Abstract
Oxidative cleavage of poly(cis-1,4-isoprene) by rubber oxygenase RoxA purified from Xanthomonas sp. was investigated in the presence of different combinations of 16O2, 18O2, H216O, and H218O. 12-Oxo-4,8-dimethyl-trideca-4,8-diene-1-al (ODTD; m/z 236) was the main cleavage product in the absence of 18O-compounds. Incorporation of one 18O atom in ODTD was found if the cleavage reaction was performed in the presence of 18O2 and H216O. Incubation of poly(cis-1,4-isoprene) (with RoxA) or of isolated unlabeled ODTD (without RoxA) with H218O in the presence of 16O2 indicated that the carbonyl oxygen atoms of ODTD significantly exchanged with oxygen atoms derived from water. The isotope exchange was avoided by simultaneous enzymatic reduction of both carbonyl functions of ODTD to the corresponding dialcohol (12-hydroxy-4,8-dimethyl-trideca-4,8-diene-1-ol (HDTD; m/z 240) during RoxA-mediated in vitro cleavage of poly(cis-1,4-isoprene). In the presence of 18O2, H216O, and alcohol dehydrogenase/NADH, incorporation of two atoms of 18O into the reduced metabolite HDTD was found (m/z 244), revealing that RoxA cleaves rubber by a dioxygenase mechanism. Based on the labeling results and the presence of two hemes in RoxA, a model of the enzymatic cleavage mechanism of poly(cis-1,4-isoprene) is proposed.
Natural rubber (NR) is a biopolymer that can be synthesized by many plants and some fungi. The most widespread source of natural rubber is the rubber tree (Hevea brasiliensis) that has been commercially exploited for more than 100 years by cultivating and tapping rubber trees. Molecules of NR (H. brasiliensis) consist of three trans-isoprene and several hundred to thousands of cis-isoprene units. Despite the development of chemosynthetic rubbers, NR is still a necessary raw material for products such as tires, latex gloves, condoms, seals, and many other things.
Many reports on the isolation and characterization of rubber-degrading microorganisms have been published (4, 6, 8, 12, and references cited therein), but the basic molecular mechanism by which poly(cis-1,4-isoprene) is degraded is unknown. Recently, we initiated analysis of rubber degradation by Xanthomonas sp. This bacterium produces translucent clearing zones during growth on solid media containing opaque latex fluid as the carbon source (12). We purified an extracellular heme-containing protein from latex-grown culture fluid of Xanthomonas sp. and characterized its gene (rubber oxygenase, roxA) (5). Purified RoxA was able to cleave poly(cis-1,4-isoprene) in vitro to 12-oxo-4,8-dimethyl-trideca-4,8-diene-1-al (ODTD) as the major cleavage product and a series of related low-molecular-weight products differing from ODTD only by the number of isoprene units as minor metabolites (2). In this contribution, we continued our studies on RoxA and analyzed the oxidative cleavage reaction of RoxA by 18O-labeling experiments.
MATERIALS AND METHODS
Bacteria, media, and culture conditions.
Xanthomonas sp. (12) was grown at 30°C in nutrient broth or in a mineral salts medium with 0.2% purified rubber latex as recently described (2). For isolation of RoxA, 24 250-ml cultures were grown for 9 days at 30°C. Purification of RoxA was performed from the combined cell-free culture fluid.
Purification of rubber oxygenase (RoxA).
The enzyme was purified at 5°C using a fast-performance liquid chromatography system consisting of an LCC 500 controller, a 500 pump, a UV-1 monitor, a Rec-482 recorder, and a FRAC autosampler (Pharmacia, Uppsala, Sweden). Cell-free supernatant of latex-grown Xanthomonas sp. cells was concentrated by ultrafiltration (30-kDa cutoff) and passed through a Q-Sepharose column (HP HR16/10; Pharmacia) preequilibrated with basic buffer (20 mM 1,3-diaminopropane/HCl [pH = 10.5]) at a flow rate of 1 ml min−1. RoxA was eluted from the column with a linear gradient of 0 to 0.5 M NaCl in basic buffer at approximately 250 mM. Fractions showing the characteristic absorption spectrum of RoxA (2) were pooled and, after desalting and changing the buffer by diafiltration (30-kDa cutoff), applied to a hydroxyapatite column (CHT 5-I; Bio-Rad) preequilibrated with 5 mM phosphate buffer (pH = 6.8) at a flow rate of 2.0 ml min−1. During elution with a linear phosphate buffer gradient up to 500 mM fractions with the characteristic spectrum of RoxA were eluted at approximately 20 mM. These fractions were pooled and passed through a Superdex 200 column (Superdex 200 Prep-grade; Pharmacia) and eluted with phosphate buffer (20 mM, pH = 7.0). Purified RoxA was stored at −20°C.
Assay of RoxA.
The following conditions were used for product high-performance liquid chromatography (HPLC) analysis of RoxA-catalyzed rubber degradation. The reaction mixture contained 100 μl of purified RoxA (10 to 15 μg/ml), rubber latex (4 μl of a 35% [wt/vol] emulsion), and bis-Tris buffer (200 mM, pH 7.0) in a total volume of 1 ml. The reaction was carried out at 40°C for 3 or 4 h in a test tube closed with Parafilm. The mixture was extracted with ethyl acetate or diethyl ether, dried, dissolved in 100 to 200 μl of methanol, and then subjected to HPLC analysis and/or to carbonyl content determination. Mixtures without RoxA and with heat-inactivated RoxA (10 min, 95°C) served as negative controls. One unit of RoxA activity corresponds to 1 μmol of generated carbonyl function per min.
Determination of carbonyl content.
The carbonyl content of rubber degradation products was determined after formation of 2,4-dinitrophenylhydrazones (in hexane) as described in reference 7 using a molar absorbance coefficient of 21,500 M−1 cm−1 at 338 nm.
Labeling experiments with 18O2 and H218O.
Isotope-labeling experiments were performed using 18O2 (97%; Sigma-Aldrich, Schnelldorf, Germany) and H218O (95%; Euriso-top, Saarbrücken, Germany). For the 18O2-labeling experiment, rubber latex (4 μl of a 35% [wt/vol] emulsion) in bis-Tris buffer (896 μl, 200 mM, pH 7.0) and purified RoxA (100 μl, 10 to 15 μg/ml) were transferred into separate 10-ml glass tubes and sealed with rubber stoppers. Oxygen was removed by repeated evacuation and flushing with nitrogen gas. The two anaerobic samples were combined under anaerobic conditions, and 18O2 was added to a final concentration of about 20% (vol/vol). The reaction mixture was incubated at 40°C for 3 h, and the degradation products were extracted and analyzed as described above. For H218O-labeling experiments 100 μl of purified RoxA (10 to 15 μg/ml in H216O-buffer), rubber latex (4 μl of a 35% [wt/vol] emulsion in H216O), and 896 μl of H218O-bis-Tris buffer (200 mM, pH 7.0) were combined (the final volume ratio of H218O to H216O was ∼85 to 15), incubated for 3 h at 40°C, extracted with ethyl acetate, and analyzed as described above. To assay the isotope exchange of (16O)-ODTD with water (H218O), HPLC-purified ODTD was dried with a rotary evaporator and dissolved in water (H218O/H216O ratio = 85:15). After incubation for 3 h at 40°C, the sample was extracted and analyzed as described above.
Enzymatic reduction of ODTD to 12-hydroxy-4,8-dimethyl-trideca-4,8-diene-1-ol (HDTD).
The reaction mixture for enzymatic reduction of ODTD contained 450 μl of purified RoxA (10 to 15 μg/ml), rubber latex (18 μl of a 35% [wt/vol] emulsion), glutathione (12 mg; reduced), NADH (50 mg), horse liver alcohol dehydrogenase (HL-ADH; 80 μl, 10 mg/ml, 2.8 U/mg), and bis-Tris buffer (200 mM, pH 7.0) in a total volume of 4.5 ml. The reaction was carried out at 40°C for 3 h in a test tube closed with Parafilm, extracted, and analyzed as described above. For the combined 18O2-labeling and reduction reaction, HL-ADH, RoxA, and the other components were transferred to two separate 10-ml glass tubes and sealed with rubber stoppers. Oxygen was removed by repeated evacuation and flushing with nitrogen gas. The two anaerobic samples were combined in the absence of oxygen, and 18O2 gas was added to about 20% (vol/vol).
Analysis of reaction products by HPLC and HPLC-MS.
Degradation products were separated by HPLC analysis and detected at 210 nm (Chromeleon Chromatography Data Systems 4.38 equipped with Dionex UV-Vis detector UVD 170S/340S, Dionex pump P 580, and Dionex autosampler Gina 50; Dionex, Isstein, Germany). Chromatographic conditions were as follows: column, Grom-Sil 100 RP-8 (125 by 4 mm; particle size, 5 μm; Grom, Herrenberg, Germany); column temperature, 25°C; flow rate, 0.7 ml/min; injection volume, 20 μl; eluent A, anhydrous methanol-H2O (50/50, vol/vol); eluent B, anhydrous methanol; gradient, % B (t [min]) 0(0) −0(5) − 100(20). Liquid chromatography-mass spectrometry (LC-MS) was run on an HP1100 HPLC system (Hewlett Packard, Waldbronn, Germany) comprising an HP1100 autosampler, an HP1100 gradient pump, an HP1100 column thermoregulator, and an HP1100 diode array detector module coupled to a Micromass VG Platform II quadrupole mass spectrometer (Micromass, Manchester, United Kingdom) equipped with an electrospray ionization (ESI) interface. ESI+-MS parameters were as follows: source temperature, 120°C; capillary, 3.5 V; HV lens, 0.5 kV; cone, 30 V; full scan range, m/z 120 to 450. Samples were resolved with the same column and mobile phase as described above. The eluent was split 10:1 before being introduced into the ion source.
RESULTS
Xanthomonas sp. was grown on latex for 9 days at 30°C, and extracellular rubber oxygenase RoxA was purified using a purification protocol that had been optimized compared to our previous procedure (2). Five milligrams of purified RoxA was obtained from ∼6 liters of culture. RoxA after hydroxyapatite chromatography was homogeneous as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (not shown). In some experiments it was necessary to include an additional purification step on Superdex 200. A solution of concentrated RoxA had a strong reddish color, and the characteristic absorption peaks for heme were present as described recently (2). To determine the specific activity of purified RoxA, a quantitative assay of poly(cis-1,4-isoprene) cleavage products was performed by hydrazone derivatization. A linear dependence of the resulting hydrazone concentration on the amount of RoxA was found in the range of 0.5 to 2.5 μg of purified RoxA/ml. At higher concentrations of RoxA, a saturation kinetic was observed (not shown). A specific activity of 300 mU/mg was determined for purified RoxA by this method.
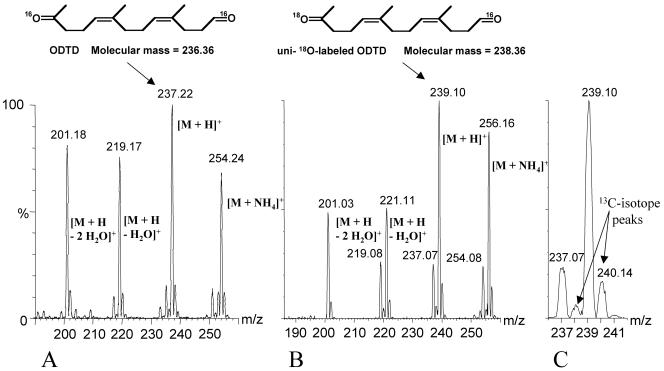
A standard experiment was set up that was used for all experiments described below. About 1 μg of purified RoxA was inoculated with latex in buffer for 3 h at 40°C (for details, see Materials and Methods). The resulting low-molecular-weight products were extracted with ethyl acetate and separated by HPLC using a C8 reverse-phase column. One major peak at 14.8 min was obtained in all experiments and subjected to ESI-MS. Using 16O2 and H216O, ODTD (m/z 236) was obtained as the main product. In ESI+ mode, unmodified ODTD shows characteristic mass peaks for the quasimolecular ion [M + H]+ m/z 237 and the corresponding ammonium adduct [M + NH4]+ m/z 254 and signals for the loss of one and two water molecules ([M + H − H216O]+ m/z 219, [M + H − 2 H216O]+ m/z 201), respectively (Fig. 1A).
FIG. 1.
HPLC-ESI+-MS analysis of latex degradation products formed by reaction with RoxA in 16O2 (A) and 18O2 (B, C) atmospheres. (A) RoxA was incubated with latex for 3 h at 40°C, pH 7.0, in a 16O2 atmosphere and in H216O buffer. Degradation products were extracted with ethyl acetate, dried, evaporated, dissolved in methanol, and loaded onto a C8 reverse-phase HPLC column. The eluate was monitored by ESI+-MS, and the figure shows the average mass spectrum summed across the 14.8-min peak representing ODTD (eight scans). (B) The same experiment as described for panel A was performed, except that the reaction was carried out in an 18O2 atmosphere and in H216O. (C) Enlargement of panel B around m/z 239. Arrows in the enlargement indicate the respective 13C isotope ions.
Cleavage of poly(cis-1,4-isoprene) in the presence of 18O2 and H216O.
Latex was cleaved by RoxA in the presence of 18O2 and H216O for 3 h at 40°C, and ODTD was isolated by extraction with ethyl acetate and HPLC. When ODTD of the 18O2 experiment was analyzed by ESI+-MS (Fig. 1B), the same mass peaks as in the 16O2/H216O experiments at m/z values of 237, 254, 219, and 201 were found. However, additional signals with higher intensity appeared at m/z 239, 256, and 221, respectively, i.e., 2 Da higher than the previous signals for the ODTD ions [M + H]+, [M + NH4]+, and [M + H − H2O]+. Apparently, these mass peaks corresponded to ODTD or its respective variant ions in which one 16O atom had been replaced by an 18O atom. Since the [M + H − 2 H2O]+ ions of ODTD did not contain any oxygen anymore, only the peak at m/z of 201 appeared. No evidence for the incorporation of two oxygen atoms (m/z values of 241) was obtained even if the exposure time of latex to RoxA in the presence of 18O2 was varied from 1 to 5 h (expansion in Fig. 1C). The experiments described above suggest that only one atom of oxygen is incorporated from 18O2 into the metabolite ODTD and that the other oxygen atom may be derived from water.
Cleavage of poly(cis-1,4-isoprene) in the presence of 16O2 and H218O.
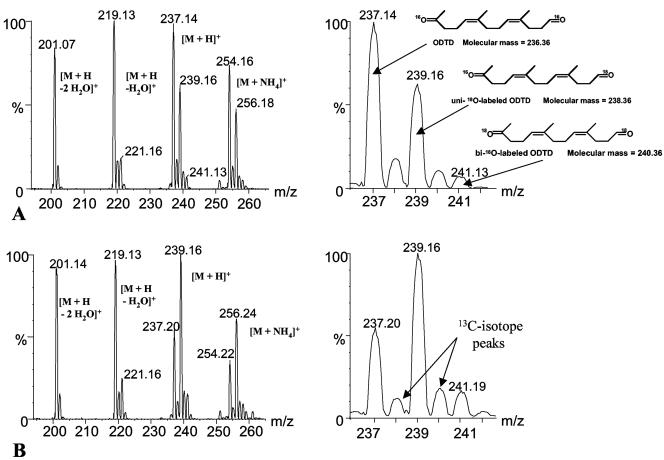
The same experiment as described above was performed in normal air (16O2) but in the presence of H218O. Since RoxA had been purified in H216O-containing buffer, the final ratio of H218O to H216O in the complete assay mixture was about 85 to 15. If water is added during the cleavage reaction, one would expect ODTD-specific signals at m/z 239 corresponding to H218O added and at m/z 237 corresponding to the addition of H216O. No signal should occur at m/z 241. When the m/z values for the isolated 14.8-min HPLC peak (ODTD) were determined, two major peaks at m/z 237 and 239 but also a clearly detectable minor peak at m/z 241 were found. Additionally, the respective variant ions (loss of one or two water molecules, ammonium adduct ion) were obtained, all of which corresponded to unlabeled, uni-18O-labeled and bi-18O-labeled ODTD ([M + H]+), respectively (Fig. 2A).
FIG. 2.
HPLC-ESI+-MS analysis of latex degradation products formed by reaction with RoxA in H218O-16O2 (A) and HPLC-ESI+-MS analysis of purified ODTD after incubation in H218O-16O2 (B). Degradation products were prepared and separated by HPLC as described in the legend to Fig. 1. The average mass spectrum of the 14.8-min peak (ODTD) is shown (A). Unlabeled ODTD was purified by HPLC and incubated with H218O-H216O (85:15, vol/vol) for 3 h at 40°C and then prepared and separated by HPLC as described in the legend to Fig. 1. The average mass spectrum of the 14.8-min peak (ODTD) is shown (B). The right parts of the figures are enlargements of the relevant m/z regions. Arrows in the enlargement of panel B indicate the respective 13C isotope ions.
Assay of isotope (16O) exchange of isolated ODTD with water (H218O).
The unexpected detection of ODTD ions at m/z 241 in addition to ions at m/z 237 and 239 after cleavage of poly(cis-1,4-isoprene) in the presence of 16O2/H218O can be explained if one assumes that carbonyl atoms of ODTD are able to exchange oxygen with oxygen from solvent water. To find definite proof for this exchange, unlabeled 16O-ODTD was isolated by HPLC after RoxA-mediated cleavage of poly(cis-1,4-isoprene) and was incubated for 3 h in the absence of RoxA but in the presence of 18O-labeled water (H218O/H216O ratio = 85:15) and analyzed by ESI-MS. As shown in Fig. 2B, the typical mass peaks of unlabeled and uni-18O-labeled ODTD and traces of bi-18O-labeled ODTD ([M + H]+, [M + NH4]+, [M + H − H2O]+, and [M + H − 2 H2O]+ ions) appeared in the ESI+ mass spectrum. Apparently, one oxygen atom of ODTD rapidly exchanged with oxygen of water (major peak at m/z 239) while the other oxygen atom slowly exchanged with water (minor peak at m/z 241). These results indicate that the interpretation of the 18O2/H216O experiment (Fig. 1B and C), which suggested that only one oxygen atom of 18O2 was incorporated into ODTD, apparently is not correct because of a rapid exchange of at least one 18O atom with water, as shown in the control experiment.
Cleavage of poly(cis-1,4-isoprene) in the presence of 18O2 and H216O and fixation of isotope label by in situ reduction of ODTD to HDTD.
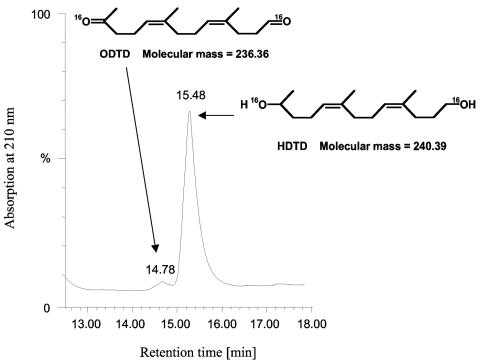
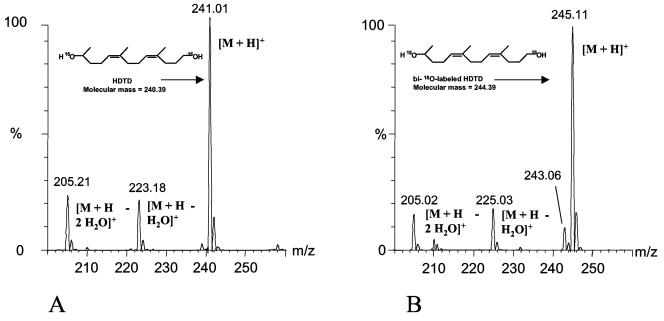
To overcome the putative artifacts by isotope exchange, it was necessary to convert the carbonyl oxygen atoms of ODTD immediately after cleavage of poly(cis-1,4-isoprene) into a more stable form that does not undergo isotope exchange. Alcohol functions are much more stable with respect to isotope exchange than carbonyl functions (3, 10). We therefore attempted to convert ODTD to the corresponding dialcohol (HDTD). This was achieved by the addition of NADH and HL-ADH directly to the cleavage reaction of poly(cis-1,4-isoprene) by RoxA (see Materials and Methods). When the products of RoxA-mediated cleavage of poly(cis-1,4-isoprene) in the presence of 16O2, H216O, and NADH/HL-ADH were separated by HPLC, the typical ODTD peak at 14.8 min was strongly reduced and one additional peak at 15.5 min appeared (Fig. 3). These peaks did not appear in the absence of RoxA. ESI+-MS analysis of the 14.8-min peak revealed m/z values undistinguishable from ODTD (m/z 237 plus the respective fragment and adduct ions; data not shown). ESI+-MS analysis of the 15.5-min peak showed m/z values of 241, 223, and 205 that correspond well to the calculated m/z values of protonated HDTD [M + H]+ and the corresponding [M + H − H216O]+ and [M + H − 2 H216O]+ ions (Fig. 4A). The ammonium adduct ions [M + NH4]+ were present only at very low intensities. We concluded that the majority of RoxA-catalyzed cleavage product ODTD is converted to HDTD in the presence of HL-ADH and NADH.
FIG. 3.
HPLC analysis of HL-ADH/NADH-reduced latex degradation products formed by reaction with RoxA in an 16O2 atmosphere. Degradation products were prepared in the presence of HL-ADH and NADH as described in Materials and Methods and separated by HPLC as described in the legend to Fig. 1. The structural formulas corresponding to the 14.8- and 15.5-min peaks as revealed by ESI+-MS (see Fig. 4) are indicated.
FIG. 4.
HPLC-ESI+-MS analysis of HL-ADH/NADH-reduced latex degradation products formed by reaction with RoxA in an 16O2 or an 18O2 atmosphere. Degradation products were prepared and separated by HPLC as described in the legend to Fig. 1. (A) Average mass spectrum of the 15.5-min peak of HDTD generated in a 16O2 atmosphere-H216O. (B) Average mass spectrum of the 15.5-min peak HDTD generated in an 18O2 atmosphere-H216O.
The RoxA-mediated cleavage reaction of poly(cis-1,4-isoprene) was repeated in the presence of 18O2/NADH/HL-ADH/H216O, and the isolated 15.5-min HPLC peak corresponding to the double-reduced metabolite (HDTD) was subjected to ESI+-MS analysis. Three major mass peaks at m/z 245, 225, and 205 and a minor peak at m/z 243 were obtained (Fig. 4B). These peaks perfectly correspond to the 18O-bilabeled HDTD ([M + H]+) and the corresponding [M + H − H218O]+ and [M + H − 2 H218O]+ ions. The minor peak at m/z 243 apparently corresponds to traces of uni-18O-labeled protonated HDTD. In conclusion, both oxygen atoms of molecular oxygen are incorporated into the product of RoxA-catalyzed cleavage of poly(cis-1,4-isoprene).
DISCUSSION
The recently described rubber-oxidizing enzyme RoxA cleaves poly(cis-1,4-isoprene) to ODTD as the principal degradation product by an unknown oxidative reaction mechanism (2, 5). Since the double bonds of poly(cis-1,4-isoprene) are cleaved by incorporation of oxygen, a monooxygenase- or dioxygenase-based reaction mechanism is possible. In chickens, cleavage of β-carotene, yielding two aldehydes, is catalyzed by a monooxygenase (13) whereas the same compound is cleaved by a dioxygenase in Arabidopsis sp. (1). Tsuchii and Takeda (12) suggested a dioxygenase cleavage mechanism of poly(cis-1,4-isoprene) based on preliminary experiments using cell-free culture fluid of Xanthomonas sp. Linos and Steinbüchel (9) assumed a common oxidative cleavage mechanism for all types of rubber-degrading organisms and proposed a metal-dependent dioxygenase mechanism; the basis of this assumption was the experiments of Tsuchii and coworkers. Recently, a gene coding for a “latex clearing protein, lcp,” was found to be involved in rubber utilization by Streptomyces sp. (11).
A monooxygenase-based mechanism of poly(cis-1,4-isoprene) cleavage appears not very likely since RoxA is an extracellular enzyme and functions in vitro without any cofactors such as NADH or related compounds. However, experimental data that confirmed the presence of a dioxygenase mechanism or excluded a monooxygenase mechanism did not exist. Due to possible exchange of carbonyl oxygen atoms in the presence of water, care has to be taken to avoid misinterpretation of labeling data (3, 10). Indeed, experiments in which we show the incorporation of 18O from H218O into isolated ODTD (Fig. 2B) confirm that a rapid exchange of at least one oxygen atom (probably the aldehyde oxygen atom of ODTD) with solvent water occurs. Therefore, it was necessary to develop a combined enzyme assay for RoxA-mediated cleavage of poly(cis-1,4-isoprene) in the presence of HL-ADH to reduce ODTD to the corresponding dialcohol (HDTD [m/z 240 Da]) immediately after release of ODTD from the polymer. The principal suitability of the combined assay was confirmed by identification of protonated HDTD in the 15.5-min HPLC peak by ESI+-MS (m/z 241). When the combined experiment was performed in the presence of 18O2 and H216O, a mass shift of m/z by 4 (m/z 245) for the protonated HDTD molecules confirmed that both oxygen atoms of 18O2 were incorporated into the cleavage product (Fig. 4B). The results are in full agreement with a dioxygenase mechanism of poly(cis-1,4-isoprene) cleavage. Based on the presence of covalently bound heme in RoxA (2) and the findings of the present study, we propose a cleavage mechanism of poly(cis-1,4-isoprene) as shown in Fig. 5.
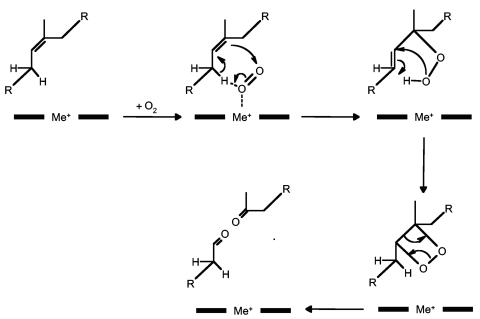
FIG. 5.
Proposed dioxygenase reaction mechanism for cleavage of natural rubber by rubber oxygenase (RoxA). Black bars and Me+ (metal) indicate the heme reaction center of RoxA.
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft to D.J.
We thank E. Chua, A. Ikram, and H. Y. Yeang (RRIM) for providing purified Hevea latex.
REFERENCES
- 1.Booker, J., M. Auldridge, S. Wills, D. McCarty, H. Klee, and O. Leyser. 2004. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 14:1232-1238. [DOI] [PubMed] [Google Scholar]
- 2.Braaz, R., P. Fischer, and D. Jendrossek. 2004. A novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl. Environ. Microbiol. 70:7388-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devery, J., and B. V. Milborrow. 1994. Beta-carotene-15,15′-dioxygenase (EC 1.13.11.21) isolation reaction mechanism and an improved assay procedure. Br. J. Nutr. 72:397-414. [DOI] [PubMed] [Google Scholar]
- 4.Heisey, R. M., and S. Papadatos. 1995. Isolation of microorganisms able to metabolize purified rubber. Appl. Environ. Microbiol. 61:3092-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jendrossek, D., and S. Reinhardt. 2003. Sequence analysis of a gene product synthesized by Xanthomonas sp. during growth on natural rubber latex. FEMS Microbiol. Lett. 224:61-65. [DOI] [PubMed] [Google Scholar]
- 6.Jendrossek, D., G. Tomasi, and R. M. Kroppenstedt. 1997. Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiol. Lett. 150:179-188. [DOI] [PubMed] [Google Scholar]
- 7.Katz, I., and M. Keeney. 1966. Quantitative micro determination and isolation of plasmalogen aldehydes as 2,4-dinitrohydrazones. J. Lipid Res. 7:170-174. [PubMed] [Google Scholar]
- 8.Linos, A., M. M. Berekaa, A. Steinbüchel, K. K. Kim, C. Sproer, and R. M. Kroppenstedt. 2002. Gordonia westfalica sp. nov., a novel rubber-degrading actinomycete. Int. J. Syst. Evol. Microbiol. 52:1133-1139. [DOI] [PubMed] [Google Scholar]
- 9.Linos, A., and A. Steinbüchel. 2001. Biodegradation of natural and synthetic rubber, p. 321-359. In T. Koyama and A. Steinbüchel (ed.), Biopolymers. Wiley-VCH, Weinheim, Germany.
- 10.Retey, J., A. Umani-Ronchi, J. Sebl, and D. Arigoni. 1966. On the mechanism of the propanediol dehydrase reaction. Experientia 22:502-503. [DOI] [PubMed] [Google Scholar]
- 11.Rose, K., K. B. Tenberge, and A. Steinbüchel. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180-188. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchii, A., and K. Takeda. 1990. Rubber-degrading enzyme from a bacterial culture. Appl. Environ. Microbiol. 56:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woggon, W. D. 2002. Oxidative cleavage of carotenoids catalyzed by enzyme models and beta-carotene 15,15′-monooxygenase. Pure Appl. Chem. 74:1397-1408. [Google Scholar]