Abstract
Invasive aspergillosis is a serious complication in immunocompromised patients. The effects of recombinant human tumor necrosis factor alpha (TNF-α) on antifungal activities of human neutrophils (polymorphonuclear leukocytes [PMNs]), human monocytes (MNCs), and rabbit pulmonary alveolar macrophages (PAMs) against Aspergillus fumigatus were studied. The percentage of PMN-induced hyphal damage was increased after 30 min of incubation of PMNs with 0.1 ng of TNF-α per ml at 37°C (P = 0.043). At 0.1 to 10 ng/ml, TNF-α also increased superoxide anion (O2−) produced by PMNs in response to phorbol myristate acetate, N-formylmethionyl leucyl phenylalanine, and unopsonized hyphae (P < 0.01) but did not exert any effect on PMN phagocytosis of conidia in the presence of serum. By comparison, TNF-α induced only a slight increase in O2− production by MNCs in response to phorbol myristate acetate (P = 0.05) and no concomitant increase in the percentage of MNC-induced hyphal damage. Incubation of MNCs with TNF-α at 0.001 to 10 ng/ml for 2 days had no effect on phagocytosis or conidiocidal activity. By contrast, incubation of PAMs with TNF-α at 0.1 to 10 ng/ml for 2 days increased phagocytosis of conidia (P = 0.03). Thus, TNF-α augments the capacity of PMNs to damage Aspergillus hyphae, possibly through enhanced oxidative mechanisms, and increases PAM phagocytic activity against conidia. As such, TNF-α may have an important role in host defense against aspergillosis, and neutralization of its activity may be complicated by increased susceptibility to aspergillosis.
As the number of immunocompromised patients has increased, invasive aspergillosis has emerged as the second most common opportunistic fungal infection (4, 19). It is associated with high morbidity and a disappointing mortality that ranges from 30% up to 90%, depending principally on host factors (10, 46). Despite recent progress, antifungal chemotherapy is frequently inadequate to eliminate infection unless there is recovery from immunosuppression.
The major host defenses against invasive aspergillosis are pulmonary alveolar macrophages (PAMs) as well as peripheral blood polymorphonuclear and mononuclear phagocytes. Macrophages have been shown to participate in the early phase of defense by ingesting inhaled airborne Aspergillus conidia and inhibiting their intracellular germination (21, 37, 45). In addition, polymorphonuclear leukocytes (PMNs), as well as circulating monocytes (MNCs), cause damage to escaping hyphae (late phase of defense) by secreting microbicidal oxidative metabolites and nonoxidative compounds, thus preventing establishment of invasive disease (12, 21, 37).
However, the role of cytokines in the modulation of responses of circulating or tissue phagocytes to Aspergillus has only recently begun to be elucidated (8, 24, 26, 29, 31, 32, 35). Elucidation of the role of these and other cytokines in invasive aspergillosis may provide further understanding of its pathogenesis and risk for infection. Tumor necrosis factor alpha (TNF-α) is a 17-kDa cytokine predominantly produced by monocytes, macrophages, and natural killer cells in response to challenges by infectious agents that exerts potent enhancing effects on inflammation and host defenses (3, 6, 13–17, 20, 25, 28, 38–40).
Little is known about the role of TNF-α in modulating host response against filamentous fungi, especially in modulating the antifungal activity of mononuclear phagocytes. Recently, Nagai et al. reported protective effects of exogenously administered TNF-α in a murine model of invasive aspergillosis (26). However, the effects of TNF-α on specific components of phagocytic host defenses against Aspergillus are unknown. We therefore investigated the effects of recombinant TNF-α on antifungal activities of human PMNs, human MNCs, and rabbit PAMs against A. fumigatus.
(Results of this study were presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September, 1996 [abstract number G25].)
MATERIALS AND METHODS
Source and preparation of effector cells. (i) Human PMNs.
Nine healthy adults served as donors for testing the effects of TNF-α on the function of PMNs. One of them donated blood twice during these studies. The PMNs were isolated from heparinized (5 to 10 U/ml) venous blood by dextran sedimentation followed by centrifugation over Ficoll and hypotonic lysis of erythrocytes (7, 36). They were then resuspended in Hanks’ balanced salt solution (HBSS) without Ca2+ or Mg2+. The resulting cell preparations consisted of more than 95% viable PMNs by trypan blue exclusion and modified May-Grunwald-Giemsa staining.
(ii) Human MNCs.
Twenty-four healthy adults served as donors of MNCs. MNCs were separated from blood buffy coats by centrifugation over Ficoll (31). They were resuspended in complete medium (CM) consisting of RPMI 1640, 25% pooled human serum (PHS) (Transfusion Medicine Department, Hippokration Hospital, Salonika, Greece), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The viability of cells was greater than 95%, and approximately 25 to 45% of them were MNCs. The concentration of MNCs was adjusted to 5 × 106 per ml. They were separated from lymphocytes by adherence as described below.
(iii) Rabbit PAMs.
Six pathogen-free female New Zealand White rabbits (Hazleton, Rockville, Md.) weighing 2.0 to 3.0 kg each were used to obtain PAMs by bronchoalveolar lavage. Rabbits were killed with pentobarbital, and lungs were excised. The edges of the lobes were cut, and phosphate-buffered saline (PBS) was injected through the trachea and bronchi. The lavage washings were filtered through sterile gauze pads, pellets were resuspended in HBSS without Ca2+ or Mg2+, and erythrocytes were disrupted by hypotonic lysis. PAMs were resuspended in CM containing 10% fetal bovine serum (FBS) (Gibco) instead of PHS at a concentration of 5 × 106 per ml.
Reagents and pretreatment of effector cells.
Recombinant human TNF-α was purchased from R+D Systems, Minneapolis, Minn. No endotoxin was detected according to the manufacturer’s assay. TNF-α was dissolved in HBSS with 0.1% albumin to a stock concentration of 1 μg/ml. Before the experiments, 2 × 107 PMNs were incubated with TNF-α at concentrations of 0.001 to 10 ng/ml in 1 ml of HBSS without Ca2+ or Mg2+ at 37°C for 30 min, as described previously (3, 15). Antifungal activity of PMNs was then evaluated without further washing. In parallel experiments, TNF-α concentrations up to 10 ng/ml did not exert any direct effect on hyphal growth. Human MNCs at a concentration of 1 × 106 to 2 × 106 per ml of CM (10 ml per flask) were incubated with TNF-α at 37°C and 5% CO2 for 2 to 3 days before the assays. Coverslip-adherent monocytes were incubated with the same range of TNF-α concentrations in 1 ml of CM for 2 to 3 days during their differentiation to monocyte-derived macrophages. MNCs were washed twice after treatment with TNF-α.
PHS was prepared from adult healthy volunteers. A random preparation of five human sera were tested for complement and found to have CH50 (153.6 ± 12.6 units), C3 (102.7 ± 9.0 mg/dl), and C4 (25.2 ± 3.3 mg/dl). On the other hand, FBS (purchased from Gibco) had undetectable CH50 activity and levels of C3 and C4.
Organism and preparation of hyphae.
Conidia from a well-characterized strain (4215) of A. fumigatus were harvested, filtered, washed, and suspended in PBS at 4°C as previously described in detail (34). Hyphae were produced from conidia for superoxide anion production assays (34) and hyphal damage assays (31) according to methods described previously. Hyphae were either used immediately or stored at 4°C for no longer than 1 to 2 h.
Superoxide anion production assay.
Production of superoxide anion (O2−) in response to soluble and fungal particulate stimuli was assessed spectrophotometrically by superoxide dismutase-inhibitable reduction of cytochrome c. One million PMNs or MNCs, which had been incubated with buffer-CM only or with buffer-CM containing TNF-α for the appropriate times (see above), were mixed with 50 μM cytochrome c (Sigma). As stimuli, 0.1 μg of phorbol myristate acetate (PMA) per ml or 0.5 μM N-formylmethionyl leucyl phenylalanine (FMLP), both from Sigma, or unopsonized hyphae of A. fumigatus at an effector-to-target cell (E/T) ratio of 1/1 were added to the phagocytes in 1 ml of HBSS and incubated on a shaker at 37°C for 15 min. Control tubes containing all of these constituents plus superoxide dismutase (40 μg/ml) were also included. Following this incubation, O2− production was assessed as described previously (34).
Hyphal damage assay.
To assess hyphal damage, the colorimetric MTT assay (22, 34) was employed. Briefly, the supernatants of the hypha-containing wells (see above) were aspirated, and phagocytes (either PMNs or MNCs) that had been incubated with or without TNF-α for 30 min or 48 h, respectively, were added to the wells at final E/T ratios of 1/1, 5/1, and 10/1. After 2 h at 37°C and in 5% CO2, supernatants were aspirated, phagocytes were lysed by adding 300 μl of 0.5% sodium deoxycholate, and hyphae were washed three times with sterile water. Subsequently, 1 ml of RPMI 1640 containing MTT (0.5 mg/ml) was added to each well, and the plates were further incubated at 37°C and in 5% CO2 for 3 h. The colorimetric reaction was measured and the percent hyphal damage was measured as previously reported (22, 34), with the following formula: percent hyphal damage = (1−X/C) × 100, where X is the optical density (OD) of test wells at 2 h and C is the OD of control wells containing hyphae only. Each condition was tested in duplicate or quadruplicate, and results were averaged.
Assays of phagocytosis of conidia by PMNs, MNCs, and PAMs.
One million phagocytes in CM (200-μl suspensions) were placed on 18-mm sterile round glass coverslips in 12-well plates (Costar, Cambridge, Mass.) and were incubated at 37°C with 5% CO2 for 45 min. The coverslips were then washed, fresh CM was added, and the glass-adherent PMNs, MNCs, and PAMs were further incubated for either 30 min (for PMNs) or 2 days (for MNCs and PAMs) with various concentrations of TNF-α. At the end of pretreatment, supernatants were removed and 1 ml of CM containing 106 A. fumigatus conidia per ml was added. CM contained 25% PHS in the case of human PMNs and MNCs and 10% FBS in the case of rabbit PAMs. After 1 h of incubation, coverslips were washed and phagocytes were fixed and stained. Phagocytic activity of PMNs, MNCs, and PAMs was assessed by light microscopy in duplicate coverslips (31).
Percent phagocytosis was the percentage of phagocytes that had one or more conidia phagocytosed or attached among 100 of them counted. Phagocytic index was the average number of conidia that had been phagocytosed or attached to each ingesting phagocyte. Total phagocytic index was the average number of conidia that had been phagocytosed or attached per phagocyte in the total population of phagocytes examined.
Inhibition of germination to hyphae by MNCs and PAMs.
Another set of coverslips identical to those for phagocytosis was incubated with conidia at 37°C and 5% CO2 for 1 h, washed, and further incubated for 8 h in CM to allow viable conidia to germinate (33). After germination of the conidia, the coverslips were fixed and stained. Coverslips having conidia but not phagocytes were used as controls. Percent inhibition of germination was determined as the percentage of intracellular conidia that had not germinated divided by a total of 100 fungi.
Conidiocidal assay.
The phagocytosis and intracellular conidiocidal activity of MNCs and PAMs against Aspergillus conidia were also assessed by use of a CFU assay (31). One million A. fumigatus conidia were mixed with 106 MNCs seeded in each of quadruplicate wells of 12-well plates (Costar), which had been incubated with or without TNF-α, in 1 ml of CM, and the mixtures were incubated for 1 h at 37°C with 5% CO2. Wells were then washed, and in two of them 0.5 ml of supernatant was replaced by 1% Triton X-100. Vigorous pipetting was performed, a portion of this suspension was mixed with sterile H2O, and dilutions were made in normal saline and plated in duplicate on Sabouraud dextrose agar plates.
The remaining two wells were further incubated with fresh CM for 6 h, 1% Triton X-100 was added, and dilutions were plated on Sabouraud dextrose agar plates. All plates were incubated at 37°C for 24 h (31). Colonies were counted, and two measurements were conducted: phagocytic activity, calculated as the number of CFU grown after the 1-h incubation and washout of extracellular conidia (C0), and intracellular conidiocidal activity, calculated according to the following formula: percent intracellular killing = (1−C6/C0) × 100, where C6 is the number of CFU at the end of the 6-h incubation and C0 is the number of CFU at the beginning of the 6-h incubation (after a 1-h incubation for phagocytosis and washing from extracellular conidia).
Statistics.
Differences between values at individual TNF-α concentrations and baseline values were assessed by use of analysis of variance (ANOVA) with the Dunnett test for correction of multiple comparisons. The paired Student t test was also used in some comparisons of means in which only two points were compared. Results were expressed as means ± standard errors of means (SEMs). All P values reported are two-sided. A P value ≤0.05 was considered to be significant.
RESULTS
Effect of TNF-α on PMN-released superoxide anion.
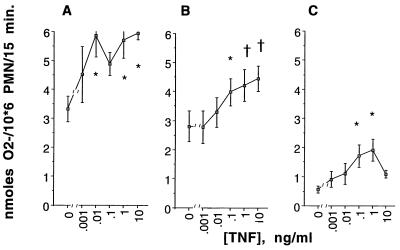
At concentrations of 0.01 to 10 ng/ml, TNF-α significantly increased O2− production by PMNs in response to PMA (by ANOVA, P = 0.046 [Fig. 1A]), FMLP (by ANOVA, P = 0.0002 [Fig. 1B]), and hyphae of A. fumigatus (by ANOVA, P = 0.024 [Fig. 1C]).
FIG. 1.
Effect of TNF-α at 0.001 to 10 ng/ml on O2− released by PMNs in response to 0.1 μg of PMA per ml (A), 0.5 μM FMLP (B), and unopsonized hyphae of A. fumigatus at an E/T ratio of 1/1 (C). Values are means ± SEMs derived from five to seven experiments performed with each concentration of TNF-α and each stimulus. PMNs were pretreated with the concentrations of TNF-α indicated on the horizontal axis for 30 min at 37°C in HBSS without Ca2+ or Mg2+. The differences between the TNF-α-treated PMNs and the buffer-pretreated PMNs (percent enhancement) are significant by the Dunnett test (∗, P < 0.05; †, P < 0.01).
Effect of TNF-α on PMN-mediated damage of A. fumigatus hyphae and phagocytosis of conidia.
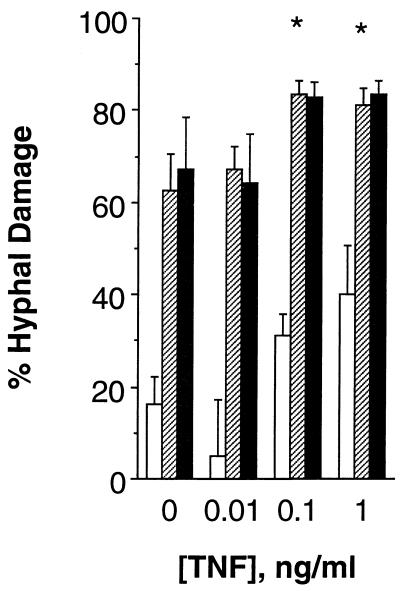
At concentrations of 0.1 to 1 ng/ml, TNF-α increased the percentage of PMN-induced hyphal damage at E/T ratios of 1/1, 5/1, and 10/1 (by ANOVA, P = 0.056, 0.022, and 0.07, respectively [Fig. 2]). However, only the results obtained with incubation of PMNs and hyphae at an E/T ratio of 5/1 were statistically significant after correction for multiple comparisons by the Dunnett test (Fig. 2; P < 0.05). In contrast, TNF-α at concentrations of 0.001 to 10 ng/ml did not exert any effect on PMN phagocytosis of conidia in the presence of PHS.
FIG. 2.
Effect of TNF-α at 0.01 to 1 ng/ml on damage of unopsonized hyphae of A. fumigatus caused by PMNs. PMNs were pretreated with the concentrations of TNF-α indicated on the horizontal axis for 30 min at 37°C in HBSS without Ca2+ or Mg2+ and were then added to wells containing 105 hyphae per well and incubated at 37°C for 2 h. The E/T ratios shown in this figure are 1/1 (□), 5/1 (▨), and 10/1 (■). Data presented have been derived from six experiments performed with each concentration of TNF-α and each E/T ratio. The vertical bars indicate SEMs. The differences between the TNF-α-treated PMNs and the buffer-pretreated PMNs (percent suppression) are significant by the Dunnett test (∗, P < 0.05).
Effect of TNF-α on MNC functions.
At 0.01 ng/ml, TNF-α slightly increased O2− production by MNCs in response to PMA (Table 1; P = 0.05) without, however, a concomitant increase in O2− production by MNCs in response to unopsonized hyphae at an E/T ratio of 1/1 (Table 1) or in the percentage of MNC-induced hyphal damage exhibited at any E/T ratio tested (1/1, 5/1, and 10/1; data not shown). In addition, incubation of MNCs with TNF-α at 0.001 to 10 ng/ml for 2 days had no effect on the percent phagocytosis of conidia, phagocytic index, or total phagocytic index in the presence of PHS. Neither the number of intracellular conidia, measured in a CFU assay (CFU phagocytosis), nor conidiocidal activity of MNCs against intracellular conidia was significantly increased by TNF-α at 0.01 to 10 ng/ml.
TABLE 1.
Effects of TNF-α on the oxidative burst of human MNCs in response to PMA and unopsonized hyphae of A. fumigatusa
| [TNF-α], ng/ml | O2 production (nmol of O2−/106 MNCs/15 min) stimulated by:
|
|
|---|---|---|
| PMA | Hyphae | |
| Control | 3.4 ± 0.4 | 1.0 ± 0.4 |
| 0.01 | 3.9 ± 0.6b | 0.8 ± 0.2 |
| 1 | 3.8 ± 0.6 | 1.3 ± 0.4 |
MNCs were pretreated with the concentrations of TNF-α indicated (0.01 and 1 ng/ml) for 2 days at 37°C. O2− released by MNCs in response to PMA (0.1 μg/ml) and unopsonized hyphae of A. fumigatus at an E/T ratio of 1/1 was determined. Means ± SEMs were derived from 8 to 14 experiments performed with each concentration of TNF-α and each stimulus.
The differences between the TNF-α-treated MNCs and the buffer-pretreated MNCs (percent enhancement) are slightly significant by the paired Student t test (∗, P = 0.05).
Effects of TNF-α on antifungal PAM functions.
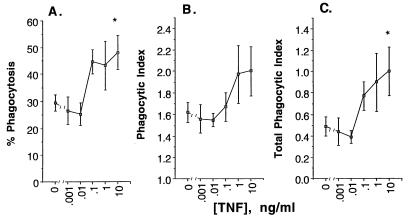
Despite the species difference, human TNF-α exhibited modulatory effects on rabbit PAMs. Incubation of PAMs with TNF-α at 1 to 10 ng/ml for 2 days significantly enhanced the percent phagocytosis of A. fumigatus (Fig. 3A), phagocytic index, (Fig. 3B; by ANOVA, P = 0.003 and 0.028, respectively), and total phagocytic index (Fig. 3C; by ANOVA, P = 0.008) in the presence of FBS.
FIG. 3.
Effect of TNF-α at 0.001 to 10 ng/ml on phagocytic activity of rabbit PAMs expressed as percent phagocytosis (A), phagocytic index (B), and total phagocytic index (C). The data are presented as means ± SEMs derived from five experiments performed with each concentration of TNF-α and an equal number of killed rabbits. The PAMs were pretreated with TNF-α as indicated on the horizontal axis for 2 days at 37°C in CM. The differences between TNF-α-treated PAMs and buffer-pretreated PAMs (percent enhancement) are significant by the Dunnett test (∗, P < 0.05).
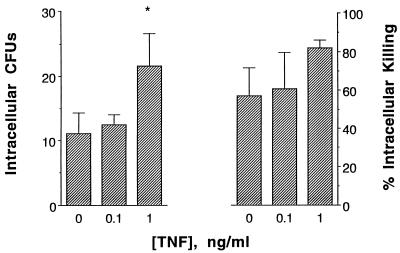
In order to study further the intracellular fate of phagocytosed conidia, intracellular killing was evaluated. Incubation of PAMs with TNF-α at 1 ng/ml for 2 days increased PAM-induced phagocytosis of conidia in the presence of FBS, as measured by growth of intracellular conidia in the CFU assay (Fig. 4, left panel; by ANOVA, P = 0.003), confirming the results of the previous studies shown in Fig. 3. In contrast, TNF-α did not cause a significant increase in the percentage of intracellular killing (Fig. 4, right panel), suggesting that TNF-α may not enhance nonoxidative microbicidal mechanisms against A. fumigatus.
FIG. 4.
Effect of TNF-α at 0.1 to 1 ng/ml on phagocytic activity (left panel) and intracellular fungicidal activity (right panel) of rabbit PAMs against conidia of A. fumigatus after incubation with either CM alone or CM with various concentrations of TNF-α for 2 days. PAMs that were preincubated with CM and TNF-α or with CM alone in wells were exposed to 106 conidia per well and were incubated at 37°C for 1 h. After this incubation, CFU phagocytosis was evaluated, and after a further 6-h incubation, the percent intracellular killing was evaluated. The difference in CFU phagocytosis between TNF-α (1 ng/ml)-treated PAMs and controls (percent enhancement) is significant by the Dunnett test (†, P < 0.01).
DISCUSSION
In this study, we demonstrated for the first time that TNF-α augments phagocytic host defenses against A. fumigatus. Specifically, TNF-α augments the capacity of human PMNs to produce oxidative burst metabolites in response to hyphae of A. fumigatus. This augmentation is associated with enhanced PMN-induced hyphal damage. Further, TNF-α upregulates phagocytosis of conidia by rabbit PAMs. In contrast, it has no effect on antifungal activity of human MNCs against conidia or hyphae or on intracellular fate of conidia in MNCs and PAMs.
Among its potent immunomodulatory effects on PMN function are the following. TNF-α increases the adherence of PMNs on endothelial membranes (17, 38), phagocytic activity of PMNs against opsonized zymosan (20), the O2− and hydrogen peroxide (H2O2) release in response to soluble stimuli and hyphae of Candida albicans (3, 13, 16, 40), degranulation of PMNs (16, 20), and antibody-dependent cell cytotoxicity (28, 39). In addition, TNF-α has been found to enhance fungicidal responses of human PMNs against blastoconidia of C. albicans (14, 15) and C. glabrata (15) but not against hyphae of C. albicans (13). However, to our knowledge no study has addressed the comparative effects of TNF-α on different phagocytic cells or against filamentous fungi.
In this study, we evaluated three major phagocytic cell types and found significant differences in their responsiveness to TNF-α. While phagocytic activity of macrophages and killing activity of PMNs were responsive to TNF-α, none of the activities of monocytes was significantly responsive. The variable effects of TNF-α on the fungicidal activity of different phagocytes or against different forms of the same organism emphasize its complicated interactions with the fungicidal mechanisms of these cells. Whether the latter difference is due to varying expression of p55 receptors or to other factors is unclear. Nevertheless, TNF-α appears to enhance both an early event during host defense against invasion by Aspergillus conidia (namely PAM phagocytosis) and a late event during this process (namely, O2− production and hyphal damage by PMNs). In contrast to the wealth of literature on various effects of TNF-α on PMNs, very few studies have reported the effects of TNF-α on monocyte or macrophage functions (43). Our results showing activation of phagocytic capacity of PAMs are novel in this regard, suggesting an autocrine function of TNF-α.
As PHS possessed intact complement activity and main components, it is possible that complement-mediated phagocytosis by PMNs and MNCs may be operational in our study. The role of antibody in this process remains to be elucidated. On the other hand, FBS contained neither CH50 activity nor C3 or C4, thus confirming that PAMs exert their defensive activity against conidia through complement-independent mechanisms (45).
TNF-α is an important proinflammatory cytokine, being an integral part of the host response to infectious challenges, including those by fungal pathogens. Its importance as a potent host defense modulator is underscored by three observations. (i) In vivo neutralization of endogenous TNF-α by administration of anti-TNF-α antibody or antagonization of its effects by TNF-α inhibitors exacerbated the fungal burden of C. albicans and increased the mortality of animals (25, 27). (ii) Together with interleukin 1, TNF-α is secreted very early during an infectious challenge, suggesting its important role as a proinflammatory cytokine (5). (iii) Administration of TNF-α to mice enhances their resistance to acute infection by C. albicans and decreases their mortality (25, 42). In addition, TNF-α plays a protective role in murine models of histoplasmosis and cryptococcosis (2, 41). These observations emphasize the key importance of TNF-α in host defense during early stages of fungal infections.
In line with these observations, we present new data demonstrating that TNF-α is not only an important cytokine in host defense against yeast like fungi but also against a medically important filamentous fungus. However, its excessive toxicities in doses required to have biologically useful effects preclude its safe administration to humans. Attempts are being undertaken to construct a TNF-α molecule that retains its biological effects without the toxic side effects. Such a nontoxic compound may be useful in management of invasive aspergillosis.
Our findings with Aspergillus hyphae and PMNs differ from the results of a previous study on the effects of TNF-α on the antifungal activity of human PMNs against hyphae of C. albicans (13). In that study, TNF-α did not enhance PMN fungicidal activity against hyphae of C. albicans but actually suppressed it, despite the fact that it enhanced O2− production in response to hyphae of the same organism. This discrepancy may be crucial in host defenses against the two fungi and may be due to differences in interaction of PMNs with the surfaces of these two organisms (11).
The concentrations used in this study were within the range of or lower than concentrations that can be measured in the early stages of infection as shown during meningitis (18, 44) and, to a lesser degree, during septicemia (30). They are also comparable to concentrations of TNF-α produced in vitro after challenge of mononuclear cells with endotoxin or C. albicans (23).
While we were undertaking our experiments, Nagai et al. published the results of a study with a murine model of invasive aspergillosis (26). In that study, exogenous administration of TNF-α protected animals from invasive aspergillosis and lowered mortality as well as the number of organs infected by Aspergillus. This protection can be explained by our in vitro findings that TNF-α broadly enhances phagocytic host defenses against A. fumigatus.
Recently, great efforts have been undertaken by many investigators to decrease mortality of septic shock. Attempts to neutralize endogenous TNF-α produced during septicemia have led to clinical trials of administration of anti-TNF-α antibody to patients with septic shock (1, 9). Neutralization of TNF-α, however, may be followed by a loss of the beneficial effects of this cytokine on host defenses. Investigators must be aware of this potential iatrogenic deficiency and proceed with caution in clinical trials of inhibitors of endogenous TNF-α.
In conclusion, this study demonstrated that TNF-α enhances specific antifungal activities during host response to invasive aspergillosis, particularly phagocytic activity of PAMs as an early event and PMN-induced hyphal injury as a late event. These findings also underscore the need for caution in utilizing anti-TNF-α compounds in immunocompromised patients.
ACKNOWLEDGMENTS
We are grateful to Sevasti Tsaparidou of the Infectious Disease Laboratory in the Third Department of Pediatrics for technical assistance and to the staff of the Laboratory Animal Facility of the Immunocompromised Host Section of the National Cancer Institute for assistance with the rabbits. We also thank Thomas A. Fleisher and the staff of the Immunology Service of the Clinical Pathology Department at the Clinical Center, National Institutes of Health, where the measurements of complement in normal serum were performed.
REFERENCES
- 1.Abraham E, Glauser M P, Butler T, Garbino J, Gelmont D, Laterre P F, Kudsk K, Bruining H A, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA. 1997;277:1531–1538. [PubMed] [Google Scholar]
- 2.Aguirre K, Havell E A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson Y H, Marasco W A, Lopez A F, Vadas A M. Recombinant human tumor necrosis factor alpha. Regulation of N-formyl methionyl leucyl phenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988;81:759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986;320:584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Milsark I W, Cerami A. Cachectin/tumor necrosis factor: production, distribution and metabolic fate in vivo. J Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- 7.Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. 97(Suppl.):77–89. [PubMed]
- 8.Cenci E, Perito S, Enssle K-H, Mosci P, Latge J-P, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 11.Diamond R D, Krzesicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro: a possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978;91:313–328. [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond R D, Krzesicki R, Jao W. Damage to pseudohyphal form of C. albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978;61:349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond R D, Lyman C A, Wysong D R. Disparate effects of interferon-gamma and tumor necrosis factor-alpha on early neutrophil respiratory burst and fungicidal responses to Candida albicans hyphae in vitro. J Clin Invest. 1991;87:711–720. doi: 10.1172/JCI115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djeu J Y, Blanchard D K, Halkias D, Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986;137:2980–2984. [PubMed] [Google Scholar]
- 15.Ferrante A. Tumor necrosis factor alpha potentiates neutrophil antimicrobial activity: increased fungicidal activity against Torulopsis glabrata and Candida albicans and associated increases in oxygen radical production and lysosomal enzyme release. Infect Immun. 1989;57:2115–2122. doi: 10.1128/iai.57.7.2115-2122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrante A, Nandoskar M, Walz A, Goh D H B, Kowanko I C. Effects of tumour necrosis factor alpha and interleukin-1 alpha and beta on human neutrophil migration, respiratory burst and degranulation. Int Arch Appl Immunol. 1988;86:82–91. doi: 10.1159/000234610. [DOI] [PubMed] [Google Scholar]
- 17.Gamble J R, Harlan J M, Klebanoff S J, Vadas N A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Inflammation/tumor resection/adherence proteins/blood vessels. Proc Natl Acad Sci USA. 1985;82:8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glimaker M, Kragsbjerg P, Forsgren M, Olcen P. Tumor necrosis factor-alpha (TNF alpha) in cerebrospinal fluid from patients with meningitis of different etiologies: high levels of TNF alpha indicate bacterial meningitis. J Infect Dis. 1993;167:882–889. doi: 10.1093/infdis/167.4.882. [DOI] [PubMed] [Google Scholar]
- 19.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a University Hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 20.Klebanoff S J, Vadas M A, Harlan J M, Sparks L H, Gamble J R, Agosti J M, Waltersdorph A M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–4225. [PubMed] [Google Scholar]
- 21.Levitz S, Selsted M E, Ganz T, Lehrer R I, Diamond R D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154:483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- 22.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 23.Levitz S M, Tabuni A, Nong S-H, Golenbock D T. Effects of interleukin-10 on human peripheral blood mononuclear cell responses to Cryptococcus neoformans, Candida albicans, and lipopolysaccharide. Infect Immun. 1996;64:945–951. doi: 10.1128/iai.64.3.945-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liles W C, Huang J E, van Burik J A, Bowden R A, Dale D C. Granulocyte colony-stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J Infect Dis. 1997;175:1012–1015. doi: 10.1086/513961. [DOI] [PubMed] [Google Scholar]
- 25.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W J, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai H, Guo J, Choi H, Kurup V. Interferon-γ and tumor necrosis factor-α protect mice from invasive aspergillosis. J Infect Dis. 1995;172:1554–1560. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 27.Netea M G, Blok W L, Kullberg B J, Bemelmans M, Vogels M T, Buurman W A, van der Meer J W. Pharmacologic inhibitors of tumor necrosis factor production exert differential effects in lethal endotoxemia and in infection with live microorganisms in mice. J Infect Dis. 1995;171:393–399. doi: 10.1093/infdis/171.2.393. [DOI] [PubMed] [Google Scholar]
- 28.Perussia B, Kobayashi M, Rossi M E, Anegon I, Trinchieri G. Immune interferon enhances properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987;138:765–774. [PubMed] [Google Scholar]
- 29.Rex J H, Bennett J E, Gallin J I, Malech H L, Decarlo E S, Melnick D A. In vivo interferon-γ therapy augments the in vitro ability of chronic granulomatous disease neutrophils to damage Aspergillus hyphae. J Infect Dis. 1991;163:849–852. doi: 10.1093/infdis/163.4.849. [DOI] [PubMed] [Google Scholar]
- 30.Riche F, Panis Y, Laisne M J, Briard C, Cholley B, Bernard-Poenaru O, Graulet A M, Gueris J, Valleur P. High tumor necrosis factor serum level is associated with increased survival in patients with abdominal septic shock: a prospective study in 59 patients. Surgery. 1996;120:801–807. doi: 10.1016/s0039-6060(96)80087-0. [DOI] [PubMed] [Google Scholar]
- 31.Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo P A, Walsh T J. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–329. [PubMed] [Google Scholar]
- 32.Roilides E, Pizzo P A. Modulation of host defenses by cytokines: evolving adjuncts in prevention and treatment of serious infections in immunocompromised hosts. Clin Infect Dis. 1992;15:508–524. doi: 10.1093/clind/15.3.508. [DOI] [PubMed] [Google Scholar]
- 33.Roilides E, Sein T, Holmes A, Blake C, Pizzo P A, Walsh T J. Effects of macrophage colony-stimulating factor on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Infect Dis. 1995;172:1028–1034. doi: 10.1093/infdis/172.4.1028. [DOI] [PubMed] [Google Scholar]
- 34.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Granulocyte colony-stimulating factor and interferon-γ enhance the oxidative responses and the damage caused by human neutrophils to Aspergillus fumigatus hyphae in vitro. Infect Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roilides E, Walsh T J, Pizzo P A. Clinical use of cytokines during fungal infections. In: van Furth R, editor. Hemopoietic growth factors and mononuclear phagocytes. S. Basel, Switzerland: Karger; 1993. pp. 90–97. [Google Scholar]
- 36.Roilides E, Walsh T J, Pizzo P A, Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163:579–583. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 37.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus: observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seow W K, Thong Y H, Ferrante A. Macrophage-neutrophil interactions: contrasting effects of the monokines interleukin-1 and tumor necrosis factor (cachectin) on human neutrophil adherence. Immunology. 1987;62:357–361. [PMC free article] [PubMed] [Google Scholar]
- 39.Shalaby M R, Aggarwal B B, Rinderknecht E, Svendersky L P, Finkle B S, Palladino M A. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factor. J Immunol. 1985;135:2069–2073. [PubMed] [Google Scholar]
- 40.Shalaby M R, Palladino M A, Hirabayashi S E, Eessalu T E, Lewis G D, Shepard H M, Aggarwal B. Receptor binding and activation of polymorphonuclear neutrophils by tumor necrosis factor-alpha. J Leukoc Biol. 1987;41:196–204. doi: 10.1002/jlb.41.3.196. [DOI] [PubMed] [Google Scholar]
- 41.Smith J G, Magee D M, Williams D M, Graybill J R. Tumor necrosis factor-alpha plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990;162:1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- 42.Steinshamn S, Bemelmans M H, van Tits L J, Bergh K, Buurman W A, Waage A. TNF receptors in murine Candida albicans infection: evidence for an important role of TNF receptor p55 in antifungal defense. J Immunol. 1996;157:2155–2159. [PubMed] [Google Scholar]
- 43.Urban J L, Shepard H M, Rothstein J L, Sugarman B J. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci USA. 1986;83:5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Furth A M, Seijmonsbergen E M, Langermans J A, Groeneveld P H, de Bel C E, van Furth R. High levels of interleukin 10 and tumor necrosis factor alpha in cerebrospinal fluid during the onset of bacterial meningitis. Clin Infect Dis. 1995;21:220–222. doi: 10.1093/clinids/21.1.220. [DOI] [PubMed] [Google Scholar]
- 45.Waldorf A R, Levitz S, Diamond R D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984;150:752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- 46.Walsh T J. Invasive aspergillosis in patients with neoplastic diseases. Semin Resp Infect. 1990;5:111–122. [PubMed] [Google Scholar]