Abstract
The Cryptosporidium spp. UV disinfection studies conducted to date have used Cryptosporidium parvum oocysts. However, Cryptosporidium hominis predominates in human cryptosporidiosis infections, so there is a critical need to assess the efficacy of UV disinfection of C. hominis. This study utilized cell culture-based methods to demonstrate that C. hominis oocysts displayed similar levels of infectivity and had the same sensitivity to UV light as C. parvum. Therefore, the water industry can be confident about extrapolating C. parvum UV disinfection data to C. hominis oocysts.
Cryptosporidium parvum is an intracellular protozoan parasite that infects the epithelial cells lining the digestive tract and is very common in many animal species (4). The resistant oocyst stage of the organism's life cycle is excreted in the feces of infected animals and can contaminate sources of drinking water. Although the disease is usually self-limiting in otherwise healthy humans, persistent infection can contribute to mortality in individuals with weakened immune systems.
There have been many outbreaks of cryptosporidiosis associated with either drinking water or recreational use of water (4). The largest waterborne outbreak occurred in 1993 in Milwaukee with estimates of the affected population ranging from 15,000 to 400,000 individuals (6, 10) and up to 100 deaths attributed to the contamination. The primary chlorine-based disinfectants used throughout the water industry have little effect on C. parvum oocysts at concentrations typically applied in drinking water treatment plants (15). Consequently, alternative disinfectants, such as UV light, have been investigated. Numerous studies have demonstrated the efficacy of UV light for disinfecting oocysts of at least six C. parvum isolates (2, 3, 11, 14, 16, 24). However, all of the UV disinfection studies conducted to date have used C. parvum type 2 oocysts. Cryptosporidium hominis (previously referred to as type 1 C. parvum) does not infect most standard animal models (21), so propagation of oocysts is difficult, limiting the number of analyses that can be accomplished with these oocysts, particularly infection and inactivation studies. Importantly, though, C. hominis predominates in most surveys of human cryptosporidiosis, accounting for 70% of infections (5, 7, 9, 19, 22). Also, C. hominis oocysts are recovered from patients more frequently than C. parvum in foodborne and waterborne outbreaks (22). Therefore, it is important to determine whether C. hominis oocysts display the same sensitivity to UV disinfection as C. parvum.
Sources and propagation of oocysts.
Oocysts of the bovine Iowa isolate of C. parvum were obtained from Sterling Parasitology Laboratories (University of Arizona, Tucson, Ariz.). They were propagated by artificial infection in Holstein calves and purified by density gradient centrifugation as described previously (13). The cervine MD isolate of C. parvum (originally obtained from S. Wright, Moredun Research Institute, Scotland) was propagated in gamma interferon knockout (17) or immunosuppressed mice. Two C. hominis isolates from human infections (TU502 and TU71) were propagated in gnotobiotic piglets (1, 21, 23).
Measuring infectivity and inactivation.
Oocyst infectivity was assessed using three cell culture-based approaches. (i) Confluent HCT-8 cell monolayers were inoculated and incubated for 72 h, and infection was detected by using RT-PCR, using C. parvum-specific primers targeting a 70-kDa heat shock protein gene (13). At least four oocyst doses were inoculated: 25 to 200 for controls and 250 to 5,000 for UV-exposed oocysts. Each dose of oocysts was inoculated onto 12 cell monolayers, and infectivity was expressed as the proportion of monolayers that developed infection. Dose response was assessed by regression of oocyst challenge dose against a logistic transformation of proportional infectivity (8, 13). (ii) HCT-8 cells were inoculated, and infection was detected by in situ hybridization (12). (iii) MDBK cells were inoculated, and following 48 h of incubation, the intensity of infection was assessed using an indirect immunofluorescence assay (18). Inoculation doses were 1 × 104 and 1 × 105 for control and UV-exposed oocysts. The extent of infection was quantified using a UV fluorescence microscope and video imaging.
Although C. hominis oocysts do not infect standard animal models, it has been demonstrated that they do infect cell cultures (13). However, comparisons of C. parvum and C. hominis infectivity in cell culture have not been performed. In the present study, the 50% infective dose of pig-propagated TU502 in HCT-8 cell culture was 75, close to the previously reported value of 77 for the Iowa isolate (13). Infectivity in HCT-8 cells was also quantified by in situ hybridization that allowed visualization of infectious foci within the cell monolayers (12). When expressed as the number of infectious foci per inoculum oocyst, average infectivity with a dose of 250 oocysts was 11% for TU502 (n = 2) and 8.8% for Iowa (n = 2), compared to a previously reported value of 8% for the Iowa isolate (12). The average number of parasite developmental stages per infectious focus was 29 and 28 for TU502 and Iowa, respectively, compared to an earlier value of 26 for the Iowa isolate (12). Therefore, based on two detection methods, the TU502 C. hominis isolate had the same level of infectivity in HCT-8 cell culture as the C. parvum Iowa isolate.
UV disinfection.
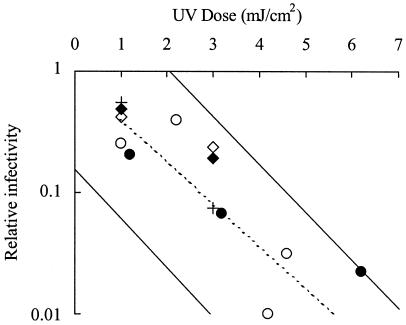
Oocysts were exposed to polychromatic UV radiation from a medium-pressure (MP) UV system using previously described equipment and procedures (11, 14). Oocyst suspensions were placed at a fixed distance from a constant-intensity UV lamp for various periods of time to achieve different UV doses. The UV dose was measured using a calibrated radiometer (11) and expressed as mJ/cm2. Oocysts were also exposed to monochromatic UV radiation at 253.7 nm from a low-pressure (LP) UV source as described previously (16). For the HCT-8/reverse transcription (RT)-PCR infectivity assay, inactivation was measured by comparing the 50% infective dose of control and UV-exposed oocysts. With the MDBK/immunofluorescence method, inactivation was measured as a reduction in parasite fluorescence relative to that of control oocysts. UV inactivation data were obtained for two C. hominis isolates (TU502 and TU71) by using LP UV and the MDBK/immunofluorescence method, and for a single isolate (TU502) by using MP UV with the HCT-8/RT-PCR method (Fig. 1). Inactivation levels for the C. hominis isolates with UV doses up to 6 mJ/cm2 were within the 90% prediction limits of previously generated UV inactivation data for the Iowa isolate (14). In Fig. 1, a relative infectivity of 0.1 is equivalent to 90% (1 log10) inactivation. With a UV dose of 3 mJ/cm2, inactivation of C. hominis oocysts averaged across both cell culture methods was 90% (1 log10). This is in close agreement with an inactivation level of 93.7% (1.2 log10) for the Iowa isolate at 3 mJ/cm2 (14). Data for a second C. parvum isolate (MD) obtained with both cell culture methods were also within the 90% prediction limits for the Iowa isolate (Fig. 1). Inactivation levels greater than 90% (1 log10) were not detected with the MDBK/immunofluorescence assay, even with LP UV doses up to 20 mJ/cm2 (data not shown), but this was most likely due to limitation of the infection detection method rather than a UV tailing effect.
FIG. 1.
Inactivation of Cryptosporidium spp. by UV light. Infectivity of C. hominis oocysts was measured in HCT-8 cells with detection by RT-PCR (MP UV, TU502 isolate, •) and in MDBK cells with immunofluorescence detection of infection (LP UV, TU502, ⧫; TU71, +). Infectivity of the C. parvum MD isolate was measured by HCT-8 cell culture/RT-PCR (MP UV, ○) and MDBK/immunofluorescence (LP UV, ◊). The solid diagonal lines represent the 90% prediction limits for UV inactivation of the Iowa isolate (n = 29) assessed in HCT-8 cells followed by RT-PCR (14). Disinfection credits provided to water treatment plants by the draft long term 2 enhanced surface water treatment rule are represented by the dashed line (20).
The results of this study clearly demonstrated that C. hominis oocysts display similar levels of infectivity in cell culture and have the same sensitivity to UV light as C. parvum. Thus, the water industry can be relatively confident about extrapolating the wealth of C. parvum UV disinfection data to the more predominant but less well-studied species of C. hominis.
Acknowledgments
This study was supported in part by funding from the Water Environment Research Foundation (grant number 99-HHE-3) and the Awwa Research Foundation (project number 2669).
C. hominis oocysts were produced in the Division of Infectious Diseases Animal Care Facility at Tufts University School of Veterinary Medicine and were kindly provided by Saul Tzipori and Don Girouard.
REFERENCES
- 1.Akiyoshi, D. E., X. Feng, M. A. Buckholt, G. Widmer, and S. Tzipori. 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect. Immun. 70:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy, J. L., M. M. Marshall, T. M. Hargy, and D. G. Korich. 2004. Susceptibility of five strains of Cryptosporidium parvum oocysts to UV light. J. Am. Water Works Assoc. 96:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craik, S. A., D. Weldon, G. R. Finch, J. R. Bolton, and M. Belosevic. 2001. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 35:1387-1398. [DOI] [PubMed] [Google Scholar]
- 4.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 5.Gatei, W., J. Greensill, R. W. Ashford, L. E. Cuevas, C. M. Parry, N. A. Cunliffe, N. J. Beeching, and C. A. Hart. 2003. Molecular analysis of the 18S rRNA gene of Cryptosporidium parasites from patients with or without human immunodeficiency virus infections living in Kenya, Malawi, Brazil, the United Kingdom, and Vietnam. J. Clin. Microbiol. 41:1458-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter, P. R., and Q. Syed. 2001. Community surveys of self-reported diarrhea can dramatically overestimate the size of outbreaks of waterborne cryptosporidiosis. Water Sci. Technol. 43:27-30. [PubMed] [Google Scholar]
- 7.Hunter, P. R., S. Hughes, S. Woodhouse, Q. Syed, N. Q. Verlander, R. M. Chalmers, K. Morgan, G. Nichols, N. Beeching, and K. Osborn. 2004. Sporadic cryptosporidiosis case-control study with genotyping. Emerg. Infect. Dis. 10:1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korich, D. G., M. M. Marshall, H. V. Smith, J. O'Grady, Z. Bukhari, C. R. Fricker, J. S. Rosen, and J. L. Clancy. 2000. Inter-laboratory comparison of the CK-1 neonatal mouse logistic dose-response model for Cryptosporidium parvum oocysts. J. Eukaryot. Microbiol. 47:294-298. [DOI] [PubMed] [Google Scholar]
- 9.Leav, B. A., M. R. Mackay, A. Anyanwu, R. M. O'Connor, A. M. Cevallos, G. Kindra, N. C. Rollins, M. L. Bennish, R. G. Nelson, and H. D. Ward. 2002. Analysis of sequence diversity at the highly polymorphic Cpgp40/15 locus among Cryptosporidium isolates from human immunodeficiency virus-infected children in South Africa. Infect. Immun. 70:3881-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, J. B. Rose, and J. P. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. New Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 11.Mofidi, A. A., H. Baribeau, P. A. Rochelle, R. De Leon, B. M. Coffey, and F. F. Green. 2001. Disinfection of Cryptosporidium parvum with polychromatic UV light. J. Am. Water Works Assoc. 93:95-109. [Google Scholar]
- 12.Rochelle, P. A., D. M. Ferguson, A. M. Johnson, and R. De Leon. 2001. Quantitation of Cryptosporidium parvum infection in cell culture using a colorimetric in situ hybridization assay. J. Eukaryot. Microbiol. 48:565-573. [DOI] [PubMed] [Google Scholar]
- 13.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochelle, P. A., D. Fallar, M. M. Marshall, B. A. Montelone, S. J. Upton, and K. Woods. 2004. Irreversible UV inactivation of Cryptosporidium sp. despite the presence of UV repair genes. J. Eukaryot. Microbiol. 51:553-562. [DOI] [PubMed] [Google Scholar]
- 15.Rose, J. B., J. T. Lisle, and M. LeChevallier. 1997. Waterborne cryptosporidiosis: incidence, outbreaks, and treatment strategies, p. 93-109. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 16.Shin, G., K. G. Linden, M. J. Arrowood, and M. D. Sobsey. 2001. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 65:4761-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, S. M. Rich, G. Widmer, X. Feng, and S. Tzipori. 2003. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am. J. Trop. Med. Hyg. 68:710-715. [PubMed] [Google Scholar]
- 20.U.S. Environmental Protection Agency. 2003. National primary drinking water regulations: long term 2 enhanced surface water treatment rule; proposed rule. Fed. Regist. 68:47640-47793.. [Google Scholar]
- 21.Widmer, G., D. Akiyoshi, M. A. Buckholt, X. Feng, S. M. Rich, K. M. Deary, C. A. Bowman, P. Xu, Y. Wang, G. A. Buck, and S. Tzipori. 2000. Animal propagation and genomic survey of a genotype 1 isolate of Cryptosporidium parvum. Mol. Biochem. Parasitol. 108:187-197. [DOI] [PubMed] [Google Scholar]
- 22.Xiao, L. C. Bern, I. M. Sulaiman, and A. A. Lal. 2003. Molecular epidemiology of human cryptosporidiosis, p. 121-146. In R. C. A. Thompson, A. Armson and U. M. Ryan (ed.), Cryptosporidium: from molecules to disease. Elsevier B.V., Amsterdam, The Netherlands.
- 23.Xu, P., G. Widmer, Y. Wang, L. S. Ozaki, J. M. Alves, M. G. Serrano, D. Puiu, P. Manque, D. Akiyoshi, A. J. Mackay, W. R. Pearson, P. H. Dear, A. T. Bankier, D. L. Peterson, M. S. Abrahamsen, V. Kapur, S. Tzipori, and G. A. Buck. 2004. The genome of Cryptosporidium hominis. Nature 431:1107-1112. [DOI] [PubMed] [Google Scholar]
- 24.Zimmer, J. L., R. M. Slawson, and P. M. Huck. 2003. Inactivation and potential repair of Cryptosporidium parvum following low- and medium-pressure ultraviolet irradiation. Water Res. 37:3517-3523. [DOI] [PubMed] [Google Scholar]