Abstract
The ability of Staphylococcus aureus to invade and survive within endothelial cells is believed to contribute to its propensity to cause persistent endovascular infection with endothelial destruction. In the present study, we show that following invasion of human umbilical vein endothelial cells, intracellular S. aureus organisms remain viable over a 72-h period and, as determined by transmission electron microscopic examination, that the bacteria exist within vacuoles and free within the cytoplasm. We also demonstrate that endothelial cell death following S. aureus invasion occurs at least in part by apoptosis as shown by DNA fragmentation and changes in nuclear morphology. Apoptotic changes were evident as early as 1 h after infection of endothelial cells. Internalization of S. aureus rather than adherence appears to be necessary, since use of the phagocytosis inhibitor cytochalasin D prevented apoptosis. UV-killed staphylococci, although retaining the capacity to be internalized, were not capable of inducing apoptosis, suggesting that apoptosis is dependent upon a factor associated with viable organisms. The studies demonstrate that viable intracellular S. aureus induces apoptosis of endothelial cells and that internalized staphylococci can exist free within the cytoplasm.
Staphylococcus aureus is a common cause of acute endocarditis which is associated with fulminant valvular destruction and metastatic infection (13, 16). The propensity of S. aureus to infect endovascular tissue and to cause disseminated infection is incompletely understood. It has been suggested that staphylococcal adherence to the endothelium can occur by at least two different mechanisms (7). S. aureus can adhere to damaged endocardium by binding to matrix proteins such as fibrinogen and to platelets. Alternatively, S. aureus can bind to normal or undamaged endothelium via specific receptors that have yet to be fully characterized. Since most cases of S. aureus endocarditis involve heart valves that exhibit normal gross morphology (17), elucidation of the interaction of this bacterial pathogen with normal endothelial cells should provide insight into the pathogenesis of this infection.
Following adherence of S. aureus to endothelial cell monolayers, invasion may occur via endothelial cell ingestion. A number of studies have reported that endothelial cells function as nonprofessional phagocytes and thus are able to ingest S. aureus organisms (5, 6, 12). Transmission electron micrographs of bovine aortic endothelial cell monolayers infected with S. aureus have demonstrated phagocytosis following a sequence of events: (i) adherence of S. aureus to the endothelial cell, (ii) formation of cup-like processes on the endothelial cell surface underlying the adherent bacteria, and (iii) elongation of the cup and engulfment of the bacteria within a phagosome (10). Despite lysosomal fusion, the endothelial cell is unable to kill the ingested S. aureus. Viable staphylococci can be isolated after treatment of infected endothelial cell monolayers with lysostaphin, which lyses extracellular staphylococci only. Uptake of S. aureus by endothelial cells offers a unique explanation for the frequency and persistence of invasive staphylococcal infections involving endothelialized surfaces such as heart valves, prosthetic vascular grafts, and catheterized vessels.
The consequences of intracellular invasion of endothelial cells by S. aureus have only recently been investigated. Vann and Proctor showed that ingestion of a high-alpha-toxin-producing S. aureus strain by bovine aortic valve endothelial cells resulted in cytotoxic damage to cell monolayers (14). In contrast, cytotoxicity was not induced with an alpha-toxin-negative derivative strain, and furthermore, cytotoxicity was reduced by anti-alpha-toxin antiserum, suggesting that alpha-toxin, a pore-forming extracellular toxin, mediates the cytotoxic effect. Investigations by Yao et al. demonstrated that internalization of S. aureus by human umbilical vein endothelial cells induced the expression of the proinflammatory cytokines interleukin-1 and interleukin-6 (18). Other evidence of potentially deleterious effects of internalization in endothelial cells has been the findings of stimulation of adhesion molecule expression and hyperadhesiveness for human monocytes and granulocytes in infected endothelial cells (3).
One consequence of invasion by an intracellular pathogen is apoptosis or programmed cell death of the host cell, which is usually observed in professional phagocytic cells such as monocytes and macrophages (19). Although S. aureus is not considered a classic intracellular pathogen as are microorganisms such as Listeria monocytogenes and Mycobacterium tuberculosis, its intracellular residence in endothelial cells could potentially elicit this type of cell death response. Recent evidence to support this hypothesis has been the finding of apoptosis in human monocytes and in bovine mammary epithelial cells that have phagocytized S. aureus (1, 2). We thus sought to determine whether apoptosis contributes to S. aureus-induced endothelial cell death. We show for the first time that cytotoxicity in human umbilical vein endothelial cells infected with S. aureus occurs via apoptosis and that internalization of metabolically active S. aureus organisms is necessary for apoptosis to occur.
MATERIALS AND METHODS
Media and chemicals.
Medium 199 (M199), Hanks’ balanced salt solution, penicillin G, streptomycin, amphotericin B, and trypsin-EDTA were purchased from Life Technologies GIBCO BRL (Gaithersburg, Md.). Fetal calf serum was purchased from Hyclone (Logan, Utah). Heparin sodium salt, lysostaphin, and cytochalasin D were obtained from Sigma Chemical Co. (St. Louis, Mo.). Endothelial cell mitogen was purchased from Biomedical Technologies, Inc. (Stoughton, Mass.).
S. aureus strains and cultivation.
S. aureus 8325-4 is a prototypic laboratory strain cured of prophages that produces hemolysins and is used for staphylococcal genetic studies (11). DK2076 is a penicillin-sensitive clinical S. aureus isolate that is virulent in animal models of infection (9). Aliquots from overnight broth cultures were grown in brain heart infusion broth at 37°C until cultures had reached an optical density at 600 nm of 0.30 (≃1.5 to 2 h), corresponding to approximately 108 organisms/ml. The staphylococci were harvested by centrifugation at 7,000 × g for 10 min, washed twice with phosphate-buffered saline (PBS), and resuspended in M199. Bacterial concentrations were estimated spectrophotometrically and confirmed by plating serial dilutions on agar plates and counting CFU. For UV-treated S. aureus, a logarithmic-phase growth culture was suspended in M199 and UV irradiated for 1 h at a wavelength of 254 nm.
Cultivation of human umbilical vein endothelial cells.
Human umbilical vein endothelial cells were purchased from Clonetics (San Diego, Calif.). Cells were grown at 37°C in 5% CO2 in medium consisting of M199 supplemented with 15% fetal bovine serum, penicillin, streptomycin, amphotericin B, endothelial cell mitogen (50 μg/ml), and heparin (10 U/ml) on gelatin-coated 100-mm-diameter tissue culture plates (Falcon, Cockeysville, Md.) or 24-well plates (Corning, Corning, N.Y.). All experiments were performed with second- to fourth-passage cells that had reached confluence.
Invasion assays.
The S. aureus inoculum was suspended in complete medium without antimicrobials and adjusted by optical density to 108 CFU/ml, and 0.4 ml was added to washed endothelial cell monolayers that had been subcultured to confluence in 24-well tissue culture plates. Human AB serum at 5% was added immediately afterwards. Following incubation of the bacteria with endothelial cells, the wells were washed three times with M199 to remove nonadherent bacteria, and then the endothelial cells were lifted with trypsin, hypotonically lysed in sterile water, and serially diluted for agar plating to determine colony counts for total numbers of adherent and internalized bacteria. For determining S. aureus invasion, the endothelial cells were treated with lysostaphin (10 μg/ml) for 20 min to lyse extracellular bacteria prior to lifting with trypsin. Assays were performed in triplicate.
Apoptosis assays.
Human umbilical vein endothelial cells were cultivated in 100-mm-diameter tissue culture plates to confluence and infected with S. aureus for at least 1 h. Lysostaphin was added to kill extracellular staphylococci. The endothelial cells were harvested for either DNA isolation or nuclear staining.
(i) DNA isolation and gel electrophoresis.
Detached endothelial cells were centrifuged at 1,000 × g for 5 min at 4°C, and the pellet was washed with ice-cold PBS and resuspended in lysis buffer. Adherent cells were lysed directly in the dish. DNA was isolated from both lysates with the Genzyme TACS Apoptotic DNA Laddering Kit according to the manufacturer’s guidelines and analyzed on an ethidium bromide-stained 1.5% TreviGel (Genzyme, Cambridge, Mass.).
(ii) Terminal deoxynucleotide transferase-mediated dUTP-biotin nick end labeling of DNA (TUNEL assay).
Detached cells were combined with adherent cells after lifting with trypsin-EDTA, centrifuged at 1,000 × g for 5 min at 4°C, and washed with ice-cold PBS. The cell pellet was resuspended in 4% methanol-free formaldehyde in PBS, fixed for 10 min, and stored in 70% ethanol at −20°C. The Genzyme TACS in situ Apoptosis Detection Kit-Blue Label was used to label apoptotic cells. Briefly, the cells were permeabilized with proteinase K, and the 3′-hydroxyl ends of DNA fragments were labeled with biotinylated dUTP by terminal deoxynucleotidyl transferase. Detection was performed with a streptavidin-horseradish peroxidase conjugate and TACS Blue Label substrate, generating a blue insoluble product. Finally, the cells were counterstained with Red Counterstain B provided by the manufacturer. Analysis was performed by oil immersion light microscopy at magnifications of ×1,000 and ×2,000.
Electron microscopy.
Endothelial cell monolayers in 24-well plates were inoculated with S. aureus and treated identically to those used in the invasion assays described above. Cells were fixed for 24 h in 2% glutaraldehyde in 0.1 M PBS (pH 7.4). Cells were then washed in 7.5% sucrose–PBS buffer and dehydrated through a graded ethanol series. The cells were then embedded in Spurrs resin, thin sectioned, stained with uranyl acetate and lead citrate, and examined with a model 300 transmission electron microscope (Philips Electronic Instruments, Mahwah, N.J.).
RESULTS
Internalization of S. aureus by endothelial cells.
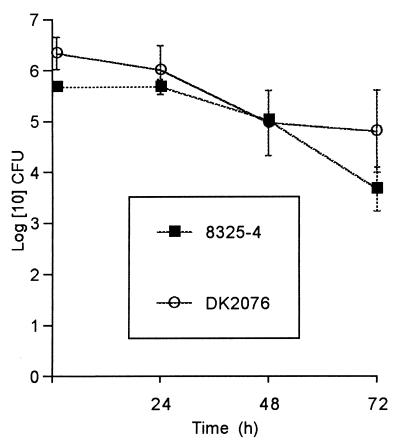
To study the consequences of S. aureus invasion of endothelial cells, we examined the ability of two S. aureus isolates, 8325-4 and DK2076, to invade human umbilical vein endothelial cells in tissue culture. The invasion assay is based upon the principle that intracellular staphylococci are protected from the lytic action of lysostaphin which is added to the medium to lyse extracellular or adherent staphylococci. Both S. aureus strains were internalized within 30 min to 1 h after infection in endothelial cells in a dose-dependent fashion up to a multiplicity of infection of 10 (data not shown). To determine survival of ingested S. aureus over time, endothelial cells harboring S. aureus were incubated in the continuous presence of lysostaphin until cells were harvested at selected time points over a 72-h period for enumeration of CFU. The numbers of internalized staphylococci decreased slightly, but a significant proportion of each strain remained viable (Fig. 1). Negative controls with uninfected cells were also performed to exclude contamination during the 72-h incubation period.
FIG. 1.
Change in mean number of S. aureus CFU in human umbilical vein endothelial cell cultures with time. Cultures were incubated with S. aureus at an infection ratio of 100:1 for 1 h, washed three times with medium, and then exposed to lysostaphin continuously until the endothelial cells were harvested for determination of intracellular CFU by colony plate counting at 1, 24, 48, and 72 h. Bars indicate the standard errors of the means for three different experiments.
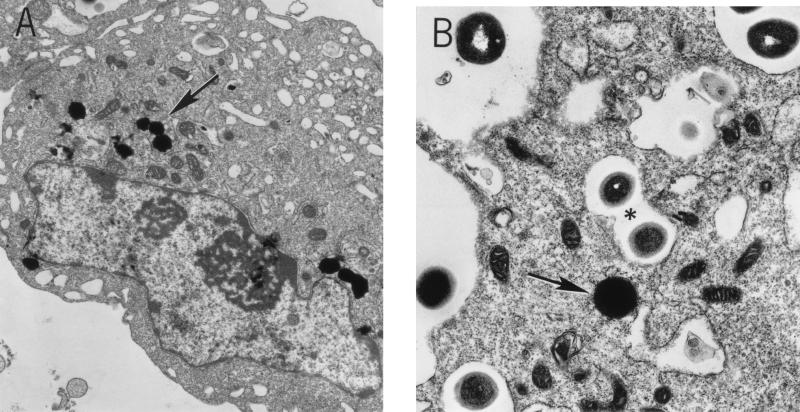
Transmission electron microscopy of endothelial cells after the invasion assay in which lysostaphin was used to remove extracellular bacteria confirmed that S. aureus organisms were internalized. Staphylococci were evident within vacuoles, as described by others (6, 12); however, it was observed that over a 4-h period an increased number appeared to be free within the cytoplasm (Fig. 2).
FIG. 2.
Transmission electron micrographs of an endothelial cell infected with S. aureus DK2076 for 4 h in the presence of lysostaphin. (A) S. aureus organisms (arrows) appear to be free within the cytoplasm. Magnification, ×14,000. (B) Higher magnification shows S. aureus free in the cytoplasm (arrows) as well as in vacuoles (asterisk). Magnification, ×17,125.
Infection by S. aureus induces apoptosis.
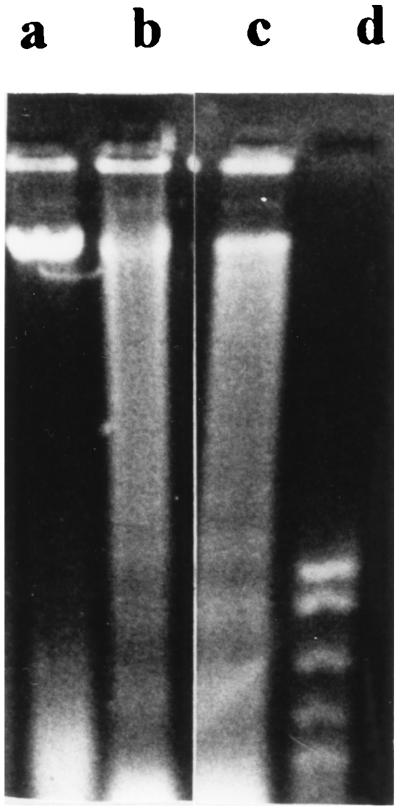
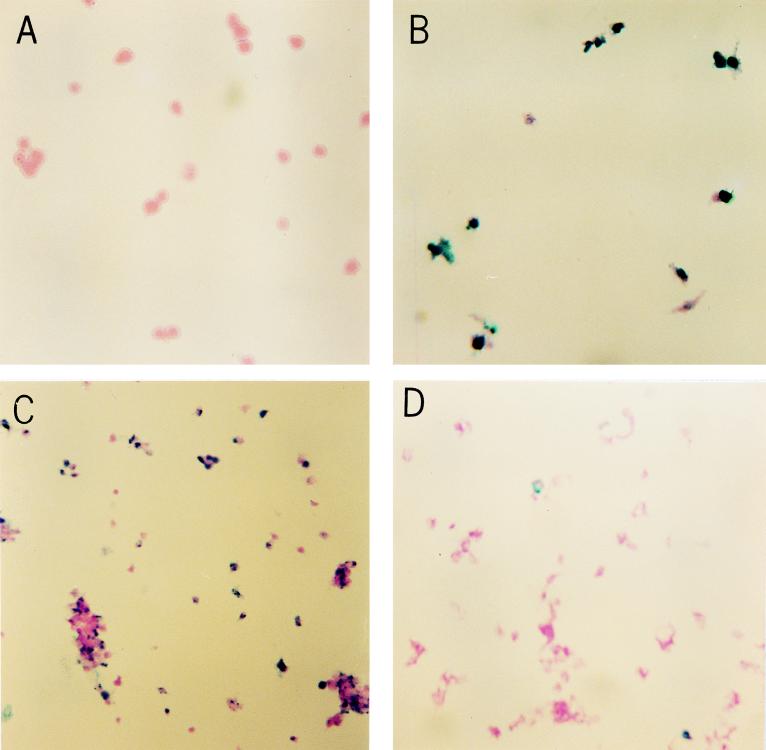
To determine whether endothelial cells undergo apoptosis after infection with S. aureus, internucleosomal DNA fragmentation, a hallmark of apoptotic cells, was assessed by examining for the characteristic 180-bp laddering pattern on agarose gels. DNA was isolated from endothelial cells which had been infected with S. aureus 8325-4 and DK2076 in M199 for 4 h and treated with lysostaphin for 1 h. DNA laddering occurred in S. aureus-infected endothelial cells but not in uninfected endothelial cells also treated with lysostaphin (Fig. 3). In addition, S. aureus-infected endothelial cells stained positively when assayed by the TUNEL method, which is based on the specific staining of 3′-hydroxyl ends of the fragmented DNA within apoptotic cells (Fig. 4). In addition, by the TUNEL technique, light microscopy of 8325- and DK2076-infected endothelial cells showed nuclear segmentation and chromatin condensation characteristic of apoptosis.
FIG. 3.
Agarose gel (1.5%) electrophoretic separation of DNA extracted from human umbilical vein endothelial cells infected with S. aureus for 4 h. Lane a, uninfected endothelial cells treated with lysostaphin (negative control); lane b, endothelial cells infected with S. aureus 8325-4; lane c, endothelial cells infected with S. aureus DK2076; lane d, 200-bp DNA ladder. Data are from a representative experiment repeated three times.
FIG. 4.
Evaluation of DNA fragmentation in human umbilical vein endothelial cells by the TUNEL method. Endothelial cells were grown in the absence or presence of S. aureus for 1 h prior to treatment with lysostaphin. The incorporation of biotinylated dUTP at the free 3′-hydroxyl ends of fragmented DNA is shown by the blue color. (A) Uninfected endothelial cells; (B) endothelial cells infected with S. aureus 8325-4; (C) endothelial cells infected with S. aureus DK2076; (D) endothelial cells preincubated with cytochalasin D for 30 min prior to infection with DK2076. Magnifications, ×2,000 (A and B) and ×1,000 (C and D).
Internalization of S. aureus by endothelial cells is required for apoptosis.
To establish whether S. aureus has to be internalized for apoptosis to be induced, we compared apoptosis induced by 8325-4 and DK2076 in the presence or absence of cytochalasin D, which inhibits actin polymerization and hence phagocytosis. Cytochalasin D at 1 μg/ml inhibited internalization in 99.9% of untreated controls by the invasion assay. This correlated with a reduced number of apoptotic cells in cytochalasin D-treated, S. aureus-infected endothelial cells (Fig. 4D).
Effect of UV-killed S. aureus on apoptosis.
To determine whether apoptosis occurs as a result of metabolically active S. aureus, the bacteria were killed by UV irradiation and endothelial cell cultures were incubated with the killed bacteria. Apoptosis was not detectable by the TUNEL assay in endothelial cells exposed to UV-killed bacteria (data not shown). Transmission electron microscopy confirmed that UV-killed bacteria retained the capacity to be internalized by endothelial cells.
DISCUSSION
Although primarily known as an extracellular pathogen, S. aureus has been shown to invade and survive within endothelial cells. We confirm this finding and show that intracellular staphylococci remain viable up to at least 72 h without evidence of intracellular multiplication. Furthermore, we show not only that S. aureus is engulfed within vacuoles but that it exists free within the cytoplasm, implying that the bacterium must be able to escape from the phagolysosome.
The fate of the host cell in the presence of viable internalized S. aureus was investigated. Previously, Vann and Proctor have shown that ingestion of S. aureus results in damage to endothelial cell monolayers by loss of membrane integrity (14). The effect was dependent on the time after exposure to S. aureus and on the inoculum size. In the present study, we show for the first time that endothelial cell toxicity mediated by S. aureus occurs through apoptosis as determined by both host cell DNA fragmentation and characteristic nuclear morphological changes. The use of the phagocytosis inhibitor cytochalasin D demonstrated that S. aureus adherence to endothelial cells does not induce apoptosis and that when internalization of adherent bacteria is inhibited, no apoptosis occurs. This indicates a requirement for intracellular residence of S. aureus for induction of apoptosis in endothelial cells and is consistent with the study results of Baran et al., who noted that apoptosis was observed predominantly in mononuclear cells that had ingested S. aureus (1). The propensity for intracellular pathogens to cause apoptosis in the host cell is well known. Other bacterial pathogens, such as Shigella flexneri, need to be within the cytoplasm to cause apoptosis in murine macrophages (19).
It is unlikely for several reasons that a staphylococcal product acting extracellularly is responsible for the apoptotic process observed in this study. The S. aureus inoculum was washed carefully to remove extracellular toxins and enzymes. In addition, the absence of apoptosis with the use of cytochalasin D during infection of endothelial cells also excludes the contribution of extracellular staphylococcal products that may be expressed during the infection or incubation period.
The mechanisms of induction of apoptosis in endothelial cells which have internalized S. aureus are likely mediated by one or more bacterially secreted staphylococcal products and not a surface component, since UV-killed S. aureus, although retaining the capacity to become internalized, was not capable of inducing apoptosis. Further studies employing isogenic S. aureus strains will be necessary for this determination.
Little is known about the nature of S. aureus gene expression or secreted gene products that would contribute to cytotoxicity during intracellular residence in the endothelial cell. From broth medium cultures, it is known that the majority of the putative virulence factors that are expressed by S. aureus are regulated by at least two distinct global regulatory loci, called agr and sar (4, 11). However, there may be altered expression of staphylococcal genes from S. aureus organisms in contact with cultured mammalian cells compared to that of genes from S. aureus cultivated in vitro within broth suspensions. Findings by Proctor and colleagues support altered S. aureus physiology during intracellular residence within endothelial cells. They cite evidence to support that the intracellular milieu of endothelial cells which have phagocytized S. aureus may induce at between 24 and 48 h the emergence of a subpopulation of metabolically hypoactive small-colony variants of S. aureus (15). Given that in the present study apoptosis was observed as early as 1 h after infection of endothelial cells, one may speculate that the small-colony variants of S. aureus as observed in other studies may arise in response to a nutritionally depleted and dying host cell.
The membrane-damaging S. aureus alpha-toxin has been implicated in triggering apoptosis in human T lymphocytes. Evidence for apoptosis was found in lymphocytes treated with low (nanomolar) concentrations of alpha-toxin and not in those treated with high toxin doses (8). On the basis of permeability studies, those authors associate the induction of apoptosis with the formation of very small alpha-toxin pores within the plasma membrane of the lymphocyte. A similar type of mechanism may be operative in endothelial cells that have ingested S. aureus. Bovine aortic endothelial cells that have ingested S. aureus show cytotoxicity as early as 3.5 h, and this appears to be attributable to intracellular production of S. aureus alpha-toxin (14). It is conceivable that very small quantities of alpha-toxin expressed by intracellular S. aureus may induce apoptosis of the endothelial cell. Certainly, further work needs to be done to investigate the role of alpha-toxin in the triggering of endothelial cell apoptosis during infection with S. aureus.
The ability of S. aureus to survive intracellularly in endothelial cells would benefit the bacterium, as it would be protected against host immune control mechanisms and most antimicrobials. Does, however, apoptosis of the endothelial cell result in any advantage for ingested S. aureus organisms? Since endothelial cells have no known significant bactericidal functions like that of macrophages, death of the endothelial cell would not compromise immune clearance of the organism. However, death of endothelial cells which have phagocytized S. aureus and detached from the endothelial surface would provide a route for dissemination of S. aureus to distant sites where foci of metastatic infection could be established.
ACKNOWLEDGMENTS
This work was funded by Minority Medical Faculty Development Program of the Robert Wood Johnson Foundation grant no. 030802 (to B.E.M.) and by grant-in-aid TN97G13 from the Southeast Affiliate of the American Heart Association (to B.E.M.).
REFERENCES
- 1.Baran J, Guzik K, Hryniewicz W, Ernst M, Flad H D, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;64:4242–4248. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beekhuizen H, van de Gevel J S, Olsson B, van Benten I J, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immun. 1997;158:774–782. [PubMed] [Google Scholar]
- 4.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of expoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 6.Hamill R J, Vann J M, Proctor R A. Phagocytosis of Staphylococcus aureus by cultured bovine endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986;54:833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ing M B, Baddour L M, Bayer A S. Bacteremia and infective endocarditis: pathogenesis, diagnosis, and complications. In: Crossley K B, Archer G L, editors. The staphylococci. New York, N.Y: Churchill Livingstone; 1997. pp. 331–353. [Google Scholar]
- 8.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernodle D S, Voladri R K R, Menzies B E, Hager C C, Edwards K M. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun. 1997;65:179–184. doi: 10.1128/iai.65.1.179-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowy F D, Fant J, Higgins L L, Ogawa S K, Hatcher V B. Staphylococcus aureus-human endothelial cell interactions. J Ultrastruct Mol Struct Res. 1988;98:137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- 11.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheagren J N. S. aureus: the persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. , 1437–1442. [DOI] [PubMed] [Google Scholar]
- 14.Vann J M, Proctor R A. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus α-hemolysin. Microb Pathog. 1988;4:443–453. doi: 10.1016/0882-4010(88)90029-0. [DOI] [PubMed] [Google Scholar]
- 15.Vesga O, Groeschel M C, Otten M F, Brar D W, Vann J M, Proctor R A. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J Infect Dis. 1996;173:739–742. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]
- 16.Watankunakorn C, Burkert T. Infective endocarditis at a large community teaching hospital, 1980–1990. A review of 210 episodes. Medicine (Baltimore) 1993;72:90–102. doi: 10.1097/00005792-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein L, Schlesinger J J. Pathoanatomic, pathophysiologic, and clinical correlation in endocarditis. N Engl J Med. 1974;291:832–837. doi: 10.1056/NEJM197410172911609. , 1122–1126. [DOI] [PubMed] [Google Scholar]
- 18.Yao L, Bengualid V, Lowy F D, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zychlinshy A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]