelength (OD640) with a Beckman model DU 530 spectrophotometer.
Copepods used in this study were collected by horizontal dragging at 1 m below the surface of Lake Garda (Italy) with a 100-μm net (32). Copepods were checked, before use, for the absence of E. faecalis by PCR amplification of a DNA tract within the pbp5 gene, as previously described (22, 32). Only copepod lots that revealed no amplification band were used in this study.
Evaluation of the time needed to enter the VBNC state by E. faecalis cells in different microcosms.
Three different laboratory microcosms were created: (i) enterococci resuspended in autoclaved water collected from Lake Garda, (ii) enteroccci adherent to copepods and resuspended in sterile lake water, and (iii) enterococci bound to purified chitin particles and resuspended in sterile lake water. To attach enterococci to copepods, 500 copepods per ml (10-ml final volume) were placed in contact with 1 × 109 bacteria per ml (10 ml) and allowed to stand for 1 h at room temperature (RT). Copepods were collected by filtration onto a piece of 64-μm net, washed thoroughly with sterile lake water, and finally resuspended in lake water. To attach enterococci to chitin particles, 150 mg per ml of sterilized chitin purified from crab shell (Sigma) (10 ml) was placed in contact with 1 × 108 enterococci per ml (10 ml), processed, and collected as copepods, except that chitin particles were collected on 8-μm membrane filters. Microcosms were maintained at 4 ± 0.5°C under illumination in a static state. Every 3 days, samples were withdrawn aseptically from the microcosms, and culturable cell numbers were evaluated as CFU on BHIA plates with suitable dilutions of the samples. For counts of enterococci adherent to copepods and chitin particles, before CFU determination, bacteria were detached from the respective substrates by 1 min of sonication in a water bath cleaner (Branson model 1210). This procedure allowed detachment of all bacteria from the zooplankton but had no effect on bacterial viability or culturability. When the culturable cell count was close to 0, 10-ml samples from the microcosms were filtered onto 0.22-μm Millipore filters, which were placed face up on BHIA plates. Cells were considered as having entered the VBNC state when the culturable cells numbered <0.1/ml.
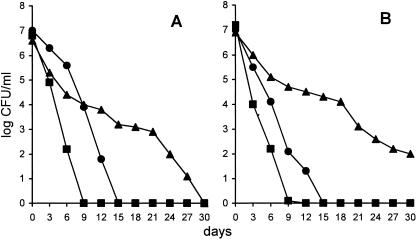
Previous results have indicated that E. faecalis, resuspended in lake water, enters the VBNC state in about 2 weeks (20, 21). In the first series of experiments, we analyzed culturability over time in a population of E. faecalis organisms which adhered to copepods or to chitin particles (the main component of the copepod carapace). Figure 1A shows that E. faecalis 56R became totally nonculturable in 9 days when it was adherent to copepods, as opposed to 15 days when it was resuspended in lake water (P < 0.05). Surprisingly, when the same bacteria were bound to chitin particles, a much longer time period (30 days) was needed to reach the nonculturable state (P was <0.01 in comparisons to bacteria that were both adherent to copepods and in water). This behavior could be easily explained by the fact that the 56R strain is a chitinase producer, which allows polymer degradation with subsequent cell growth or, at least, the maintenance of cell culturability, as a result of nutrient availability (1). To test this possibility, we used an E. faecalis strain (JH2-2) which is a nonproducer of chitin-degrading enzyme. Figure 1B shows that this strain behaved like 56R but that, in addition, after 30 days, 102 cells were still culturable when cells adhered to chitin particles. To explain this discrepancy, the presence of an additional chitin-degrading enzyme(s) should be postulated; alternatively, an amount of chitinase should be present in JH2-2 cells but at an undetectable level when ethylene glycol chitin is used as the substrate in the test performed in this study (11). The major discrepancy between enterococci adherent to copepods and those attached to chitin particles may be explained by the fact that the surface of the copepod carapace is layered with a proteinaceous cuticle that prevents access to the lower chitin layer. Alternatively, a signal triggered by adhesion to the living surface might induce the rapid entry of enterococci into the VBNC state. The ease of its entry into this state when adherent to planktonic organisms might explain why E. faecalis was found in both lake water and seawater adherent to copepods and mainly in the VBNC state (32).
FIG. 1.
Decline of E. faecalis 56R (A) and JH2-2 (B) when inoculated at 4°C in lake water in free form (•), adherent to copepods (▪), and adherent to chitin particles (▴). Each point is the mean from triplicate samples. The standard error averaged 15% of the value indicated when the number of CFU/ml was greater than 50.
To provide evidence that the reduction in CFU counts was really due to bacteria entering the nonculturable state rather than the simple loss of the microorganisms, we enumerated enterococci (56R strain) adherent to copepods or chitin particles at the time of entering the nonculturable state (i.e., 9 and 30 days later) by means of a quantitative PCR method. The E. faecalis pbp5 gene was used as the amplification target. The competitive-PCR protocol used in this study was the one previously described (22, 32). The same cell numbers or slight reductions (though invariably less than 10%) were detected in all samples.
The cell viability of E. faecalis was determined with the Live/Dead Baclight bacterial viability kit (Molecular Probes), which evaluates cell membrane integrity (31). Data revealed that 84% ± 4% to 93% ± 4% of the cells were still fluorescent green (viable) when E. faecalis cells became totally nonculturable. This result provides evidence that the loss of culturability was really due to entry of the majority of the cells into the VBNC state.
Evaluation of binding ability to copepods and chitin particles by E. faecalis 56R in different states.
Since it is impossible to evaluate the VBNC cells adherent to copepods or chitin particles with the culture method, we were forced to apply the strategy of using radiolabeled bacteria. Radioactive bacteria were obtained as previously described (28) by external labeling of bacteria with N-succinimidyl [2,3-3H]propionate. This procedure allowed the binding of the same amount of radioactivity (ca. 13,500 cpm/1 × 107 bacteria), whether these cells were exponentially growing, stationary, or in the VBNC state.
Bacterial attachment to chitin particles was evaluated as previously described by Tarsi and Pruzzo (35). Briefly, 1 volume of labeled bacterial suspension (about 1 × 108 bacteria/ml) was added to 1 volume of phosphate-buffered 3% NaCl solution (pH 8) containing chitin (2.5 mg/ml), and the mixture was incubated at 20°C with shaking. A control sample without chitin was also prepared. At timed intervals, three replicate samples from each treatment were filtered onto 8-μm-pore-size filters (25-mm polycarbonate membranes; Bio-Rad Laboratories), which were then rinsed with phosphate-buffered 3% NaCl solution (pH 8) and radioassayed with an LS 7000 scintillation counter (Beckman Instruments, Inc.). The total number of bacteria attached to chitin particles was calculated using a cell labeling efficiency method. To evaluate background counts due to bacterial attachment to filtration membranes, triplicate samples for each treatment were incubated without chitin and filtered to correct for unattached cells left on the filter. The values from these control filters were subtracted from the sample values.
To evaluate the adherence of E. faecalis to copepods, 1 ml of radiolabeled bacterial suspension (about 2 × 108 bacteria/ml) was added to 1 ml of lake water containing 100 copepods and incubated at 20°C. Three replicates of each treatment were prepared. At timed intervals, copepods were collected and gently washed three times to remove nonadherent bacteria. Copepod-bound radioactivity was evaluated as described above. The total number of bacteria per copepod was calculated using the cell labeling efficiency method (35).
Table 1 shows the time course of attachment to chitin particles and adherence to copepods of enterococci harvested in different growth phases and states (exponentially growing [OD640 = 0.35], stationary [48 h old], and VBNC cells). The best binding efficiency was obtained at 60 min for both copepods and chitin particles. In addition, it was found that bacteria in the stationary growth phase interacted more efficiently with both copepods and chitin particles than exponentially growing and VBNC cells. VBNC cells of E. faecalis, though reduced in numbers, still maintained their binding capability. A control consisting of UV-killed cells of E. faecalis and incubated at 4°C in lake water for 15 days was used to evaluate the residual binding ability to both copepods and chitin particles. Table 1 shows that the binding ability of UV-killed cells was drastically impaired, as shown by a roughly 3-log decrease, in comparison with the binding ability of stationary-phase enterococci. This clearly indicates that only live enterococci are capable of binding copepods or chitin particles.
TABLE 1.
Effect of growth phase on E. faecalis 56R interactions with copepod surface or chitin particles
| Growth phase or state | Mean no. of enterococci (105)/copepod (±SD) at:
|
Mean no. of enterococci (106)/2.5 mg of chitin (±SD) at:
|
||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 120 min | 30 min | 60 min | 120 min | |
| Exponential | 1.8 ± 0.1 | 2.2 ± 0.3 | 2.1 ± 0.2 | 1.3 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.3 |
| Stationary | 5.4 ± 0.4 | 7.9 ± 0.6 | 7.5 ± 0.7 | 4.6 ± 0.4 | 7.1 ± 0.5 | 6.8 ± 0.5 |
| VBNC | 0.61 ± 0.01 | 0.89 ± 0.02 | 0.84 ± 0.2 | 1.7 ± 0.2 | 2.9 ± 0.1 | 3.0 ± 0.2 |
| UV killeda | NDb | 0.0076 ± 0.0002 | ND | ND | 0.032 ± 0.0001 | ND |
Exposed to a UV lamp for 15 min and subsequently incubated for 15 days at 4°C, as previously described (33).
ND, not determined.
In order to establish that the evaluation of the radioactivity bound to chitin or copepods corresponded to the real determination of the adherent bacterial count, the number of bacteria bound to chitin particles was determined in one experiment by CFU counting on BHIA plates. This experiment was performed only for exponentially growing and stationary cells. The same numbers as those reported in Table 1 were obtained when a cell number evaluation was performed by counting CFU. Although we are unable, at present, to completely rule out the possibility that the labeling process has no effect on the cell's ability to attach to the various substrates, it must be stressed that this is not the case for bacteria in the exponential and stationary phases.
Table 2 shows the effects of ions and sugars on the binding efficiency of E. faecalis to copepods or chitin particles. The following salts at the concentrations indicated were separately added to the lake water: NaCl, 0.9%; MgCl2, 1 mM; MgCl2, 30 mM; CaCl2, 1 mM; and CaCl2, 30 mM. When sugar competition was evaluated, N-acetylglucosamine, d-glucose, d-fructose, and d-mannose were used, each at the concentration of 10 mg/ml. As far as ions are concerned, no relevant effects were observed on the adhesion of either stationary or VBNC cells. On the other hand, both bivalent cations at the concentration of 30 mM substantially improved the adhesion of exponentially growing cells of E. faecalis 56R to both copepods and chitin, thus increasing the absolute cell numbers of adherent bacteria to the same level as in stationary cells. These differences were statistically significant (P < 0.05). This may be due to the fact that the surfaces of exponentially growing cells bear a higher negative charge than those of old cells (i.e., stationary cells and cells in the VBNC state), which exerts a repulsive force when bacteria approach copepods. By masking the negative charges of exponentially growing cells, the bivalent cations may encourage the two surfaces to approach one another, with a consequent net increase in enterococcal adhesion.
TABLE 2.
Effect of ions and sugars on enterococcal interactions with copepod surface and chitin particles
| Substance added | Mean no. (±SD) of enterococci (105)/copepoda
|
Mean no. (±SD) of enterococci (106)/2.5 mg of chitin
|
||||
|---|---|---|---|---|---|---|
| Exponential | Stationary | VBNC | Exponential | Stationary | VBNC | |
| Noneb | 2.20 ± 0.3 (100) | 7.90 ± 0.6 (100) | 0.89 ± 0.02 (100) | 2.40 ± 0.2 (100) | 7.10 ± 0.5 (100) | 2.90 ± 0.1 (100) |
| NaCl (0.9%) | 2.86 ± 0.3 (131) | 6.79 ± 0.6 (86) | 0.79 ± 0.01 (89) | 3.00 ± 0.2 (125) | 6.46 ± 0.4 (91) | 2.49 ± 0.2 (86) |
| Mg2+ (1 mM) | 3.30 ± 0.4 (150) | 6.47 ± 0.5 (82) | 1.08 ± 0.02 (122) | 2.88 ± 0.3 (120) | 5.68 ± 0.4 (80) | 2.96 ± 0.2 (102) |
| Mg2+ (30 mM) | 9.46 ± 0.8 (430) | 7.29 ± 0.6 (92) | 1.15 ± 0.03 (129) | 7.82 ± 0.5 (326) | 6.03 ± 0.4 (85) | 2.95 ± 0.1 (102) |
| Ca2+ (1 mM) | 2.53 ± 0.4 (115) | 7.26 ± 0.5 (92) | 0.83 ± 0.01 (93) | 2.61 ± 0.2 (109) | 6.46 ± 0.5 (91) | 2.46 ± 0.2 (85) |
| Ca2+ (30 mM) | 8.60 ± 0.7 (391) | 7.28 ± 0.6 (92) | 0.80 ± 0.02 (90) | 5.76 ± 0.4 (240) | 6.11 ± 0.5 (86) | 2.44 ± 02 (84) |
| GlcNAc | 1.28 ± 0.2 (58) | 3.79 ± 0.3 (58) | 0.27 ± 0.009 (30) | 1.20 ± 0.27 (53) | 3.34 ± 0.2 (57) | 0.81 ± 0.01 (28) |
| d-(+)-Glucose | 2.55 ± 03 (116) | 8.29 ± 0.6 (105) | 0.83 ± 0.02 (93) | 2.09 ± 0.3 (87) | 6.82 ± 0.5 (96) | 2.58 ± 0.1 (89) |
| d-Fructose | 2.09 ± 0.4 (95) | 7.26 ± 0.6 (92) | 0.87 ± 0.03 (98) | 2.15 ± 0.2 (88) | 6.46 ± 0.5 (91) | 2.48 ± 0.1 (86) |
| d-Mannose | 1.36 ± 0.1 (62) | 1.66 ± 0.2 (21) | 0.11 ± 0.004 (12) | 2.73 ± 0.3 (114) | 8.94 ± 0.6 (126) | 3.13 ± 0.2 (108) |
Values in parentheses are percentages of the values for the respective controls.
Lake water.
As far as the effects of sugars are concerned (Table 2), the only marked effect on the inhibition of interaction with both copepods and chitin particles was observed for GlcNAc, with reduction rates ranging from 40 to 82%, as opposed to the nonefficacy of d-glucose and d-fructose. d-Mannose exerted significant inhibitory activity (P < 0.05) only on the adhesion of enterococci to copepods, irrespective of the growth phase and state of the cells, while no effect on cell adhesion to purified chitin particles was detected. This suggests a role for bacterial surface lectins in adhesion to copepods. That chitin may be involved in the adhesion of enterococci is documented by the inhibition exerted by GlcNAc on adhesion to either copepods or purified chitin, while d-mannose probably acts on a receptor located in the mucilaginous materials present on the carapace surface (7).
Surface hydrophobicities of E. faecalis cells under different growth conditions.
In a further experiment, we evaluated whether the differences in adhesion capabilities of E. faecalis cells were due to differences in surface hydrophobicity. Cell surface hydrophobicity was measured as described by Rosenberg et al. (30). Briefly, 300 μl of n-hexadecane was added to 3 ml of exponentially growing, stationary, and VBNC cells at an OD470 of 1.0. After a 10-min incubation at 37°C and a 30-s shaking, the OD470 was evaluated in the aqueous phase. The percentage of bacterial adhesion to hydrocarbon (BATH) was calculated by applying the following equation: [1 − (ODF/ODI)] × 100, where ODI and ODF were the ODs of cells at the beginning and at the end of the experiment, respectively. The percentages of enterococcal adhesion to hydrocarbon slightly increased from exponentially growing (BATH = 1.48% ± 0.21%), to stationary (BATH = 2.29% ± 0.31%), and to VBNC cells (BATH = 3.0% ± 0.29%). These differences, however, were not statistically significant (P > 0.05).
E. faecalis cell wall molecules that interact with chitin.
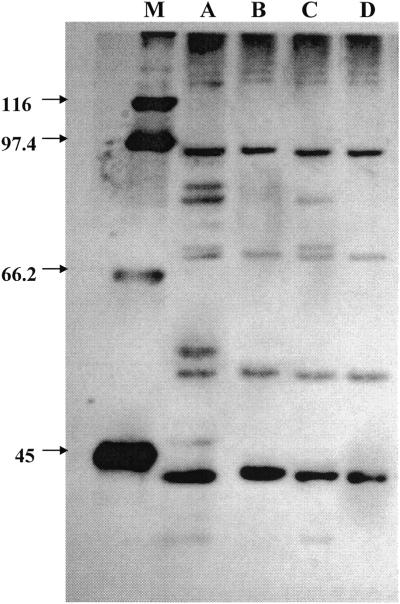
Because the copepod carapace is essentially made up of chitin, in a second phase of the research, we explored the possibility that E. faecalis surface molecules could be involved in chitin binding. Protein extraction from the E. faecalis cell wall by lithium chloride was performed as described previously (19). Briefly, 1 liter each of a stationary-phase and a VBNC culture of E. faecalis 56R was collected by centrifugation at 4°C, and the resulting pellet was washed twice with cold Na-phosphate buffer (0.01 M, pH 7.2). The pellet was resuspended in 2 M LiCl and incubated at RT for 60 min under gentle agitation. Particulate material was sedimented out by centrifugation at 8,000 × g for 15 min at 4°C. Supernatants were thoroughly dialyzed against sterilized lake water with four changes. The extracts were concentrated by ultrafiltration using a Vivascience VIVASPIN concentrator equipped with a 5,000-molecular-weight-cutoff polyethersulfone membrane. Two hundred microliters of a suspension containing 40 μg/ml of proteins was placed in contact with 25 mg of chitin and allowed to stand at RT for 1 h under gentle agitation. Chitin was sedimented by centrifugation and the supernatant removed with a pipette. Chitin was washed four times with lake water. CBPs were solubilized in boiling sodium dodecyl sulfate (SDS) in loading buffer and separated by SDS-polyacrylamide gel electrophoresis (29). After migration, proteins were detected by silver staining (Bio-Rad Laboratories). Figure 2 shows the electropherogram of cell wall proteins and the corresponding CBPs of stationary and VBNC cells. Few proteins were present in the stationary-growth-phase E. faecalis 56R wall, and the following molecular masses were calculated: 91, 84, 80, 74, 71, 55, 49, 43, 39, and 35 kDa. Six of 10 proteins present in the stationary cells were displayed by the E. faecalis VBNC cells. The molecular masses were 91, 80, 71, 49, 39, and 35 kDa. Of these wall proteins, only four displayed chitin-binding capability, whether they were in stationary or VBNC cells. Their molecular masses were 90, 71, 49, and 39 kDa.
FIG. 2.
SDS-polyacrylamide gel electrophoresis of cell wall proteins and the corresponding CBPs of E. faecalis 56R grown to the stationary phase and in the VBNC state. Lane A, cell wall proteins of stationary-phase cells; lane B, CBPs corresponding to those in lane A; lane C, cell wall proteins of VBNC cells; lane D, CBPs corresponding to those in lane C; lane M, molecular mass markers (in kilodaltons) as indicated on the left.
Extraction of lipoteichoic acid (LTA) was done with 45% aqueous phenol at 68°C, as described by Kessler and Shockman (18), with modifications introduced by Signoretto et al. (33). Purified LTA was placed in contact with 25 mg of chitin and allowed to stand at RT for 60 min under gentle agitation. The supernatant was removed as stated above for CBPs, chitin was washed four times with lake water, and total bound phosphorus was evaluated (6). Table 3 shows the results of these experiments. In all cases, i.e., with exponentially growing, stationary, and VBNC cells, roughly 25 to 30% of LTA was tightly bound to purified chitin.
TABLE 3.
Determination of E. faecalis 56R LTA bound by chitin particles
| Type of cells | Phosphorous in LTA (mg/dl)
|
||
|---|---|---|---|
| Total | Bound to chitin | Surnatanta | |
| Exponential | 7.92 ± 0.13 | 2.44 ± 0.06 | 5.72 ± 0.11 |
| Stationary | 6.18 ± 0.09 | 2.01 ± 0.09 | 4.45 ± 0.06 |
| VBNC | 5.73 ± 0.11 | 1.66 ± 0.05 | 3.83 ± 0.05 |
Liquid fraction after chitin removal. Values correspond to the unbound LTA fractions.
The finding that either some cell surface proteins or LTA is capable of in vitro chitin-binding activity supports the direct involvement of chitin as a target for enterococcal adhesion to copepods. In particular, we have found four proteins located in the cell wall of E. faecalis which are present in both stationary and VBNC cells and act as CBPs. This situation may be reminiscent of that of V. cholerae (29, 35). The 90-kDa cell wall protein may correspond to E. faecalis autolysin (4), a peptidoglycan lytic enzyme involved in remodeling peptidoglycan for cell surface extension during cell growth and division (12). The proteins with molecular masses of 39 and 49 kDa may be the result of a proteolytic cleavage of autolysin. That a bacterial surface muramidase may be involved in chitin binding is not surprising due to a number of structural similarities between peptidoglycan and chitin. We evaluated the adhesion capability of a Lyt mutant of E. faecalis (3), but no impairment of cell adhesion was observed (data not shown). This suggests that multiple factors may be involved in copepod and chitin binding and is in accordance with the identification of four cell wall proteins and with LTA as being involved. Finally, it is reasonable to assume that LTA may be involved in binding to both biotic and abiotic surfaces, inasmuch as involvement of this wall polymer has been previously demonstrated in adhesion to mammalian cells (25).
Our results as a whole indicate that copepods may constitute an additional environmental reservoir of enterococci, thus suggesting the advisability of redesigning the protocols currently used for microbial detection during the evaluation of the microbiological quality of environmental samples.
Acknowledgments
The help of Giancesare Guidi (Laboratorio Analisi Policlinico Università di Verona) with phosphorus determination is greatly appreciated.
This study was supported by Cofin2003 from the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Rome, Italy, and by Fondi ex 60% from the Università di Verona, Verona, Italy.
REFERENCES
- 1.Amako, K., S. Shimodori, T. Imoto, S. Miake, and A. Umeda. 1987. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperature. Appl. Environ. Microbiol. 53:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcina, I., P. Lebaron, and J. Vives-Rego. 1997. Survival of allochthonous bacteria in aquatic systems: a biological approach. FEMS Microbiol. Ecol. 23:1-9. [Google Scholar]
- 3.Barrett, J. F., V. L. Schramm, and G. D. Shockman. 1984. Hydrolysis of soluble, linear, un-cross-linked peptidoglycans by endogenous bacterial N-acetylmuramoylhydrolases. J. Bacteriol. 159:520-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Béliveau, C., C. Potvin, J. Trudel, A. Asselin, and G. Bellemare. 1991. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J. Bacteriol. 173:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carli, A., L. Pane, L. Casareto, S. Bertone, and C. Pruzzo. 1993. Occurrence of Vibrio alginolyticus in Ligurian coast rock pools (Tyrrhenian Sea, Italy) and its association with the copepod Tigriopus fulvus (Fisher 1860). Appl. Environ. Microbiol. 59:1960-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 7.Chiavelli, D. A., J. W. Marsh, and R. K. Taylor. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 9.Colwell, R. R. 2000. Bacterial death revisited, p. 325-342. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 10.Colwell, R. R., P. R. Brayton, A. Huq, B. Tall, P. Harrington, and M. Levine. 1996. Viable but non-culturable Vibrio cholerae O1 revert to a culturable state in the human intestine. World J. Microbiol. Biotechnol. 12:28-31. [DOI] [PubMed] [Google Scholar]
- 11.Connell, T. D., D. J. Metzger, J. Lynch, and J. P. Folster. 1998. Endochitinase is transported to the extracellular milieu by the eps-encoded general secretory pathway of Vibio cholerae. J. Bacteriol. 180:5591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneo-Moore, L., and G. D. Shockman. 1977. The bacterial cell surface in growth and division, p. 597-715. In G. Poste (ed.), Cell surface reviews, vol. 4. Elsevier/North Holland Biochemical Press, Amsterdam, The Netherlands.
- 13.Hood, M. A., and P. A. Winter. 1997. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol. Ecol. 22:215-223. [Google Scholar]
- 14.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosmos. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, X., and T.-J. Chai. 1996. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl. Environ. Microbiol. 62:1300-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler, R. E., and G. D. Shockman. 1979. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J. Bacteriol. 137:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, O. D., F. Ascencio, L.-Å. Fransson, and T. Wadström. 1992. Binding of heparan sulfate to Staphylococcus aureus. Infect. Immun. 60:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lleò, M. M., S. Pierobon, M. C. Tafi, C. Signoretto, and P. Canepari. 2000. mRNA detection by reverse transcription-PCR for monitoring viability over time in an Enterococcus faecalis viable but nonculturable population maintained in a laboratory microcosm. Appl. Environ. Microbiol. 66:4564-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lleò, M. M., M. C. Tafi, and P. Canepari. 1998. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst. Appl. Microbiol. 21:333-339. [DOI] [PubMed] [Google Scholar]
- 22.Lleò, M. M., M. C. Tafi, C. Signoretto, C. Dal Cero, and P. Canepari. 1999. Competitive polymerase chain reaction for quantification of nonculturable Enterococcus faecalis cells in lake water. FEMS Microbiol. Ecol. 30:345-353. [DOI] [PubMed] [Google Scholar]
- 23.Montanari, M. P., C. Pruzzo, L. Pane, and R. R. Colwell. 1999. Vibrios associated with plankton in a coastal zone of the Adriatic Sea (Italy). FEMS Microbiol. Ecol. 29:241-247. [Google Scholar]
- 24.Montgomery, M. T., and D. L. Kirchman. 1993. Role of chitin-binding proteins in the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl. Environ. Microbiol. 59:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofek, I., E. H. Beachey, W. Jefferson, and G. L. Campbell. 1975. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J. Exp. Med. 141:990-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver, J. D. 2000. The public health significance of viable but nonculturable bacteria, p. 277-300. In R. R. Colwell and D. J. Grimes (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 27.Pruzzo, C., A. Crippa, S. Bertone, L. Pane, and A. Carli. 1996. Attachment of Vibrio alginolyticus to chitin mediated by chitin-binding proteins. Microbiology 142:2181-2186. [DOI] [PubMed] [Google Scholar]
- 28.Pruzzo, C., R. Tarsi, M. M. Lleò, C. Signoretto, M. Zampini, R. R. Colwell, and P. Canepari. 2002. In vitro adhesion to human cells by viable but nonculturable Enterococcus faecalis. Curr. Microbiol. 45:105-110. [DOI] [PubMed] [Google Scholar]
- 29.Pruzzo, C., R. Tarsi, M. M. Lleò, C. Signoretto, M. Zampini, L. Pane, R. R. Colwell, and P. Canepari. 2003. Persistence of adhesive properties in Vibrio cholerae after long-term exposure to sea water. Environ. Microbiol. 5:850-858. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg, M., D. Gutnik, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 31.Roth, B. L., M. Poot, S. T. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63:2421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Signoretto, C., G. Burlacchini, M. M. Lleò, C. Pruzzo, M. Zampini, L. Pane, G. Franzini, and P. Canepari. 2004. Adhesion of Enterococcus faecalis in the nonculturable state to plankton is the main mechanism responsible for persistence of this bacterium in both lake and seawater. Appl. Environ. Microbiol. 70:6892-6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signoretto, C., M. M. Lleò, M. C. Tafi, and P. Canepari. 2000. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl. Environ. Microbiol. 66:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarsi, R., and C. Pruzzo. 1999. Role of surface proteins in Vibrio cholerae attachment to chitin. Appl. Environ. Microbiol. 65:1348-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, C., A. M. Lee, and S. Roseman. 1987. The sugar-specific adhesion/deadhesion apparatus of the marine bacterium Vibrio furnissii is a sensorium that continuously monitors nutrient levels in the environment. Biochem. Biophys. Res. Commun. 149:86-92. [DOI] [PubMed] [Google Scholar]