Abstract
BACKGROUND:
Neonates admitted to the neonatal intensive care unit (NICU) are at risk for healthcare-associated infections, including central line-associated bloodstream infections. We aimed to characterize the epidemiology of bloodstream infections among neonates with central venous catheters admitted to three Indian NICUs.
METHODS:
We conducted a prospective cohort study in three tertiary NICUs, from May 1, 2017 until July 31, 2019. All neonates admitted to the NICU were enrolled and followed until discharge, transfer, or death. Cases were defined as positive blood cultures in neonates with a central venous catheter in place for greater than 2 days or within 2 days of catheter removal.
RESULTS:
During the study period, 140 bloodstream infections were identified in 131 neonates with a central venous catheter. The bloodstream infection rate was 11.9 per 1000 central line-days. Gram-negative organisms predominated, with 38.6% of cases caused by Klebsiella spp. and 14.9% by Acinetobacter spp. Antimicrobial resistance was prevalent among Gram-negative isolates, with 86.9% resistant to third- or fourth-generation cephalosporins, 63.1% to aminoglycosides, 61.9% to fluoroquinolones, and 42.0% to carbapenems. Mortality and length of stay were greater in neonates with bloodstream infection than in neonates without bloodstream infection (unadjusted analysis, p < 0.001).
CONCLUSIONS:
We report a high bloodstream infection rate among neonates with central venous catheters admitted to three tertiary care NICUs in India. Action to improve infection prevention and control practices in the NICU is needed to reduce the morbidity and mortality associated with BSI in this high-risk population.
Keywords: Antimicrobial resistance, bloodstream infection, central line-associated bloodstream infection, healthcare-associated infection
1. Introduction
Hospitalized neonates are at increased risk of healthcare-associated infection (HAI) due to pre-maturity, an immature immune system, poor skin integrity, and prolonged length of stay [1, 2]. Central line-associated bloodstream infections (CLABSI) account for significant morbidity and mortality among hospitalized neonates [3]. Central venous catheter (CVC) use for medication administration and total parental nutrition (TPN) is often medically necessary in preterm and critically ill neonates but may be complicated by CLABSI [1, 3]. BSI in neonates are not only associated with adverse short-term outcomes such as mortality, prolonged length of stay, and increased healthcare costs, but also with negative long-term consequences such as neurodevelopmental delay [4, 5]. Among United States neonatal intensive care units (NICU) reporting to the National Healthcare Safety Network (NHSN) in 2020, the rate of CLABSI was 0.81 per 1000 central line-days [6]. HAI surveillance in NICUs is limited or non-existent in many resource-limited settings, and data on CLABSI in this population are scarce. A systematic review and meta-analysis of HAI in low- and middle-income countries (LMIC) reported infection rates more than two-fold higher than in high income countries [7]. Limited data are available on HAI risk in Indian NICUs, though a study of Indian healthcare facilities participating in International Nosocomial Infection Control Consortium surveillance reported a pooled CLABSI rate of 36.21 per 1000 line-days for the five participant NICUs [8, 9].
Higher HAI rates in LMIC settings, including in India, are complicated by the high prevalence of antimicrobial resistance (AMR) among pathogens most commonly associated with these infections [9-12]. In particular, multidrug resistant Gram-negative infections pose a challenge in neonates, with limited treatment options and high burden of associated morbidity and mortality [8, 13-15].
We performed a secondary data analysis of a subset of neonates with CVCs enrolled in a prospective cohort study conducted to describe the epidemiology of BSI in three Indian tertiary NICUs [16]. The primary objective was to describe the incidence of BSI in patients with CVCs. The secondary objectives were to describe the pathogen distribution and associated AMR.
2. Methods
2.1. Study design
The parent multi-center prospective cohort study enrolled all neonates admitted to three tertiary care NICUs at Byramjee Jeejeebhoy Government Medical College, Dr. D. Y. Patil Medical College, and King Edward Memorial Hospital in Pune, India, from May 1, 2017, until July 31, 2019 [16]. Byramjee Jeejeebhoy Government Medical College is affiliated with Sassoon Hospital, which has a 60-bed NICU. Dr. D. Y. Patil Medical College is private medical college and has a 26-bed NICU. King Edward Memorial Hospital is operated by a charitable trust and has a 46-bed NICU. All NICUs have an open ward structure and care for inborn and outborn neonates, including extremely preterm infants. All sites have the capacity to provide mechanical ventilation, administer intravenous fluids and medications, and insert and maintain CVCs.
Study participants were followed from NICU admission until hospital discharge, transfer, or death. This secondary data analysis included all neonates in whom a CVC was placed during NICU admission and were admitted for greater than one calendar day. During the parent prospective cohort study, clinical care was provided at the discretion of the clinical teams, including decisions to obtain blood cultures and to initiate antibiotic therapy. All blood cultures obtained were processed at site microbiology laboratories, accredited by the Indian National Accreditation Board for Testing & Calibration Laboratories. Laboratory methods included use of VITEK for organism identification and antimicrobial susceptibility testing (AST), as well as manual methods by agar plate and biochemical workup, per clinical routine at each site.
2.2. Study definitions
BSI with CVC was defined as a single positive blood culture with a known neonatal pathogen in neonates with a CVC in place for greater than 2 days or within 2 days of catheter removal. Repeat cultures positive for the same organism within 7 days were considered as the same BSI episode. Information on CVC presence was collected through daily observations, and line days were those for which CVC was documented as present. Daily observations were not systematically collected on weekends and holidays, resulting in information gaps in CVC presence for 25.5% of patient days (n = 8529/33398 days). The vast majority (86.0%) of information gaps lasted for one day; an additional 10.5% lasted two days. Unobserved days were only considered as line days if CVC presence was documented both immediately before and immediately after the information gap.
Other variables of interest included maternal age, sex, gestational age, birth weight, multiple gestation, birth location, delivery mode, and positive pressure ventilation (PPV), mechanical ventilation, and antibiotic administration on admission. Gestational age was categorized in two ways: 1) extremely preterm (<28 weeks), very preterm (28–31 weeks), moderate to late preterm (32–36 weeks) and term (>37 weeks); and 2) dichotomized as preterm (≤36 weeks) and term (≥37 weeks). Birth weight also was categorized in two ways: 1) extremely low birth weight (ELBW, <1000 g), very low birth weight (VLBW, 1000 g–1499 g), low birth weight (LBW, 1500 g – 2499 g) and non-low birth weight (non-LBW, ≥2500 g); and 2) dichotomized as LBW (<2499 g) and non-LBW (≥2500 g). For neonates with missing birth weight, admission weight was used for analysis if obtained within the first seven days of life.
Among Gram-negative BSI cases, difficult-to-treat resistance (DTR) was defined as isolates identified intermediate or resistant on AST to all reported agents among the following: (1) carbapenems, (2) β-lactams, and (3) fluoroquinolones, as well as (4) piperacillin-tazobactam and ampicillin-sulbactam (Acinetobacter spp. only), and (5) aztreonam (organisms other than Acinetobacter spp.), consistent with the definition proposed by Kadri et al. [17]
2.3. Statistical analysis
Summary statistics for characteristics of neonates with CVC were generated. BSI incidence was calculated as the number of BSI cases per 1000 line-days overall and by sex, gestational age, birth weight, and delivery mode. Cumulative incidence by birth weight category was calculated using the Kaplan-Meier method. Among neonates with multiple episodes of BSI, time to the first BSI episode was used to calculate BSI incidence. Incidence rate ratios (IRR) and 95% confidence intervals (CI) were calculated using Poisson regression with clustered standard errors to account for clustering by tertiary NICU site. Adjusted models to explore the relationship between BSI and length of stay and BSI and mortality were performed using Poisson regression with cluster robust standard errors; neonates with missing information were excluded from adjusted analysis. All analyses were conducted using Stata version 16.1 (Stata Corp, College Station, TX).
2.4. Ethics committee approval
The study was approved by the ethics committees of all sites, the Indian Council of Medical Research, and the Institutional Review Board of Johns Hopkins Medicine.
3. Results
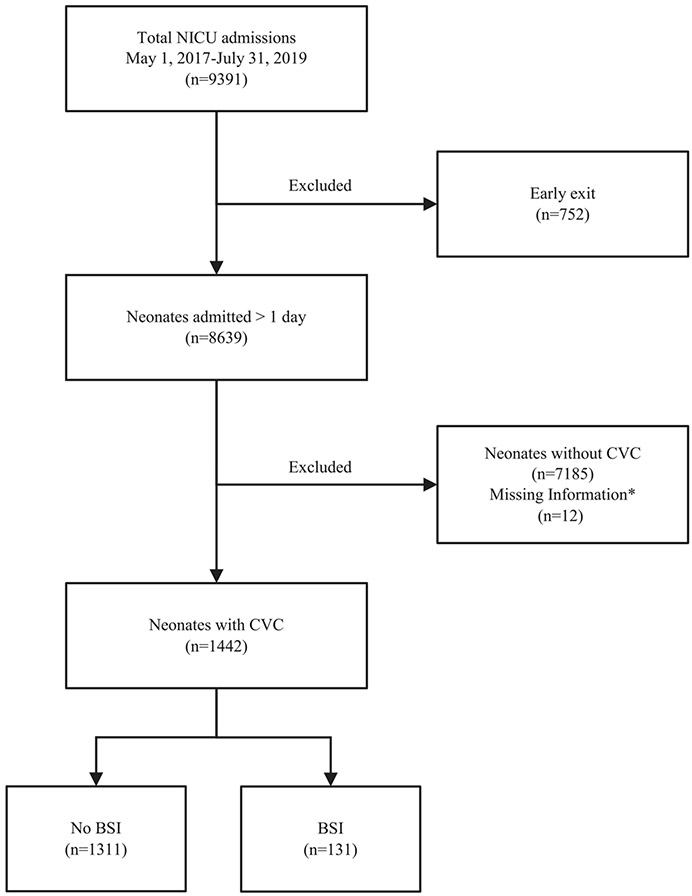
Over the course of the study period, 9391 neonates were admitted to the NICU, of whom 8639 were admitted >1 calendar day. Among these 8639 neonates, 1442 (16.7%) had a CVC placed during their admission (Fig. 1). The median gestational age was 32 weeks (interquartile range (IQR) 29–36), with a median birth weight of 1358 grams (1030–2200) (Table 1). There were 1180 neonates (82.2%) who were LBW (<2500 grams), 291 of whom (20.3%) were ELBW (<1000 grams). Most neonates (88.2%) were inborn, and 52.8% were delivered via Cesarean. On admission, there were high rates of mechanical ventilation (37.3%), pressors (35.0%), and antibiotic administration (75.6%). The median duration of CVC presence was 6 days (IQR 4–9). The median length of stay was 16 days (IQR 8–32), and 363 (25.2%) of neonates died prior to NICU discharge or transfer.
Fig. 1.
Study flow diagram. *Neonates with no daily observation data (n = 12).
Table 1.
Demographic and clinical characteristics of neonates with central venous catheters, with and without bloodstream infection, in three tertiary care neonatal intensive care units in Pune, India, May 1, 2017 - July 31, 2019
| Neonates without BSI n = 1311 |
Neonates with BSI n = 131 |
Total n = 1442 |
|
|---|---|---|---|
| Pregnancy and birth characteristics | |||
| Maternal age in years, median (IQR) | 25 (22–30) | 26 (23–29) | 25 (22–30) |
| Male sex, n (%) | 788 (60.1) | 72 (55.0) | 860 (59.6) |
| Multiple gestation, n (%) | 235 (17.9) | 26 (19.8) | 261 (18.1) |
| Gestational age in weeks, median (IQR) | 32 (29–36) | 31 (28–34) | 32 (29–36) |
| Preterm (<37 weeks), n (%) | 970 (78.3) | 107 (87.0) | 1077 (79.1) |
| Extremely preterm (<28 weeks), n (%) | 166 (13.4) | 25 (20.3) | 191 (14.0) |
| Very preterm (28–31 weeks), n (%) | 428 (34.5) | 44 (35.8) | 472 (34.7) |
| Moderate to late preterm (32–36 weeks), n (%) | 376 (30.3) | 38 (30.9) | 414 (30.4) |
| Term (≥37 weeks) | 269 (21.7) | 16 (13.0) | 285 (20.9) |
| Birth weight in grams, median (IQR) | 1358 (1030–2200) | 1250 (1000–1726) | 1340 (1020–2150) |
| ELBW (<1000 g) | 259 (19.9) | 32 (24.4) | 291 (20.3) |
| VLBW (1000–1499 g) | 467 (35.8) | 52 (39.7) | 519 (36.2) |
| LBW (1500–2499 g) | 336 (25.8) | 34 (26.0) | 370 (25.8) |
| Non-LBW (≥2500 g) | 242 (18.6) | 13 (9.9) | 255 (17.8) |
| Inborn, n (%) | 1157 (88.3) | 115 (87.8) | 1272 (88.2) |
| Cesarean delivery, n (%) | 675 (52.7) | 69 (53.9) | 744 (52.8) |
| PPV at delivery, n (%) | 427 (34.4) | 40 (32.0) | 467 (34.1) |
| NICU course and disposition | |||
| Mechanical ventilation on admission, n (%) | 486 (37.1) | 52 (39.7) | 538 (37.3) |
| Antibiotic administration on admission, n (%) | 983 (75.0) | 107 (81.7) | 1090 (75.6) |
| Duration of CVC presence in days, median (IQR) | 6 (4–9) | 11 (7–17) | 6 (4–9) |
| Length of stay in days, median (IQR) | 16 (8–33) | 24 (12–41) | 16 (8–32) |
| Died, n (%) | 310 (23.6) | 53 (40.5) | 363 (25.2) |
Data not available for all neonates for select variables. Denominator for presented data by variable are as follows (without BSI, with BSI, total presented for each): Maternal age 1129, 110, 1239; gestational age 1239, 123, 1362; birth weight 1304, 131, 1435; delivery mode 1275, 133, 1408; PPV at delivery 1243, 125, 1368. Abbreviations: BSI – bloodstream infection; CVC – central venous catheter; ELBW – extremely low birth weight; IQR – interquartile range; LBW – low birth weight; NICU – neonatal intensive care unit; PPV – positive pressure ventilation; VLBW – very low birth weight.
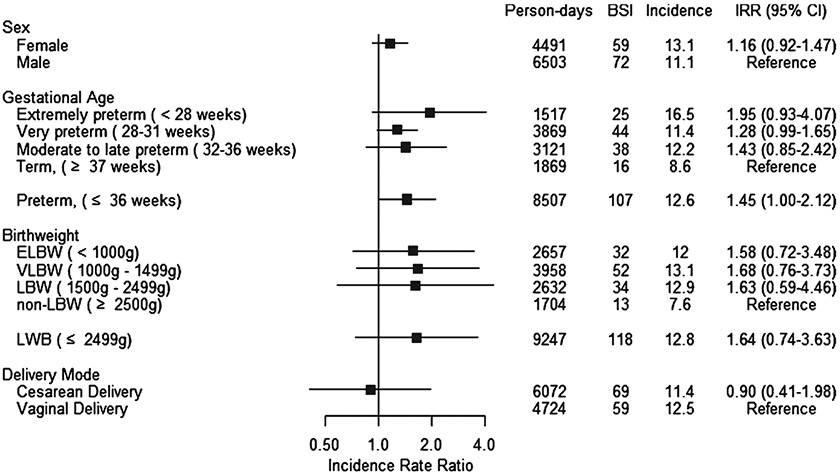
After exclusion of contaminants (Sphingomonas spp., n = 2, Pentoea spp., n = 1, Roseomonas spp., n = 1, Rhyzobium spp., n = 1), there were 140 unique BSI cases over the course of our study, occurring in 131 neonates (9.1% of neonates with CVC). The overall incidence of BSI was 11.9 per 1000 line-days (Table 2). The was no statistically significant difference in incidence by gestational age: among term neonates (≥37 weeks gestation at birth), the incidence was 8.6 per 1000 line-days (reference group), 12.2 per 1000 line-days (IRR 1.43, 95% CI 0.84–2.42) in moderate to late preterm neonates (32–36 weeks gestation), 11.4 per 1000 line-days (IRR 1.28, 95% CI 0.99–1.65), and 16.5 per 1000 line-days (IRR 1.95, 95% CI 0.93–4.07) in extremely preterm neonates (<28 weeks gestation). There were no statistically significant differences in BSI incidence by sex, gestational age, birth weight, or delivery mode (Table 2, Fig. 2). BSI rates were compared by two-day intervals and there were no significant differences in rates by dwell time (Table 3). Very few lines remained in place >30 days and no BSI events occurred after 30 days.
Table 2.
Incidence of bloodstream infection among neonates with central venous catheters in three tertiary care neonatal intensive care units in Pune, India, May 1, 2017 - July 31, 2019
| BSI | Line-days | Incidence per 1000 line-days |
IRR | 95% CI | |
|---|---|---|---|---|---|
| Total | 131 | 11018 | 11.9 | ||
| Sex | |||||
| Female | 59 | 4491 | 13.1 | 1.16 | (0.92–1.47) |
| Male | 72 | 6503 | 11.1 | ||
| Gestational age | |||||
| Extremely preterm (<28 weeks) | 25 | 1517 | 16.5 | 1.95 | (0.93–4.07) |
| Very preterm (28–31 weeks) | 44 | 3869 | 11.4 | 1.28 | (0.99–1.65) |
| Moderate to late preterm (32–36 weeks) | 38 | 3121 | 12.2 | 1.43 | (0.85–2.42) |
| All preterm (<37 weeks) | 107 | 8507 | 12.6 | 1.45 | (1.00–2.12) |
| Term (≥37 weeks) | 16 | 1869 | 8.6 | ||
| Birth weight | |||||
| ELBW (<1000 g) | 32 | 2657 | 12.0 | 1.58 | (0.72–3.48) |
| VLBW (1000–1499 g) | 52 | 3958 | 13.1 | 1.68 | (0.76–3.73) |
| LBW (1500–2499 g) | 34 | 2632 | 12.9 | 1.63 | (0.59–4.64) |
| All LBW (<2500 g) | 118 | 9247 | 12.7 | 1.64 | (0.74–3.63) |
| Non-LBW (≥2500 g) | 13 | 1704 | 7.2 | ||
| Delivery mode | |||||
| Cesarean delivery | 69 | 6072 | 11.4 | 0.90 | (0.41–1.98) |
| Vaginal delivery | 59 | 4724 | 12.5 |
Incidence rate ratios calculated using Poisson regression with clustered standard errors to account for clustering by tertiary NICU site. Abbreviations: BSI – bloodstream infection; CI – confidence interval; ELBW – extremely low birth weight; IRR – incidence rate ratio; LBW – low birth weight; VLBW – very low birth weight.
Fig. 2.
Forrest plots, incidence of bloodstream infections among neonates with central venous catheters by clinical characteristic in three tertiary care neonatal intensive care units in Pune, India, May 1, 2017 - July 31, 2019.
Table 3.
Incidence of bloodstream infections among neonates with central venous catheters by line-days in three tertiary care neonatal intensive care units in Pune, India, May 1, 2017 - July 31, 2019
| Line day | BSI | Line-days | Incidence per 1000 line-days |
IRR | 95% CI |
|---|---|---|---|---|---|
| 3–4 | 40 | 2449 | 16.3 | REF | |
| 5–6 | 33 | 1884 | 17.5 | 1.07 | (0.66–1.74) |
| 7–8 | 19 | 1247 | 15.2 | 0.93 | (0.51–1.65) |
| 9–10 | 13 | 793 | 16.4 | 1.00 | (0.49–1.92) |
| 11–12 | 10 | 522 | 19.2 | 1.17 | (0.52–2.39) |
| 13–14 | 4 | 350 | 11.4 | 0.70 | (0.18–1.94) |
| 15–16 | 2 | 246 | 8.1 | 0.50 | (0.06–1.92) |
| 17–18 | 4 | 176 | 22.7 | 1.39 | (0.36–3.85) |
| 19–20 | 1 | 130 | 7.7 | 0.47 | (0.01–2.78) |
| 21–22 | 0 | 94 | 0 | — | |
| 23–24 | 0 | 74 | 0 | — | |
| 25–26 | 1 | 56 | 17.9 | 1.09 | (0.27–6.45) |
| 27–28 | 0 | 37 | 0 | — | |
| 29–30 | 1 | 32 | 31.3 | 1.91 | (0.05–11.29) |
Comparison of BSI incidence by two-day interval in neonates with a CVC in place. Day of insertion is day 1; risk for BSI with CVC begins at line day 3. Analysis is censored at day 30; very few lines remained in place for >30 days and no BSI events occurred. Abbreviations: BSI – bloodstream infection; CI – confidence interval; IRR – incidence rate ratio.
There was a considerable Gram-negative predominance in BSI cases, with 105 Gram-negative BSI (75.0%) (Table 4). Klebsiella spp. (n = 54, 38.6% of all BSI, % of Gram-negative BSI), Acinetobacter spp. (n = 21, 15.0% of all BSI, % of Gram-negative BSI), Escherichia spp. (n = 9, 6.4% of all BSI, % of Gram-negative BSI), and Citrobacter spp. (n = 7, 5.0% of all BSI, % of Gram-negative BSI) were the mostly commonly identified Gram-negative pathogens. Resistance to third- or fourth-generation cephalosporins was ubiquitous, with 86/99 (86.9%) of Gram-negative organisms tested demonstrating resistance (Table 3). Aminoglycoside resistance (63.1%) and fluoroquinolone resistance (61.9%) were highly prevalent among Gram-negative isolates. Forty-two percent of Gram-negatives were resistant to carbapenems, including 26.4% of Klebsiella isolates and 80.0% of Acinetobacter isolates, the two most common causative pathogens of BSI among neonates with CVC. Among 42 Gram-negative isolates for which AST was performed for colistin, 3 (7.1%) were resistant, including two Klebsiella isolates. Among Gram-negative isolates, 32/105 (30.5%) met criteria for DTR: 4/6 (66.7%) of Enterobacter spp., 13/21 (61.9%) of Acinetobacter spp., 4/9 (44.4%) of Escherichia spp., 10/54 (18.5%) of Klebsiella spp., and 1/7 (14.3%) of Citrobacter spp. No Elizabethkingia (n = 5), Pseudomonas (n = 2), or Serratia (n = 1) isolates met criteria for DTR.
Table 4.
Pathogen distribution and Gram-negative antimicrobial resistance for bloodstream infections among neonates with central venous catheters in three tertiary care neonatal intensive care units in Pune, India, May 1, 2017 - July 31, 2019
| Total n = 140 n (%) |
Aminoglycoside resistance n/n tested (%) |
Carbapenem resistance n/n tested (%) |
3rd/4th generation cephalosporin resistance n/n tested (%) |
Colistin resistance n/n tested (%) |
Fluoroquinolone resistance n/n tested (%) |
Difficult-to- treat resistance n (%) |
|
|---|---|---|---|---|---|---|---|
| Gram-negative organisms | 105 (75.0) | 65/103 (63.1) | 42/100 (42.0) | 86/99 (86.9) | 3/42 (7.1) | 60/97 (61.9) | 30 (30.5) |
| Klebsiella spp. | 54 (38.6) | 26/53 (49.1) | 14/53 (26.4) | 46/52 (88.5) | 2/15 (13.3) | 26/53 (49.1) | 10 (18.5) |
| Acinetobacter spp. | 21 (15.0) | 19/21 (90.5) | 16/20 (80.0) | 21/21 (100) | 0/16 (0) | 18/19 (94.7) | 13 (61.9) |
| Escherichia spp. | 9 (6.4) | 5/9 (55.6) | 4/9 (44.4) | 6/8 (75.0) | 0/5 (0) | 7/9 (77.8) | 4 (44.4) |
| Citrobacter spp. | 7 (5.0) | 3/7 (42.9) | 1/6 (16.7) | 4/7 (57.1) | 0/2 (0) | 2/7 (28.6) | 1 (14.3) |
| Enterobacter spp. | 6 (4.3) | 5/5 (100) | 2/4 (50.0) | 2/3 (66.7) | 0/3 (0) | 1/1 (100) | 4 (66.7) |
| Elizabethkingia spp. | 5 (3.6) | 5/5 (100) | 5/5 (100) | 5/5 (100) | 1/1 (100) | 4/5 (80.0) | 0 |
| Pseudomonas spp. | 2 (1.4) | 2/2 (100) | 0/2 (0) | 2/2 (100) | – | 1/2 (50.0) | 0 |
| Serratia spp. | 1 (0.7) | 0/1 (0) | 0/1 (0) | 0/1 (0) | – | 1/1 (100) | 0 |
| Gram-positive organisms | 24 (17.1) | ||||||
| Staphylococcus spp. | 18 (12.9) | ||||||
| Enterococcus spp. | 4 (2.9) | ||||||
| Bacillus spp. | 2 (1.4) | ||||||
| Yeast | 11 (7.9) | ||||||
| Candida spp. | 8 (5.7) | ||||||
| Other | 3 (2.1) |
Difficult-to-treat resistance was defined as isolates identified intermediate or resistant on AST to all reported agents among the following: (1) carbapenems, (2) β-lactams, and (3) fluoroquinolones, as well as (4) piperacillin-tazobactam and ampicillin-sulbactam (Acinetobacter spp. only), and (5) aztreonam (organisms other than Acinetobacter spp.) [17]. For Staphylococcus spp. (n = 18), five isolates (27.8%) were identified as S. aureus, eight (44.4%) were identified as CONS, and five (27.8%) were identified only at the genus level. Abbreviations: AST = antimicrobial susceptibility testing; CONS = coagulase-negative Staphylococcus; spp. = species.
Staphylococcus spp. (n = 18, 12.9%) were the most common Gram-positive pathogens; species-level identification was available for 13 isolates, which includes eight BSI cases with coagulase-negative Staphylococcus (CONS) and five cases with S. aureus. Fungal pathogens accounted for 11 (7.9%) BSI cases, of which the majority were Candida spp. (n = 8, 5.7%).
Length of stay was greater in neonates with BSI than in those without BSI, 24 days (IQR 12–41) versus 16 days (IQR 8–33) (p < 0.001) (Table 1). Similarly, mortality was higher in those with BSI, with 53 deaths (40.5%) compared to 310 deaths (23.6%) in the non-BSI group (unadjusted analysis, p < 0.001). After adjusting for birth weight, sex, delivery mode, and mechanical ventilation at admission, mortality continued to be higher among those with BSI (adjusted incidence ratio 2.14, 95% CI 1.38–3.31, p < 0.01). Forty-four of 99 (44.4%) neonates with Gram-negative BSI died, whereas five of 22 (22.7%) neonates with Gram-positive BSI died (p = 0.091).
Characteristics of the eight neonates with multiple BSI events are summarized in Supplemental Table 1. All neonates were preterm (26–33 weeks gestation at birth, two neonates with unknown gestational age). Seven of eight (87.5%) neonates were inborn and admitted to the NICU on the day of life (DOL) 0, and one neonate was outborn and transferred on DOL 1. CVCs were placed on hospital day 1–7, and first BSI event occurred on hospital day 3–20. There were six neonates with two BSI events and two neonates with three BSI events. Among neonates with two BSI events, one neonate had two blood cultures positive for the same organism, Klebsiella spp., on hospital days 15 and 25, whereas five neonates had two blood cultures positive for different organisms. The first neonate with three BSI events had blood cultures positive for Acinetobacter spp. on hospital day 30 and two blood cultures positive for Candida spp. on hospital days 38 and 58; the second had blood cultures positive for Klebsiella spp. on hospital days 9 and 19 and for Escherichia spp. on hospital day 103. The median duration of CVC presence among neonates with multiple BSI events was 26.5 days (IQR 16.5–39), whereas median line duration among neonates with one BSI events was 11 days (IQR 7–17). Two of eight (25%) died, five (62.5%) were discharged home, and one was transferred to a ward at the same hospital.
4. Discussion
Preterm and sick neonates requiring prolonged hospital admission are at increased risk for HAI, including CLABSI. These risks are augmented in low resource settings due to factors such as overcrowding, understaffing, limited capacity for isolation or cohorting, and inadequate supply of personal protective equipment and hand hygiene products [2, 18, 19]. In our cohort, BSI occurred in greater than 9% of neonates with a CVC in place over the course of their NICU admission, and these infections were associated with greater length of stay and increased mortality. These data are consistent with prior reports of increased mortality associated with neonatal Gram-negative infections, highlighting the impact of these infections in vulnerable preterm and sick neonates [13].
There are limited data on incidence of CLABSI in Indian NICUs. A quality improvement initiative to reduce CLABSI incidence in a NICU in Bangalore, India, noted a pre-intervention incidence of 31.7 per 1000 line-days, comparable to rates reported by five Indian NICUs reporting to the International Nosocomial Infection Control Consortium, but significantly higher than the incidence in our cohort [8, 20]. Our incidence was more comparable to pre-intervention CLABSI rates described in a quality improvement initiative in a NICU in Pakistan, 17.1 per 1000 line-days [21]. However, our rates exceeded those typically described in high income settings, including in the United States [6]. It is worth noting that these studies have slightly different definitions used for CLABSI surveillance, such as by hospitals reporting to NHSN in the United States, and study definitions for CLABSI or BSI with CVC, which might account for some of the differences in reported rates.
The pathogen distribution for BSI cases among neonates with CVCs was similar to that observed in our parent cohort of all neonates admitted to the three study site NICUs, though with an even greater Gram-negative predominance [16]. K. pneumoniae, in particular, is a pathogen of great concern in India, including in NICUs, and has been repeatedly associated with neonatal sepsis and outbreaks in the NICU setting [16, 22-25]. In a 2017 scoping report of AMR in India, carbapenem resistance was described in greater than 50% of K. pneumoniae isolates, and resistance to third-generation cephalosporins was ubiquitous [12]. Antibiotic decision-making in NICUs with high rates of AMR is complicated by limited treatment options in neonates and potential adverse effects of broad-spectrum antibiotics in this population. Antibiotics of so-called last resort, such as colistin, are increasingly used for empiric treatment of neonatal sepsis in India due to endemic multidrug resistance [26]. Outbreaks of colistin resistant K. pneumoniae have been described in NICUs in India, raising concern for lack of effective treatment options for such infections in the future [24, 27].
A high incidence of these HAI in our cohort, as well as increased length of stay and mortality among affected neonates, highlights the importance of identifying effective preventive mechanisms to reduce the burden of these infections. In a 2022 systematic review of IPC interventions targeting neonatal HAI, bundled interventions targeting CLABSI and other device-associated infections were most successful [28]. The World Health Organization recommends a multimodal improvement strategy to optimize IPC in healthcare facilities, consisting of five elements: (1) system change, (2) training and education, (3) monitoring and feedback, (4) reminders and communications, and (5) culture of safety [29]. Such multimodal improvement strategies have been successfully implemented in LMIC NICUs to improve key IPC practices, including in our 2021 quasi-experimental study that implemented the Comprehensive Unit-based Safety Programme to improve hand hygiene, aseptic technique for invasive procedures, and medication and intravenous fluid administration safety in four Indian NICUs [30]. In a 2019 survey on IPC in healthcare facilities participating in a national HAI surveillance network in India, training and education were reported as the most commonly implemented element of a multimodal IPC improvement strategy and interventions focused on safety culture were least commonly reported [31]. Meaningful, sustained reductions in neonatal HAI in LMIC settings rely on successful introduction of bundled interventions using multimodal improvement strategies to optimize IPC practices.
Strengths of this study include its prospective nature and large sample size. Limitations of this study include the inability to attribute BSI cause to CVCs, as we did not perform root cause analysis for each of these BSI cases. We may be overestimating incidence as BSI cases may be due to other causes, such as necrotizing enterocolitis. Root cause analysis is an integral component of HAI surveillance and should be considered in future initiatives to reduce CLABSI and other HAI risk. We may have underestimated the number of line days due to information gaps on weekends or holidays, which also may have led to overestimation of BSI incidence. However, sensitivity analyses showed findings were robust to different assumptions about central line presence on unobserved days. Additionally, we may be overestimating BSI incidence as we considered a single blood culture positive for CONS as a BSI case. It is not standard practice to obtain a second confirmatory culture in our study sites, and we did not exclude single cultures positive for CONS due to its known importance as a neonatal pathogen, particularly for late onset sepsis and CLABSI [1].
Multidrug-resistant Gram-negative pathogens were common BSI causing organisms among neonates with CVCs in our cohort. Prolonged duration of CVC presence was associated with increased CLABSI risk, underscoring the importance of line removal as soon as feasible. The BSI rate among neonates with CVCs in our study exceeds CLABSI rates seen in most high-income setting NICUs, highlighting a need to prioritize IPC measures in India and other LMIC settings. Although prevention and treatment of nosocomial infection is a complex process requiring vigilant surveillance and multi-modal interventions at multiple levels, these data indicate that such efforts are warranted.
Supplementary Material
Acknowledgments
The authors wish to thank the NICU staff who supported this study.
Funding
This work was supported by the United States Centers for Disease Control and Prevention (Safe Healthcare, Epidemiology, and Prevention Research Development Program Domain 7, Contract 200-2016-91781 Task Order 1) and the National Institutes of Health [UM1AI104681 to M.L.R., K23HD100594 to J.J., UM1AI069465-13 and 1R01AI43748-01A1 to V.M., UM1AI069465, UM1AI068632, and UM1AI068636 to A.G., and K24AI141580 to A.M.M.].
Footnotes
CDC disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Human research statement
Research involving human subjects was conducted in accordance with the ethical standards of all applicable national and institutional committees and the World Medical Association’s Helsinki Declaration.
Financial disclosure statement
The authors declare no relevant conflicts of interest.
References
- [1].Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–80. [DOI] [PubMed] [Google Scholar]
- [2].Johnson J, Akinboyo IC, Schaffzin JK. Infection prevention in the neonatal intensive care unit. Clin Perinatol. 2021;48(2):413–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hooven TA, Polin RA. Healthcare-associated infections in the hospitalized neonate: A review. Early Hum Dev. 2014;90(Suppl 1):S4–6. [DOI] [PubMed] [Google Scholar]
- [4].Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–91. [DOI] [PubMed] [Google Scholar]
- [5].Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–65. [DOI] [PubMed] [Google Scholar]
- [6].Centers for Disease Control and Prevention National Health Surveillance Network. 2020. National and State Healthcare-Associated Infections Progress Report 2020 [Available from: https://www.cdc.gov/hai/data/portal/progress-report.html. [Google Scholar]
- [7].Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377(9761):228–41. [DOI] [PubMed] [Google Scholar]
- [8].Mehta Y, Jaggi N, Rosenthal VD, Kavathekar M, Sakle A, Munshi N, et al. Device-associated infection rates in 20 cities of India, data summary for 2004-2013: Findings of the International Nosocomial Infection Control Consortium. Infect Control Hosp Epidemiol. 2016;37(2):172–81. [DOI] [PubMed] [Google Scholar]
- [9].Singhal T, Shah S, Thakkar P, Naik R. The incidence, aetiology and antimicrobial susceptibility of central line-associated bloodstream infections in intensive care unit patients at a private tertiary care hospital in Mumbai, India. Indian J Med Microbiol. 2019;37(4):521–6. [DOI] [PubMed] [Google Scholar]
- [10].Datta P, Rani H, Chauhan R, Gombar S, Chander J. Device-associated nosocomial infection in the intensive care units of a tertiary care hospital in northern India. J Hosp Infect. 2010;76(2):184–5. [DOI] [PubMed] [Google Scholar]
- [11].Saharman YR, Karuniawati A, Severin JA, Verbrugh HA. Infections and antimicrobial resistance in intensive care units in lower-middle income countries: A scoping review. Antimicrob Resist Infect Control. 2021;10(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].GandraS JJ, Trett A, Lamkang AS, Laxminarayan R. Scoping report on antimicrobial resistance in India. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2017. [Google Scholar]
- [13].Tsai MH, Chu SM, Hsu JF, Lien R, Huang HR, Chiang MC, et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacteremia in the NICU. Pediatrics. 2014;133(2):e322–9. [DOI] [PubMed] [Google Scholar]
- [14].Hsu AJ, Tamma PD. Treatment of multidrug-resistant Gram-negative infections in children. Clin Infect Dis. 2014;58(10):1439–48. [DOI] [PubMed] [Google Scholar]
- [15].Annamalai A, Gupta V, Jain S, Datta P. Increasing resistance to reserve antibiotics: The experience of a tertiary level neonatal intensive care unit. J Trop Pediatr. 2021;67(1). [DOI] [PubMed] [Google Scholar]
- [16].Johnson J, Robinson ML, Rajput UC, Valvi C, Kinikar A, Parikh TB, et al. High burden of bloodstream infections associated with antimicrobial resistance and mortality in the neonatal intensive care unit in Pune, India. Clin Infect Dis. 2021;73(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramasethu J. Prevention and treatment of neonatal nosocomial infections. Matern Health Neonatol Perinatol. 2017;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weinshel K, Dramowski A, Hajdu Á, Jacob S, Khanal B, Zoltán M, et al. Gap analysis of infection control practices in low- and middle-income countries. Infect Control Hosp Epidemiol. 2015;36(10):1208–14. [DOI] [PubMed] [Google Scholar]
- [20].Balla KC, Rao SP, Arul C, Shashidhar A, Prashantha YN, Nagaraj S, et al. Decreasing central line-associated blood-stream infections through quality improvement initiative. Indian Pediatr. 2018;55(9):753–6. [PubMed] [Google Scholar]
- [21].Hussain AS, Ahmed AM, Arbab S, Ariff S, Ali R, Demas S, et al. CLABSI reduction using evidence based interventions and nurse empowerment: A quality improvement initiative from a tertiary care NICU in Pakistan. Arch Dis Child. 2021;106(4):394–400. [DOI] [PubMed] [Google Scholar]
- [22].Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: A cohort study. Lancet Glob Health. 2016;4(10):e752–60. [DOI] [PubMed] [Google Scholar]
- [23].Chaurasia S, Sivanandan S, Agarwal R, Ellis S, Sharland M, Sankar MJ. Neonatal sepsis in South Asia: Huge burden and spiralling antimicrobial resistance. BMJ. 2019;364:k5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sharma S, Banerjee T, Kumar A, Yadav G, Basu S. Extensive outbreak of colistin resistant, carbapenemase (bla(OXA-48), bla(NDM)) producing Klebsiella pneumoniae in a large tertiary care hospital, India. Antimicrob Resist Infect Control. 2022;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Banerjee T, Bhattacharjee A, Upadhyay S, Mishra S, Tiwari K, Anupurba S, et al. Long-term outbreak of Klebsiella pneumoniae & third generation cephalosporin use in a neonatal intensive care unit in north India. Indian J Med Res. 2016;144(4):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jajoo M, Kumar V, Jain M, Kumari S, Manchanda V. Intravenous colistin administration in neonates. Pediatr Infect Dis J. 2011;30(3):218–21. [DOI] [PubMed] [Google Scholar]
- [27].Singh SK, Gupta M. blaOXA-48 carrying clonal colistin resistant-carbapenem resistant Klebsiella pneumoniae in neonate intensive care unit, India. Microb Pathog. 2016;100:75–7. [DOI] [PubMed] [Google Scholar]
- [28].Fitzgerald FC, Zingg W, Chimhini G, Chimhuya S, Wittmann S, Brotherton H, et al. The impact of interventions to prevent neonatal healthcare-associated infections in low- and middle-income countries: A systematic review. Pediatr Infect Dis J. 2022;41(3s):S26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- [30].Johnson J, Latif A, Randive B, Kadam A, Rajput U, Kinikar A, et al. Implementation of the Comprehensive Unit-Based Safety Program to improve infection prevention and control practices in four neonatal intensive care units in Pune, India. Front Pediatr. 2021;9:794637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Katoch O, Katyal S, Srivastav S, Rodrigues C, Rupali P, Chakrabarti A, et al. Self-reported survey on infection prevention and control structures in healthcare facilities part of a national level healthcare associated infection surveillance network in India, 2019. Am J Infect Control. 2022;50(4):390–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.