Abstract
N-Acylhomoserine lactones (AHLs) play an important role in regulating virulence factors in pathogenic bacteria. Recently, the enzymatic inactivation of AHLs, which can be used as antibacterial targets, has been identified in several soil bacteria. In this study, strain M664, identified as a Streptomyces sp., was found to secrete an AHL-degrading enzyme into a culture medium. The ahlM gene for AHL degradation from Streptomyces sp. strain M664 was cloned, expressed heterologously in Streptomyces lividans, and purified. The enzyme was found to be a heterodimeric protein with subunits of approximately 60 kDa and 23 kDa. A comparison of AhlM with known AHL-acylases, Ralstonia strain XJ12B AiiD and Pseudomonas aeruginosa PAO1 PvdQ, revealed 35% and 32% identities in the deduced amino acid sequences, respectively. However, AhlM was most similar to the cyclic lipopeptide acylase from Streptomyces sp. strain FERM BP-5809, exhibiting 93% identity. A mass spectrometry analysis demonstrated that AhlM hydrolyzed the amide bond of AHL, releasing homoserine lactone. AhlM exhibited a higher deacylation activity toward AHLs with long acyl chains rather than short acyl chains. Interestingly, AhlM was also found to be capable of degrading penicillin G by deacylation, showing that AhlM has a broad substrate specificity. The addition of AhlM to the growth medium reduced the accumulation of AHLs and decreased the production of virulence factors, including elastase, total protease, and LasA, in P. aeruginosa. Accordingly, these results suggest that AHL-acylase, AhlM could be effectively applied to the control of AHL-mediated pathogenicity.
Many bacterial species employ complex communication systems that link cell density and gene expression to regulate a broad range of biological functions (11, 27). Such cell-to-cell communication, termed quorum sensing (QS), depends on the production, diffusion, and recognition of small signal molecules. In gram-negative bacteria, the most intensively studied QS systems rely on the interaction of N-acylhomoserine lactones (AHLs), whose synthesis is directed by LuxI-type AHL synthases, with LuxR-type transcriptional regulators. AHLs share identical homoserine lactone rings yet vary in length and the substitution of an acyl side chain, which determines the signal specificities in different bacterial species. At low population densities, AHLs are present at a basal concentration level. As the population density increases, the accumulated AHLs reach concentrations that allow binding to regulators, and the complexes then activate or inactivate the expression of target genes. In particular, AHL-mediated QS systems play a key role in controlling a range of activities implicated in pathogen and host interactions, such as antibiotic production and the expression of virulence factors (4, 5, 28, 30).
Recently, AHLs have been found to be subject to biological inactivation, thereby attracting attention as QS signal interference. Two groups of AHL-degrading enzymes, classified according to the AHL cleavage site, are produced by soil bacteria. AHL-lactonases, first identified in the Bacillus cereus group, inactivate AHLs by hydrolyzing their lactone rings and produce the corresponding acylhomoserine molecules (8). It has been found that AHL-lactonases share a zinc-binding motif essential for AHL-lactonase activity that is identical to several metallohydrolases (9, 29), yet the conserved HXDH∼H motif of AHL-lactonase is a novel catalytic motif different from the zinc-binding motif, HXHXDH, of metallohydrolases (39). In another enzymatic mechanism for AHL inactivation, the amide bond of AHLs is broken by AHL-acylases involved in the utilization of AHLs as growth nutrients in Variovorax paradoxus (20), a Ralstonia isolate (23), and Pseudomonas aeruginosa PAO1 (14). In this process, homoserine lactone (HSL) is released as a product of these reactions, and the acyl chain is further metabolized (20). To date, only two AHL-acylase genes have been identified, from a Ralstonia isolate (23) and P. aeruginosa PAO1 (14). Sequence analyses indicate that AHL-acylase genes encode the polypeptides predicted to be posttranslationally cleaved into two subunits, which is characteristic of N-terminal nucleophilic (Ntn) hydrolases (13). However, the posttranslational processing of precursor polypeptides has not been demonstrated clearly.
Until now, several strategies for biocontrol have been postulated on the basis of AHL inactivation among QS interference mechanisms. One strategy is to express an AHL-degrading enzyme in bacteria or a host and disrupt the bacterial QS. The expression of AHL-degrading enzymes in pathogenic bacteria shows that AHL inactivation is a promising strategy to attenuate AHL-mediated virulence (9, 34). In practice, when expressed in transgenic plants, AHL-lactonase successfully interrupted the QS system of Erwinia carotovora and provided resistance to bacterial infection (8). Another approach to biocontrol is the use of natural bacterial isolates to competitively counteract the QS-regulated functions of pathogenic bacteria. The soil isolates Bacillus thuringiensis (10) and Rhodococcus erythropolis W2 (37), which produce an AHL-degrading enzyme, significantly reduced the pathogenicity of E. carotovora in plants. These facts strongly indicate that AHL inactivation could be developed as a potent tool for biocontrol, but the direct application of an AHL-degrading enzyme of bacterial infection has not yet been tried.
Previously, we reported that AHL-lactonase genes are distributed in many bacterial species, including B. thuringiensis subspecies, Arthrobacter strains, and Klebsiella pneumoniae, and we also showed the possibility of controlling plant pathogenic bacteria with these bacteria (21, 29). In addition to AHL degradation, we are also interested in AHL antagonists that compete for the AHL-binding site in the regulator and disrupt the QS system. Accordingly, in the present study, Streptomyces species were targeted as sources of diverse quorum-quenching agents, since these bacteria possess the ability to synthesize and secrete a variety of secondary metabolites, as well as various extracellular hydrolytic enzymes (6, 35). Interestingly, a Streptomyces sp. was found to interfere with quorum sensing by means of an extracellular AHL-degrading enzyme rather than an AHL competitor. The ahlM gene encoding the AHL-acylase was cloned from Streptomyces sp. strain M664 and expressed in Streptomyces lividans. Also, the effect of AhlM on QS-mediated gene expression was investigated using P. aeruginosa as the target pathogen.
MATERIALS AND METHODS
Bacterial strains, culture media, and conditions.
Streptomyces strains from different soil samples were isolated on a humic acid vitamin plate and grown at 30°C for 7 days in GSM medium (1% soluble starch, 2% glucose, 2.5% soybean meal, 0.1% beef extract, 0.4% yeast extract, 0.2% NaCl, 0.025% K2HPO4, 0.2% CaCO3 [pH 7.2]) and GSS medium (1% soluble starch, 2% glucose, 0.5% molasses, 0.5% yeast extract, 0.5% peptone, 0.2% CaCO3 [pH 7.2]). To purify the AHL-degrading enzyme, S. lividans was cultivated in a YEME medium (16). Pseudomonas aeruginosa PAO1, supplied by Y.-H. Cho (Sogang University, Seoul, Korea), was grown on a nutrient agar plate or in a nutrient yeast broth at 37°C. Chromobacterium violaceum CV026 (19, 36) and Agrobacterium tumefaciens NT1 (pDCI41E33) (3), reporter strains for the bioassay, were cultivated at 30°C in Luria-Bertani and minimal medium, respectively (41). For a bioassay plate to measure the inhibition of violacein synthesis, an overnight culture of C. violaceum CV026 was added to LB soft agar (0.8% agar) containing 50 nM N-(hexanoyl)-l-homoserine lactone (C6-HSL) at an optical density at 600 nm (OD600) of 0.1, and then 5 ml of the mixture was overlaid on the surface of an LB agar plate. When necessary, antibiotics were added at the following concentrations: ampicillin, 100 μg/ml for Escherichia coli; carbenicillin, 100 μg/ml for A. tumefaciens NT1 (pDCI41E33); thiostreptone, 5 μg/ml for S. lividans.
Screening and isolation.
Violacein production in C. violaceum CV026 is induced by AHLs with a short chain (C4 to C8) of acyl or a 3-oxo-acyl side chain, while the addition of AHLs with a long side chain (C10 to C12) completely inhibits the violacein synthesis induced by short-side-chain AHLs, such as C6-HSL (2, 25). Therefore, C. violaceum CV026 was used as the reporter strain to screen a wide range of QS-interfering substances. To screen for Streptomyces strains that inhibited AHL-mediated violacein production, the Streptomyces strains were cultivated in GSS and GSM media for 7 days, the Streptomyces cells were then removed by centrifugation (12,000 × g, 15 min, 4°C), and the resulting supernatant was collected. Because AHLs are easily hydrolyzed under alkaline conditions, the pH of the culture supernatants was adjusted to 7.0. Fifty microliters of each supernatant was loaded into the hole of a bioassay plate overlaid with C. violaceum CV026 and C6-HSL, and the plates were incubated at 30°C for 24 h for color development. To test whether the Streptomyces strains could prevent the N-(decanoyl)-l-homoserine lactone (C10-HSL)-mediated inhibition of C6-HSL-induced violacein synthesis, 50 μl of each supernatant was also mixed with 100 pmol of C10-HSL and then loaded into the hole of a bioassay plate overlaid with C. violaceum CV026 and C6-HSL.
Strain identification.
The 16S rRNA gene of strain M664 was amplified from a colony by PCR using the primers 9f (AGAGTTTGATCCTGGCTCAG) and 1542r (AGAAAGGAGGTGATCCAGCC). The PCR conditions involved denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, 45°C for 30 s, and 72°C for 60 s using a PCR Master Mix (Roche Applied Science, Mannheim, Germany). The resulting PCR products were sequenced by an ABI 3700 automatic sequencer (Applied Biosystems, Foster City, CA), and the sequence identification was performed using the BLATAN facility of the National Center for Biotechnology Information and the sequence-matching facility of the Ribosomal Database Project (24).
Cloning of ahlM gene.
The total DNA from Streptomyces sp. strain M664 was isolated using the method described by Kieser et al. (16) and used as the template for the PCR. The PCR primers were designed on the basis of the most conserved amino acid sequences of AHL-acylases and acylase homologues (see Fig. 2). The sequences of the degenerated primers were as follows: N1, 5′-GSIAAYCCNCAYTTYCCNTGG, and C1, 5′-TCIGCNGCDATIGTRTTNACCCANGG. The PCR amplification was performed for 30 cycles, consisting of 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s. The PCR products were then subcloned into a pT7Blue T vector (Takara, Tokyo, Japan), sequenced, and used as probes for colony hybridization, as described below.
FIG. 2.
Alignment of amino acid sequences of AhlM, other AHL-acylases, and putative homologues. The black and gray shading indicates identical and similar amino acids, respectively. The amino acid residues involved in substrate specificity are indicated by asterisks. The amino-terminal sequences determined by amino acid sequencing of the recombinant AhlM are shown by solid lines. The amino acid sequences corresponding to the degenerated primers are boxed. St AhlM, AhlM of Streptomyces sp. strain M664; St CLA, cyclic lipopeptide acylase of Streptomyces sp. strain FERM BP-5809; Ra AiiD, AiiD of Ralstonia strain XJ12B; Pa PvdQ, PvdQ of P. aeruginosa PAO1; Au AAC, aculeacin A acylase of A. utahensis; Dr AAC, aculeacin A acylase of D. radiodurans; Rs AAC, aculeacin A acylase of R. solanacearum.
To construct the genomic library of Streptomyces sp. strain M664, the total DNA was digested with SalI. Fractions ranging from 2 to 6 kb were then eluted using a QIAquick Gel Extraction kit (QIAGEN, Santa Clarita, CA), ligated into the dephosphorylated SalI site of pUC18, and transformed into E. coli DH5α. The library was screened by colony hybridization using a DIG DNA Labeling and Detection kit (Roche Applied Science). From candidates showing a positive signal plasmid, pUahlM_S2.0 was then isolated and sequenced. However, the 2.0-kb fragment of pUahlM_S2.0 was found to contain only a partial open reading frame (ORF) and lacked a C-terminal region compared with the AHL-acylases and acylase homologues. Thus, based on the sequence analysis of pUahlM_S2.0, an MluI-digested DNA library was constructed. The total DNA of strain M664 was digested with MluI and filled in with the Klenow enzyme (Promega, Madison, WI). Fragments ranging from 3 to 6 kb were then eluted from the gel, ligated into SmaI-digested pUC18, and screened using the 0.5-kb fragment as a probe. One positive clone harboring a 3.5-kb insert was isolated, and the plasmid pUahlM_M3.5 was sequenced. To create a clone containing a complete ORF of a putative AHL-acylase, pUahlM_S2.0 was digested with HindIII and XmaI and pUahlM_M3.5 was digested with XmaI and BamHI. The two resulting fragments, 1.7 kb and 2.3 kb, were then ligated into pUC18 digested with HindIII and BamHI, yielding pUahlM. The sequence of pUahlM was analyzed using the BLASTX facility and aligned using the cluster alignment algorithm of Vector NTI (Informax Inc. Bethesda, Md.).
Purification of recombinant AhlM from S. lividans.
To express AhlM in S. lividans, the ahlM gene containing the promoter and six-His tag was amplified by a PCR using the following primers: EF, 5′-GTGAATTCGTCGACTGTGCTGCTGCGTACACCTGTCAT, and ER, 5′-GAAAGCTTCTAGTGGTGGTGGTGGTGGTGCCGCCGCTCGTGCACCCG (the EcoRI and HindIII sites are underlined; nucleotides encoding histidine are in boldface). The PCR products were then subcloned into an E. coli and S. lividans shuttle vector, pWHM3 (38), and digested with HindIII and EcoRI. The resulting plasmid, pEahlM, was transformed into an S. lividans protoplast. Standard protocols were used for the transformation of S. lividans, plasmid isolation, and colony selection (16).
To purify the recombinant AhlM, S. lividans carrying pEahlM was cultivated in YEME medium containing thiostreptone for 3 days, and then the culture supernatant was precipitated by a 60% saturation of ammonium sulfate. The precipitated proteins were collected, dissolved in 20 mM sodium phosphate (pH 7.0), and dialyzed against the same buffer. The six-His-tagged AHL-acylase was then purified using Ni-NTA Superflow (QIAGEN) under native conditions according to the manufacturer's instructions. Next, the AHL-degrading active fractions were pooled, and the purity was analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To determine the amino termini of two subunits, the protein bands were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) after the SDS-PAGE and sequenced by automatic Edman degradation (Applied Biosystems Inc.).
Assay of AHL-degrading activity.
The AHL substrates, N-(butanoyl)-l-homoserine lactone (C4-HSL), N-(octanoyl)-l-homoserine lactone (C8-HSL), N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL), N-(dodecanoyl)-l-homoserine lactone (C12-HSL), and N-(3-oxo-dodecanoyl)-l-homoserine lactone (3-oxo-C12-HSL), were all purchased from Quorum Sciences Inc. and Sigma-Aldrich (St. Louis, MO). The N-(hexanoyl)-l-homoserine lactone (C6-HSL), N-(3-oxohexanoyl)-l-homoserine lactone (3-oxo-C6-HSL), and N-(decanoyl)-l-homoserine lactone (C10-HSL) were synthesized as previously described (41). For the bioassay of AHL-degrading activity, 50 μl of the supernatant from strain M664 was mixed with an equal volume of 100 mM Tris (pH 7.0) containing 40 μM C10-HSL. Thereafter, the reaction mixture was incubated at 30°C for 1 h with gentle agitation, followed by incubation at 95°C for 5 min to stop the reaction, and then 10 μl of the reaction mixture was loaded into the hole of a bioassay plate overlaid with A. tumefaciens NT1 (pDCI41E33).
The AHL-degrading activity of purified AhlM was assayed by measuring the HSL released during deacylation, as described by Xu et al. (40), with a slight modification. Ten micrograms of AhlM was mixed with 0.5 mM AHLs in 100 μl of 100 mM sodium phosphate (pH 7.0) and incubated at 30°C for 20 min or 1 h. To make a fluorescent derivative of the released HSL, 40 μl of the reaction sample was then immediately mixed with 60 μl of the phosphate buffer and 100 μl of the o-phthaldialdehyde (OPA) stock, as described previously (40), in a 96-well plate, and the absorbance was measured at OD340 using a SPECTRAMAX190 (Molecular Devices, Sunnyvale, CA). Pure HSL (Sigma) within the range of 0.1 mM to 1 mM was used to calibrate the assay and gave the following linear correlation: OD340 = 0.93[HS] + 0.0184 (r2 = 0.997).
HPLC and ESI-MS analyses.
The reaction of AHL digestion was carried out in 200 μl of 100 mM sodium phosphate (pH 7.0) containing 30 μg of AhlM and 2 mM C10-HSL. The reaction mixture was incubated at 30°C for 1 h with gentle shaking and analyzed by reverse-phase high-pressure liquid chromatography (HPLC) with precolumn derivatization by using a Vydac 218 TP C18 reverse-phase column (4.6 by 250 mm; Hesperia, CA). The reaction mixture was derivatized with OPA, as provided for the AminoQuant column (Hewlett-Packard, Wilmington, DE), where 0.2 M MES (morpholineethanesulfonic acid; pH 5.5) was used to reduce the conversion of HSL to homoserine (HS) at a high pH instead of 0.4 M borate (pH 10.0). The initial mobile phase was 20 mM sodium acetate (pH 7.2) containing 0.018% triethylamine, and the elution was created by 20 mM sodium acetate (pH 7.2) containing 40% acetonitrile and 40% methanol running at 0.45 ml/min according to the following profile: 0 min, 0%; 17 min, 60%; 18 to 25 min, 100%. As standards, 2 mM HSL and HS were also reacted with the OPA reagent. The actions of AhlM on penicillin G, cephalosporin C (Fluka), and glutaryl-7-aminocephalosporanic acid, provided by Young-Soo Kim (Seoul National University, Seoul, Korea), were examined by incubating 30 μg of AhlM with 2 mM (each) of the antibiotics for 1 h, and then the corresponding products were analyzed by HPLC, as described above. The electrospray ionization mass spectrometry (ESI-MS) was performed using an LCQ DECA XP (Thermo Finnigan) at the Korea Basic Science Institute, where the sample was dissolved in distilled water and ionized by a negative electrospray.
Effect of AhlM on AHL production in P. aeruginosa.
To investigate the effect of AhlM on the accumulation of AHLs, P. aeruginosa PAO1 was cultivated in the presence of AhlM. Overnight cultures of P. aeruginosa PAO1 were inoculated at a concentration of 1% into a nutrient yeast broth medium. Since strain PAO1 has been reported to produce approximately 1 μM 3-oxo-C12-HSL during the late stationary phase (32, 34), purified AhlM, sterilized through 0.2 μm-pore-size filters (Millipore, Bedford, MA), was added to the growth medium to achieve final concentrations of 2.0 μg/ml and 20 μg/ml, respectively, based on which AhlM can degrade 1 μM 3-oxo-C12-HSL per min (Table 1). Heat-inactivated AhlM (final concentration, 20 μg/ml) was used as the control. The growth was measured at OD600 using a SPECTRAMAX190 during cultivation. The bacterial cells were then removed by centrifugation (12,000 × g; 10 min), and the resulting supernatant was sterilized through 0.2-μm-pore-size filters. For AHL quantification, the filtered supernatants were first acidified at pH 5.0 with HCl and incubated at 100°C for 10 min to inactivate the AhlM. After the culture supernatants were extracted with ethyl acetate, the presence of AHLs in the extracts was tested using C18 reverse-phase thin-layer chromatography, developed with 60% methanol-water (60:40 [vol/vol]), and revealed by the indicator strains C. violaceum CV026 and A. tumefaciens NT1 (pDCI41E33). The amounts of 3-oxo-C12-HSL and C4-HSL were estimated by comparing different dilutions of the respective standards.
TABLE 1.
Substrate specificities of AhlM against AHLsa
| Substrate | Sp act (pmol · min−1 · μg−1) | Relative activity (%) |
|---|---|---|
| C4-HSL | 0.0 ± 0.1 | 0 |
| C6-HSL | 1.1 ± 0.2 | 1.0 |
| C8-HSL | 105.2 ± 12.0 | 100.0 |
| C10-HSL | 61.1 ± 6.0 | 58.1 |
| C12-HSL | NDb | ND |
| 3-oxo-C6-HSL | 1.4 ± 0.1 | 1.3 |
| 3-oxo-C8-HSL | 15.4 ± 1.5 | 14.6 |
| 3-oxo-C12-HSL | 50.9 ± 1.4 | 48.4 |
The data present the mean values of three independent experiments.
ND, not determined due to poor solubility of the substrate.
Assay for virulence factors.
The elastolytic activities of the cell-free culture supernatants were determined as previously described (1) with elastin Congo red (ECR; Sigma) as the substrate. One hundred microliters of each supernatant was mixed with 900 μl of an ECR buffer (100 mM Tris, 1 mM CaCl2, pH 7.5) containing 20 mg of ECR and incubated with shaking at 37°C for 3 h. The insoluble ECR was removed by centrifugation, and then the absorbance of the mixture was measured at OD495 using a SPECTRAMAX190, and elastase activity was expressed as the increase at OD495. The casein-hydrolyzing proteolytic activity was determined as previously described with 0.3% azocasein (Sigma) as the substrate, and the proteolytic activity was expressed as the increase at OD400 (1). The LasA activity was measured based on the staphylolytic activity, which was determined by the rate of lysis of a standard suspension of boiled Staphylococcus aureus, as previously described (7). LasA activity was expressed as the rate of decrease at OD600 for 30 min.
Nucleotide sequence accession numbers.
The partial 16S rRNA gene sequence of Streptomyces sp. strain M664 was assigned GenBank accession number AY561760, and the nucleotide sequence of ahlM was submitted to the GenBank-EMBL database under accession number AY561759.
RESULTS
Isolation of AHL-degrading Streptomyces sp. strain M664.
QS-interfering compounds were screened from the culture supernatants of 500 Streptomyces spp. isolated from soil samples, based on their abilities to inhibit AHL-mediated violacein production. Among five supernatants visibly inhibiting C6-HSL-induced violacein synthesis and leaving a colorless area around the hole of a bioassay plate, the GSM supernatant from strain M664 strongly prevented the C10-HSL-mediated inhibition of C6-HSL-induced violacein synthesis. No inhibition of bacterial growth was observed around the hole. Interestingly, solvent extraction showed that the substance from M664 that inhibited AHL-induced violacein synthesis was still present in an aqueous phase after butanol and ethyl acetate extraction. To examine the possibility of an enzyme, the supernatant from strain M664 was reacted with C10-HSL as the substrate. A bioassay of the AHL-degrading activity showed that C10-HSL was rapidly degraded by the supernatant, compatible with its ability to strongly prevent the C10-HSL-mediated inhibition of AHL-induced violacein synthesis. When M664 was cultivated in YEME medium for 6 days, most of the AHL-degrading activity was detected in the culture supernatant, which dramatically increased at the early exponential phase and maintained this level until the late stationary phase (data not shown). Therefore, these results indicate that strain M664 secretes an extracellular AHL-degrading enzyme into the culture medium, and the inhibition of AHL-mediated violacein synthesis results from AHL degradation rather than AHL competition. The 16S rRNA gene sequence of strain M664 exhibited 99% identity with those of Streptomyces griseus and Streptomyces argenteolus. Thus, the AHL-degrading isolate M664 was named Streptomyces sp. strain M664.
Cloning of ahlM gene encoding AHL-degrading enzyme.
The AHL-acylase AiiD has been reported to contain the signal sequences of and be most similar to the aculeacin A acylase (AAC) from Actinoplanes utahensis, which belongs to the genera of actinomycetes, on an amino sequence level (23). Based on these facts, degenerated primers were designed for the well-conserved regions of acylase homologues from Ralstonia strain XJ12B (23), P. aeruginosa PAO1 (14), A. utahensis (15), and Deinococcus radiodurans (AAF12385.1) to clone the AHL-degrading enzyme gene from Streptomyces sp. strain M664. The PCR amplification was carried out using the degenerated primers and the total DNA of Streptomyces sp. strain M664 as the template. As a result, a single band of 0.5-kb fragments was obtained and sequenced. A BLAST search revealed that the amino acid sequences deduced for the PCR products showed identity with AHL-acylases, AAC, and other penicillin acylase families. To identify that the full ORF of the 0.5-kb fragment encoded a putative AHL-acylase, SalI-digested and MluI-digested DNA libraries were constructed and screened using a 0.5-kb fragment as the probe. Consequently, positive clones carrying pUahlM_S2.0 and pUahlM_M3.5 were isolated from the two libraries, respectively, and sequenced. On the basis of a sequence and restriction enzyme site analysis, the subcloning of pUahlM_S2.0 and pUahlM_M3.5 led to the creation of plasmid pUahlM containing a 4.0-kb insert in pUC18.
A further sequence analysis showed that the 4.0-kb DNA fragment of pUahlM contained a complete ORF of 2,415 nucleotides with a TTG start codon and TGA stop codon, which was designated ahlM. A putative ribosome binding site, GGAGG, was identified 6 bp upstream of the TTG start codon of ahlM. The ahlM gene was predicted to encode a polypeptide with a molecular mass of 85,633 Da, corresponding to a polypeptide of 804 amino acids. A putative acyl-coenzyme A synthetase gene, transcribed with the opposite orientation to ahlM, was identified downstream of ahlM and revealed 99% identity with that (NP_822434.1) in Streptomyces avermitilis MA-4680 on the amino acid sequence level.
Posttranslational processing of AhlM to active form in S. lividans.
Even though the full gene for a putative AHL-acylase was cloned, the E. coli clone harboring pUahlM did not show any AHL-degrading activity. Furthermore, the ahlM gene was not expressed under the control of the T7 promoter in E. coli (data not shown). Thus, to investigate whether the ahlM gene indeed encoded an AHL-degrading enzyme, the expression of ahlM was carried out in S. lividans, an actinomycete species closely related to our strain. The ahlM gene containing the putative promoter and six-His tag was subcloned into a shuttle vector, pWHM3, and the resulting plasmid, pEAhlM, was transformed into S. lividans. The recombinant S. lividans carrying pEAhlM was then cultivated in YEME medium for 3 days. Most of the AHL-degrading activity was observed in the culture supernatant of the recombinant S. lividans, as with Streptomyces sp. strain M664, while S. lividans carrying pWHM3, as the control, was unable to degrade C10-HSL. Therefore, these results verify that the ahlM gene from Streptomyces sp. strain M664 encodes an AHL-degrading enzyme.
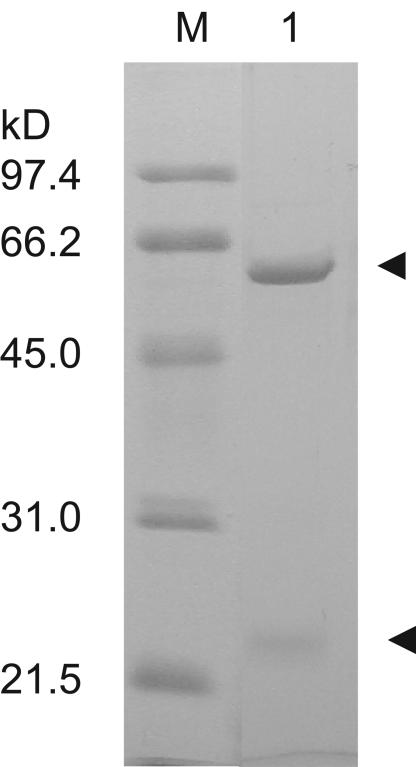
AhlM was successfully purified from the culture supernatant of the recombinant S. lividans using ammonium sulfate precipitation and an Ni affinity column. SDS-PAGE analysis showed that AhlM consisted of two subunits, corresponding to approximately 60 and 23 kDa (Fig. 1). The amino-terminal sequence of the small (α) subunit was determined to be RHPSG, found at positions 36 to 41, indicating that the first 35 amino acids of the protein were cleaved off during maturation (Fig. 2). Meanwhile, the amino-terminal sequence of the large (β) subunit was SNAVA, which contained the conserved amino-terminal Ser-Asp doublet that plays a critical role in both the processing and activity of the penicillin acylase family (13, 22). Therefore, these results indicate that AhlM is synthesized as a precursor polypeptide and modified into an active enzyme by posttranslational processing.
FIG. 1.
SDS-PAGE analysis of purified AhlM. The enzyme was purified from recombinant S. lividans, as described in Materials and Methods. The pooled active fractions were loaded on SDS-12% PAGE after Ni affinity chromatography. The arrowheads indicate the bands corresponding to two subunits of AhlM. Lane M, size marker; lane 1, purified AhlM.
Sequence analysis of AhlM.
In agreement with the SDS-PAGE (Fig. 1) and amino-terminal sequence analyses, the deduced amino acid sequences of AhlM showed the typical polypeptide organization (signal sequence, α subunit, spacer, and β subunit) observed for the penicillin acylase family, including penicillin acylases, cephalosporin acylases, and aculeacin A acylases. The first 35 residues of AhlM had the properties of a signal peptide, consistent with the presence of AHL-degrading activity in the culture media of Streptomyces sp. strain M664 and the recombinant S. lividans. A Gly-Ser pair, representing the cleavage site between the spacer peptide and the β subunit, was well conserved. In addition, the generation of free N-terminal nucleophile Ser was demonstrated by the determined amino-terminal sequences of the β subunit. Therefore, these facts indicate that the AHL-acylase AhlM is a class within the penicillin acylase family, which structurally belongs to the Ntn hydrolase superfamily.
The amino acid sequences of AhlM were compared with the sequences of known AHL-acylases and other members of the penicillin acylase family (Fig. 2). The comparison of AhlM with Ralstonia strain XJ12B AiiD (23) and P. aeruginosa PAO1 PvdQ (14) exhibited 35% and 30% identities in the deduced amino acid sequences, respectively. However, AhlM was most similar to the cyclic lipopeptide acylases (AB158476 [unpublished]) from the high-G+C gram-positive Streptomyces sp. strain FERM BP-5809 and A. utahensis (15), showing 93% and 36% identities on the amino acid sequence level, respectively. This suggests that AhlM may have the ability to degrade cyclic lipopeptides, as well as AHLs. In addition, AhlM revealed 32 to 34% identities on the amino acid sequence level with homologues from D. radiodurans, Ralstonia solanacearum, Ralstonia metallidurans, and Pseudomonas putida, all of which are deduced proteins with undemonstrated functions yet are classified as belonging to the penicillin acylase family.
Extracellular AhlM is AHL-acylase.
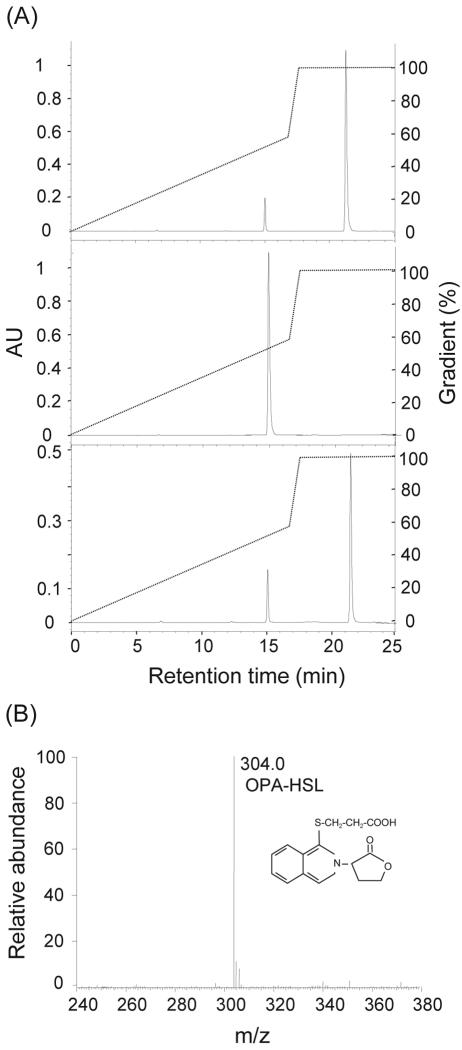
The sequence similarity of AhlM to known acylases suggests that AhlM may inactivate AHL by hydrolyzing the amide bond of AHL. To test this possibility, purified AhlM was reacted with C10-HSL for 1 h. The OPA derivation of the reaction mixture produced an OPA-active amine, indicating the release of free HSL from C10-HSL. The identity of the product of the enzymatic reaction was verified by HPLC and ESI-MS. As shown in Fig. 3A, the HPLC analysis demonstrated that the OPA-active amine with a retention time of 21.3 min was identical to the OPA-derivatized HSL. The minor peak, identical to that of the OPA-derivatized HS standard, may have resulted from the hydrolysis of HSL by the high pH in the OPA derivation reaction, which was also generated in the OPA-derivatized HSL as the standard. Furthermore, the ESI-MS analysis of the 21.3-min HPLC fraction revealed an M-H ion at m/z 304, corresponding to the OPA-derivatized HSL (Fig. 3B). Conversely, a peak corresponding to the OPA-derivatized HSL was not detected in the HPLC analysis of C10-HSL and the reaction mixture of the heat-treated AhlM and C10-HSL as controls (data not shown). Consequently, these results strongly suggest that the extracellular AhlM of Streptomyces sp. strain M664 is an AHL-acylase that hydrolyzes the amide bond between HSL and the acyl side chain of AHL.
FIG. 3.
HPLC and ESI-MS spectrometry analyses of C10-HSL degradation product. (A) The purified AhlM was reacted with 2 mM C10-HSL for 1 h. The reaction mixture was separated by HPLC after OPA derivation (bottom). HSL (top) and HS (middle) were used as the standards. The initial mobile phase was 20 mM sodium acetate (pH 7.2) containing 0.018% triethylamine, and the elution gradient was made by 20 mM sodium acetate (pH 7.2) containing 40% acetonitrile and 40% methanol. The hatch marker corresponds to changes in mobile phase ratios during the course of the run. AU, absorbance units. (B) The HPLC fraction at 21.3 min was collected and analyzed by ESI-MS.
Substrate specificity of AhlM.
The substrate specificity of AhlM was studied by determining its enzymatic activity against a range of AHLs. A mixture of the purified AhlM and AHL was incubated at 30°C, and the release of free HSL was assayed by monitoring the absorption of the OPA-active amine at OD340. Table 1 shows that AhlM effectively degraded C8-HSL, C10-HSL and 3-oxo-C12-HSL with different acyl chain substitutions. However, AhlM exhibited a relatively low activity of short-acyl-chain AHLs, C6-HSL and 3-oxo-C6-HSL, and did not degrade detectable amounts of C4-HSL. In addition to HPLC and ESI-MS analyses, these results show that AhlM is an AHL-acylase that exhibits high deacylation activity against AHLs with long acyl chains.
The sequence similarity of AhlM to the penicillin acylase family led to an examination of the deacylation activity of penicillin G, cephalosporin C, and 7-glutaryl aminocephalosporanic acid. The reaction of penicillin G with AhlM produced an OPA-active amine, identical to OPA-derivatized 6-aminopenicillanic acid, as determined by HPLC (Fig. 4), while heat-inactivated AhlM as the control did not produce an OPA-active amine (data not shown). However, AhlM did not catalyze the release of OPA-active materials from cephalosporin C and 7-glutaryl aminocephalosporanic acid, which are the substrates for cephalosporin acylases.
FIG. 4.
HPLC analysis of penicillin G degradation product. AhlM was reacted with 2 mM penicillin G for 1 h and separated by HPLC after OPA derivation (bottom). 6-Aminopenicillanic acid was used as the standard (top). The elution gradient was made as described in the legend to Fig. 3. The hatch marker corresponds to changes in the mobile phase ratios during the course of the run. AU, absorbance units.
AhlM reduces production of AHLs and virulence factors in P. aeruginosa.
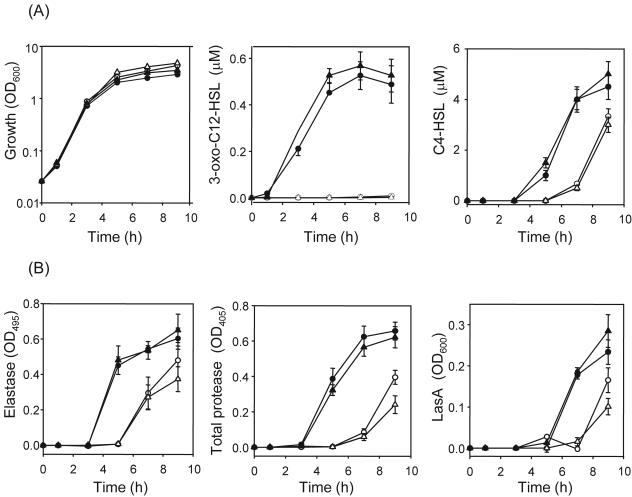
To test the potential of AhlM as an anti-quorum-sensing agent, an opportunistic pathogen, P. aeruginosa, which makes and responds to two major signal molecules, 3-oxo-C12-HSL and C4-HSL, was selected as the target organism. First, we examined the effect of AhlM on growth and the production of AHLs (Fig. 5A). The presence of AhlM at final concentrations of 2.0 and 20 μg/ml did not influence the growth of P. aeruginosa PAO1 during the late exponential phase, but a slight increase was observed in the stationary phase. Meanwhile, the accumulation of 3-oxo-C12-HSL was strongly reduced even with 2.0 μg/ml AhlM, whereas P. aeruginosa PAO1 alone and cultivated with heat-inactivated AhlM showed a population density-dependent accumulation of 3-oxo-C12-HSL. Although AhlM did not degrade C4-HSL (Table 1), it significantly reduced the accumulation of C4-HSL during early stationary phase compared to that with just strain PAO1. However, the accumulation of C4-HSL did not fall dramatically during the late stationary phase, even though the concentration of AhlM increased 10-fold, showing 30% reduction. In contrast, heat-inactivated AhlM as the control had no effect on the production of C4-HSL.
FIG. 5.
Effects of AhlM on AHL accumulation and virulence factor production in P. aeruginosa PAO1. The cultures were grown in the absence (•) or presence of 2.0 μg/ml (○) and 20 μg/ml (▵) AhlM. Heat-inactivated AhlM (final concentration, 20 μg/ml) was used as the control (▴). (A) The OD600 of the cultures was measured, and their supernatants were extracted and analyzed by thin-layer chromatography for 3-oxo-C12-HSL and C4-HSL. (B) The elastase, total protease, and LasA activities were determined spectrophotometrically during growth. The data represent the mean values of three independent experiments.
Since AhlM decreased the accumulation of AHLs in strain PAO1, its impact on major targets of QS (such as elastase, casein-hydrolyzing protease, and LasA protease) was examined (Fig. 5B). The presence of 2.0 and 20 μg/ml AhlM significantly reduced the production of elastase during the early stationary phase, yet the degree of reduction became lower, showing 23 and 43% reductions, respectively, after 9 h of growth compared with P. aeruginosa PAO1 alone. In contrast, P. aeruginosa PAO1 cultivated together with the inactivated AhlM showed an elastase production similar to that of P. aeruginosa PAO1 grown in the absence of AhlM. Similar results were also obtained in the production of total casein-hydrolyzing protease and LasA protease. Compared with P. aeruginosa PAO1 alone, the presence of 2.0 and 20 μg/ml AhlM completely blocked the production of proteases during the early stationary phase and led to 40 and 60% reductions in total protease activity, respectively, and 30 and 50% reductions in LasA protease activity, respectively, after 9 h of growth. Consequently, these results show that virulence factor production in P. aeruginosa is reduced by the application of AhlM, although a 10-fold increase in the AhlM concentration has no additional effect. To investigate whether AhlM would also prevent virulence factor production when the QS system was already activated, it was added to the medium after 4 h of growth (at a higher population density). As expected, AhlM significantly reduced the amount of 3-oxo-C12-HSL 2 h after the addition and was still effective in decreasing the virulence factor production at a higher population density, although the relative reduction was lower than that at a low population density (data not shown). Accordingly, these results suggest that AhlM has the potential to attenuate the AHL-mediated virulence of pathogenic bacteria.
DISCUSSION
We screened and isolated a novel Streptomyces strain, M664, which produces an extracellular AHL-degrading enzyme, based on its ability to inhibit AHL-dependent violacein production. The ahlM gene from Streptomyces sp. strain M664, encoding a potent AHL-acylase, was then cloned and expressed in S. lividans. The secretion of AhlM was also confirmed in a recombinant S. lividans expressing AhlM. Recently, the ability to degrade AHL has been identified in unrelated genera of soil bacteria, including proteobacteria (14, 20, 23), low-G+C gram-positive bacteria (9, 21), and high-G+C gram-positive bacteria (29, 37). However, no extracellular AHL-degrading enzyme has yet been reported. Furthermore, an ESI-MS analysis demonstrated that the extracellular AhlM released HSL by hydrolyzing AHL, indicating that AhlM is an AHL-acylase. Two AHL-acylases, AiiD and PvdQ, and a putative acylase have already been identified in gram-negative bacteria, including a Ralstonia isolate, P. aeruginosa, and V. paradoxus. However, this is the first report of an AHL-acylase in gram-positive bacteria, indicating that AHL-acylases are also widely distributed across many species, like AHL-lactonases.
Since the AHL-acylases AiiD and PvdQ share many of the known characteristics of Ntn hydrolases, including a signal peptide followed by an α subunit, spacer sequence, and β subunit (Fig. 2), their posttranslational modification into active enzymes has been predicted. However, the expression of two acylase genes in E. coli has shown that the polypeptides encoded by these genes are mainly recovered as unprocessed propeptides (14, 23). Although the site-directed mutagenesis of AiiD has shown that the conserved Gly-Ser pair is important in AHL-acylase activity (23), its maturation process remains unclear. In contrast to aiiD and pvdQ, no AHL-degrading activity was detected when ahlM was expressed with or without its signal sequences under the strong promoter in E. coli (data not shown). Because of a high G+C content or inaccurate processing, AhlM may not have been expressed properly in E. coli. However, the expression of ahlM in S. lividans both resulted in the detection of AHL-degrading activity and proved to be a posttranslational process that generated a mature protein with an active catalytic center (Fig. 1). Furthermore, the determined amino-terminal sequences showed that the first residue of the β subunit was the nucleophile Ser, essential for processing and catalysis. Thus, the current results verified that the AHL-acylase AhlM is a member of the Ntn hydrolase family.
There are notable differences in the catalytic spectrum toward AHLs among AHL-acylases. PvdQ from P. aeruginosa has been reported to be unable to degrade AHLs with acyl chains shorter than eight carbons (14), yet AiiD from a Ralstonia isolate has been reported to rapidly degrade C4-HSL and 3-oxo-C12-HSL with equal efficiencies, while exhibiting a significantly low activity toward 3-oxo-C6-HSL (23). In this study, AhlM from Streptomyces sp. strain M664 was found to be more effective in degrading AHLs with acyl chains longer than six carbons, regardless of the substitution and acyl chain length. AhlM appeared to be more active against unsubstituted rather than 3-oxo-substituted AHLs, e.g., the rate of hydrolysis of C8-HSL was sevenfold higher than that of 3-oxo-C8-HSL, yet this difference in susceptibility was reduced as the acyl side chain was lengthened, based on comparing the activities of 3-oxo-C12 and 3-oxo-C8-HSL (Table 1). In contrast to AHLs with long acyl chains, the degrading activity of AhlM dramatically decreased for AHLs with acyl chains shorter than eight carbons and was not detected with C4-HSL, which has an AHL substrate preference quite similar to that of P. aeruginosa PAO1 PvdQ (14). A comparison of the two residues known to be involved in the substrate specificity of acylases (17) has shown that the three AHL-acylases have different residues (Leu-50β and Ser-57β in AhlM; Leu-50β and Asp-57β in PvdQ; Ile-50β and Ser-57β in AiiD) (Fig. 2). Site-directed mutation of these residues may provide clues about the substrate specificity of AHL-acylases.
The penicillin acylase family, including penicillin G acylases, cephalosporin acylases, and aculeacin A acylases, is a member of the Ntn-hydrolase superfamily structurally yet has a high degree of substrate specificity (17, 26). AiiD from a Ralstonia isolate has also been reported to serve as an AHL-acylase for several reasons, such as no activity on penicillin G and AHL-degrading activity at a nanomolar concentration of AHLs (23). However, it is still unclear whether AHLs are the true substrates of Streptomyces sp. strain M664 AhlM. Surprisingly, AhlM showed a higher sequence identity with the cyclic lipopeptide acylases from Streptomyces than with the AHL-acylases AiiD and PvdQ (Fig. 2). In particular, the cyclic lipopeptide acylase (AB158476 [unpublished]) from Streptomyces sp. strain FERM BP-5809 exhibited 93% identity with AhlM, indicating that AhlM may be capable of degrading cyclic lipopeptides composed of a hexapeptide moiety and acyl chains longer than C16. Furthermore, in contrast to AiiD, AhlM catalyzed the hydrolysis of penicillin G and released 6-aminopenicillanic acid (Fig. 4). Accordingly, these results indicate that AhlM is an AHL-acylase with a broad substrate specificity, in contrast to the penicillin acylase family. Thus, further studies should focus on determining the primary substrate for AhlM.
To test the potential of AhlM as an anti-quorum-sensing agent, the purified AhlM was added to the growth medium of P. aeruginosa PAO1, where two AHLs, 3-oxo-C12-HSL and C4-HSL, play key roles in regulating the production of virulence factors, such as elastase, proteases, and swarming motility (5). AhlM eliminated 3-oxo-C12-HSL in a growth medium of P. aeruginosa PAO1 during the late stationary phase (Fig. 5A), compatible with its high degrading activity against 3-oxo-C12-HSL (Table 1). Even though AhlM was unable to degrade C4-HSL (Table 1), the addition of AhlM also delayed and reduced the accumulation of C4-HSL compared with P. aeruginosa PAO1 alone. Similar to the accumulation of C4-HSL, the production of virulence factors was also strongly decreased by the presence of AhlM during the early stationary phase, and the degree of reduction became lower during the late stationary phase (Fig. 5B). Accordingly, the current results support the notion that the expression of virulence factors in P. aeruginosa is regulated by two major signal molecules, and the two quorum circuits, the LasRI and RhlRI systems, form a regulatory cascade, in which C4-HSL-mediated quorum sensing is dependent on 3-oxo-C12-HSL (19, 31). In this study, the LasRI system regulates RhlRI systems, yet the degradation of 3-oxo-C12-HSL by AhlM results in merely a short delay and low reduction of C4-HSL levels. Previously, Latifi et al. reported that expression of RhlI, which directs the synthesis of C4-HSL, depends to a greater extend on RhlR and C4-HSL than LasR and 3-oxo-C12-HSL (18). In addition, the GacA response regulator of the two-component system has been known to positively regulate the production of C4-HSL by activating the expression of RhlR and LasR directly or indirectly (33). Furthermore, our current results also provide additional indirect evidence that in P. aeruginosa, the RhlRI system is not totally dependent on the LasRI system for its expression.
The application of AhlM in quorum-sensing bacteria showed the potential of virulence attenuation (Fig. 5), parallel to the previous results showing that the expression of AHL-degrading enzymes interferes with the quorum-sensing signaling and attenuates the virulence of E. carotovora (9) and P. aeruginosa (23). Recently, Hentzer et al. reported that a halogenated furanone compound blocked P. aeruginosa quorum sensing and rapidly cleared bacteria in a mouse pulmonary model (12), implying that bacterial infections can be controlled by substances that specifically disrupt bacterial quorum sensing. Thus, similar to an AHL antagonist, the enzymatic attenuation of bacterial infection, that is, the direct application of AhlM, may be developed as a highly attractive quorum-quenching agent.
Acknowledgments
We thank the Microbial Resources Data Base in Korea for providing the culture supernatants of 400 Streptomyces spp., as well as Sun-Mi Kang and Eun-Ju Chang for their technical assistance.
This work was supported by grants from the Ministry of Commerce, Industry and Energy and the Rural Development Administration of Korea (BioGreen21 Project).
REFERENCES
- 1.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe. Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 3.Cook, D. M., P. L. Li, F. Ruchaud, S. Padden, and S. K. Farrand. 1997. Ti plasmid conjugation is independent of vir: reconstitution of the tra functions from pTiC58 as a binary system. J. Bacteriol. 179:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dangl, J. L., and J. D. Jones. 2001. Plant pathogens and integrated defense responses to infection. Nature 411:826-833. [DOI] [PubMed] [Google Scholar]
- 5.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demain, A. L., and A. Fang. 2000. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 69:1-39. [DOI] [PubMed] [Google Scholar]
- 7.Diggle, S. P., K. Winzer, A. Lazdunski, P. Williams, and M. Camara. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J. Bacteriol. 184:2576-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 9.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong, Y. H., X. F. Zhang, J. L. Xu, and L. H. Zhang. 2004. Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol. 70:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 12.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt, L., V. Kasche, K. Lummer, R. J. Lewis, G. N. Murshudov, C. S. Verma, G. G. Dodson, and K. S. Wilson. 2000. Structure of a slow processing precursor penicillin acylase from Escherichia coli reveals the linker peptide blocking the active-site cleft. J. Mol. Biol. 302:887-898. [DOI] [PubMed] [Google Scholar]
- 14.Huang, J. J., J. I. Han, L. H. Zhang, and J. R. Leadbetter. 2003. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 69:5941-5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inokoshi, J., H. Takeshima, H. Ikeda, and S. Omura. 1992. Cloning and sequencing of the aculeacin A acylase-encoding gene from Actinoplanes utahensis and expression in Streptomyces lividans. Gene 119:29-35. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Charter, and D. A. Hopwood. 2000. Practical Streptomyces genetics. a laboratory manual. John Innes Foundation, Norwich, England.
- 17.Kim, Y., K. Yoon, Y. Khang, S. Turley, and W. G. Hol. 2000. The 2.0 Å crystal structure of cephalosporin acylase. Structure 8:1059-1068. [DOI] [PubMed] [Google Scholar]
- 18.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 19.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, S. J., S. Y. Park, J. J. Lee, D. Y. Yum, B. T. Koo, and J. K. Lee. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., J. Chen, W. Jiang, X. Mao, G. Zhao, and E. Wang. 1999. In vivo post-translational processing and subunit reconstitution of cephalosporin acylase from Pseudomonas sp. 130. Eur. J. Biochem. 262:713-719. [DOI] [PubMed] [Google Scholar]
- 23.Lin, Y. H., J. L. Xu, J. Hu, L. H. Wang, S. L. Ong, J. R. Leadbetter, and L. H. Zhang. 2003. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 47:849-860. [DOI] [PubMed] [Google Scholar]
- 24.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 26.McVey, C. E., M. A. Walsh, G. G. Dodson, K. S. Wilson, and J. A. Brannigan. 2001. Crystal structures of penicillin acylase enzyme-substrate complexes: structural insights into the catalytic mechanism. J. Mol. Biol. 313:139-150. [DOI] [PubMed] [Google Scholar]
- 27.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 28.Nasser, W., M. L. Bouillant, G. Salmond, and S. Reverchon. 1998. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol. Microbiol. 29:1391-1405. [DOI] [PubMed] [Google Scholar]
- 29.Park, S. Y., S. J. Lee, T. K. Oh, J. W. Oh, B. T. Koo, D. Y. Yum, and J. K. Lee. 2003. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 149:1541-1550. [DOI] [PubMed] [Google Scholar]
- 30.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 31.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 34.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Defago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 35.Strickler, J. E., T. R. Berka, J. Gorniak, J. Fornwald, R. Keys, J. J. Rowland, M. Rosenberg, and D. P. Taylor. 1992. Two novel Streptomyces protein protease inhibitors. Purification, activity, cloning, and expression. J. Biol. Chem. 267:3236-3241. [PubMed] [Google Scholar]
- 36.Throup, J. P., M. Camara, G. S. Briggs, M. K. Winson, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1995. Characterization of the yenI/yenR locus from Yersinia enterocolitica mediating the synthesis of two N-acylhomoserine lactone signal molecules. Mol. Microbiol. 17:345-356. [DOI] [PubMed] [Google Scholar]
- 37.Uroz, S., C. D'Angelo-Picard, A. Carlier, M. Elasri, C. Sicot, A. Petit, P. Oger, D. Faure, and Y. Dessaux. 2003. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 149:1981-1989. [DOI] [PubMed] [Google Scholar]
- 38.Vara, J., M. Lewandowska-Skarbek, Y. G. Wang, S. Donadio, and C. R. Hutchinson. 1989. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J. Bacteriol. 171:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L. H., L. X. Weng, Y. H. Dong, and L. H. Zhang. 2004. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem. 279:13645-13651. [DOI] [PubMed] [Google Scholar]
- 40.Xu, F., T. Byun, H. J. Deussen, K. R. Duke, and H. J. Dussen. 2003. Degradation of N-acylhomoserine lactones, the bacterial quorum-sensing molecules, by acylase. J. Biotechnol. 101:89-96. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]