Abstract
Background
Previous research suggests a decline in body mass index (BMI) among older adults is associated with negative health outcomes, including mild cognitive impairment (MCI) and incident dementia. However, no studies have examined the effects of education or developing MCI on BMI trajectories over time. The purpose of this investigation was to characterize trajectories of change in BMI among older adults who develop MCI.
Methods
Participants were from the Minority Aging Research Study (MARS), a longitudinal cohort study of cognitive decline and Alzheimer’s disease in older African Americans living in the greater Chicago, Illinois, area. The study included annual clinical evaluations of cognitive status, as well as measurements of height and weight for BMI calculation. Older African American participants without cognitive impairment at baseline were included in the present analysis (N = 436, 78% women, mean baseline age = 72 [SD = 5.7], mean education = 15 [SD = 3.5]).
Results
In piecewise linear mixed-effects models that included a random intercept and 2 random slopes, BMI declined over time (B = −0.20, SE = 0.02, p < .001), with a faster decline after MCI diagnosis (additional decline, B = −0.15, SE = 0.06, p = .019). Older age was associated with lower baseline BMI (B = −0.19, SE = 0.05, p < .001), as was higher education (B = −0.34, SE = 0.09, p < .001). Further, higher education was associated with a slower decline in BMI before MCI (B = 0.02, SE = 0.006, p = .001), but a faster decline after MCI (B = −0.06, SE = 0.022, p = .003).
Conclusions
These results suggest an accelerated decline in BMI following an MCI diagnosis, with higher education related to an even faster BMI decline.
Keywords: Cognitive aging, Obesity, Resilience
The relationship between body mass index (BMI) and cognition is complex. Although high BMI in midlife is typically a risk factor for poor cognitive outcomes in later life (1), associations often differ for BMI measured in later life. Unlike the literature on BMI in midlife, lower BMI at older age is related to faster cognitive decline (2) and higher incidence of mild cognitive impairment (MCI) and dementia (3). In fact, some studies have demonstrated that higher BMI in late life is actually protective—that is, it is associated with less rapid cognitive decline (4–6) and reduced risk of developing dementia (5) or its precursor, MCI (6). Still others have not found a relationship between BMI and late-life cognition (7,8). In addition, most studies measure BMI at one point in time to see how it relates to changes in cognition over time. Given that BMI tends to decline with age (9,10), it is important to understand how changes in BMI relate to changes in cognition at older age.
There is evidence that greater weight loss between mid and late life is associated with increased MCI risk (6), and changes in BMI over time among older adults is associated with an accelerated rate of cognitive decline and risk of MCI and dementia (eg, [3,11]). But all of these studies, except one (3), have been conducted with predominantly White samples. African Americans, particularly African American women, have the highest rates of overweight/obesity overall in the United States (12). Older African Americans also have nearly two times the risk of developing cognitive impairment and Alzheimer’s disease or other dementias in comparison to their White counterparts (13). Yet, despite the disparities in weight and cognition, few studies have included sufficient numbers of African Americans to examine the relationship between BMI and cognitive impairment. In a prior study, using participants from the same cohort examined in the present study, we reported that BMI fluctuations were related to cognitive decline in African Americans (14). Higher BMI at baseline was related to slower cognitive decline as in previous literature, but greater fluctuations in BMI were related to faster decline, especially among African American women. In that study, however, we did not examine how BMI changes in those who develop cognitive impairment compared to those who remain cognitively intact over time.
Another factor that may influence the association of late-life BMI with cognition is education. Education is widely cited as an important contributor to both cognitive function (15,16), cognitive reserve and resilience (17–19), and risk for dementia in late life (20,21). Similarly, previous studies have linked education and BMI: higher education is associated with lower BMI and lower obesity risk (22,23). Few studies have linked these literatures to examine the extent to which education might modify the relation of BMI to late-life cognition in older adults. One study tested whether education moderated the association of midlife BMI and dementia risk, but the sample consisted of older Finnish twins (24). We are not aware of any studies that have examined this question in older African Americans.
To address some of these gaps in our understanding, the present study examined changes in BMI the years before and after an MCI diagnosis in older African American participants from the Minority Aging Research Study (MARS). We also assessed the extent to which education might moderate this relationship. First, we hypothesized that persons diagnosed with MCI would show a decline in BMI prior to diagnosis, given evidence that weight loss may be an early sign of dementia. Second, we hypothesized that education would moderate the relationship between BMI and MCI. Like the study of Finnish twins (24) and similar to the idea of cognitive reserve, we hypothesized that education could be protective against decline in BMI prior to an MCI diagnosis but would be associated with a faster decline in BMI once MCI develops. Our hypothesis is based on the pattern predicted for typical cognitive reserve components such as education and cognitive activity (25), in which case higher cognitive reserve (as indicated by higher education) will help delay dementia-related BMI decline until cognitive impairment (MCI) develops, after which BMI will decline faster in highly educated MCI persons because of the negative effect of disease coupled with the loss of the positive effect of higher reserve.
Method
Participants
Details about the MARS have been published elsewhere (26). The MARS is an ongoing, longitudinal cohort study that began in 2004. It was designed to study risk factors for cognitive decline and Alzheimer’s disease among older African American adults living in the greater Chicago, Illinois, area and includes annual clinical evaluations. All study participants were without known dementia at baseline. This study was approved by the Institutional Review Board of Rush University Medical Center, and all participants gave written informed consent.
To be included in the present analysis, participants had to be without cognitive impairment at baseline and have at least 2 BMI measurements (ie, at baseline and at least one follow-up). In addition, individuals who developed MCI had to have at least 2 valid BMI measurements starting at the time of MCI diagnosis. This allowed post-MCI change in BMI to be modeled. According to these criteria, there were 436 participants (78% women; mean baseline age = 72 [SD = 5.7] years; mean education = 15 [SD = 3.5] years) included in the current analysis. Participants ineligible for the analysis (n = 358) did not differ by sex (p = .24) but were older (mean baseline age = 75 [SD = 6.8] years; p < .001) and slightly less educated (mean education = 14 [SD = 3.4]; p < .01).
Demographic Variables
Race was self-reported using the 1990 US Census categories. Age (years based on the date of birth), gender (male or female), and self-reported years of education were collected at baseline.
Clinical Evaluations
Clinical evaluations were conducted at baseline and annually with all follow-up evaluations identical to the baseline assessment. Evaluations included the following elements: (1) a physical examination, which included direct measurement of height and weight; (2) a detailed medical history; and (3) a battery of 19 neuropsychological tests (26).
Assessment of BMI
Body mass index values were calculated by dividing weight (kilograms) by median height (meters) squared (kg/m2).
Assessment of Cognitive Function/MCI
The battery of 19 neurocognitive tests was administered annually to all participants. As described in a previous publication (27), composite measures of global cognition and 5 cognitive domains were calculated from the battery of tests by converting raw scores to z-scores (using baseline mean and standard deviation for the full study cohort). The z-scores were then averaged to form each composite score: episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), perceptual speed (4 tests), and visuospatial ability (2 tests).
At each annual assessment, participants were given a clinical diagnosis of either Alzheimer’s dementia, dementia, or MCI. This clinical diagnosis was made utilizing computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification made by an experienced clinician. A dementia diagnosis was made using the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) Work Group criteria (28), which required impairment in 2 cognitive domains. For Alzheimer’s dementia, one cognitive domain had to be memory. Participants were diagnosed with MCI if they had cognitive impairment but did not meet the criteria for dementia, which is consistent with the criteria for a diagnosis of cognitive impairment, no dementia (CIND) (29).
Statistical Analyses
To characterize the sample, we first ran descriptive statistics. Next, to investigate trajectories of change in BMI, we constructed piecewise linear mixed-effects models that included a random intercept and 2 random slopes. The first slope began at baseline and measured the change between baseline and MCI diagnosis. The second slope began at MCI diagnosis and measured change after diagnosis. The second slope allowed for an accelerated decline in BMI after the MCI diagnosis. Models were adjusted for age and education and age by time and education by time interactions. Gender was found to not be a significant covariate and so was excluded from the final models. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used for analytic programming. Statistical models were validated graphically and analytically. A significance level of .05 and 2-sided tests were used throughout the analyses.
Results
Sample Characteristics
Table 1 presents the baseline characteristics for the sample (n = 436), which consisted of mostly women (78%) with an average baseline age of 72 years (SD = 5.76) and average baseline education of 15 years (SD = 3.45). The sample was classified as normal at baseline, and 27.5% (n = 120) developed MCI during follow-up. The total median time of follow-up was about 8 years. The median time of MCI onset was 4 years from baseline. In addition, at baseline, the majority of the sample reported having hypertension (76%; n = 333) and taking antihypertensive medications (90%; n = 392), and 27% (n = 116) of the sample reported having diabetes with 9% taking insulin. Finally, the mean baseline BMI was 30.9 (SD = 6.68).
Table 1.
Baseline Sample Characteristics
| Total n = 436 | NCI n = 316 | MCI n = 120 | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 94 (22) | 67 (21%) | 27 (23%) |
| Age at baseline, mean (SD) | 72.19 (5.67) | 71.72 (5.46) | 73.45 (6.03%) |
| Education, mean (SD) | 15.19 (3.45) | 15.34 (3.53) | 14.79 (3.21%) |
| Clinical variables at baseline | |||
| Body mass index, mean (SD) | 30.86 (6.68) | 31.14 (6.75) | 30.11 (6.46) |
| Diabetes, n (%) | 116 (27) | 80 (25%) | 36 (30%) |
| Stroke, n (%) | 12 (3%) | 7 (2%) | 5 (4%) |
| Cancer, n (%) | 106 (24%) | 75 (24%) | 31 (26%) |
| Hypertension, n (%) | 333 (76%) | 242 (77%) | 91 (76%) |
| Heart, n (%) | 36 (8%) | 28 (9%) | 8 (7%) |
| Claudication, n (%) | 26 (6%) | 16 (5%) | 10 (8%) |
| Smoking, n (%) | 208 (50%) | 157 (53%) | 51 (44%) |
| Systolic blood pressure, mean (SD) | 135.29 (19.43%) | 136.71 (20.51%) | 131.55 (15.73%) |
| Diastolic blood pressure, mean (SD) | 79.78 (11.73%) | 81.12 (11.98%) | 76.26 (10.31%) |
| CES-D, median (IQR) | 1 (0.00–2.00) | 1 (0.00–2.00) | 1 (0.00–2.00) |
| Medical conditions, median (IQR) | 1 (1.00–2.00) | 1 (1.00–2.00) | 2 (1.00–2.00) |
| Medications | |||
| Insulin, n (%) | 39 (9%) | 28 (9%) | 11 (9%) |
| Aspirin, n (%) | 314 (72%) | 223 (71%) | 91 (76%) |
| Statin, n (%) | 272 (62%) | 188 (59%) | 84 (70%) |
| Antihypertensive, n (%) | 392 (90%) | 280 (89%) | 112 (93%) |
Note: CES-D = Center for Epidemiologic Studies Depression scale; IQR = interquartile range; MCI = mild cognitive impairment; n = sample size; NCI = no cognitive impairment; SD = standard deviation.
Piecewise Linear Mixed-Effects Model
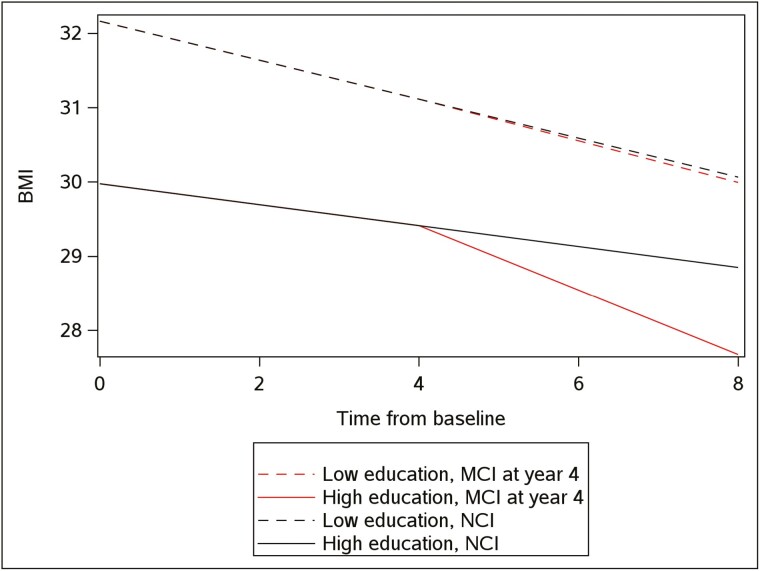
Results from the piecewise linear mixed-effects model are shown in Table 2. The model includes terms for time from baseline, time from MCI, as well as age and education, and their interaction with time from baseline and time from MCI. Older age and higher education were associated with lower baseline BMI levels at baseline (respectively B = −0.19, SE = 0.05, p < .001, and B = −0.34, SE = 0.09, p < .001). Overall, there was a significant decline in BMI over time (B = −0.20, SE = 0.02, p < .001). After MCI diagnosis, there was a 75% acceleration in the decline (B = −0.15, SE = 0.06, p = .019). Older age was associated with a faster decline in BMI (B = −0.15, SE = 0.004, p < .001) during no cognitive impairment (NCI) but was not associated with an additional decline after MCI diagnosis (B = −0.0001, SE = 0.01, p = .99). In contrast, higher education was associated with a slower decline in BMI before MCI (B = 0.02, SE = 0.006, p = .001) and a faster decline after MCI (B = −0.06, SE = 0.022, p = .003). These results are depicted in Figure 1, which illustrates acceleration as a function of education level and MCI status over time for individuals with low education (25th percentile) versus high education (75th percentile), and for individuals that were classified as MCI during the study (assuming median starting point of the MCI) versus individuals that remained NCI.
Table 2.
Mixed Effect Model With Two Random Times
| Terms | Estimate (SE), p Value |
|---|---|
| Time from baseline | −0.2041 (0.02252), <.0001 |
| Time from MCI | −0.1516 (0.06464), .0191 |
| Age at baseline, y | −0.1882 (0.05482), .0007 |
| Education, y | −0.3398 (0.09007), .0002 |
| Age × time from baseline | −0.01524 (0.004298), .0004 |
| Age × time from MCI | 0.000109 (0.01230), .9929 |
| Education × time from baseline | 0.01963 (0.006465), .0024 |
| Education × time from MCI | −0.04740 (0.02099), .0240 |
Notes: MCI = mild cognitive impairment; SE = standard error.
× Represents the interaction between 2 predictors. Using one piecewise linear double-lag mixed-effect model with random intercept and times with body mass index as the outcome.
Figure 1.
Individuals with low education (25th percentile, 12 years) maintained high BMI over time and did not change BMI trajectory after MCI (median time of MCI illustrated, 4 years). However, individuals with high education (75th percentile, 18 years) started with overweight BMI (edge of obese BMI = 30+) and showed a slower BMI decline until MCI. At this point, individuals who developed MCI had accelerated BMI decline relative to those with high education who did not develop MCI. BMI = body mass index; MCI = mild cognitive impairment; NCI = no cognitive impairment.
Discussion
The present study’s objective was to understand how BMI changes as a function of MCI diagnosis and the extent to which education moderates these relationships among a cohort of African American older adults living in the Chicago, Illinois, metropolitan area. Results demonstrated that BMI declined significantly over time, with an accelerated decline noted for those who developed MCI. With increasing age, BMI declined faster between baseline and MCI diagnosis, but not after MCI diagnosis. In contrast, individuals with higher education showed a slower decline in BMI between baseline and MCI diagnosis, but an accelerated decline in BMI post-MCI diagnosis.
The literature on BMI and cognition has been mixed, primarily as a function of when during the life course this relationship is measured (eg, midlife or old age). For example, in middle age, having a BMI in the underweight or obese categories is associated with worse cognitive performance and a greater risk for dementia (7,30). Instead, overweight/obese BMI in late life has been associated with better cognitive performance, slower cognitive decline, and lower dementia risk (3,31). Yet, the vast majority of previous research on this topic has been conducted in predominantly White populations, despite the fact that older African Americans have a disproportionate burden of cognitive impairment and are more likely to be obese or overweight. Our study extends the only other study to focus on older African Americans (3) by using a change-point model to determine how the development of MCI influences change in BMI over time. Gao et al. (3) found participants with MCI or incident dementia had a greater decline in BMI than those with normal cognition. At 6 years prior to diagnosis, participants with MCI had significantly lower BMI than participants with normal cognition, as did participants who went on to develop incident dementia. However, they included persons with MCI in the analyses making it difficult to determine whether early loss of cognition may have affected the results. They also did not conduct follow-up evaluations on an annual basis to detect year-to-year changes in BMI and cognition. Like Gao et al., we found that BMI declined more with a diagnosis of MCI, but unlike Gao et al., BMI change was modeled before and after diagnosis. In addition, unlike Gao et al., we did not find that the effects were modified by gender (data not shown). Instead, we found an effect modification by education, such that individuals with high education (75th percentile) started with overweight BMI and showed a slower BMI decline before MCI. However, among those who developed MCI, persons with higher education showed accelerated BMI decline relative to those with lower education (25th percentile).
The underlying mechanism for the education effects after MCI is unknown but is at least consistent with the concept of cognitive reserve. Cognitive reserve is a theoretical construct that posits lifetime experiences, such as education, occupation, socioeconomic status, and engagement in stimulating activities (eg, social, physical, cognitive), especially in early life but also across the lifespan, serve to influence brain health (32,33). These lifetime experiences also affect the manner in which the brain adjusts to normal and pathological changes related to aging. A key outcome of higher cognitive reserve is the ability for individuals to compensate for such aging-related brain changes as demonstrated by preserved cognitive function and delayed dementia onset (33). Nonetheless, higher cognitive reserve is also associated with a faster rate of cognitive decline once pathological brain changes have reached a certain threshold (ie, a clinical diagnosis) where compensatory mechanisms fail (33).
In fact, previous cross-sectional studies have used cognitive reserve as a framework for conceptualizing findings regarding how age, education, BMI, and cognitive functioning are associated among young and older adults (34), how estimated premorbid intelligence moderates the relationship between BMI and cognitive function among obese adults (35), and the extent to which the association between BMI and cognition varies according to educational attainment, leisure activity, and cognitive and physical job demands (35). Whereas Galioto et al. (35) and Ihle et al. (36) supported the view that cognitive reserve preserved cognitive function among older adults with obese BMI (greater than or equal to 30 individuals), Kirton and Dotson (34) only found evidence for cognitive reserve among younger adults. Notable about these cross-sectional samples is that none included substantial numbers of participants from racially or ethnically diverse backgrounds. In addition, these studies included focal, rather than comprehensive, neurocognitive assessment batteries. The present study extends the literature in that it demonstrates evidence for cognitive reserve using a longitudinal sample of African American older adults.
The present findings showing accelerated BMI decline after MCI among individuals with higher education also align with a “physical” reserve framework. In the aging literature, the construct that has emerged is “physical resilience” (37). Similar to psychological resilience, physical resilience refers to the ability to maintain physical health and functional status in response to stressors (ie, changes in physiologic reserve, life events, environmental; [37]). In the case of the present study, it may be that functional status is maintained until weight-related compensatory mechanisms fail as a result of pathological brain changes reaching a critical threshold. Regarding the broader literature, physical resilience may help explain the “obesity paradox” observed: the apparent benefit of maintaining higher BMI in late life could be a function of one’s ability to physically weather the impact of stressors that inevitably come with physical aging.
Strengths and Limitations
The present study has several strengths that make it an important contribution to the existing literature. First, we used data from a large, longitudinal cohort of African American older adults with comprehensive cognitive data, which allowed us to make reliable estimates of cognition and MCI diagnoses. Both cognition and BMI were measured objectively on an annual basis, which permitted us to conduct the piecewise mixed-effects model that reliably measured changes in BMI over time (across 6 years on average). Limitations of the study include the use of BMI as the only measure of adiposity. Other measures, such as waist circumference and waist-to-hip ratio, that assess central adiposity have been found to be more sensitive than BMI, especially among older persons (38). Like other cohort studies of aging, the present sample was mostly female, which may have influenced the ability to observe the role of gender in the analyses.
Taken together, the present research adds to the few studies that have examined the relationships between BMI and MCI among African Americans, who experience higher rates of overweight/obesity and dementia. This study demonstrates the trajectory of BMI in old age is one of accelerated decline after MCI diagnosis. Additionally, present findings suggest higher education significantly predicts an accelerated weight loss, potentially as a consequence of cognitive reserve. Future research should take a lifespan approach by studying individuals earlier in their adult years. Such longitudinal research will aid our understanding of how BMI trajectories map onto cognitive trajectories.
Contributor Information
Adrienne T Aiken-Morgan, Campbell University Divinity School, Campbell University, Buies Creek, North Carolina, USA; Center on Health and Society, Duke University, Durham, North Carolina, USA.
Ana W Capuano, Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA; Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA.
Robert S Wilson, Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA; Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA.
Lisa L Barnes, Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA; Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, Illinois, USA.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
The MARS was funded by National Institute on Aging (NIA) [grant #RF1AG22018] (PI—L.L.B.). A.T.A.-M. was supported by a NIA Diversity Supplement Award #3RF1AG022018-11S2 (PI—L.L.B.). A.W.C. was funded in part by R01 NS084965 and RF1 AG059621.
Conflict of Interest
None.
Author Contributions
A.T.A.-M. contributed to the conceptualization and design of the study, data analyses and interpretation of results, and writing and revision of the manuscript. A.W.C. assisted in the conceptualization and design of the study, data analyses and interpretation of results, and writing and revision of the manuscript. R.S.W. contributed to data analyses and interpretation of results, and revision of the manuscript. L.L.B. led the conceptualization, design, and implementation of the parent study and contributed to the present data analyses and interpretation of results, as well as the writing and revision of the manuscript.
Sponsor’s Role
The sponsor (NIH) provided funding for the study but did not have a role in the design, methods, participant recruitment, data collection/analysis, or preparation of this manuscript.
References
- 1. Emmerzaal TL, Kiliaan AJ, Gustafson DR.. 2003-2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43(3):739–755. 10.3233/JAD-141086 [DOI] [PubMed] [Google Scholar]
- 2. Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL.. Body mass index and decline in cognitive function in older Black and White persons. J Gerontol A Biol Sci Med Sci. 2018;73(2):198–203. 10.1093/gerona/glx152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao S, Nguyen JT, Hendrie HC, et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc. 2011;59(1):18–25. 10.1111/j.1532-5415.2010.03169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luchsinger JA, Biggs ML, Kizer JR, et al. Adiposity and cognitive decline in the cardiovascular health study. Neuroepidemiology. 2013;40(4):274–281. 10.1159/000345136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim S, Kim Y, Park SM.. Body mass index and decline of cognitive function. PLoS One. 2016;11(2):e0148908. 10.1371/journal.pone.0148908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim G, Choi S, Lyu J.. Body mass index and trajectories of cognitive decline among older Korean adults. Aging Ment Health. 2020;24(5):758–764. 10.1080/13607863.2018.1550628 [DOI] [PubMed] [Google Scholar]
- 7. Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB.. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34(4):222–229. 10.1159/000297742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA.. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70(5):360–367. 10.1212/01.wnl.0000285081.04409.bb [DOI] [PubMed] [Google Scholar]
- 9. Capuano AW, Wilson RS, Leurgans SE, Dawson JD, Bennett DA, Hedeker D.. Sigmoidal mixed models for longitudinal data. Stat Methods Med Res. 2016;27:863–875. pii: 0962280216645632. 10.1177/0962280216645632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuchibhatla MN, Fillenbaum GG, Kraus WE, Cohen HJ, Blazer DG.. Trajectory classes of body mass index in a representative elderly community sample. J Gerontol A Biol Sci Med Sci. 2013;68:699–704. 10.1093/gerona/gls215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beeri MS, et al. “Stability in BMI over time is associated with a better cognitive trajectory in older adults”. Alzheimers Dement. 2022;18(11):2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Center for Health Statistics. Health, United States, 2018. MD, USA. 2019. Accessed June 7, 2021. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25 [Google Scholar]
- 13. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA.. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aiken-Morgan AT, Capuano AW, Arvanitakis Z, Barnes LL.. Changes in body mass index are related to faster cognitive decline among African American older adults. J Am Geriatr Soc. 2020;68(11):2662–2667. 10.1111/jgs.16814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards M, Sacker A.. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):614–624. 10.1076/jcen.25.5.614.14581 [DOI] [PubMed] [Google Scholar]
- 16. Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA.. Educational attainment and cognitive decline in old age. Neurology. 2009;72(5):460–465. 10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner M, Wilson RS, Leurgans SE, et al. Quantifying longitudinal cognitive resilience to Alzheimer’s disease and other neuropathologies. Alzheimers Dement. 2022;18(11):2252–2261. 10.1002/alz.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bocancea DI, van Loenhoud AC, Groot C, Barkhof F, van der Flier WM, Ossenkoppele R.. Measuring resilience and resistance in aging and Alzheimer disease using residual methods: a systematic review and meta-analysis. Neurology. 2021;97(10):474–488. 10.1212/WNL.0000000000012499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA.. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041–e1050. 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R.. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 21. Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol. 1997;54(11):1399–1405. 10.1001/archneur.1997.00550230066019 [DOI] [PubMed] [Google Scholar]
- 22. Della Bella S, Lucchini M.. Education and BMI: a genetic informed analysis. Qual Quant. 2015;49:2577–2593. 10.1007/s11135-014-0129-1 [DOI] [Google Scholar]
- 23. Devaux M, Sassi F, Church J, Cecchini M, Borgonovi F.. Exploring the relationship between education and obesity. OECD J: Econ Stud. 2011;2011(1):1–40. 10.1787/eco_studies-2011-5kg5825v1k23 [DOI] [Google Scholar]
- 24. Iso-Markku P, Kaprio J, Lindgrén N, Rinne JO, Vuoksimaa E.. Education as a moderator of middle-age cardiovascular risk factor-old-age cognition relationships: testing cognitive reserve hypothesis in epidemiological study. Age Ageing. February 2, 2022;51(2):afab228. 10.1093/ageing/afab228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. September 14, 2010;75(11):990–996. 10.1212/WNL.0b013e3181f25b5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA.. The minority aging research study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer’s Res. 2012;9:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA.. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11(4):400–407. [PubMed] [Google Scholar]
- 28. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM.. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. 10.1212/wnl.34.7.939 [PubMed: 6610841]. [DOI] [PubMed] [Google Scholar]
- 29. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA.. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. February 2010;58(2):248–255. 10.1111/j.1532-5415.2009.02671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H.. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 31. Smith E, Hay P, Campbell L, Trollor JN.. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- 32. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–S74. 10.1097/00002093-200607001-00010 [DOI] [PubMed] [Google Scholar]
- 33. Pettigrew C, Soldan A, Brichko R, et al. ; BIOCARD Research Team. Computerized paired associate learning performance and imaging biomarkers in older adults without dementia. Brain Imaging Behav. 2022;16(2):921–929. 10.1007/s11682-021-00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirton JW, Dotson VM.. The interactive effects of age, education, and BMI on cognitive functioning. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23(2):253–262. 10.1080/13825585.2015.1082531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galioto RM, Alosco ML, Spitznagel MB, Stanek KM, Gunstad J.. Cognitive reserve preserves cognitive function in obese individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(6):684–699. 10.1080/13825585.2012.762972 [DOI] [PubMed] [Google Scholar]
- 36. Ihle A, Mons U, Perna L, et al. The relation of obesity to performance in verbal abilities, processing speed, and cognitive flexibility in old age: the role of cognitive reserve. Dement Geriatr Cogn Disord. 2016;42(1–2):117–126. 10.1159/000448916 [DOI] [PubMed] [Google Scholar]
- 37. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS.. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. April 2016;71(4):489–495. 10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R.. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64(3):392–398. 10.1001/archneur.64.3.392 [DOI] [PMC free article] [PubMed] [Google Scholar]