Dear Editor,
For thousands of years, medicinal plants have played vital roles in treating and preventing diseases in the world. Enhancing the active ingredients of medicinal plants through modern biotechnological methods holds great promise for maximizing the therapeutic potential of these plants. However, genetic transformation has only been feasible for a very limited number of medicinal plant species, hampering the effort to improve these plants through genetic engineering or gene editing. Recently, we developed the cut-dip-budding (CDB) gene delivery system for plant transformation, which uses Agrobacteria rhizogenes K599 to induce transformed roots from explants and then obtain transformed buds from the transformed roots because of the root suckering ability [1]. Given that many medicinal plants also possess the characteristics of root suckering, we explored the applicability of CDB system in medicinal plants that are difficult or impossible to transform. Compared to the published CDB method, we optimized the infection procedure by suspending cultured Agrobacteria in MES buffer (pH 5.6), which contained 10 mM 2-(N-morpholino) ethanesulfonic acid (MES), 10 mM MgCl2, and 100 μM Acetosyringone (AS). We adapted the CDB method to transform medicinal plants as depicted (Fig. 1A).
Figure 1.
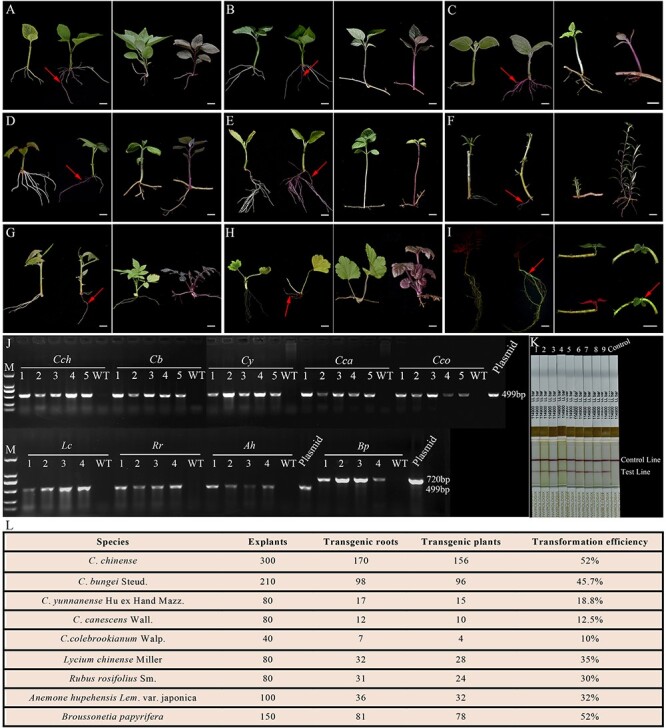
Transformation of medicinal plants using the CDB system. A CDB delivery system workflow. B RUBY-positive hairy roots and RUBY-positive Clerodendrum chinense (Cch) plant generated from the transformed roots. C RUBY-positive hairy roots and RUBY-positive Clerodendrum bungei (Cb) plant. D RUBY-positive hairy roots and RUBY-positive Clerodendrum yunnanense (Cy) plant. E RUBY-positive hairy roots and RUBY-positive Clerodendrum canescens (Cca) plant. F RUBY-positive hairy roots and RUBY-positive Clerodendrum colebrookianum (Cco) plant. G RUBY-positive hairy roots and RUBY-positive Lycium chinense (Lc) plant. H RUBY-positive hairy roots and RUBY-positive Rubus rosifolius (Rr) plant. I RUBY-positive hairy roots and RUBY-positive Anemone hupehensis (Ah) plant. J EGFP-positive hairy roots and EGFP-positive B. papyrifera (Bp) plant. K PCR results showing the presence of EGFP or RUBY reporter gene in positive shoots. L Bar protein detection results. M Statistics for the transformation efficiency of the nine medicinal plant species. In panels A–I, the plant on the left is an untransformed control. Red arrows point to a RUBY or EGFP-positive signal. Bar: 1 cm.
Clerodendrum, an important genus within the Verbenaceae family, has medicinal, ornamental, and research values [2–4]. Lack of a genetic transformation system has limited the improvement of these plant species. Considering that most Clerodendrum species have the root suckering ability, there is potential to establish a transformation system for these plants using the CDB approach. We used the RUBY reporter to test the applicability of the CDB gene delivery system in five different Clerodendrum species, including Clerodendrum chinense, Clerodendrum bungei Steud., Clerodendrum yunnanense Hu ex Hand Mazz., Clerodendrum canescens Wall., and Clerodendrum colebrookianum Walp. By infecting the young terminal buds or stems of these species with A. rhizogenes K599, hairy roots were formed approximately three weeks later. Some of these hairy roots were visually positive for RUBY (Fig. 1). Transgene positive RUBY red buds were generated from these RUBY roots after the roots were cultured in soil for 2–3 months. Finally, we obtained transgene positive shoots for C. chinense (Fig. 1B), C. bungei (Fig. 1C), C. yunnanense (Fig. 1D), C. canescens (Fig. 1E), and C. colebrookianum (Fig. 1F). These RUBY red shoots developed into normally growing plants that displayed the RUBY red color in all plant parts.
Having successfully applied the CDB gene delivery method to five species of Clerodendrum plants, we extended our efforts to transform four other medicinal plant species: Lycium chinense Miller, Rubus rosifolius Sm., Anemone hupehensis Lem. var. japonica and Broussonetia papyrifera Linnaeus. These selections were based on their root suckering ability, medicinal value and the difficulties in genetic transformation using traditional tissue culture-based methods [5–8]. Notably, genetic modification had not been reported for A. hupehensis and R. rosifolius. While transformation protocols exist for L. chinense and B. papyrifera, they are cumbersome and genotype dependent [9, 10]. We carried out the CDB protocol using RUBY as the reporter for the transformation of L. chinense, R. rosifolius, and A. hupehensis, and EGFP as the reporter for the transformation of B. papyrifera. Transgenic hairy roots formed approximately one month after infection with Agrobacteria. These transgene-positive hairy roots grew into healthy, normal-looking roots. Similar to the Clerodendrum species, transgene positive buds formed naturally on these roots without the need for root cutting (Fig. 1G–I). As B. papyrifera is a tall tree with a relatively long root suckering process, we expedited the generation of transgenic buds by cutting the transgene-positive roots into segments once they reached a certain thickness, and placing the segments on nutrient soil. Subsequently, transgenic buds emerged in 2–4 weeks (Fig. 1J).
All transgenic lines of the nine species of medicinal plants were confirmed to be transgene-positive through PCR and Bar (Bialaphos resistance/phosphinothricin acetyltransferase, a herbicide resistance gene) protein detection (Fig. 1K and L). To assess the efficiency of the CDB system, we conducted larger scale transformation experiments on these plants, yielding transformation efficiencies of 52%, 45.7%, 18.8%, 12.5%, 10%, 35%, 30%, 32%, and 52% for C. chinense, C. bungei, C. yunnanense, C. canescens, C. colebrookianum, L. chinense, R. rosifolius, A. hupehensis and B. papyrifera, respectively (Fig. 1M). These findings underscore the broad applicability and high efficiency of the CDB delivery system for the transformation of medicinal plants with root suckering capability.
In summary, we have successfully transformed nine species of traditional medicinal plants with root suckering capability using the CDB gene delivery system, providing compelling support to our hypothesis that this gene delivery system can be used to modify many plants with root suckering capability [1]. The application of the CDB system in the realm of medicinal plants carries substantial significance, as it opens up new avenues for enhancing these important species through transgenic or gene editing techniques. Future genetic improvements of medicinal plants have the potential to elevate the quality and consistency of herbal remedies, thereby increasing their efficacy for patients, and benefiting human health and well-being.
Acknowledgements
This work was supported by Shandong Shunfeng Biotechnology Co. Ltd., Jinan, China, project of Sanya Yazhou Bay Science and Technology City (Grant No. SCKJ-JYRC-2023-72) and Hainan Yazhou Bay Seed Laboratory (Grant No. B22C10305).
Author contributions
J.L., J.-K.Z. designed and supervised the research. J.L., S.L., C.S., S.D., and H.T. carried out the experiments. J.L., M.W., Z.W., Z.L., and J.-K.Z. discussed the data. J.L., G.L. Z.L., and J.-K.Z. wrote the paper.
Data availability
All the data supporting the findings of this study are available in the paper, and the plasmid is available upon request.
Conflict of interest statement
None declared.
Contributor Information
Jinghua Lu, Institute of Tropical Bioscience and Biotechnology/Sanya Research Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou 570102, China; Institute of Crop Sciences/National Nanfan Research Institute, Chinese Academy of Agricultural Sciences, and Key Laboratory of Gene Editing Technologies, Ministry of Agriculture and Rural Affairs, Sanya 572024, China.
Suhui Lu, Shandong Shunfeng Biotechnology Co. Ltd., Jinan 250000, China.
Chunli Su, Shandong Shunfeng Biotechnology Co. Ltd., Jinan 250000, China.
Shuai Deng, Shandong Shunfeng Biotechnology Co. Ltd., Jinan 250000, China.
Mugui Wang, Institute of Crop Sciences/National Nanfan Research Institute, Chinese Academy of Agricultural Sciences, and Key Laboratory of Gene Editing Technologies, Ministry of Agriculture and Rural Affairs, Sanya 572024, China.
Huan Tang, Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou 570102, China.
Zhunian Wang, Sanya Research Institute & Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences, Sanya 572025, China.
Guofu Li, Shandong Shunfeng Biotechnology Co. Ltd., Jinan 250000, China.
Zhaobo Lang, Institute of Advanced Biotechnology and School of Life Sciences, Southern University of Science and Technology, Shenzhen 518055, China.
Jian-Kang Zhu, Institute of Crop Sciences/National Nanfan Research Institute, Chinese Academy of Agricultural Sciences, and Key Laboratory of Gene Editing Technologies, Ministry of Agriculture and Rural Affairs, Sanya 572024, China; Institute of Advanced Biotechnology and School of Life Sciences, Southern University of Science and Technology, Shenzhen 518055, China.
References
- 1. Cao X, Xie H, Song M. et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation (Camb). 2023;4:100345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu S, Zhou F, Gao X. et al. Advances in research on common medicinal plants of Clerodendrum. Drug Eval Res. 2011;34:469–73 [Google Scholar]
- 3. Mu K, Liu Y, Liu G. et al. A review of hemostatic chemical components and their mechanisms in traditional Chinese medicine and ethnic medicine. J Ethnopharmacol. 2023;307:116200 [DOI] [PubMed] [Google Scholar]
- 4. Wahba HM, AbouZid SF, Sleem AA. et al. Chemical and biological investigation of some Clerodendrum species cultivated in Egypt. Pharm Biol. 2011;49:66–72 [DOI] [PubMed] [Google Scholar]
- 5. Oğuz İ, Kafkas E. Determination of the total phenolic and anthocyanin contents, as well as the total antioxidant capacity, of black wolfberry (Lycium ruthenicum) fruits. J Process Energy Agric. 2019;23:158–61 [Google Scholar]
- 6. Yokosuka A, Sano T, Hashimoto K. et al. Triterpene glycosides from the whole plant of Anemone hupehensis var. japonica and their cytotoxic activity. Chem Pharm Bull. 2009;57:1425–30 [DOI] [PubMed] [Google Scholar]
- 7. Biondo E, Corrêa APF, Brandelli A. et al. Wild strawberries (Rubus rosifolius Sm.) from southern Brazil: centesimal and mineral composition, total polyphenols, antioxidant, antibacterial and anti-hypertensive actives. Revista Ciência Agrícola. 2021;19:71–8 [Google Scholar]
- 8. Zhang N, Zhang C, Xiao X. et al. New cytotoxic compounds of endophytic fungus Alternaria sp. isolated from Broussonetia papyrifera (L.) vent. Fitoterapia. 2016;110:173–80 [DOI] [PubMed] [Google Scholar]
- 9. Zhang H, An Y, Zhang J. et al. Establishment of efficient plant regeneration and genetic transformation system of Lycium ruthenicum. J Nuclear Agric Sci. 2022;36:929–36 [Google Scholar]
- 10. Li M, Li H, Jiang H. et al. Establishment of a highly efficient Agrobacterium tumefaciens-mediated leaf disc transformation method for Broussonetia papyrifera. Plant Cell Tissue Organ Cult. 2008;93:249–55 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the findings of this study are available in the paper, and the plasmid is available upon request.


