Abstract
Chronic obstructive pulmonary disease (COPD) kills millions of people annually and patients suffering from exacerbations of this disorder display high morbidity and mortality. The clinical course of COPD is associated with dysbiosis and infections, but the underlying mechanisms are poorly understood. Glycosylation of proteins play roles in regulating interactions between microbes and immune cells, and knowledge on airway glycans therefore contribute to the understanding of infections. Furthermore, glycans have biomarker potential for identifying smokers with enhanced risk for developing COPD as well as COPD subgroups. Here, we characterized the N-glycosylation in the lower airways of healthy never-smokers (HNS, n = 5) and long-term smokers (LTS) with (LTS+, n = 4) and without COPD (LTS−, n = 8). Using mass spectrometry, we identified 57 highly confident N-glycan structures whereof 38 oligomannose, complex, and paucimannose type glycans were common to BAL samples from HNS, LTS− and LTS+ groups. Hybrid type N-glycans were identified only in the LTS+ group. Qualitatively and quantitatively, HNS had lower inter-individual variation between samples compared to LTS− or LTS+. Cluster analysis of BAL N-glycosylation distinguished LTS from HNS. Correlation analysis with clinical parameters revealed that complex N-glycans were associated with health and absence of smoking whereas oligomannose N-glycans were associated with smoking and disease. The N-glycan profile from monocyte-derived macrophages differed from the BAL N-glycan profiles. In conclusion, long-term smokers display substantial alterations of N-glycosylation in the bronchoalveolar space, and the hybrid N-glycans identified only in long-term smokers with COPD deserve to be further studied as potential biomarkers.
Keywords: airway, COPD, glycosylation, macrophage, N-glycans
Introduction
Chronic obstructive pulmonary disease (COPD) affects around 8% of the adult population and is the 4th most common cause of disease-related mortality in Sweden (Danielsson et al. 2012; Backman et al. 2016). Here, COPD is predominately caused by long-term cigarette smoking, and the clinical course is often characterized by a slowly progressing airflow limitation, which is irreversible (Donaldson et al. 2002). The clinical long-term prognosis in COPD, including the decline in lung function, relates strongly to the occurrence of COPD exacerbations, a complication with very high morbidity and mortality (Donaldson et al. 2002; Anzueto 2010). Exacerbation, in turn, relate to the occurrence of bacterial dysbiosis and infection (Anzueto 2010).
The alveolae in the lungs are covered by a thin liquid film, which contains inflammatory cells, epithelial and endothelial cells, soluble glycoproteins, lipids and other biochemical components (Balbi et al. 2007). The fluid can be accessed via bronchoscopy using bronchoalveolar lavage (BAL). Currently, analyses of BAL serve predominantly as a research tool for the evaluation of lower respiratory tract pathology in COPD (Reynolds 2000; Balbi et al. 2007). Numerous studies of differential cell counts in BAL samples have demonstrated an overall increase in the total number of leukocytes (Padra et al. 2020), an increase both in polymorphonuclear leukocytes (PMNs) and alveolar macrophages (AMs) among tobacco smokers (Stockley 2002; Barnes 2004; Hodge et al. 2007). The AMs, derived largely from blood monocytes, are major effector cells in the lung, and provide the first line of defense against pathogens (Rubins 2003; Fingleton 2017). AMs also regulate adaptive immune response and inflammation, including the production of cytokines and metalloproteinases, and these phagocytes also present antigens (Senior and Anthonisen 1998; Atkinson and Senior 2003; Fingleton 2017).
The field of glycobiology has grown tremendously due to the discovery and potential of glycan-based biomarkers for a range of conditions, including disease related to chronic infection and inflammation as well as cancer (Dube and Bertozzi 2005; Fuster and Esko 2005; Arnold et al. 2011). Given this, it is especially interesting that BAL samples are rich in proteins and immune cells and can therefore be expected to provide a source of potential glycan-based biomarkers.
We recently demonstrated that the O-glycosylation of mucin glycoproteins from BAL samples obtained from long-term tobacco smokers with and without COPD is altered (Padra et al. 2021). The composition and quantity of BAL mucin glycoproteins also differ between smokers (with or without COPD) and healthy never-smokers (Padra et al. 2020). Furthermore, one study has shown an increase in defective N-glycosylated, carbohydrate-deficient transferrin in COPD patient serum compared to non-COPD subjects (Nihlén et al. 2001). O- and N-glycosylation on macrophage and neutrophil derived proteins have also been documented in previous studies (Hare et al. 2017; Venkatakrishnan et al. 2020; Reiding et al. 2021).
In the current study we characterized the N-glycosylation of proteins in the bronchoalveolar space of long-term tobacco smokers with (LTS+) and without COPD (LTS−), compared with healthy never-smokers (HNS), using a patient material previously used for analyzing mucin composition, complex size, O-glycosylation and Moraxella catarrhalis binding (Padra et al. 2021). By comparing the N-glycome of BAL proteins in the HNS and LTS− groups and between LTS− and LTS+ groups we were able to associate smoking exposure and disease (COPD) with N-glycosylation of BAL proteins. Furthermore, we analyzed N-glycans from in vitro-generated monocyte-derived macrophages (MDM) and compared them with N-glycans from BAL proteins.
Results
Clinical characteristics
We characterized the BAL protein N-glycosylation profile obtained from HNS (n = 5), LTS− (n = 8) and LTS+ (n = 4) groups and the general clinical characteristics of the utilized material have been described in previous publications (Padra et al. 2020, 2021). Briefly, the study material available for the current analyses included both genders, with a similar and substantial tobacco load for both the LTS− and LTS+ group (Table 1). The presence of pathogenic bacteria was detected in one of the included HNS samples only (Padra et al. 2020). Macrophages dominated among BAL leukocytes in all three study groups (Table 1).
Table 1.
Overview of BAL samples used in this study.
| Variable | HNS | LTS− | LTS+ |
|---|---|---|---|
|
Subject Characteristics No. of individuals Gender (Male/Female) Age (Year)a Pack-Yearsa FVC FEV1 FEV1 (% predicted) FEV1/FVC Serum cotinine (pg/mL) |
5 4/1 45 (41–66) – 0 (0–0) 4.7 (2.6–5.1) 108 (100–127) 74 (68–83) 0 (0–0) |
8 3/5 67.5 (45–72) 33 (21–51) 3.2 (2.5–5.4) 2.4 (2.0–3.9) 100 (68–116) 73 (70–82) 376 (19–500) |
4 2/2 57 (52–64) 32.5 (20–40) 4.4 (3.5–6.3) 2.9 (2.1–3.2) 81 (75–105) 61 (50–68) 306 (184–420) |
|
BAL Specificities
a
BAL recovery (%)b Cell conc. (106/liter)c Macrophages (%) Neutrophils (%) Lymphocytes (%) |
57 (25–74) 98.9 (44.3–221) 93 (70–97) 1 (1–4) 3 (2–29) |
53 (32–69) 218 (157–503) 98 (95–99) 1 (0–2) 1(0–3) |
36.8 (22–60) 292.6 (216–433) 86 (69–96) 8.5 (2–18) 1.5 (0–14) |
Summary of key clinical characteristics for the current study, described in detail in previous publications (Padra et al. 2020, 2021).
aData presented as median (min-max).
bBAL recovery is the ratio between the return volume and instillation volume during the BAL procedure.
cCell concentration in BAL samples. FVC, forced vital capacity; FEV1, forced expiratory volume during 1 s.
Qualitative overview of the BAL N-glycome
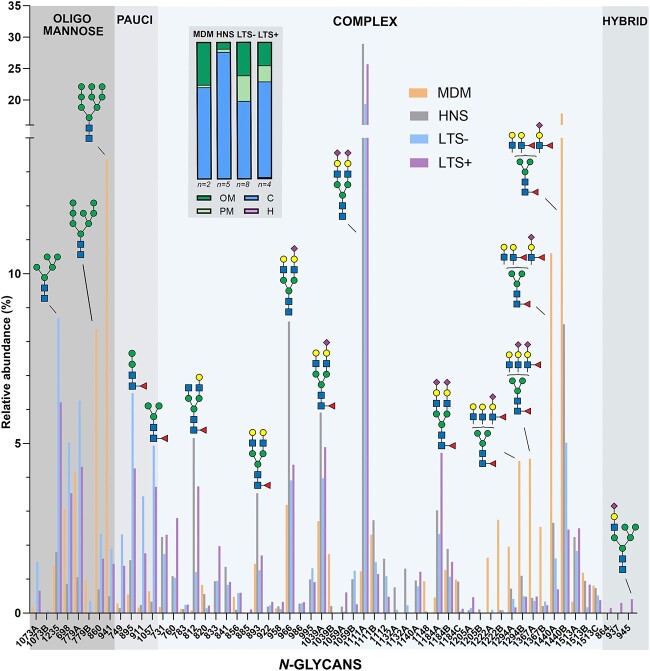
N-glycans were released from soluble proteins isolated from BAL samples from each patient and analyzed as reduced non-derivatized alditols using graphitized carbon chromatography and mass spectrometry. Figure 1 presents representative combined mass spectra over the LC–MS range. A qualitative observation was that the N-glycans in the BAL fluid from HNS and LTS− groups displayed different mass spectral profiles. To identify the nature of the differences, the first step was to group the N-glycans according to their types, i.e. oligomannose, paucimannose, hybrid, and complex N-glycans. By this approach, we found that mannosylated glycans (oligomannose and paucimannose) dominated the spectral profiles in the LTS− group, whereas complex N-glycans dominated the profiles in the HNS group. The spectral profiles from the LTS+ group were relatively similar to those from the LTS− group. By LC separation we could identify 26 isomeric structures giving a total of 57 N-glycans from the 41 masses (monosaccharide compositions) detected in the samples (Supplementary Table S1).
Fig. 1.
LC/MS analyses of N-glycans from BAL proteins. Combined mass spectral profiles (15–24 min) from LC/MS analyses of a never-smoker, an asymptomatic long-term smoker and a COPD patient, representative of the three patient groups in this study (HNS, LTS− and LTS+). The most abundant N-glycans are annotated using the SNFG nomenclature [26].
Cluster analysis of BAL protein N-glycosylation distinguishes smokers (LTS−/+) from never-smokers (HNS)
The relationship of the BAL protein N-glycosylation profiles between the HNS, LTS− and LTS+ groups was further assessed by a cluster dendrogram using the relative abundances of the N-glycans (Fig. 2). The limited intra-group N-glycome variation among HNS samples was illustrated by tight clustering of four out of five samples. The separation in the clustering between HNS and the smoker groups (LTS− and LTS+) supports a potential role played by smoking in regulating the BAL protein N-glycosylation.
Fig. 2.

Relatedness of the BAL protein N-glycosylation. Cluster dendrogram of the BAL protein N-glycomes of healthy never-smokers (HNS), long-term smokers without (LTS−) and with (COPD (LTS+), measured using the relative abundances of the observed N-glycans as detected with liquid chromatography-mass spectrometry.
Hybrid type N-glycans were identified in the COPD (LTS+) group only
Out of the 57 N-glycans characterized, 38 oligomannose, complex, and paucimannose type glycans were common to all three patient groups. An overview of characterized N-glycans in all samples is shown in Fig. 3. 1, 3 and 4 structures were unique to HNS, LTS− and LTS+ groups, respectively, of which three hybrid type N-glycans were identified only in the LTS+ group (Fig. 3). The hybrid glycans constituted between 0.7%–2% of the N-glycans in three of the four patients analyzed (Fig. 4A and B). Two examples of MS/MS spectra of hybrid glycans detected at m/z 937.4 and m/z 945.6 from patient LTS4+ are shown in Supplementary Figs S1 and S2. Sub-structural features, such as NeuAc linked to Gal and α1,6 linked core fucosylation were the most abundant glyco-epitopes identified in all the sample groups, and β1,4 linked bisecting GlcNAcylation was detected in all groups (Fig. 3 and Supplementary Table S1). Qualitatively and quantitatively, the HNS group had very low inter-individual variation between samples compared to the LTS− or LTS+ groups (Fig. 4A and B).
Fig. 3.
Structural characterization of N-glycans on BAL proteins by LC–MS and MS/MS. Venn diagram of the distribution of N-glycans detected in BAL from healthy never-smokers (HNS), long-term smokers without (LTS−) and with COPD (LTS+).
Fig. 4.

Relative abundance (%) of N-glycan types on BAL proteins from individual subjects as determined by LC/MS. A) Glycan data is presented as horizontal bars for the individual patients to illustrate the proportion and inter-individual distribution of the glycans. B) Grouped glycan data for healthy never-smokers (HNS), long-term smokers without (LTS−) and with COPD (LTS+). The horizontal bars represent the median for the groups (**P < 0.01, Kruskal-Wallis with Dunn’s multiple comparison test).
Increased mannosylation and decreased relative amounts of complex glycans in BAL samples from long-term smokers (LTS−/+) compared to never-smokers (HNS)
The inter-individual variation among HNS samples was low and predominantly consisted of complex N-glycans (93.6% [89.5–95.5]; median [interquartile range]) and less abundant oligomannose (4.7% [3.2–6.9]) and paucimannose (1.2% [1.2–3.6]) type N-glycans (Fig. 4). Although the LTS− group also had a high abundance of complex N-glycans, their relative abundance was lower (54.8 [39.3–73.5], p = 0.003, Kruskal-Wallis with Dunn’s multiple comparison test) than in the HNS group. The decrease in complex glycans was compensated by the subsequent increase in oligomannose (25.3% [16.9–33.3], p = 0.003) and paucimannose glycans (19.0% [9.2–28.8], p = 0.006). The BAL N-glycosylation pattern in the LTS+ group was relatively similar to that of the LTS− group, with complex N-glycans dominating the spectra (69.1% [59.5–82.2]), followed by oligomannose (17.9% [11.5–22.0]) and paucimannose (12.7% [4.7–18.0]) type N-glycans. Hybrid glycans were identified in three out of four LTS+ samples, albeit with a low relative abundance (0.7% [0.2–1.7]).
Differences in BAL sialylation and fucosylation in the N-glycome of long-term smokers (LTS−/+) compared to never-smokers (HNS)
A statistically significant decrease in sialylation was observed in the LTS− group compared to the HNS control group, where HNS had a median of 75.0% (69.5–81.8) and LTS− of 45.3% (30.8–61.7, Fig. 5A, p = 0.01). The change was explained by the overall decrease of complex glycans compared to mannosylated glycans (Fig. 4), which are not sialylated.
Fig. 5.

Sialylation and fucosylation of N-glycans from BAL proteins from individual subjects as determined by LC/MS. Relative abundance of A) sialylation, B) fucosylation (sum of glycans with core and terminal fucosylation, C) core fucosylation and D) terminal fucosylation identified on N-glycans in BAL samples from for healthy never-smokers (HNS), long-term smokers without (LTS−) or with COPD (LTS+). The bars indicate the median. (**P < 0.01, *P < 0.05, Kruskal-Wallis with Dunns multiple comparison test).
The relative abundance of fucosylated N-glycans (sum of core Fuc and Fuc present on the outer branches, “terminal Fuc”) in the HNS, LTS− and LTS+ groups, were 50.3% (48.9–52.8), 38.9% (36.4–44.8) and 42.5% (40.8–44.1), respectively, being significantly lower in the LTS− group compared to HNS (p = 0.006, Fig. 5B). This decrease was reflected by a lower relative abundance of both core fucosylated N-glycans (Fig. 5C, p = 0.03) and N-glycans with terminal Fuc (Fig. 5D, p = 0.05). N-glycans with terminal Fuc were also significantly decreased in LTS+ compared to HNS (Fig. 5D, p = 0.02).
A total of nine structures were identified to be complex bisecting N-glycans, based on their early retention on PGC column and the presence of the diagnostic [D—221] ion in the MS/MS spectra (Harvey et al. 2008). The relative abundance of bisecting N-glycans was similar in all groups (HNS: 9.0% [4.5–10.7], LTS−: 5.7% [4.4–6.9] and LTS+: 5.5% [3.4–16.5].
The proportion of Man4, Man3, and M2F within the oligomannose and paucimannose groups differ in smokers (LTS−/+) compared to never-smokers (HNS)
The BAL N-glycome from the three groups was investigated to identify further variations of the oligomannose and paucimannose N-glycans on the sub-structural level. The oligomannose type N-glycans were classified between Man4 to Man9 glycans and although the overall abundance of oligomannose glycans compared to the other N-glycan types was higher among smokers (LTS−/+) compared to never-smokers (HNS), the relative distribution of Man5 to Man9 structures within the oligomannose N-glycan pool were found to be similar in all three groups (Fig. 6). Man4 oligomannose glycans were identified only in one HNS sample out of five but were present in all samples in the LTS− (p = 0.01) and LTS+ groups. Man5 was the most abundant oligomannose N-glycan across the sample types, comprising 38.3% (29.8–46.2) (median, (IQR)), 33.2% (29.0–35.4) and 35.8% (32.8–37.5) of the total oligomannose pool in HNS, LTS− and LTS+ groups, respectively (Fig. 6).
Fig. 6.

Relative distribution of mannosylated glycans within the groups defined as “Oligomannose” and “Paucimannose” in BAL samples from healthy never-smokers (HNS), long-term smokers without (LTS−) and with COPD (LTS+). The median (IQR) is listed. Statistical significance for differences between LTS− or LTS+ and HNS are indicated (**P < 0.01, *P < 0.05, Kruskal-Wallis with Dunn’s multiple comparison test [compared to HNS]). 1Man4 and Man7 oligomannose isomers were pooled in this table, but the relative abundances of them can be seen in Supplementary Table S1.
The LTS− had a higher relative abundance of total paucimannose than the HNS group, and a similar trend was found for the LTS+ (Fig. 4). We identified four paucimannosylated glycans in the BAL samples: Man2, Man3, Man2-core fucose (M2F) and Man3-core fucose (M3F). In BAL from HNS, about two-thirds of the paucimannose glycans consisted of the M2F structure. The proportion of M2F was higher in HNS than in LTS− (p = 0.001, Fig. 6). The proportion of Man3 was also higher in the LTS− group compared to the HNS group (p = 0.04).
Complex N-glycans are associated with health and absence of smoking whereas oligomannose N-glycans are associated with smoking and disease
For the current material, we have previously demonstrated that the level of serum cotinine (metabolite of nicotine) was higher in the pooled group of LTS+ and LTS− than in the HNS group, in which there was no detectable levels, thereby proving the presence and absence of ongoing tobacco use (Padra et al. 2020). As shown in Fig. 4, the relative abundance of complex N-glycans is higher in the HNS group than in the LTS groups combined (LTS+ and LTS−, Mann Whitney test, p = 0.0003). Furthermore, among individuals in the LTS groups (n = 12), the relative abundance of complex N-glycans displayed a negative correlation with historic and current exposure to tobacco smoke, in terms of the number of pack-years (r = −0.55; p = 0.025) and urine cotinine (r = −0.58; p = 0.049) respectively. In contrast, the relative abundance of oligomannose N-glycans displayed a positive correlation with the exposure to tobacco smoke, both in terms of the number of pack years (r = 0.55; p = 0.023) and terms of the level of urine cotinine (r = 0.55; p = 0.015). A trend toward similar correlations was observed for paucimannose (number of pack years: r = 0.45; p = 0.069; level of urine cotinine: r = 0.53; p = 0.080).
The relative abundance of complex N-glycans displayed a positive correlation with lung function outcomes, including forced expiratory volume during 1 s (FEV1, r = 0.53; p = 0.030) and forced vital capacity (FVC, r = 0.52; p = 0.035). In contrast, the relative abundance of oligomannose and paucimannose N-glycans displayed a negative correlation with these parameters (high mannose r = −0.51 and −0.50, p = 0.036 and 0.041, paucimannose r = −0.50 and −0.50, p = 0.045 and 0.041). The relative abundance of these glycans did not show significant statistical correlation with FEV% (FEV1/FVC). We do not propose that the glycan level is an effect of the above clinical parameters, but rather that they are both linked to a third parameter, such as smoking and inflammation.
MDM N-glycosylation differ from BAL protein N-glycosylation
In the BAL samples from all study groups, macrophages constituted the most frequent type of leukocyte (Table 1). Furthermore, a positive correlation was detected between the concentration of macrophages in each sample and the relative abundances of oligomannose (r = 0.49, p = 0.048) and paucimannose glycans (r = 0.55, p = 0.025). We therefore set out to investigate whether the N-glycosylation profile of proteins from macrophages was similar to that of BAL proteins. To do this, we used LC/MS and MS/MS to characterize the N-glycosylation of MDM, an in vitro model of macrophages. N-glycans from soluble MDM proteins were analyzed in duplicate, and the results are compiled in Supplementary Table S1. The MDM N-glycosylation consisted of approximately 67% complex and 31% oligomannose and only 2% paucimannose glycans, which distinguished these from BAL fluids from both HNS, LTS− and LTS+, all displaying different average distributions (Fig. 7, insert). For oligomannose glycans, MDM N-glycans were dominated by Man8 and Man9, whereas among BAL proteins, Man5, Man6 and Man7 dominated. For the complex glycans, Fig. 7 illustrates the high abundance of large N-glycans (above m/z 1,222) on MDM compared to BAL, indicating that the average size of complex N-glycans is larger than in BAL samples. No bisecting glycans were detected on MDM (3%–20% in BAL samples, Supplementary Table S1).
Fig. 7.
Relative abundance of N-glycans on proteins from monocyte-derived macrophages (MDM) cultured in vitro and BAL proteins harvested from human subjects in vivo. The BAL samples were harvested from healthy never-smokers (HNS, n = 5), and long-term smokers without (LTS− n = 8) and with COPD (LTS+, n = 4) and analyzed with LC/MS. The insert shows the average relative abundance of glycan types for MDM and BAL samples, represented as vertical bars. Data are presented as mean values for each study group. The individual measurements are summarized in Supplementary Table S1, data from individual BAL samples are shown in Fig. 4.
Discussion
This study is the first to characterize and compare N-glycan profiles of BAL proteins from the study groups HNS, LTS−, and LTS+. In all, 57 different N-glycans were identified of which the majority were of complex type, the remaining mostly high mannose and paucimannose types. The LTS− group differed from HNS by having higher relative abundances of oligomannose and paucimannose. Differences were also detected on a structural level, where both LTS groups displayed differences in the relative N-glycan levels within the oligomannose and paucimannose groups, and a decreased overall fucosylation, core and terminal fucosylation as well as sialylation, compared to the HNS group. Furthermore, three out of four LTS+ patients had low levels of hybrid glycans, which were detected only in this group. The HNS group had lower inter-individual variation between samples compared to LTS− or LTS+. The relative abundance of paucimannose and oligomannose glycans correlated with the macrophage concentration in BAL samples, however, although these N-glycans were present on MDM, the proportion of them differed between MDM and BAL.
Our results showed that the relative abundance of paucimannose glycans in BAL proteins increased from on average 2.5% in the NHS group, to 20% in the LTS− and 12% in the LTS+ group, respectively, and this is a striking increase. Paucimannose glycans in vertebrates have until recently been quite rarely reported in the literature. Paucimannose glycans are defined as truncated N-glycans, consisting of Man1–3GlcNAc2 Fuc0–1, and may have been observed but not reported due to that they appear to rather be a degradation product, or of non-human origin. Since 2015, the number of studies reporting paucimannose glycans of mammalian origin has increased, and the structure and functional aspects of these truncated glycans has been characterized (Venkatakrishnan et al. 2015, 2020; Loke et al. 2017; Reiding et al. 2019, 2021). Interestingly, a recent study on N-glycomics from tissue from patients with lung cancer and healthy controls revealed an increase of paucimannose glycans in cancerous tissues and long-term smokers with COPD are known to have a five to ten-fold higher risk for lung cancer (Mouronte-Roibás et al. 2016; Lattová et al. 2020). Our analyses of leukocyte concentrations in BAL samples confirmed that macrophages constitute the dominating leukocyte in the bronchoalveolar space and we found a positive correlation between the concentration of macrophages in each sample and the relative abundances of paucimannose glycans. We therefore anticipated that the paucimannose N-glycans on BAL proteins may originate from alveolar macrophages but did not have access to this cell type. Instead we analyzed in vitro generated MDM from primary peripheral monocytes but concluded that paucimannose glycans were only present at low levels on in vitro generated MDM. These MDM are not identical to alveolar macrophages. Earlier studies have, however, indeed found paucimannose glycans on a cell line (THP-1) differentiated into macrophage-like cells (Hare et al. 2017) and also on MDM generated rather similarly to our MDM (Hinneburg et al. 2020). Our data suggests that MDMs may be generated with differing N-glycan profiles, most likely linked to varying experimental factors.
Proteomics studies have provided insights to the protein content of BAL samples, including that BAL from smokers and patients diagnosed with COPD contained on average twice as many proteins as never-smokers, with a higher inter-individual variation (Pastor et al. 2013; Fernández et al. 2018; Machata et al. 2020). This matches the increased inter-individual variation in N-glycan groups observed in our data set. Moreover, we previously characterized the mucin content in this patient cohort and determined that the MUC5AC mucin levels were almost 10-fold higher in BAL fluids from LTS compared to HNS (; Padra et al. 2020, 2021). The airway mucins MUC5AC and MUC5B have numerous potential N-glycosylation sites, although these are poorly characterized. Another study has shown that alveolar macrophages purified from BAL fluids from COPD patients, asymptomatic smokers and non-smokers have different expression of surface receptors (Pons et al. 2005). Thus, it may be expected that a varying composition of protein content in BAL samples between the study groups may contribute to the N-glycan pool. In our study we found an increased intra individual variation in the N-glycosylation in both the LTS− and LTS+ group compared to the HNS group. Although we do not know the reason for why this is, this is an interesting finding that might relate to activation of different inflammatory pathways. Further studies with a much larger patient material is clearly needed to elucidate if certain N-glycan profiles relate to different subgroups, with for example increased risk for developing severe disease. Furthermore, it would be interesting to perform glycoproteomics on the glycans that differ between patient groups to identify which proteins they originate from, although we expect many of the glycans to be present on several proteins and many proteins may carry several N-glycans.
The α1,6 fucosyl transferase gene (Fut8) encodes an enzyme responsible for adding fucose to the N-glycan core and Fut8-null mice have been shown to spontaneously develop pathological phenotypes of pulmonary emphysema (Yamada et al. 2011). Furthermore, a correlation between single nucleotide polymorphism of human FUT8 gene and pulmonary emphysema has also been found, pointing to a relevance of FUT8 in COPD pathology (Yamada et al. 2011). Another study showed that heterozygous Fut8 knock-out mice developed emphysematous lesions in the alveolar wall after only a 3 month cigarette smoke exposure, and earlier than wild type mice, further emphasizing that mice with low expression levels of Fut8 develops emphysema (Gao et al. 2012). However, although core fucosylation was lower in smokers (LTS− and LTS+) than in HNS in our study, we did not find any differences in core fucosylation between LTS+ and LTS−.
In general, the N-glycan profiles of LTS+ and LTS− were relatively similar, possibly due to the fact that the LTS+ and LTS− in the current patient group were unusually well-matched for smoking exposure, with only a trend toward more current smoking (higher serum-cotinine) in the LTS− group than in the LTS+ group. Given this, differences in tobacco load is unlikely to account for the fact that the hybrid glycans were detected exclusively in the LTS+ group. Clearly, this finding deserves further investigation, both to identify which proteins these glycans are present on and to investigate if they have a potential as biomarkers for COPD or sub-classification of the COPD diagnosis in a larger patient study. MS traces indicating hybrid glycans were also observed in some samples in the LTS− group, although the levels were so low that they did not trigger an MS2 collision spectra. It would be interesting to investigate in a prospective study if LTS− with MS traces of hybrid glycans later develop COPD with clearly identifiable levels of hybrid glycans, and hybrid glycans thus could act as a warning sign for upcoming COPD.
To conclude, the results of our current study suggest that long term smoking can cause pronounced alterations in the N-glycosylation on proteins in the bronchoalveolar space of humans without COPD. Moreover, the results suggest that the overall N-glycan profile is similar for these long-term smokers and those with COPD, being characterized by high levels of oligomannose and paucimannose glycans. In contrast, healthy never-smokers display proteins in the bronchoalveolar space that are characterized by high levels of complex glycans. The molecular nature of glycosylation, such as the glycan structures introduced above, may provide further clues as to how glycans may contribute to disease pathogenesis, given that even long-term smokers without COPD are known to display micropathology in lung and airway tissue, although these individuals do not fulfill spirometry criteria for COPD per se. Finally, the hybrid N-glycans identified exclusively in long-term smokers with COPD constitutes a target with biomarker potential and deserves to be investigated in a larger patient material than the current one.
Materials and methods
Detailed protocols are found in the online supplement.
Ethics approval and consent to participate
This study was based on samples collected from healthy never-smokers and long-term tobacco smokers with and without COPD at Sahlgrenska University Hospital, Gothenburg, Sweden. The study populations were recruited and characterized after review and approval by the regional committee for ethical review in Gothenburg (Diary No. 968-11). All participating subjects gave oral and written informed consent, in accordance with the recommendations of the code of ethics of the World Medical Association (Declaration of Helsinki). Buffy-coats were obtained from the Sahlgrenska hospital blood bank after de-identification and therefore no informed consent is needed according to Swedish law (Swedish legislation section code 4§ 3p SFS 2003:460).
BAL sample processing
Bronchoscopy was performed by an experienced pulmonologist at Sahlgrenska University Hospital, Gothenburg, Sweden, in accordance with clinical protocols. Details of the sample collection protocol and the demographic and clinical data of the patients has been described previously in (Padra et al. 2020), as (‘Materials 2’).
Clinical parameters
Clinical parameters, cell counts in BAL samples and cotinine levels (determined in serum samples) were performed as described and previously presented (Padra et al. 2020).
Culture of monocyte-derived macrophages (MDM)
Buffy coat cells were separated by dextran sedimentation and Ficoll-Paque gradient centrifugation (Bøyum et al. 1991). After density centrifugation, 300 x g, 30 min at 4 °C, peripheral blood mononuclear cells (PBMCs) were collected from the top of the Ficoll, washed once in Krebs-Ringer glucose buffer (KRG), and remaining red blood cells were lysed with dH2O. The PBMC’s were washed in KRG and suspended to a concentration of 4 x 106 cells/mL in RPMI (Gibco) supplemented with 10% fetal calf serum (FCS, GE Hyclone) and 1% penicillium-streptomycin (PEST, Gibco). MDM were essentially cultured as described previously (Karlsson et al. 2009).
Analyses of N-glycans from BAL and macrophage derived proteins
N-glycans were released from proteins and analyzed in their nonderivatized reduced form using liquid chromatography mass spectrometry kept in the negative ion mode (Jensen et al. 2012).
Author contributions
SKL, AL and VV, Study Concept and Design. VV Performed the Experiments and Interpreted the Data. MP, AA, BB and KC, Sample Collection and Preparation. VV, KAT and SKL, Writing. KAT, Illustrations, JB and NGK, Methodology, SKL, Supervision. All authors reviewed, commented and approved the manuscript.
CRediT author statement
Vignesh Venkatakrishnan (Conceptualization [equal], Data curation [equal], Formal analysis [lead], Investigation [lead], Visualization [equal], Writing—original draft [equal]), Kristina A. Thomsson (Validation [lead], Visualization [lead], Writing—original draft [equal], Writing—review & editing [equal]), Medea Padra (Investigation [supporting]), Anders Andersson (Investigation [supporting]), Bettina Brundin (Investigation [supporting]), Karin Christenson (Investigation [supporting]), Johan Bylund (Methodology [supporting]), Niclas Karlsson (Methodology [supporting]), Anders Lindén (Conceptualization [equal], Funding acquisition [equal], Resources [equal], Writing—review & editing [supporting]) and Sara Lindén (Conceptualization [equal], Funding acquisition [equal], Project administration [lead], Resources [equal], Supervision [lead], Writing—review & editing [equal])
Funding
This work was supported by the Swedish Research Council to SL [2019-01152, 2021-02542]; Swedish Heart-Lung Foundation [20200579 to SL, 20210286 to AL]; federal funding (ALF grants) from Västra Götaland Region to AL [141851] and Region Stockholm to AL [018-0088].
Conflict of interest statement: None declared.
Data availability
Raw MS data for BAL and MDM N-glycan analyses are available on glycopost (https://glycopost.glycosmos.org/) using the project ID: GPST000266 and GPST000267, respectively. MSMS data with tentative structures are available https://unicarb-dr.glycosmos.org/references/530.
Supplementary Material
Contributor Information
Vignesh Venkatakrishnan, Department of Medical Chemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9C, 41390, Gothenburg, Sweden.
Kristina A Thomsson, Department of Medical Chemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9C, 41390, Gothenburg, Sweden.
Médea Padra, Department of Medical Chemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9C, 41390, Gothenburg, Sweden.
Anders Andersson, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 3, 41390, Gothenburg, Sweden.
Bettina Brundin, Division of Lung and Airway Research, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, 17177, Stockholm, Sweden.
Karin Christenson, Department of Oral Microbiology and Immunology, Institute of Odontology, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 12F, 41390, Gothenburg, Sweden.
Johan Bylund, Department of Oral Microbiology and Immunology, Institute of Odontology, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 12F, 41390, Gothenburg, Sweden.
Niclas G Karlsson, Department of Medical Chemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9C, 41390, Gothenburg, Sweden.
Anders Lindén, Division of Lung and Airway Research, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, 17177, Stockholm, Sweden; Department Respiratory Medicine and Allergy, Karolinska Severe COPD Center, Karolinska University Hospital, Solna, Eugeniavägen 3, 171 76 Stockholm, Sweden.
Sara K Lindén, Department of Medical Chemistry and Cell Biology, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Medicinaregatan 9C, 41390, Gothenburg, Sweden.
References
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010:19(116):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, Godwin AK, Rudd PM. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011:10(4):1755–1764. [DOI] [PubMed] [Google Scholar]
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003:28(1):12–24. [DOI] [PubMed] [Google Scholar]
- Backman H, Eriksson B, Rönmark E, Hedman L, Stridsman C, Jansson SA, Lindberg A, Lundbäck B. Decreased prevalence of moderate to severe COPD over 15 years in northern Sweden. Respir Med. 2016:114:103–110. [DOI] [PubMed] [Google Scholar]
- Balbi B, Pignatti P, Corradi M, Baiardi P, Bianchi L, Brunetti G, Radaeli A, Moscato G, Mutti A, Spanevello A, et al. Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adults. Eur Respir J. 2007:30(4):769–781. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004:1(1):59–70. [DOI] [PubMed] [Google Scholar]
- Bøyum A, Løvhaug D, Tresland L, Nordlie EM. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol. 1991:34(6):697–712. [DOI] [PubMed] [Google Scholar]
- Danielsson P, Ólafsdóttir IS, Benediktsdóttir B, Gíslason T, Janson C. The prevalence of chronic obstructive pulmonary disease in Uppsala, Sweden—the burden of obstructive lung disease (BOLD) study: cross-sectional population-based study. Clin Respir J. 2012:6(2):120–127. [DOI] [PubMed] [Google Scholar]
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002:57(10):847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005:4(6):477–488. [DOI] [PubMed] [Google Scholar]
- Fernández-Blanco JA, Fakih D, Arike L, Rodríguez-Piñeiro AM, Martínez-Abad B, Skansebo E, Jackson S, Root J, Singh D, McCrae C, et al. Attached stratified mucus separates bacteria from the epithelial cells in COPD lungs. JCI Insight. 2018:3(17):e120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta Mol Cell Res. 2017:1864(11 Pt A):2036–2042. [DOI] [PubMed] [Google Scholar]
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005:5(7):526–542. [DOI] [PubMed] [Google Scholar]
- Gao C, Maeno T, Ota F, Ueno M, Korekane H, Takamatsu S, Shirato K, Matsumoto A, Kobayashi S, Yoshida K, et al. Sensitivity of heterozygous α1,6-fucosyltransferase knock-out mice to cigarette smoke-induced emphysema: implication of aberrant transforming growth factor-β signaling and matrix metalloproteinase gene expression. J Biol Chem. 2012:287(20):16699–16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare NJ, Lee LY, Loke I, Britton WJ, Saunders BM, Thaysen-Andersen M. Mycobacterium tuberculosis infection manipulates the glycosylation machinery and the N-glycoproteome of human macrophages and their microparticles. J Proteome Res. 2017:16(1):247–263. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Royle L, Radcliffe CM, Rudd PM, Dwek RA. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry. Anal Biochem. 2008:376(1):44–60. [DOI] [PubMed] [Google Scholar]
- Hinneburg H, Pedersen JL, Bokil NJ, Pralow A, Schirmeister F, Kawahara R, Rapp E, Saunders BM, Thaysen-Andersen M. High-resolution longitudinal N- and O-glycoprofiling of human monocyte-to-macrophage transition. Glycobiology. 2020:30(9):679–694. [DOI] [PubMed] [Google Scholar]
- Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2007:37(6):748–755. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Karlsson NG, Kolarich D, Packer NH. Structural analysis of N- and O-glycans released from glycoproteins. Nat Protoc. 2012:7(7):1299–1310. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Christenson K, Matlak M, Björstad A, Brown KL, Telemo E, Salomonsson E, Leffler H, Bylund J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009:19(1):16–20. [DOI] [PubMed] [Google Scholar]
- Lattová E, Skřičková J, Hausnerová J, Frola L, Křen L, Ihnatová I, Zdráhal Z, Bryant J, Popovič M. N-glycan profiling of lung adenocarcinoma in patients at different stages of disease. Mod Pathol. 2020:33(6):1146–1156. [DOI] [PubMed] [Google Scholar]
- Loke I, Østergaard O, Heegaard NHH, Packer NH, Thaysen-Andersen M. Paucimannose-rich N-glycosylation of spatiotemporally regulated human neutrophil elastase modulates its immune functions. Mol Cell Proteomics. 2017:16:1507–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machata S, Müller MM, Lehmann R, Sieber P, Panagiotou G, Carvalho A, Cunha C, Lagrou K, Maertens J, Slevogt H, et al. Proteome analysis of bronchoalveolar lavage fluids reveals host and fungal proteins highly expressed during invasive pulmonary aspergillosis in mice and humans. Virulence. 2020:11(1):1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouronte-Roibás C, Leiro-Fernández V, Fernández-Villar A, Botana-Rial M, Ramos-Hernández C, Ruano-Ravina A. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett. 2016:382(2):240–244. [DOI] [PubMed] [Google Scholar]
- Nihlén U, Montnémery P, Lindholm LH, Löfdahl CG. Increased serum levels of carbohydrate-deficient transferrin in patients with chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2001:61(5):341–347. [DOI] [PubMed] [Google Scholar]
- Padra M, Andersson A, Levänen B, Premaratne P, Asgeirsdottir H, Tengvall S, Christenson K, Stockfelt M, Bozinovski S, Yoshihara S, et al. Increased MUC1 plus a larger quantity and complex size for MUC5AC in the peripheral airway lumen of long-term tobacco smokers. Clin Sci (Lond). 2020:134(10):1107–1125. [DOI] [PubMed] [Google Scholar]
- Padra M, Benktander J, Padra JT, Andersson A, Brundin B, Tengvall S, Christenson K, Qvarfordt I, Gad R, Paulsson M, et al. Mucin binding to Moraxella catarrhalis during airway inflammation is dependent on sialic acid. Am J Respir Cell Mol Biol. 2021:65(6):593–602. [DOI] [PubMed] [Google Scholar]
- Pastor MD, Nogal A, Molina-Pinelo S, Meléndez R, Salinas A, González de la Peña M, Martín-Juan J, Corral J, García-Carbonero R, Carnero A, et al. Identification of proteomic signatures associated with lung cancer and COPD. J Proteome. 2013:89:227–237. [DOI] [PubMed] [Google Scholar]
- Pons AR, Noguera A, Blanquer D, Sauleda J, Pons J, Agustí AG. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. Eur Respir J. 2005:25(4):647–652. [DOI] [PubMed] [Google Scholar]
- Reiding KR, Franc V, Huitema MG, Brouwer E, Heeringa P, Heck AJR. Neutrophil myeloperoxidase harbors distinct site-specific peculiarities in its glycosylation. J Biol Chem. 2019:294(52):20233–20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding KR, Lin YH, van Alphen FPJ, Meijer AB, Heck AJR. Neutrophil azurophilic granule glycoproteins are distinctively decorated by atypical pauci- and phosphomannose glycans. Commun Biol. 2021:4(1):1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HY. Use of bronchoalveolar lavage in humans—past necessity and future imperative. Lung. 2000:178(5):271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins JB. Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med. 2003:167(2):103–104. [DOI] [PubMed] [Google Scholar]
- Senior RM, Anthonisen NR. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 1998:157(4):S139–S147. [DOI] [PubMed] [Google Scholar]
- Stockley RA. Neutrophils and the pathogenesis of COPD. Chest. 2002:121(5):151s–155s. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan V, Thaysen-Andersen M, Chen SC, Nevalainen H, Packer NH. Cystic fibrosis and bacterial colonization define the sputum N-glycosylation phenotype. Glycobiology. 2015:25(1):88–100. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan V, Dieckmann R, Loke I, Tjondro HC, Chatterjee S, Bylund J, Thaysen-Andersen M, Karlsson NG, Karlsson-Bengtsson A. Glycan analysis of human neutrophil granules implicates a maturation-dependent glycosylation machinery. J Biol Chem. 2020:295(36):12648–12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ishii T, Ikeda S, Naka-Mieno M, Tanaka N, Arai T, Kumasaka T, Gemma A, Kida K, Muramatsu M, et al. Association of fucosyltransferase 8 (FUT8) polymorphism Thr267Lys with pulmonary emphysema. J Hum Genet. 2011:56(12):857–860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw MS data for BAL and MDM N-glycan analyses are available on glycopost (https://glycopost.glycosmos.org/) using the project ID: GPST000266 and GPST000267, respectively. MSMS data with tentative structures are available https://unicarb-dr.glycosmos.org/references/530.