Abstract
The potential for repeated ovulation and menstruation is thought to have provided a Darwinian advantage during the Palaeolithic. Reproductive conditions remained relatively stable until the pre-industrial era, characterized by late menarche, very young age at first birth, multiple pregnancies, and prolonged periods of lactational amenorrhoea. For hundreds of thousands of years, menstruators experienced few ovulatory cycles, even though they were genetically adapted to ovulate and menstruate every month. In the post-industrial era, the age at menarche gradually declined, the age at first birth progressively increased, and breastfeeding became optional and often of short duration. This created a mismatch between genetic adaptation and socio-environmental evolution, so that what was initially a probable reproductive advantage subsequently contributed to increased susceptibility to diseases associated with lifetime oestrogen exposure, such as ovarian, endometrial and breast cancer and, hypothetically, also those associated with the number of ovulatory menstruations, such as endometriosis and adenomyosis. The incidence of endometriosis shows a steep and progressive increase around the age of 25 years, but given the consistently reported delay in diagnosis, the actual incidence curve should be shifted to the left, supporting the possibility that the disease has its roots in adolescence. This raises the question of whether, from an evolutionary point of view, anovulation and amenorrhoea should not still be considered the physiological state, especially in the postmenarchal period. However, an increase in the frequency of endometriosis in recent decades has not been demonstrated, although this deserves further epidemiological investigation. In addition, as endometriosis occurs in a minority of individuals exposed to retrograde menstruation, other important pathogenic factors should be scrutinised. Research should be resumed to explore in more detail the transtubal reflux of not only blood, but also endometrial cells, and whether they are systematically present in the peritoneal fluid after menstruation. If repetitive ovulatory menstruation during the early reproductive years is shown to increase the risk of endometriosis and adenomyosis development and progression in susceptible individuals, hormonal interventions could be used as secondary prevention in symptomatic adolescents.
Keywords: endometriosis, adenomyosis, menstruation, ovulation, menarche, adolescence, ovarian cancer
Introduction: connecting the dots
Endometriosis is defined as ‘a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process’ (Becker et al., 2022). According to the findings of systematic reviews, the prevalence of endometriosis is around 3–5% in the general female population of reproductive age, but around 30% in patients with infertility, 50% in patients with pelvic pain (Ghiasi et al., 2020; Parazzini et al., 2020; Sarria-Santamera et al., 2020), and as high as 65–75% in symptomatic adolescents who do not respond to medical treatment (Janssen et al., 2013; Hirsch et al., 2020). The disease has a detrimental effect on health-related quality of life, psychological mood, sexual function, interpersonal and social relationships, working capacity, use of healthcare resources, and overall societal costs (Zondervan et al., 2018, 2020; Bulun et al., 2019; Taylor et al., 2021; Horne and Missmer, 2022).
Adenomyosis is the presence of foci of endometrium within the myometrium, usually surrounded by hypertrophic smooth muscle cells and areas of fibrosis (Bulun et al., 2021; Guo, 2023). Estimates of the prevalence of adenomyosis in menstruators of different ages vary widely, from <10% to >60%, depending on the presence of typical symptoms such as heavy menorrhagia and dysmenorrhoea, physician awareness of the condition, the accuracy of the diagnostic techniques adopted, and the interpretation of imaging findings (Vercellini et al., 2006; Upson and Missmer, 2020).
Endometriosis and adenomyosis often co-exist (Bulun et al., 2023), can be diagnosed clinically (i.e. non-surgically) with the support of transvaginal ultrasonography or, in selected circumstances, pelvic MRI (Vercellini et al., 2006; Upson and Missmer, 2020), and are associated with adverse obstetric outcomes (Vercellini et al., 2023).
Various theories have been proposed to explain the development of endometriosis, but the metastatic model, based on the implantation of endometrial cells reaching the pelvis via transtubal retrograde flow during menstruation, is supported by the largest body of evidence (Vercellini et al., 1998, 2007, 2014; Zondervan et al., 2018, 2020; Bulun et al., 2019; Taylor et al., 2021; Horne and Missmer, 2022). Different pathogenic hypotheses have also been suggested for adenomyosis, but awareness and interest in the condition have only recently increased and diagnostic capabilities are rapidly improving. This has led to a change in the epidemiological scenario and adenomyosis is now considered to be a disease not only of parous menstruators in their forties, but also of young menstruators (Martire et al., 2020; Bulun et al., 2021; Guo 2023; Millischer et al., 2023).
Although additional factors must play a role in the progression of the two diseases, a high number of consecutive ovulatory menses could theoretically contribute to the early phase of development of both conditions by facilitating (i) endometrial pelvic contamination via transtubal retrograde blood flow and (ii) endometrial invasion of the inner myometrium via recurrent trauma and inflammation at the endo-myometrial junction (Bulun et al., 2019, 2021, 2023; Guo, 2020; Maruyama et al., 2020; Bulun, 2022). According to some investigators, adenomyosis and endometriosis may be different phenotypes of a single disorder resulting from inappropriate and dysfunctional myometrial contractions during menstruation caused by a chronic intrauterine inflammatory state (Leyendecker et al., 1998, 2009, 2015, 2023; Maruyama et al., 2020).
Several years ago, our group hypothesized a common pathogenesis for sporadic serous, endometrioid, and clear cell ovarian cancer, proposing as a shared carcinogenic pathway the oxidative stress induced by iron overload resulting from reiterative retrograde menstruation (Vercellini et al., 2011). Our model, initially based mainly on epidemiological evidence, was subsequently shown to be compatible with laboratory findings reported by independent investigators (Seidman, 2013; Lattuada et al., 2015; Huang et al., 2016; Uberti et al., 2016; Rockfield et al., 2019; Chhabra et al., 2021). Indeed, the presence of blood in the peritoneal fluid has been recognized as a likely oncogenic factor (Lin et al., 2017; Rockfield et al., 2017; Dawson et al., 2018; Otsuka, 2021).
We now revisit our hypothesis with the aim of proposing a unifying theory that includes endometriosis and adenomyosis in addition to the above ovarian cancer histotypes. The underlying concept remains the notion of repetitive ovulatory menstruation (ROM) as a source of reiterative bleeding and thus as a direct or indirect spring of iron-induced oxidative stress and secondary chronic inflammation (Van Langendonckt et al., 2002a,b; Lousse et al., 2009, 2012; Donnez et al., 2016; Bulun et al., 2019, 2021; Wyatt et al., 2023). However, whereas the ovarian cancer model takes into account the total number of ovulatory menstrual cycles over the entire reproductive period (Eaton et al., 1994, 2002; Fu et al., 2022), and thus the lifetime pelvic exposure to blood, in the case of endometriosis and adenomyosis the focus is on early postmenarchal exposure (Kvaskoff et al., 2013; Martire et al., 2020; Bulun et al., 2021; Millischer et al., 2023). Epidemiological and endocrinological factors will be interpreted here in the light of recent changes in menstrual and reproductive patterns (Short, 1976, 1994; Thomas and Ellertson, 2000; Renfree, 2012; Fathalla, 2019).
In the first part of this opinion piece, we hypothesize that endometriosis and adenomyosis may partly result from a mismatch between Darwinian genetic adaptation and global social evolution (Thomas, 1993; Eaton et al., 1994, 2002; Fathalla, 2019; Pei et al., 2022). As a consequence of an unprecedented increase in the number of ovulatory menstruations in the postmenarchal years, iron overload caused by repetitive bleeding episodes would act as a major trigger for chronic inflammation as well as tissue injury and repair by fibrosis, thus providing a common thread for the aetiology, pathogenesis, clinical manifestations, and potential complications of early-onset endometriosis and adenomyosis (Lousse et al., 2012; Donnez et al., 2016; Bulun et al., 2019, 2021, 2023; Guo 2020, 2023; Maruyama et al., 2020; Bulun, 2022; Kobayashi et al., 2023; Wyatt et al., 2023).
Information was identified by searching PubMed using the MESH terms ‘endometriosis’ and ‘adenomyosis’ in combination with ‘menstruation’, ‘epidemiology’, ‘etiology’, ‘pathogenesis’, ‘infertility’, ‘pain’, ‘oral contraceptives’, ‘progestins’, and ‘ovarian cancer’. References from relevant publications were systematically screened and further articles identified using PubMed’s ‘similar articles’ and ‘cited by’ functions. The search was limited to peer-reviewed, full-text, and English language articles. For this opinion article, priority was given to systematic reviews and meta-analyses, as well as original studies published in journals currently rated as Q1 (i.e. first quartile or the top 25%) by ISI Web of Science/Clarivate in the category ‘Clinical Medicine’ and the subcategory ‘Obstetrics & Gynaecology’. Specific international guidelines were also consulted.
A detailed critical review of the many pathogenic mechanisms that may underlie the development and progression of endometriosis and adenomyosis is beyond the scope of this article, and readers are referred to the several excellent publications available on the subject (Zondervan et al., 2018, 2020; Bulun et al., 2019, 2021, 2023; Guo 2020, 2023; WaNg et al., 2020; Taylor et al., 2021; Horne and Missmer, 2022).
What does social evolution have to do with endometriosis and adenomyosis?
From a health care perspective, the transition from the pre-industrial to the post-industrial era has been characterised by a shift from a predominantly acute disease pattern (e.g. infections) to a predominantly chronic disease pattern (i.e. degenerative diseases) (Eaton et al., 2002). Many anthropologists, evolutionary biologists, and geneticists support the concept that much of this is due to a mismatch between extremely slow Darwinian genetic adaptation on the one hand, and recent extremely rapid environmental and social evolution on the other (Short, 1976; Strassmann, 1996, 1997; Eaton et al., 1994, 2002; Thomas and Ellertson, 2000; Renfree, 2012; Fathalla, 2019; Pei et al., 2022). In short, it took millions of years for humans to undergo selective genetic adaptation that favoured specific traits that were advantageous in those environmental and social conditions. However, when living conditions changed rapidly, the same genetic characteristics became disadvantageous and contributed to the spread of previously uncommon diseases (Eaton et al., 1994, 2002).
The potential for repeated ovulation and menstruation probably provided a Darwinian advantage during the Palaeolithic (Jarrell, 2018), although the real reason why people began to menstruate regularly is still debated (Finn, 1996). In fact, only humans and a few other mammals (e.g. apes, Old World monkeys, and shrews) menstruate (Critchley et al., 2020a; Strassmann, 1996). Reproductive conditions (i.e. the environment) remained relatively stable until the pre-industrial times, and were characterized by late menarche, very young age at first birth, multiple pregnancies, and prolonged periods of lactational amenorrhoea (Short, 1976, 1994; Eaton et al., 1994, 2002; Strassmann, 1996, 1997). As a result, for hundreds of thousands of years, menstruators experienced few ovulatory menses, even though they were genetically adapted to potentially ovulate and menstruate every month. In a traditional West African population in Mali, regular menstruation is still a sign of sterility, not fertility (Strassman, 1999).
In the post-industrial era, living conditions improved greatly and the role of menstruators in modern societies evolved rapidly. Thus, in less than two centuries, the age at menarche gradually declined, the age at first birth progressively increased, and breastfeeding became optional and often of short duration (Haines and Guest, 2008; Table 1). This created a mismatch between genetic adaptation and socio-environmental evolution, so that what was initially a reproductive advantage (Finn, 1996; Jarrell, 2018) subsequently contributed to increased susceptibility to diseases associated with lifetime oestrogen exposure, such as ovarian, endometrial, and breast cancer (Jordan et al., 2012; Schüler et al., 2013; Bieuville et al., 2023).
Table 1.
Variation in reproductive pattern and estimated number of ovulatory menstruations in different periods of reproductive life during the last two centuries in Western countries. Literature data.*
| Variable | Nineteenth century | Twenty-first century |
|---|---|---|
| Menarche | 16 years | 12.5 years |
| Mean no. of children per woman | 5–6 | 1–2 |
| Mean duration of exclusive breast lactation | 1–2 years | 4–6 months |
| Mean no. of menstruations between menarche and 25 years of age | ∼50 | ∼150 |
| Mean no. of menstruations during the menarche-to-FFTP interval | ∼20 | ∼200 |
| Mean no. of lifetime ovulatory menstruations | 40–160 | 400–460 |
* Data from Short (1976, 1994), Wyshak and Frisch (1982), Eaton (1994, 2002), Thomas (1993), Strassmann (1996, 1997), Haines and Guest (2008), Fathalla (2019), InterLACE Study Team (2019), and Gottschalk et al. (2020).
FFTP, first full-term pregnancy.
In this context, repetitive ovulation and menstruation without prolonged periods of amenorrhoea induced by multiple pregnancies and prolonged lactation may be considered a maladaptive reproductive trait that may also increase the risk of diseases such as endometriosis and adenomyosis (Renfree, 2012; Fathalla, 2019; Pei et al., 2022). In other words, it cannot be excluded that the exposure to repeated menstruation-related and blood-mediated tissue injury and repair and monthly transtubal mentrual reflux may be greater than the female immune system has genetically evolved to handle at the endo-myometrial junction or within the pelvic environment, respectively (Guo, 2020; Maruyama et al., 2020; Kobayashi, 2023).
Is monthly menstruation for years on end the physiological norm?
In 1976, Roger Short reviewed the available evidence on the evolution of human reproduction and the secular changes in environmental and nutritional conditions, sexual behaviours, social organization, and biological events that regulate age at menarche, conception, and lactation, i.e. the factors that control the interval between successive births and the lifetime menstrual pattern over subsequent periods of human history (Short, 1976). Based on anthropological and evolutionary data, the author reported that in hunter-gatherer communities a girl became pregnant ∼3 years after puberty. Lactational amenorrhoea lasted ∼3 years, and another pregnancy usually occurred after a few ovulatory cycles. Because of limited life expectancy, a menstruator generally gave birth to an average of five children and spent most of their reproductive life without menstruation (Short, 1976, 1994). Nowadays, individuals from Western, industrialized, high-income nations give birth to an average of two children or less, with limited breastfeeding, and life expectancy has largely overcome the menopause insurgency. As a result, 35 years of their reproductive life are characterized by regular menstrual cycles. Short (1976) concluded: ‘There can be no doubt that this ninefold increase in the time spent having menstrual cycles poses a number of new problems for us; it is something of which we have had no prior evolutionary experience, and hence we are not genetically adapted to cope with the situation’. Italy is a case in point: the average age at first birth rose by 7.5 years between 1974 and 2022, and the average number of children per menstruator gradually fell from 2.7 in 1964 to 1.2 in 2022. The average duration of exclusive breastfeeding is now ∼4 months (Istituto Nazionale di Statistica, 2023). However, in non-Western, industrialized, high-income societies, it is still common for more than two children to be the norm.
The evolutionary view of Short (1976, 1994), has lately been shared by several other researchers (Eaton et al., 1994, 2002; Strassmann, 1996, 1997; Thomas and Ellertson, 2000; Renfree, 2012; Fathalla, 2019), including Pei et al. (2022), who also reviewed more recent evidence on the potential effects of ROM and excess oestrogen exposure. However, while the associations between ROM and risk of endometrial, ovarian, and breast cancer are strong and support causation (Jordan et al., 2012; Schüler et al., 2013; Fu et al, 2022; Bieuville et al., 2023), the relationship with endometriosis is less clear.
An inverse association between higher parity and the risk of endometriosis has been consistently observed (Missmer and Cramer, 2003; Viganò et al., 2004; Parazzini et al., 2017; Shafrir et al., 2018). In a case–control study conducted in Lombardy, northern Italy (Parazzini et al., 1995), the punctual odds ratio (OR) of endometriosis compared with nulliparous menstruators was 0.4 in menstruators reporting 1 birth and 0.2 in those reporting ≥2 births, thus confirming the results of another case–control study previously conducted in the same region (Candiani et al., 1991). Analysing data from 473 patients who underwent surgery for various indications, Peterson et al. (2013) observed a reduced risk of endometriosis in parous compared to nulliparous individuals (adjusted OR 0.27; 95% CI 0.15–0.49). The risk was even lower when the analysis was restricted to patients with moderate or severe endometriosis only (adjusted OR 0.19; 95% CI 0.10–0.37).
The effect of reproductive events on the risk of endometriosis was investigated by Missmer et al. (2004) using prospective data from the Nurses’ Health Study II cohort. A higher incidence of laparoscopically diagnosed endometriosis was observed in individuals with an earlier age at menarche and shorter cycle length during adolescence. A linear decrease in risk was also observed with the number of live births and lifetime breastfeeding duration. There was also a significant association between the number of lifetime ovulatory cycles and the risk of endometriosis. Importantly, among people who had never used combined oral contraceptives, the risk of endometriosis was 6-fold in the highest quartile of lifetime cycles (>291) compared with people in the lowest quartile (<174) (Missmer et al., 2004).
The inverse association between duration of total and exclusive breastfeeding and incident endometriosis was further confirmed by Farland et al. (2017a), who observed an 8% reduction in endometriosis risk for each additional 3 months of total breastfeeding and a 14% lower risk for each additional 3 months of exclusive breastfeeding. Compared with individuals who never breastfed, those who breastfed for more than 3 years had a 40% reduced risk of endometriosis. However, the association was only partly influenced by postpartum amenorrhoea. This period is characterized by several metabolic phenomena that may play a role, including a trend towards postpartum weight loss, a recognized maternal health benefit of breastfeeding (Hart et al., 2022).
Regarding menstrual characteristics, Cramer et al. (1986) first reported that surgical patients with short cycle length (≤27 days) and long flow (≥7 days) had more than double the risk of endometriosis compared to patients with longer cycle length and shorter flow. Also in the study by Darrow et al. (1993), when the analysis was restricted to subjects <30 years of age and using age-matched friend controls, menstruators with flow ≥6 days per month and those with heavy flows had more than a 2-fold increased risk of endometriosis (OR 2.5; 95% CI 1.1–5.9 and OR 2.5; 95% CI 1.1–6.3, respectively). Parazzini et al. (1995) observed that, compared with patients who reported lifelong regular menstrual cycles, the OR in those with irregular cycles was 0.4 (95% CI 0.2–0.8). In the case–control study by Sangi-Haghpeykar and Poindexter (1995), a long duration of uninterrupted menstrual cycles was a risk factor for endometriosis (OR 2.9; 95% CI 1.3–6.4). Similar results were observed in the Yale series, where patients with endometriosis reported significantly shorter cycles and longer and heavier flow than controls (Matalliotakis et al., 2008).
Overall, the available evidence on reproductive and menstrual patterns supports the notion that augmented exposure to ROM increases the risk of endometriosis. This is compatible with increased pelvic contamination by refluxed endometrium (Shafrir et al., 2018; Missmer and Cramer, 2003). The effect of pregnancy may also be explained by the protective action of high progesterone levels in addition to the prolonged period of amenorrhoea (Parazzini et al., 2017). In the pre-industrial era, multiple pregnancies meant the creation of a progesterone-dominant environment for much of the decade following menarche (Short, 1976, 1994; Renfree, 2012). The effect of progesterone may be particularly important in the early phase of endometriosis development (WaNg et al., 2020; Shafrir et al., 2023), as it may inhibit the sequence of events leading to lesion development and progression.
Are endometriosis and adenomyosis rooted in adolescence?
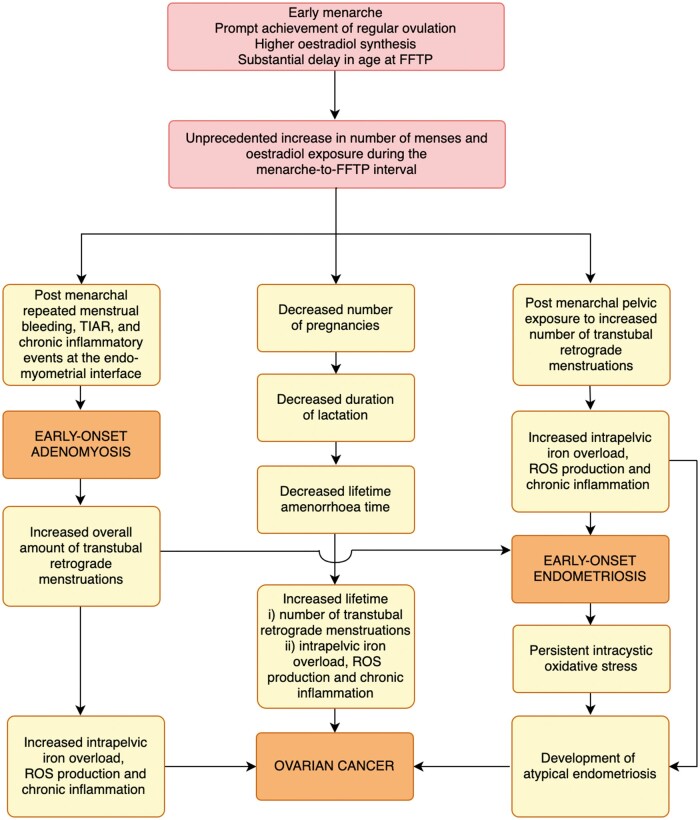
The potential impact of the substantial increase in the number of ovulatory menstrual cycles over the past two centuries should be considered separately for oncological and benign diseases (Fig. 1). The risk of ovarian, endometrial, and breast cancer, the peak incidence of which occurs after menopause, is influenced by the cumulative number of ovulations and menstruations over a lifetime, which is also a reasonable proxy for lifetime oestrogen exposure (Eaton et al., 1994, 2002; Jordan et al., 2012; Schüler et al., 2013; Bieuville et al., 2023).
Figure 1.
The repetitive menstruation hypothesis revisited. A neo-evolutionary theoretical framework based on the effect of a common pathogenic pathway on the risk of endometriosis, adenomyosis and ovarian cancer. FFTP, first full-term pregnancy; TIAR, tissue injury and repair; ROS, reactive oxygen species.
However, the peak incidence of oestrogen-dependent benign diseases such as endometriosis and adenomyosis occurs much earlier (Missmer and Cramer, 2003; Shafrir et al., 2018; Parazzini et al., 2020; Upson and Missmer, 2020). In particular, after a steep and progressive increase around the age of 25 years, the incidence curve of endometriosis tends to plateau after 5 years (Parazzini et al., 2017, 2020). Such a curve represents cases when they are diagnosed, not when they actually develop. Thus, given the consistently reported delay in diagnosis (Davenport et al., 2023), the actual incidence curve should realistically be shifted to the left (Kvaskoff et al., 2013). Therefore, the period of highest risk appears to be from menarche to the age of 25 years, i.e. the years preceding the detection of the majority of full-blown endometriosis cases (Viganò et al., 2004; Kvaskoff et al., 2013; Parazzini et al., 2017, 2020; Shafrir et al., 2018; Koninckx et al., 2021b).
Based on available data on the prevalence of endometriosis in the general population, menstruators could be divided into susceptible (∼5%) and non-susceptible (∼95%) subgroups (Parazzini et al., 2017, 2020; Koninckx et al., 2021b). In light of the above observations, susceptible individuals may develop endometriosis after a limited period of pelvic exposure to retrograde menstruation (Kvaskoff et al., 2013; Koninckx et al., 2021b). Thus, over time, predisposed persons would be selectively excluded from the average-risk general female population, which would become increasingly composed of non-susceptible individuals. This would explain the non-linearity of the incidence curve and its plateau after the age of 30 years (Parazzini et al., 2017, 2020; Koninckx et al., 2021b).
In the same vein, Koninckx et al. (2021b) suggests that the growth period of endometriotic lesions is rather short. In a series of 2086 patients who underwent laparoscopy for endometriosis at the University Hospital Gasthuisberg between 1988 and 2011, the number of surgical patients increased up to the age of 28 years and decreased thereafter. In addition, the severity and relative frequency of different endometriotic lesions did not vary in patients aged 24–44 years.
If endometriosis has its roots in adolescence, efforts to prevent the onset or progression of the disease should focus on the first few postmenarchal years (Kvaskoff et al., 2013). In Western countries, the average age at menarche decreased from 15 to 17 years for menstruators born in the early 1800s to 12.6 years for those born between 1970 and 1984 (Wyshak and Frisch, 1982; InterLACE Study Team, 2019; Gottschalk et al., 2020). As nowadays the average age at first birth is well over 25 years in most nations (United Nations Department of Economic and Social Affairs, Population Division, 2022), the number of ovulatory menstruations during the interval between menarche and peak endometriosis incidence in individuals not using hormonal contraception has approximately tripled (Table 1). Indeed, in European countries, the time from menarche to first full-term pregnancy (menarche-to-FFTP interval) has increased in two centuries from only 3 years (∼16 years at menarche and ∼19 years at first birth; Short, 1976, 1994; Eaton et al., 2002) to almost 20 years. In Italy, for example, the age at menarche is 12.4 years (De Sanctis et al., 2019; Piras et al., 2020) and the age at first birth is 32.4 years (Istituto Nazionale di Statistica, 2023).
Moreover, given the inverse relationship between decreasing age at menarche and the number of ovulatory menstrual cycles, as well as the amount of gonadal oestradiol (E2) synthesis in the postmenarchal period (Macmahon et al., 1982; Vihko and Apter, 1984; Farland et al., 2017b), such an impressive increase in the length of the menarche-to-FFTP interval may have an even greater effect on the risk of developing endometriosis than simply augmenting the number of menstruations. In fact, compared with late menarche, early menarche is associated with higher serum FSH and E2 concentrations and more frequent ovulatory cycles (Vihko and Apter, 1984). Thus, not only are individuals with early menarche exposed to oestrogen for a longer period of time, but this excess exposure is characterised by particularly high serum oestrogen levels (Macmahon et al., 1982; Emaus et al., 2008; Farland et al., 2017b).
According to Lund et al. (2022), early menarche can be seen as a proxy measure for increased oestrogen exposure during adolescence. Even in the case of partially anovulatory cycles, proliferation of refluxed endometrial cells would be favoured by unopposed exposure to E2 action that is not counterbalanced by progesterone action (Gunn et al., 2018; Carlson and Shaw, 2019). In addition to local stimulation of eutopic and ectopic endometrium, oestrogens also exert a systemic pro-inflammatory effect (Straub, 2007; Oertelt-Prigione, 2012a,b; Cutolo et al., 2014; Dodd and Menon, 2022).
Along this line, a recent meta-analysis of data published over the last two decades showed that menarche before the age of 12 years was associated with a one-third increased risk of endometriosis (OR 1.34; 95% CI 1.16–1.54) (Lu et al., 2023). When only studies started after 2000 were considered, the risk increased further. The risk was particularly high in low-resource countries (OR 2.11; 95% CI 1.55–2.87). These results confirm the findings of a previous systematic review and meta-analysis showing that, based on selected studies with rigorous control of confounders, early age at menarche was significantly associated with a higher risk of endometriosis (Nnoaham et al., 2012). In the case–control study by Treloar et al. (2010), menarche after the age of 14 years of age was strongly and inversely associated with endometriosis (OR 0.3; 95% CI 0.1–0.6).
African-American girls experience menarche at an earlier age than Caucasians. In the study conducted by Reagan et al. (2012) on a sample of 23 337 girls drawn from the US National Longitudinal Survey of Youth Child–Mother file, the mean age at menarche was 144 months for African-American adolescents and 150 months for whites. This would disproportionately expose the former subpopulation to the possible deleterious effects of early ROM, potentially exacerbating the racial and ethnic health disparities in endometriosis diagnosis and management already observed in the USA (Bougie et al., 2019, 2022; Orlando et al., 2022; Westwood et al., 2023). In this context, given the difficulties in diagnosis, the apparent lack of differences in the incidence of endometriosis between ethnic groups (Katon et al., 2023) may actually reflect differences in access to care (Williams et al., 2019; Westwood et al., 2023).
A word of caution
There are three ways to interpret the presented epidemiological evidence: (i) there are direct biological links between early menarche, late age at first birth, low total number of live births, and shortened breastfeeding to endometriosis and adenomyosis, independently; (ii) each or some of these factors are proxies for higher ROM, which is the causal biological link to endometriosis and adenomyosis; or (iii) most likely, there is a combination of (i) and (ii). In the following sections, we will present biological and molecular arguments as to why these epidemiological factors may be proxies for ROM, which in turn acts as the triggering mechanistic event for the onset and progression of endometriosis and adenomyosis.
It should be noted, however, that the social evolution referred to here is specific to post-industrialized, generally high-income, nations. It is therefore critical not to overgeneralize this theory to the whole world, but to limit it to the specific circumstances (i.e. societies) in which it is relevant. In this regard, Henrich et al. (2010) coined the acronym WEIRD (i.e. Western, Educated, Industrialised, Rich, and Democratic) with the aim of raising awareness of humanity’s cultural diversity and encouraging scientists to differentiate their sampling. Recognizing that WEIRD populations are a limited subset of the total world population could avoid sampling bias and inappropriate generalisations.
In addition, despite the above findings, it may still be difficult to disentangle the evolutionary issues from other epidemiological aspects (Eaton et al., 2002), and since retrograde menstruation is reported to be a common phenomenon in most menstruators (Halme et al., 1984; Liu and Hitchcock, 1986; Tang et al., 2022), multiple factors in addition to ROM are likely to play a role in the development of endometriosis and adenomyosis (Shafrir et al., 2018; Zondervan et al., 2018, 2020; Koninckx et al., 2019; Leonardi et al, 2020; Olšarová and Mishra, 2020; WaNg et al., 2020; Kuan et al., 2021; Salliss et al., 2021; Taylor et al., 2021; Horne and Missmer, 2022; Marroquin et al., 2023; Muraoka et al., 2023; Vallée et al., 2023; Yang et al., 2023). Even an increase in endocrine-disrupting chemicals due to air and water pollution and occupational hazards can potentially lead to the onset and progression of endometriosis (Vallée et al., 2023). Exposure to these contributors, including environmental, sociological, and microbiological risk factors, may change over time or vary between geographical regions, potentially influencing the development of endometriosis (Ghiasi et al., 2020). Furthermore, several morbidities are associated with early menarche, some of which may be explained by more menstrual cycles, but also by other conditions that would cause early menarche, such as elevated leptin levels, particularly in adolescents with a metabolically unhealthy phenotype (Gamble, 2017).
Ovulation and menstruation as inflammatory events
The hypothesis of an effect of ROM on the risk of endometriosis and adenomyosis maybe even more plausible if one considers not only the number of events, in terms of the mere total amount of displaced endometrial tissue and thus its probabilistic chance of survival at ectopic sites, but also the quality of such events, in terms of a possible transition from a state of acute and self-limited inflammation to a state of chronic and self-perpetuating inflammation (Guo, 2018, 2020, 2023; Bulun et al., 2019, 2021; Kobayashi, 2023; Kobayashi et al., 2023). In this context, both ovulation and menstruation can be considered as a source of acute inflammation with a risk of chronicity if relentlessy repeated (Jarrell and Arendt-Nielsen, 2016; Jarrell, 2018).
An LH-triggered inflammatory process is the mechanism by which the connective tissue layers of the tunica albuginea and theca externa at the apex of a pre-ovulatory follicle are loosened, allowing the follicle wall to migrate towards the ovarian surface and then rupture due to intrafollicular fluid pressure (Espey, 1994; Richards et al., 2002; Gérard et al., 2004). The fundamental role of inflammation during ovulation is supported by the observation that pre-ovulatory use of non-steroidal anti-inflammatory drugs and cyclo-oxygenase (COX)-2 inhibitors can interfere with this process to the point of reducing the likelihood of conception (Weiss and Ghandi, 2016; Duffy et al., 2019). Li et al. (2023) recently demonstrated that piroxicam, a COX-2 inhibitor, confers a significant synergistic contraceptive effect to levonorgestrel used for emergency contraception.
The potential impact of repeated, ovulation-associated local inflammation on ectopic endometrial pelvic implants is currently unknown. However, with each ovulation, large amounts of intrafollicular fluid E2 are released directly onto pelvic lesions, potentially promoting disease progression (Bulun et al., 2019; Koninckx et al., 2021c; Koninckx et al., 2022). The role of ovulation in the pathogenesis of ovarian endometriomas is indirectly confirmed by the remarkable protective effect of prolonged postoperative hormonal therapy on endometrioma recurrence rates. In the systematic review and network meta-analysis by Chiu et al. (2022), the OR of cyst recurrence in patients who underwent ovulation suppression compared with those who chose expectant management varied from 0.04 to 0.21, depending on the type of therapy used.
The relationship between lifetime ovulatory years and risk of epithelial ovarian cancer has also been interpreted in terms of reiterative inflammatory events (Ness and Cottreau, 1999). This may be particularly relevant for patients with endometriosis as, according to the results of a meta-analysis of 24 cohort and case–control studies in which endometriosis-related tumours where overrepresented (Kvaskoff et al., 2021), the absolute lifetime risk of developing ovarian cancer was 2.5% (1 in 40 individuals) in patients with endometriosis, compared with 1.3% (1 in 75 individuals) observed in the general population (Reid et al., 2017). Indeed, based on the findings of another systematic review and meta-analysis (Fu et al., 2022), the strength of the association between lifetime ovulations and ovarian cancer was higher for endometrioid tumours (pooled OR 3.05; 95% CI 2.08–4.45) than for serous tumours (pooled OR 2.31, 95% CI 1.60–3.33). According to the dualistic model of epithelial carcinogenesis, endometrioid tumours of the ovary are included in the group of tumours arising from endometriosis (Kurman and Shih, 2016).
Menstruation should also be considered an inflammatory phenomenon (Jarrell, 2018), with histological and biological features similar to those classically observed when inflammation occurs at any other site in the body (Finn, 1986; Critchley et al., 2020b; Jain et al., 2022). The decline in ovarian steroid concentration prior to the onset of menses leads to increased synthesis of ROS, prostaglandins, cytokines, chemochines, and matrix metalloproteinases (Evans and Salamonsen, 2012), as well as interleukin (IL)-1β and IL-18, which can also lead to systemic inflammatory events (Azlan et al., 2020).
Under physiological conditions, menstruation-associated inflammation is self-limited and does not lead to persistent inflammation with the consequences of tissue destruction, fibrotic replacement, and potential loss of function (Critchley et al., 2020b). However, some researchers have suggested that ROM may sometimes induce chronic inflammation, uterine hypercontractility, and fibrotic scarring due to repeated myometrial injury and repair (Bulun et al., 2021; Guo, 2023; Kobayashi, 2023).
Thus, if ovulation and menstruation are acute inflammatory events, their endless repetition may not necessarily be considered physiological, and the question may arise as to whether their incessant occurrence in the early reproductive period may favour the onset of endometriosis and adenomyosis, and through what pathogenic pathways (Bulun et al., 2019, 2021, 2023).
Does ROM contribute to the early-onset and progression of endometriosis and adenomyosis?
The group of Jacques Donnez carried out pioneering studies on dysregulated iron homeostasis in the pelvic cavity, which would promote oxidative stress and inflammation (Van Langendonckt et al., 2002a,b; Lousse et al., 2009). Erythrocytes refluxed into the pelvis during menstruation are normally phagocytized and lysed by local macrophages (Lousse et al., 2012; Donnez et al., 2016). However, in an oversimplified synthesis, when the capacity of pelvic macrophages to eliminate erythrocytes and haemoglobin is overwhelmed, or the homeostatic processes are defective, toxic by-products such as haem and free iron are released (Lousse et al., 2012; Donnez et al., 2016; Kobayashi et al., 2023; Wyatt et al., 2023). The resulting Fenton reaction and generation of ROS promotes an inflammatory state, with damage to the peritoneal mesothelium, exposure of the underlying mesenchymal tissue, and the possibility of implantation of regurgitated endometrial cells (Lousse et al., 2009, 2012; Donnez et al., 2016; Ng et al., 2020; Ansariniya et al., 2022). This may be particularly likely to occur in menstruators with increased pelvic exposure to blood and regurgitated endometrial glands, such as those with regular cycles and abundant menstrual flow (Darrow et al., 1993; Vercellini et al., 1997; Missmer and Cramer, 2003; Parazzini et al., 2017), or in those with inadequate pelvic scavenging systems (e.g. haptoglobin, which binds extracellular haemoglobin and facilitates its clearance by macrophages, and haem oxygenase, which degrades haemoglobin and haem with incorporation of iron into ferritin in pelvic macrophages) (Van Langendonckt et al., 2002a; Lousse et al., 2009, 2012; Pirdel and Pirdel, 2014; Donnez et al., 2016; Wyatt et al., 2023). Indeed, the presence of haemosiderin-laden macrophages (siderophages) is a histological hallmark of endometriosis and supports the hypothesis that peritoneal antioxidant mechanisms may have been overwhelmed (Lousse et al., 2009, 2012; Donnez et al., 2016). The recent systematic review by Wyatt et al. (2023) summarizes the available evidence on the role of abnormal pelvic iron homeostasis due to retrograde menstruation in the development and progression of endometriosis.
Furthermore, oestrogens are a biological driver of inflammation in endometriosis, as E2 is crucial for endometrial gland survival, with subsequent production of a number of inflammatory molecules leading to peritoneal and subperitoneal tissue remodelling, adhesions, and fibrosis (Bulun et al., 2012, 2019; Reis et al., 2013; Clemenza et al., 2022). In this regard, using data from the Nurses’ Health Study II, Shafrir et al. (2023) found that menstruators with higher early follicular phase free or total plasma E2 levels had an increased risk of surgically confirmed endometriosis at least 1 year after blood sampling. In addition, higher mid-luteal plasma progesterone levels were associated with a lower risk of endometriosis.
These findings support the notion that repeated retrograde menstruation may act as an initiating factor in the pathogenesis of endometriosis (Jarrell, 2018), and that promoting factors, including dysfunctional pelvic iron homeostasis, high oestrogen exposure, and reduced progesterone exposure, may determine disease development and progression in genetically susceptible individuals (Parazzini et al., 2017).
Repetitious menstrual episodes also imply reiterative intrauterine tissue hypoxia and necrosis, myometrial contractions, and rapid angiogenesis and regenerative processes (Bulun et al., 2021; Guo, 2023; Kobayashi, 2023). These events may disrupt the endo-myometrial junction, favouring intra-myometrial invagination of basal endometrium fragments and the development of adenomyosis (Jarrell, 2018; Bulun et al., 2021). Platelet aggregation and macrophages recruitment would contribute to inflammation, local oestrogen synthesis, tissue remodelling, and ultimately fibrogenesis (Guo, 2028, 2020, 2023).
In summary, repetitious ovulatory menses may favour the displacement of endometrial fragments in the pelvis via transtubal retrograde flow and into the myometrium via the opening of a ‘denuded intrauterine terrain’. The excess of free iron resulting from repetitive bleeding episodes may damage the mesothelial lining, which would facilitate pelvic implantation of endometrial glands, and may induce resistance to ferroptosis, which would trigger lesion progression and eventually fibrosis (Li et al., 2023; Wyatt et al., 2023; Wu et al., 2023). In addition, relentless ovulation increases oestrogen exposure, with the consequent stimulation to proliferation and infiltration of ectopic endometrium.
Criticisms of the repetitive ovulatory menstruation hypothesis
There is a large body of evidence to support the hypothesis that ROM contributes to the development of endometriosis and adenomyosis, but we did not conduct a formal systematic review of the literature, so our search may have missed important studies. Some of the publications considered are old, and the quality of the included reports was not assessed. Moreover, we disclose our intellectual conflict of interest related to the belief that retrograde menstruation is the necessary, although not sufficient, condition for the development of most cases of endometriosis. Finally, our hypothesis is based on the change in reproductive patterns observed in Western countries. The situation may be different in countries with different ethnic groups (Somigliana et al., 2012; Ohayi et al., 2022). In addition to the these methodological issues, there are three main criticisms that can be made of our ROM hypothesis.
(i) First, there is no evidence of an increase in the incidence of endometriosis and adenomyosis following the demographic transition. Neither disease could be diagnosed in the pre-industrial era, but an increase in the incidence of endometriosis as a result of an increase in the number of ovulatory menstruations remains unproven even in recent decades (Christ et al., 2021). In fact, in a systematic review of data published between 1989 and 2019, Ghiasi et al. (2020) observed no time trend in the incidence and prevalence of endometriosis in the general population. Methodological limitations prevented the authors from drawing conclusions about a possible change in the severity of incident cases over the past 30 years.
However, several biases preclude an accurate definition of the true frequency of endometriosis, including diagnostic bias leading to underestimation in population studies, and health inequalities influencing access to care and assessment by physicians with sufficient knowledge of the condition. Geographical, economic, and social factors also affect the possibility of being evaluated by an experienced imaging specialist (Ghiasi et al., 2020). An excellent overview of the dynamics involved in the pathways to endometriosis diagnosis has been proposed by Shafrir et al. (2018).
Moreover, it seems reasonable to assume that most of the changes in reproductive patterns in Western countries in the post-industrial era have already occurred half a century ago (Short, 1976, 2002; Eaton et al., 1994; Strassmann, 1996, 1997). In fact, compared with the changes that had taken place over the previous two centuries, there have been only minor variations over the last 50 years, as the fertility rate has remained essentially stable. For example, the total fertility rate in the USA was 7 in 1800, 3.9 in 1900, fell below the replacement level in the early 1970s, and has remained at around 2 ever since (United Nations Department of Economic and Social Affairs, Population Division, 2019). Thus, it cannot be excluded that the effect of an increase in the number of ovulatory menstruations was already almost fully established when awareness of endometriosis and adenomyosis increased and diagnostic motivation and competence improved in the 1980s. If this is the case, contemporary epidemiological studies would not be expected to reveal major changes in the incidence of these diseases over time, but this would not per se discredit the possibility of an earlier and therefore undetected effect of an increased number of ovulatory menstruations on the risk of endometriosis and adenomyosis.
(ii) Second, in addition to repetitious ovulatory cycles, several other factors appear to play a role in the pathogenesis of endometriosis and adenomyosis, including genetic predisposition, epigenetic profile, constitutional variables, immunological and hormonal factors, and individual lifestyle (Shafrir et al., 2018; Zondervan et al., 2018, 2020; Bulun et al., 2019, 2021, 2023; Guo, 2020, 2023; WaNg et al., 2020; Taylor et al., 2021; Horne and Missmer, 2022; Vallée et al., 2023). Thus, even if there is an actual progressive increase in frequency, this could be explained by augmented exposure to toxic environmental chemicals and endocrine disruptors (Sirohi et al., 2021; Matta et al., 2021; Vallée et al., 2023; Marroquin et al., 2023), high trans-unsaturated fat intake (Missmer et al., 2010), altered microbiome (Koninckx et al., 2019; Leonardi et al, 2020; Salliss et al., 2021; Muraoka et al., 2023; Yang et al., 2023), or other features of modern life that may influence the risk of these diseases (Missmer and Cramer, 2003; Viganò et al., 2004; Ottolina et al., 2020; Sasamoto et al., 2020; Shafrir et al., 2018; Koninckx et al., 2021a). More in general, since endometriosis and adenomyosis appear as multistep phenomena in which, after an establishment phase, a proliferation and invasion phase occurs, accompanied or followed by an inflammatory reaction phase, each phase may be subject to the influence of different contributing factors (Parazzini et al., 2017).
Furthermore, the real question here may not necessarily be: ‘Are there other factors that could explain an [unobserved] increase in the incidence of endometriosis and adenomyosis in WEIRD societies?’ But rather, ‘Are there confounding factors of the demographic transition that could explain the link between ROM and endometriosis and adenomyosis, thus negating the causal relationship between ROM and endometriosis and adenomyosis?’ As a theoretical example, a contemporary change in diet could have led on the one hand to an earlier menarche (Gamble, 2017) and, on the other hand, to an increased risk of endometriosis due to a higher intake of selected nutrients such as red meat, trans fatty acids, and saturated fatty acids (Missmer et al., 2010; Arab et al., 2022). Thus, a rise in the number of ROMs is identified, but the pathogenic factor causing the increase in the onset of endometriosis is actually the change in diet over time. A different epidemiological context is represented by the increasing use over the last century (Barton, 1942; Bullough, 1985; Liberty et al., 2023) of thousands of menstrual pads and tampons per person, as these disposable products have been shown to contain and release several endocrine disruptors, such as phthalates, phenols, and parabens, which may promote the development of endometriosis and adenomyosis (Marroquin et al., 2023). In this case, the causative factor would not be the recent increase in ROM per se, but the resulting contemporary increased exposure of the average menstruator to chemicals that can be absorbed by the vulvar and vaginal mucosa without undergoing first-pass metabolism (Marroquin et al., 2023). In addition, heavy menstrual flow has been consistently associated with the risk of endometriosis (Cramer et al., 1986; Parazzini et al., 1995; Matalliotakis et al., 2008). At the same time, women with menorrhagia tend to use higher absorbency products and more protective disposables per cycle, thus potentially increasing their exposure to endocrine-disrupting chemicals (Marroquin et al., 2023).
(iii) Third, probably the most important weakness of our theory is that exposure to retrograde menstruation appears to be the norm for most women (Halme et al., 1984; Liu and Hitchcock, 1986; Kruitwagen, 1993; Tang et al., 2022), but the prevalence of endometriosis and adenomyosis is relatively low (Missmer and Cramer, 2003; Parazzini et al., 2017, 2020; Ghiasi et al., 2020; Sarria-Santamera et al., 2020; Christ et al., 2021). Thus, it seems logical to accept that protective mechanisms against the potential effects of ROM are likely to be at play, otherwise almost all menstruators would sooner or later develop endometriosis during the reproductive period. Presumably, the variable individual level of antioxidant potential of pelvic macrophages may determine whether a given amount of refluxed erythrocytes and endometrial glands can overwhelm the scavenging capacity, thus allowing damage to the mesothelial lining and implantation of the refluxed endometrium (Van Langendonckt et al., 2002a,b; Lousse et al., 2009, 2012; Donnez et al., 2016; Ng et al., 2020; Ansariniya et al., 2022; Wyatt et al., 2023). Furthermore, different patterns of crosstalk between pelvic NK lymphocytes and the local microenvironment may determine whether refluxed endometrial epithelial cells harbouring somatic cancer driver mutations (Praetorius et al, 2022) are systematically eliminated or undergo immune escape (Liang et al., 2019; Fukui et al., 2021).
However, some more clarity on this point might also be useful to put the ROM hypothesis in the right context. Apart from the seminal articles by Sampson (1927, 1940), which still form the solid foundation of the implantation theory (Yovich et al., 2020), and some anecdotal reports published before the 1980s (for review, see Kruitwagen, 1993; D’Hooghe and Debrock, 2002), the first study to document the systematic and not occasional occurrence of retrograde menstruation, was published by Blumenkrantz et al. (1981). The authors reviewed data from 11 menstruators between the ages of 15 and 44 who were undergoing periodic peritoneal dialysis for end-stage renal failure. In nine of them, blood was regularly observed in the Silastic peritoneal dialysis catheter just before menses and on the first day of menstruation. Bloody peritoneal fluid was never observed at any other time during the menstrual cycle. Six patients underwent laparotomy for various reasons, but no endometriosis was found. Numerous attempts to identify endometrial cells or glands in the peritoneal effluent of three patients were unsuccessful.
Halme et al. (1984) aspirated peritoneal fluid from 331 patients during laparoscopy and classified it as straw-coloured or pink or bloody. Of the 65 patients who underwent surgery on perimenstrual days (1–6 and 27–30 of the cycle), 49 (75%) had pink or bloody fluid (38/42 without endometriosis and with open tubes; 2/11 without endometriosis and with closed tubes; and 9/10 with endometriosis). Of relevance, eight of the nine patients who underwent laparoscopy in the perimenstrual phase while on cyclic oral contraceptives had bloody peritoneal fluid. However, peritoneal fluid samples were examined for the presence of blood, but not for endometrial cells or glands.
Inspired by these landmark studies, which showed that transtubal blood reflux during menses was very common, several investigators began to focus on the identification of endometrial cells or tissue structures in the peritoneal fluid in addition to the presence of blood (Kruitwagen, 1993; D’Hooghe and Debrock, 2002).
Reti et al. (1983) performed laparoscopy during menstruation in 15 symptomatic (mean age 26.2 years) and 31 asymptomatic (35.4 years) individuals. Bloodstained peritoneal fluid was aspirated in 10 (67%) subjects of the former group and in 23 (74%) of the latter. Small dense clusters of cells resembling endometrial glandular and stromal material were identified cytologically in five (33%) and three (10%) cases, respectively. Endometriosis was detected in only one symptomatic patient.
In subsequent studies, different techniques were used, including not only cytology, but also cell block analysis, immunocytochemistry, and cell culture (Kruitwagen, 1993), and high variability was observed in the occurrence of peritoneal fluid samples positive for endometrial cells or tissue fragments, with percentages varying from 19% to 75% when endometriosis was present and from 10% to 92% when it was not (Kruitwagen, 1993).
This inconsistency in the observed frequencies, together with the limited sample size of most studies, the heterogeneity of the methodological approaches used, the collection of samples at different phases of the cycle, and the choice of highly selected participants who may not be representative of the general population (e.g. infertile patients with patent and blocked fallopian tubes, or parous people undergoing tubal sterilization), may limit the validity of the available data. In fact, the frequency of the perimenstrual presence of endometrial cells or glands, and not merely ‘blood’, in the peritoneal fluid of menstruators in the general populations, and not just in selected patients undergoing surgery for different indications, is currently undefined (Bokor et al., 2009; Dorien et al., 2017).
Thus, the idea that true retrograde menstruation is the norm during the reproductive years has not been conclusively proven and cannot be taken for granted (Bartosik et al., 1986). It would be extremely helpful to know how many menstruators have transtubal reflux containing endometrial cells or glands, whether this occurs occasionally or every month, how many days it lasts, what the absolute amount of refluxed endometrial cells is, whether the cytological characteristics remain stable over time or vary, and what, if any, differences there are between individuals who develop endometriosis and those who do not, both in the erythrocyte component (potentially responsible for the oxidative stress) and in the mucosal component. The mere observation of perimenstrual transtubal blood reflux cannot be considered synonymous with demonstrating the validity of the metastatic theory (Kruitwagen, 1993; van der Linden et al., 1995) but, with few exceptions (e.g. Bulletti et al., 2002; Bokor et al., 2009; Dorien et al., 2017; Masuda et al., 2021; Tang et al., 2022), research into this pathogenic aspect seems to have rather waned in the last two decades.
Overall, it seems premature to exclude the possibility that elementary mechanistic factors (e.g. calibre of the cervical canal, internal cervical os stiffness, calibre of the tubal ostia, diameter and course of the intramural tubal portion, function of the utero-tubal junction, amount of menstrual flow, frequency and strength of myometrial contractions with loss of fundocervical polarity) (Bartosik et al., 1986; Cramer et al., 1986; Barbieri et al., 1992; Vercellini et al., 1997; Barbieri, 1998; Leyendecker et al., 2004; Bulletti et al., 2002; Missmer et al., 2004; Rozewicki et al., 2005; Munro, 2023; XholLi et al., 2023) together with non-modifiable genetic determinants (Liang et al., 2023) govern the amount of refluxed blood and endometrium per cycle in the general population of menstruators and thus contribute substantially to the chance of developing endometriosis in specific subgroups (D’Hooghe and Debrock, 2002; Yovich et al., 2020). Indeed, endometriosis is significantly more common in patients with obstructive Müllerian anomalies than in those with non-obstructive anomalies (Pinsonneault and Goldstein, 1985; Sanfilippo et al., 1986; Olive and Henderson, 1987). In addressing the question ‘Why do not all women develop endometriosis?”, ’ D’Hooghe and Debrock (2002) stated, after reviewing published epidemiological and experimental data, that ‘it can be hypothesised that the quantity of retrogradely flushed endometrial cells may be important in the development of endometriosis and in the spontaneous evolution of the disease’.
Ultimately, it remains uncertain whether the association between ROM and endometriosis-adenomyosis can be interpreted as causal. In an attempt to disentangle this issue, the Bradford Hill criteria (1965) could be used as a framework. However, these criteria, defined as ‘viewpoints’ by the author himself (Hill, 1965), are not intended to be a checklist to support causation, and meeting all the criteria is neither necessary nor sufficient to establish it. Furthermore, alternative hypotheses to explain the association presented here cannot be ruled out (Table 2). In fact, especially in multifactorial diseases, causation can rarely be established irrefutably, and a range from ‘highly unlikely’ to ‘highly likely’ is more often contemplated (Nowinski et al., 2022).
Table 2.
Applying the Bradford Hill (1965) criteria for causation to reiterative ovulatory menstruation and endometriosis-adenomyosis.
| Criterion | Strength of evidence* | Argument for fulfilment | Supporting citations |
|---|---|---|---|
| Strength | ++ | Consistent associations have been observed between age at menarche, regular cycles, and heavy menstrual flow, i.e. indicators of pelvic exposure to refluxed endometrium, and endometriosis | Cramer et al. (1986), Darrow et al. (1993), Parazzini et al. (1995, 2017), Vercellini et al. (1997), Missmer and Cramer (2003), Missmer et al. (2004), Viganò et al. (2004), Treloar et al. (2010), Nnoaham et al. (2012), Hoppenbrouwers et al. (2016), Shafrir et al. (2018), and Lu et al. (2023). |
| Consistency | ++ | The above associations have been observed by independent groups in different countries | Missmer and Cramer (2003), Missmer et al. (2004), Viganò et al. (2004), Nnoaham et al. (2012), Hoppenbrouwers et al. (2016), Parazzini et al. (2017), Shafrir et al. (2018), and Lu et al. (2023). |
| Specificity | ++ | Although exceptions have been described, in the vast majority of cases adenomyosis and endometriosis develop and progress in menstruators. The distribution of endometriotic lesions is consistent with the dissemination of refluxed endometrial glands according to anatomical and physiological determinants | Vercellini et al. (1998, 2007, 2014), Missmer and Cramer (2003), Viganò et al. (2004), Parazzini et al. (2017, 2020), and Shafrir et al. (2018). |
| Temporality | +++ | Excluding anecdotal cases, adenomyosis and endometriosis are diagnosed after a variable number of years of repetitive ovulatory menstruations | Missmer and Cramer (2003), Missmer et al. (2004), Parazzini et al. (2017), Shafrir et al. (2018), Zondervan et al. (2018, 2020), Bulun et al. (2019, 2021, 2023), Guo (2020, 2023), Wang et al. (2020), Koninckx et al. (2021b), Taylor et al. (2021), Horne and Missmer (2022), and Kobayashi (2023a). |
| Biological gradient | ++ | A dose–response relationship has been observed between the number of ovulatory menses and the amount of menstrual flow and the risk of endometriosis | Darrow et al. (1993), Vercellini et al. (1997), Missmer and Cramer (2003), Missmer et al. (2004), Viganò et al. (2004), Matalliotakis et al. (2008), and Parazzini et al. (2017). |
| Plausibility | +++ | An large amount of data supports the mechanistic role of reiterative ovulatory menstruation in the pathogenesis of both adenomyosis and endometriosis | Zondervan et al. (2018, 2020), Bulun et al. (2019, 2021, 2023), Guo (2020, 2023), Wang et al. (2020), Koninckx et al. (2021b), Taylor et al. (2021), Horne and Missmer (2022), and Kobayashi (2023a). |
| Coherence | ++ | Epidemiological observations are consistent with several laboratory studies | Greaves et al. (2014), Shen et al. (2016), Zhang et al. (2017), Kusama et al. (2021), and Cordeiro et al., (2022). |
| Experiment | + | Experimental evidence exists for the validity of pelvic iron overload and secondary oxidative stress as determinants of chronic inflammation and fibrosis. No randomized, controlled trials have been conducted on the effect of early menstrual suppression and risk of endometriosis and adenomyosis | Van Langendonckt et al. (2002a,b), Lousse et al. (2009, 2012), Donnez et al. (2016), Ng et al. (2020), Ansariniya et al. (2022), Kobayashi (2023b), and Wyatt et al. (2023). |
| Analogy | ++ |
|
Macmahon et al. (1982), Vihko and Apter (1984), Viganò et al. (2004), Vercellini et al. (2006, 2011), Seidman (2013), Lattuada et al. (2015), Huang et al. (2016), Uberti et al. (2016), Farland et al. (2017b), Rockfield et al. (2019), Chhabra et al. (2021), and Bieuville et al. (2023). |
* Strength of evidence: + weak; ++ moderate; +++ strong.
With due regard to the above caveats, based on the available evidence, the association between ROM and endometriosis-adenomyosis appears to be moderately skewed towards ‘highly likely’ on the continuum between the two extremes of the causation scale (Table 2). Moreover, even if additional pathogenic causes are eventually identified, they would not necessarily diminish the importance of the relationship between ROM and endometriosis-adenomyosis.
Against this background, the next key question is whether medical interventions with an appropriate balance of potential benefits, potential harms, and costs could be implemented in practice to try to limit the risk of progression of early endometriotic and adenomyotic lesions to more extensive forms, with the consequent potential detrimental effects on health-related quality of life and fertility.
Prospectus: preventive interventional endocrinology?
The available epidemiological evidence on the possible effect of ROM on the frequency of endometriosis may appear puzzling, as on the one hand it consistently shows convincing associations between menstrual patterns suggesting increased retrograde pelvic contamination (e.g. early menarche, short and regular cycles, abundant menses) and the disease (Missmer and Cramer, 2003; Missmer et al., 2004; Viganò et al., 2004; Parazzini et al., 2017; Shafrir et al., 2018), but on the other hand it does not indicate an increase in the incidence and prevalence of endometriosis over time in regular menstruators (Ghiasi et al., 2020; Parazzini et al., 2020; Christ et al., 2021; Sarria-Santamera et al., 2020).
Indeed, research into retrograde menstruation should be resumed, as it seems to have been somewhat disregarded, despite the possible fundamental pathogenic insights that may be derived from it (Wyatt et al., 2023). In fact, uncertainties about the potential impact of the substantial increase in the number of ovulatory menstruations in the post-industrial era on the risk of endometriosis appear to be partly determined by our limited knowledge of what actually happens in the pelvis of individuals in the general population during the perimenstrual days.
However, despite the criticisms of our view described above, it is undeniable that menstruators from Palaeolithic to pre-industrial times spent the first decade after menarche in a relatively stable hormonal environment, characterized by infrequent ovulatory menstruation, prolonged progesterone exposure and long periods of hypoestrogenism, whereas menstruators from Western, industrialized, high-income nations now experience recurrent gonadal hormone fluctuations, characterized by repetitious ovulatory menstruation, prolonged oestrogen exposure, and the absence of hypoestrogenic phases. Indeed, Renfree (2012) suggests that reiterative menstruation is an iatrogenic disorder of modern societies, potentially causing ‘diseases of nulliparity’, and several investigators support the notion of endometriosis and adenomyosis as disorders initiated by ROM in susceptible individuals, promoted by a mitogenic and pro-inflammatory hyper-oestrogenic local and systemic milieu, and alleviated by amenorrhoea and long-term, stable, low-oestrogen, and high-progesterone exposure (Kruitwagen, 1993; D’Hooghe and Debrock, 2002; Lousse et al., 2009; Donnez et al., 2016; Bulun et al., 2019, 2021, 2023; Guo, 2020, 2023; Maruyama et al., 2020; Koninckx et al., 2021c; Bulun, 2022; Yovich et al., 2020; Kobayashi, 2023; Kobayashi et al., 2023; Wyatt et al., 2023).
Given these premises, should we ultimately accept or reject the null hypothesis that ROM has no effect on disease development and progression? From an evolutionary point of view, should not anovulation and amenorrhoea still be considered the physiological state at least during the early reproductive years? If ROM, especially during adolescence, defined here as the period between 12 and 20 years of age (Martire et al., 2020; Millischer et al., 2023), has an impact on the likelihood of the development and progression of endometriosis and adenomyosis, then there could be an opportunity to use hormonal interventions in secondary prevention selectively in individuals with disabling pelvic pain to reduce the burden of exposure to one of the well-established risk factors for these two conditions (Fathalla, 2019; Bulun et al., 2019, 2021; Munro, 2023).
Notwithstanding foreseeable problems with diagnostic modalities, recruitment, and long-term treatment adherence, randomized trials could be designed to provide mechanistic insight into whether inhibiting ovulation and menstruation can protect adolescents with severe dysmenorrhoea and heavy monthly bleeding from progression of early-onset endometriosis and adenomyosis. However, this would take many years and, in the meantime, the ethical question may emerge as to whether, in the absence of definitive epidemiological evidence of causation and results from formal RCTs, we are justified in not suppressing a hypothetical source of lesions and an undeniable cause of symptoms.
In the words of Nowinski et al. (2022) ‘any actions are risky, including decisions to do nothing. When thinking of causation as a continuum from highly unlikely to highly likely, we must consider the expected net harm of doing something vs. doing nothing. If we do something, and we learn later that the hazard was not really a hazard, there may be harm done. If we do nothing, and the hazard was in fact a hazard, there will be harm done of a very different sort’.
The issue may have important clinical implications given that (i) most adolescents with severe pelvic pain symptoms have endometriosis (Janssen et al., 2013); (ii) most lesions visualised at laparoscopy are limited superficial peritoneal implants (Hirsch et al., 2020); (iii) a not negligeable proportion of these superficial lesions will progress to more severe and fibrotic forms (Koninckx et al., 2021b); (iv) the consequences of disease progression are potentially severe.
Accordingly, in the second part of this opinion piece, a proposal for possible individualized secondary prevention of early-onset endometriosis and adenomyosis is presented based on the evolutionary considerations expressed above.
Acknowledgements
We are extremely grateful to the anonymous reviewers and the Associate Editor, who provided essential analytical criticism and key constructive suggestions, and clearly spent much of their valuable time helping improve the overall structure and logical framework of this article.
Contributor Information
Paolo Vercellini, Department of Clinical Sciences and Community Health, Academic Centre for Research on Adenomyosis and Endometriosis, Università degli Studi, Milano, Italy; Gynecology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Veronica Bandini, Department of Clinical Sciences and Community Health, Academic Centre for Research on Adenomyosis and Endometriosis, Università degli Studi, Milano, Italy.
Paola Viganò, Department of Clinical Sciences and Community Health, Academic Centre for Research on Adenomyosis and Endometriosis, Università degli Studi, Milano, Italy; Gynecology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Giorgia Di Stefano, Gynecology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Camilla Erminia Maria Merli, Gynecology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Edgardo Somigliana, Department of Clinical Sciences and Community Health, Academic Centre for Research on Adenomyosis and Endometriosis, Università degli Studi, Milano, Italy; Gynecology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Data availability
The data included in this article were extracted as published in the available original articles. No new data were generated or analysed to support this article.
Authors’ roles
P.Ve. conceived the study and drafted the original version of the article. V.B. and P.Vi. contributed to data collection and drafting of the manuscript. G.D.S and C.E.M.M. drafted part of the article. E.S. participated in conceiving and drafting the article and critically revising the paper. All authors approved the final version of the manuscript.
Funding
The open access facility of this paper was funded by Italian Ministry of Health, Current Research IRCCS Ca’Granda Ospedale Maggiore Policlinico di Milano.
Conflict of interest
P.Ve. serves as Associate Editor for Human Reproduction; is a member of the Editorial Board of the Journal of Obstetrics and Gynaecology Canada, of the Italian Journal of Obstetrics and Gynaecology, and of the International Editorial Board of Acta Obstetricia et Gynecologica Scandinavica; has received royalties from Wolters Kluwer for chapters on endometriosis management in the clinical decision support resource UpToDate; and maintains both a public and private gynaecological practice. E.S. discloses payments from Ferring for research grants, as well as receipt of equipment and honoraria from Merck-Serono for lectures. All other authors declare they have no conflict of interest.
References
- Ansariniya H, Yavari A, Javaheri A, Zare F.. Oxidative stress-related effects on various aspects of endometriosis. Am J Reprod Immunol 2022;88:e13593. [DOI] [PubMed] [Google Scholar]
- Arab A, Karimi E, Vingrys K, Kelishadi MR, Mehrabani S, Askari G.. Food groups and nutrients consumption and risk of endometriosis: a systematic review and meta-analysis of observational studies. Nutr J 2022;21:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azlan A, Salamonsen LA, Hutchison J, Evans J.. Endometrial inflammasome activation accompanies menstruation and may have implications for systemic inflammatory events of the menstrual cycle. Hum Reprod 2020;35:1363–1376. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Stenosis of the external cervical os: an association with endometriosis in women with chronic pelvic pain. Fertil Steril 1998;70:571–573. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Callery M, Perez SE.. Directionality of menstrual flow: cervical os diameter as a determinant of retrograde menstruation. Fertil Steril 1992;57:727–730. [DOI] [PubMed] [Google Scholar]
- Barton M. Intravaginal packs. Br Med J 1942;1:524–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosik D, Jacobs SL, Kelly LJ.. Endometrial tissue in peritoneal fluid. Fertil Steril 1986;46:796–800. [DOI] [PubMed] [Google Scholar]
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K. et al. ; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum Reprod Open 2022;2022:hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieuville M, Faugère D, Galibert V, Henard M, Dujon AM, Ujvari B, Pujol P, Roche B, Thomas F.. Number of lifetime menses increases breast cancer occurrence in postmenopausal women at high familial risk. Front Ecol Evol 2023;11:912083. doi: 10.3389/fevo.2023.912083 [DOI] [Google Scholar]
- Blumenkrantz MJ, Gallagher N, Bashore RA, Tenckhoff H.. Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstet Gynecol 1981;57:667–670. [PubMed] [Google Scholar]
- Bokor A, Debrock S, Drijkoningen M, Goossens W, Fülöp V, D'Hooghe T.. Quantity and quality of retrograde menstruation: a case control study. Reprod Biol Endocrinol 2009;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougie O, Nwosu I, Warshafsky C.. Revisiting the impact of race/ethnicity in endometriosis. Reprod Fertil 2022;3:R34–R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougie O, Yap MI, Sikora L, Flaxman T, Singh S.. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG 2019;126:1104–1115. [DOI] [PubMed] [Google Scholar]
- Bulletti C, De Ziegler D, Polli V, Del Ferro E, Palini S, Flamigni C.. Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertil Steril 2002;77:1156–1161. [DOI] [PubMed] [Google Scholar]
- Bullough VL. Merchandising the sanitary napkin: Lillian Gilbreth's 1927 survey. Signs (Chic) 1985;10:615–627. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis caused by retrograde menstruation: now demonstrated by DNA evidence. Fertil Steril 2022;118:535–536. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ.. Role of estrogen receptor-b in endometriosis. Semin Reprod Med 2012;30:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Yildiz S, Adli M, Chakravarti D, Parker JB, Milad M, Yang L, Chaudhari A, Tsai S, Wei JJ. et al. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril 2023;119:746–750. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Yildiz S, Adli M, Wei JJ.. Adenomyosis pathogenesis: insights from next-generation sequencing. Hum Reprod Update 2021;27:1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J.. Endometriosis. Endocr Rev 2019;40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiani GB, Danesino V, Gastaldi A, Parazzini F, Ferraroni M.. Reproductive and menstrual factors and risk of peritoneal and ovarian endometriosis. Fertil Steril 1991;56:230–234. [PubMed] [Google Scholar]
- Carlson LJ, Shaw ND.. Development of ovulatory menstrual cycles in adolescent girls. J Pediatr Adolesc Gynecol 2019;32:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R, Rockfield S, Guergues J, Nadeau OW, Hill R, Stevens SM Jr, Nanjundan M.. Global miRNA/proteomic analyses identify miRNAs at 14q32 and 3p21, which contribute to features of chronic iron-exposed fallopian tube epithelial cells. Sci Rep 2021;11:6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Hsu TF, Jiang LY, Chan IS, Shih YC, Chang YH, Wang PH, Chen YJ.. Maintenance therapy for preventing endometrioma recurrence after endometriosis resection surgery—a systematic review and network meta-analysis. J Minim Invasive Gynecol 2022;29:602–612. [DOI] [PubMed] [Google Scholar]
- Christ JP, Yu O, Schulze-Rath R, Grafton J, Hansen K, Reed SD.. Incidence, prevalence, and trends in endometriosis diagnosis: a United States population-based study from 2006 to 2015. Am J Obstet Gynecol 2021;225:500.e1–500-e9. [DOI] [PubMed] [Google Scholar]
- Clemenza S, Vannuccini S, Ruotolo A, Capezzuoli T, Petraglia F.. Advances in targeting estrogen synthesis and receptors in patients with endometriosis. Expert Opin Investig Drugs 2022;31:1227–1238. [DOI] [PubMed] [Google Scholar]
- Cordeiro MR, Carvalhos CA, Figueiredo-Dias M.. The emerging role of menstrual-blood-derived stem cells in endometriosis. Biomedicines 2022;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC.. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986;255:1904–1908. [PubMed] [Google Scholar]
- Critchley HOD, Babayev E, Bulun SE, Clark S, Garcia-Grau I, Gregersen PK, Kilcoyne A, Kim J-YJ, Lavender M, Marsh EE. et al. Menstruation: science and society. Am J Obstet Gynecol 2020a;223:624–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW.. Physiology of the endometrium and regulation of menstruation. Physiol Rev 2020b;100:1149–1179. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Straub RH.. Estrogen's effects in chronic autoimmune/inflammatory diseases and progression to cancer. Expert Rev Clin Immunol 2014;10:31–39. [DOI] [PubMed] [Google Scholar]
- Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S.. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology 1993;4:135–142. [DOI] [PubMed] [Google Scholar]
- Davenport S, Smith D, Green DJ.. Barriers to a timely diagnosis of endometriosis: a qualitative systematic review. Obstet Gynecol 2023;142:571–583. doi: 10.1097/AOG.0000000000005255. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Dawson A, Llauradó Fernandez M, Anglesio M, Yong PJ, Carey MS.. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018;12:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis V, Rigon F, Bernasconi S, Bianchin L, Bona G, Bozzola M, Buzi F, Sanctis D, Tonini C, Radetti G. et al. Age at menarche and menstrual abnormalities in adolescence: does it matter? The evidence from a large survey among Italian Secondary Schoolgirls. Indian J Pediatr 2019;86:34–41. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Debrock S.. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update 2002;8:84–88. [DOI] [PubMed] [Google Scholar]
- Dodd KC, Menon M.. Sex bias in lymphocytes: Implications for autoimmune diseases. Front Immunol 2022;13:945762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Binda MM, Donnez O, Dolmans MM.. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril 2016;106:1011–1017. [DOI] [PubMed] [Google Scholar]
- Dorien FO, Roskams T, Van den Eynde K, Vanhie A, Peterse DP, Meuleman C, Tomassetti C, Peeraer K, D'Hooghe TM, Fassbender A.. The presence of endometrial cells in peritoneal fluid of women with and without endometriosis. Reprod Sci 2017;24:242–251. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE.. Ovulation: parallels with inflammatory processes. Endocr Rev 2019;40:369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SB, Pike MC, Short RV, Lee NC, Trussell J, Hatcher RA, Wood JW, Worthman CM, Jones NG, Konner MJ.. Women's reproductive cancers in evolutionary context. Q Rev Biol 1994;69:353–367. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Strassman BI, Nesse RM, Neel JV, Ewald PW, Williams GC, Weder AB, Eaton SB, Lindeberg S, Konner MJ. et al. Evolutionary health promotion. Prev Med 2002;34:109–118. [DOI] [PubMed] [Google Scholar]
- Emaus A, Espetvedt S, Veierød MB, Ballard-Barbash R, Furberg AS, Ellison PT, Jasienska G, Hjartåker A, Thune I.. 17-Beta-estradiol in relation to age at menarche and adult obesity in premenopausal women. Hum Reprod 2008;23:919–927. [DOI] [PubMed] [Google Scholar]
- Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod 1994;50:233–238. [DOI] [PubMed] [Google Scholar]
- Evans J, Salamonsen LA.. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord 2012;13:277–288. [DOI] [PubMed] [Google Scholar]
- Farland LV, Eliassen AH, Tamimi RM, Spiegelman D, Michels KB, Missmer SA.. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ 2017a;358:j3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farland LV, Mu F, Eliassen AH, Hankinson SE, Tworoger SS, Barbieri RL, Dowsett M, Pollak MN, Missmer SA.. Menstrual cycle characteristics and steroid hormone, prolactin, and growth factor levels in premenopausal women. Cancer Causes Control 2017b;28:1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathalla MF. Impact of reproductive evolutionary mismatch on women's health and the need for action and research. Int J Gynaecol Obstet 2019;144:129–134. [DOI] [PubMed] [Google Scholar]
- Finn CA. Implantation, menstruation and inflammation. Biol Rev Camb Philos Soc 1986;61:313–328. [DOI] [PubMed] [Google Scholar]
- Finn CA. Why do women menstruate? Historical and evolutionary review. Eur J Obstet Gynecol Reprod Biol 1996;70:3–8. [DOI] [PubMed] [Google Scholar]
- Fu Z, Taylor S, Modugno F.. Lifetime ovulations and epithelial ovarian cancer risk and survival: a systematic review and meta-analysis. Gynecol Oncol 2022;165:650–663. [DOI] [PubMed] [Google Scholar]
- Fukui A, Mai C, Saeki S, Yamamoto M, Takeyama R, Kato T, Ukita Y, Wakimoto Y, Yamaya A, Shibahara H.. Pelvic endometriosis and natural killer cell immunity. Am J Reprod Immunol 2021;85:e13342. [DOI] [PubMed] [Google Scholar]
- Gamble J. Puberty: early starters. Nature 2017;550:S10–S11. [DOI] [PubMed] [Google Scholar]
- Gérard N, Caillaud M, Martoriati A, Goudet G, Lalmanach AC.. The interleukin-1 system and female reproduction. J Endocrinol 2004;180:203–212. [DOI] [PubMed] [Google Scholar]
- Ghiasi M, Kulkarni MT, Missmer SA.. Is endometriosis more common and more severe than it was 30 years ago? J Minim Invasive Gynecol 2020;27:452–461. [DOI] [PubMed] [Google Scholar]
- Gottschalk MS, Eskild A, Hofvind S, Gran JM, Bjelland EK.. Temporal trends in age at menarche and age at menopause: a population study of 312 656 women in Norway. Hum Reprod 2020;35:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PT.. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol 2014;184:1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn HM, Tsai MC, McRae A, Steinbeck KS.. Menstrual patterns in the first gynecological year: a systematic review. J Pediatr Adolesc Gynecol 2018;31:557–565.e6. [DOI] [PubMed] [Google Scholar]
- Guo SW. Fibrogenesis resulting from cyclic bleeding: the Holy Grail of the natural history of ectopic endometrium. Hum Reprod 2018;33:353–356. [DOI] [PubMed] [Google Scholar]
- Guo SW. The pathogenesis of adenomyosis vis-à-vis endometriosis. J Clin Med 2020;9:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SW. The role of platelets in the pathogenesis and pathophysiology of adenomyosis. J Clin Med 2023;12:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines MR, Guest AM.. Fertility in New York state in the pre-civil war era. Demography 2008;45:345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM.. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151–154. [PubMed] [Google Scholar]
- Hart TL, Petersen KS, Kris-Etherton PM.. Nutrition recommendations for a healthy pregnancy and lactation in women with overweight and obesity—strategies for weight loss before and after pregnancy. Fertil Steril 2022;118:434–446. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A.. The weirdest people in the world? Behav Brain Sci 2010;33:61–83. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc Roy Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M, Dhillon-Smith R, Cutner AS, Yap M, Creighton SM.. The prevalence of endometriosis in adolescents with pelvic pain: a systematic review. J Pediatr Adolesc Gynecol 2020;33:623–630. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers K, Roelants M, Meuleman C, Rijkers A, Van Leeuwen K, Desoete A, D'Hooghe T.. Characteristics of the menstrual cycle in 13-year-old Flemish girls and the impact of menstrual symptoms on social life. Eur J Pediatr 2016;175:623–630. [DOI] [PubMed] [Google Scholar]
- Horne AW, Missmer SA.. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022;379:e070750. [DOI] [PubMed] [Google Scholar]
- Huang HS, Hsu CF, Chu SC, Chen PC, Ding DC, Chang MY, Chu TY.. Haemoglobin in pelvic fluid rescues Fallopian tube epithelial cells from reactive oxygen species stress and apoptosis. J Pathol 2016;240:484–494. [DOI] [PubMed] [Google Scholar]
- InterLACE Study Team. Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum Reprod 2019;34:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istituto Nazionale di Statistica. La dinamica demografica—anno 2022. https://www.istat.it/it/files//2023/03/Dinamica-demografica2022.pdf; http://dati.istat.it/ (8 April 2023, date last accessed).
- Jain V, Chodankar RR, Maybin JA, Critchley HOD.. Uterine bleeding: how understanding endometrial physiology underpins menstrual health. Nat Rev Endocrinol 2022;18:290–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EB, Rijkers AC, Hoppenbrouwers K, Meuleman C, D'Hooghe TM.. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update 2013;19:570–582. [DOI] [PubMed] [Google Scholar]
- Jarrell J. The significance and evolution of menstruation. Best Pract Res Clin Obstet Gynaecol 2018;50:18–26. [DOI] [PubMed] [Google Scholar]
- Jarrell J, Arendt-Nielsen L.. Evolutionary considerations in the development of chronic pelvic pain. Am J Obstet Gynecol 2016;215:201.e1–201.e4. [DOI] [PubMed] [Google Scholar]
- Jordan SJ, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA.. Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes Control 2012;23:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon JG, Plowden TC, Marsh EE.. Racial disparities in uterine fibroids and endometriosis: a systematic review and application of social, structural, and political context. Fertil Steril 2023;119:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Endometrial inflammation and impaired spontaneous decidualization: insights into the pathogenesis of adenomyosis. Int J Environ Res Public Health 2023;20:3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Yoshimoto C, Matsubara S, Shigetomi H, Imanaka S.. Current understanding of and future directions for endometriosis-related infertility research with a focus on ferroptosis. Diagnostics 2023;13:1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninckx PR, Fernandes R, Ussia A, Schindler L, Wattiez A, Al-Suwaidi S, Amro B, Al-Maamari B, Hakim Z, Tahlak M. et al. Pathogenesis based diagnosis and treatment of endometriosis. Front Endocrinol (Lausanne) 2021c;12:745548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Adamyan L, Gomel V, Martin DC.. Peritoneal fluid progesterone and progesterone resistance in superficial endometriosis lesions. Hum Reprod 2022;37:203–211. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Adamyan L, Tahlak M, Keckstein J, Wattiez A, Martin DC.. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract Res Clin Obstet Gynaecol 2021a;71:14–26. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Tahlak M, Adamyan L, Wattiez A, Martin DC, Gomel V.. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: a systematic review. Facts Views Vis Obgyn 2019;11:209–216. [PMC free article] [PubMed] [Google Scholar]
- Koninckx PR, Ussia A, Wattiez A, Adamyan L, Martin DC, Gordts S.. The severity and frequency distribution of endometriosis subtypes at different ages: a model to understand the natural history of endometriosis based on single centre/single surgeon data. Facts Views Vis Obgyn 2021b;13:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruitwagen RF. Menstruation as the pelvic aggressor. Baillieres Clin Obstet Gynaecol 1993;7:687–700. [DOI] [PubMed] [Google Scholar]
- Kuan KKW, Gibson DA, Whitaker LHR, Horne AW.. Menstruation dysregulation and endometriosis development. Front Reprod Health 2021;3:756704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman RJ, Shih IM.. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 2016;186:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama K, Fukushima Y, Yoshida K, Sakakibara H, Tsubata N, Yoshie M, Kojima J, Nishi H, Tamura K.. Endometrial epithelial-mesenchymal transition (EMT) by menstruation-related inflammatory factors during hypoxia. Mol Hum Reprod 2021;27:gaab036. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Bijon A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC.. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology 2013;24:261–269. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, Roman H, Becker CM, As-Sanie S, Zondervan KT. et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update 2021;27:393–420. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Uberti F, Colciaghi B, Morsanuto V, Maldi E, Squarzanti DF, Molinari C, Boldorini R, Bulfoni A, Colombo P. et al. Fimbrial cells exposure to catalytic iron mimics carcinogenic changes. Int J Gynecol Cancer 2015;25:389–398. [DOI] [PubMed] [Google Scholar]
- Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G.. Endometriosis and the microbiome: a systematic review. BJOG 2020;127:239–249. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, Bottcher B, Wildt L.. Adenomyosis and endometriosis. Arch Gynecol Obstet 2015;291:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendecker G, Kunz G, Herbertz M, Beil D, Huppert P, Mall G, Kissler S, Noe M, Wildt L.. Uterine peristaltic activity and the development of endometriosis. Ann N Y Acad Sci 2004;1034:338–355. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Kunz G, Noe M, Herbertz M, Mall G.. Endometriosis: a dysfunction and disease of the archimetra. Hum Reprod Update 1998;4:752–762. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Wildt L, Laschke MW, Mall G.. Archimetrosis: the evolution of a disease and its extant presentation: pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch Gynecol Obstet 2023;307:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendecker G, Wildt L, Mall G.. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 2009;280:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RHW, Lo SST, Gemzell-Danielsson K, Fong CHY, Ho PC, Ng EHY.. Oral emergency contraception with levonorgestrel plus piroxicam: a randomised double-blind placebo-controlled trial. Lancet 2023;402:851–858. 10.1016/0. [DOI] [PubMed] [Google Scholar]
- Li Y, He Y, Cheng W, Zhou Z, Ni Z, Yu C.. Double-edged roles of ferroptosis in endometriosis and endometriosis-related infertility. Cell Death Discov 2023;9:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Ali F, Ramaiyer M, Borahay MA.. Determinants and assessment of menstrual blood flow. Curr Epidemiol Rep 2023;1:1. doi: 10.1007/s40471-023-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Wu J, Wang W, Xie H, Yao S.. Pro-endometriotic niche in endometriosis. Reprod Biomed Online 2019;38:549–559. [DOI] [PubMed] [Google Scholar]
- Liberty A, Samuelson Bannow B, Matteson K, Edelman A, Colwill A.. Menstrual technology innovations and the implications for heavy menstrual bleeding. Obstet Gynecol 2023;141:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SF, Gerry E, Shih IM.. Tubal origin of ovarian cancer—the double-edged sword of haemoglobin. J Pathol 2017;242:3–6. [DOI] [PubMed] [Google Scholar]
- Liu DT, Hitchcock A.. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br J Obstet Gynaecol 1986;93:859–862. [DOI] [PubMed] [Google Scholar]
- Lousse JC, Defrère S, Van Langendonckt A, Gras J, González-Ramos R, Colette S, Donnez J.. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil Steril 2009;91:1668–1675. [DOI] [PubMed] [Google Scholar]
- Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J.. Peritoneal endometriosis is an inflammatory disease. Front Biosci (Elite Ed) 2012;4:23–40. [DOI] [PubMed] [Google Scholar]
- Lu MY, Niu JL, Liu B.. The risk of endometriosis by early menarche is recently increased: a meta-analysis of literature published from 2000 to 2020. Arch Gynecol Obstet 2023;307:59–69. [DOI] [PubMed] [Google Scholar]
- Lund CI, Engdahl B, Rosseland LA, Stubhaug A, Grimnes G, Furberg AS, Steingrímsdóttir ÓA, Nielsen CS.. The association between age at menarche and chronic pain outcomes in women: the Tromsø Study, 2007 to 2016. Pain 2022;163:1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmahon B, Trichopoulos D, Brown J, Andersen AP, Cole P, Dewaard F, Kauraniemi T, Polychronopoulou A, Ravnihar B, Stormby N. et al. Age at menarche, urine estrogens and breast cancer risk. Int J Cancer 1982;30:427–431. [DOI] [PubMed] [Google Scholar]
- Marroquin J, Kiomourtzoglou MA, Scranton A, Pollack AZ.. Chemicals in menstrual products: a systematic review. BJOG 2023:1–10. doi: 10.1111/1471-0528.17668. [DOI] [PubMed] [Google Scholar]
- Martire FG, Lazzeri L, Conway F, Siciliano T, Pietropolli A, Piccione E, Solima E, Centini G, Zupi E, Exacoustos C.. Adolescence and endometriosis: symptoms, ultrasound sign and early diagnosis. Fertil Steril 2020;114:1049–1057. [DOI] [PubMed] [Google Scholar]
- Maruyama S, Imanaka S, Nagayasu M, Kimura M, Kobayashi H.. Relationship between adenomyosis and endometriosis; different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol 2020;253:191–197. [DOI] [PubMed] [Google Scholar]
- Masuda H, Schwab KE, Filby CE, Tan CSC, Tsaltas J, Weston GC, Gargett CE.. Endometrial stem/progenitor cells in menstrual blood and peritoneal fluid of women with and without endometriosis. Reprod Biomed Online 2021;43:3–13. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A.. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet 2008;277:389–393. [DOI] [PubMed] [Google Scholar]
- Matta K, Koual M, Ploteau S, Coumoul X, Audouze K, Le Bizec B, Antignac JP, Cano-Sancho G.. Associations between exposure to organochlorine chemicals and endometriosis: a systematic review of experimental studies and integration of epidemiological evidence. Environ Health Perspect 2021;129:76003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millischer AE, Santulli P, Da Costa S, Bordonne C, Cazaubon E, Marcellin L, Chapron C.. Adolescent endometriosis: prevalence increases with age on magnetic resonance imaging scan. Fertil Steril 2023;119:626–633. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE.. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod 2010;25:1528–e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Cramer DW.. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30:1–19. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ.. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol 2004;104:965–974. [DOI] [PubMed] [Google Scholar]
- Munro MG. Adenomyosis and uterine stiffness: what is the chicken? Which is the egg? Hum Reprod 2023;38:527–528. [DOI] [PubMed] [Google Scholar]
- Muraoka A, Suzuki M, Hamaguchi T, Watanabe S, Iijima K, Murofushi Y, Shinjo K, Osuka S, Hariyama Y, Ito M. et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci Transl Med 2023;15:eadd1531. [DOI] [PubMed] [Google Scholar]
- Ness RB, Cottreau C.. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst 1999;91:1459–1467. [DOI] [PubMed] [Google Scholar]
- Ng SW, Norwitz SG, Taylor HS, Norwitz ER.. Endometriosis: the role of iron overload and ferroptosis. Reprod Sci 2020;27:1383–1390. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT.. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril 2012;98:702–712.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski CJ, Bureau SC, Buckland ME, Curtis MA, Daneshvar DH, Faull RLM, Grinberg LT, Hill-Yardin EL, Murray HC, Pearce AJ. et al. Applying the Bradford Hill criteria for causation to repetitive head impacts and chronic traumatic encephalopathy. Front Neurol 2022;13:938163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 2012a;11:A479–85. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev 2012b;11:A486–92. [DOI] [PubMed] [Google Scholar]
- Ohayi S, Onyishi N, Mbah S.. Endometriosis in an indigenous African women population. Afr Health Sci 2022;22:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive DL, Henderson DY.. Endometriosis and mullerian anomalies. Obstet Gynecol 1987;69:412–415. [PubMed] [Google Scholar]
- Olšarová K, Mishra GD.. Early life factors for endometriosis: a systematic review. Hum Reprod Update 2020;26:412–422. [DOI] [PubMed] [Google Scholar]
- Orlando MS, Luna Russo MA, Richards EG, King CR, Park AJ, Bradley LD, Chapman GC.. Racial and ethnic disparities in surgical care for endometriosis across the United States. Am J Obstet Gynecol 2022;226:824.e1–824.e11. [DOI] [PubMed] [Google Scholar]
- Otsuka I. Mechanisms of high-grade serous carcinogenesis in the fallopian tube and ovary: current hypotheses, etiologic factors, and molecular alterations. Int J Mol Sci 2021;22:4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolina J, Schimberni M, Makieva S, Bartiromo L, Fazia T, Bernardinelli L, Viganò P, Candiani M, Gentilini D.. Early-life factors, in-utero exposures and endometriosis risk: a meta-analysis. Reprod Biomed Online 2020;41:279–289. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S.. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017;209:3–7. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Ferraroni M, Fedele L, Bocciolone L, Rubessa S, Riccardi A.. Pelvic endometriosis: reproductive and menstrual risk factors at different stages in Lombardy, northern Italy. J Epidemiol Community Health 1995;49:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini F, Roncella E, Cipriani S, Trojano G, Barbera V, Herranz B, Colli E.. The frequency of endometriosis in the general and selected populations: a systematic review. J Endometriosis Pelvic Pain Disorders 2020;12:176–189. [Google Scholar]
- Pei Z, Lu W, Feng Y, Xu C, Hsueh AJW.. Out of step societal and Darwinian adaptation during evolution is the cause of multiple women's health issues. Hum Reprod 2022;37:1959–1969. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Johnstone EB, Hammoud AO, Stanford JB, Varner MW, Kennedy A, Chen Z, Sun L, Fujimoto VY, Hediger ML. et al. ; ENDO Study Working Group. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am J Obstet Gynecol 2013;208:451.e1–451.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault O, Goldstein DP.. Obstructing malformations of the uterus and vagina. Fertil Steril 1985;44:241–247. [PubMed] [Google Scholar]
- Piras GN, Bozzola M, Bianchin L, Bernasconi S, Bona G, Lorenzoni G, Buzi F, Rigon F, Tonini G, De Sanctis V. et al. The levelling-off of the secular trend of age at menarche among Italian girls. Heliyon 2020;6:e04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirdel L, Pirdel M.. Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis. Reproduction 2014;147:R199–207. [DOI] [PubMed] [Google Scholar]
- Praetorius TH, Leonova A, Lac V, Senz J, Tessier-Cloutier B, Nazeran TM, Köbel M, Grube M, Kraemer B, Yong PJ. et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil Steril 2022;118:524–534. [DOI] [PubMed] [Google Scholar]
- Reagan PB, Salsberry PJ, Fang MZ, Gardner WP, Pajer K.. African-American/white differences in the age of menarche: accounting for the difference. Soc Sci Med 2012;75:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BM, Permuth JB, Sellers TA.. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14:9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis FM, Petraglia F, Taylor RN.. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update 2013;19:406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree MB. Why menstruate? Bioessays 2012;34:1. [DOI] [PubMed] [Google Scholar]
- Reti LL, Byrne GD, Davoren RA.. The acute clinical features of retrograde menstruation. Aust N Z J Obstet Gynaecol 1983;23:51–52. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL.. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 2002;64:69–92. [DOI] [PubMed] [Google Scholar]
- Rockfield S, Kee Y, Nanjundan M.. Chronic iron exposure and c-Myc/H-ras-mediated transformation in fallopian tube cells alter the expression of EVI1, amplified at 3q26.2 in ovarian cancer. Oncogenesis 2019;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockfield S, Raffel J, Mehta R, Rehman N, Nanjundan M.. Iron overload and altered iron metabolism in ovarian cancer. Biol Chem 2017;398:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozewicki S, Radomska A, Kurzawa R.. Relation between anatomical courses of the intramural portions of the uterine tubes and pelvic endometriosis. Fertil Steril 2005;84:60–66. [DOI] [PubMed] [Google Scholar]
- Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM.. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update 2021;28:92–131. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Obstet Gynecol 1927;14:422–469. [PMC free article] [PubMed] [Google Scholar]
- Sampson JA. The development of the implantation thory for the origin of peritoneal endometriosis. Am J Obstet Gynecol 1940;40:549–557. [Google Scholar]
- Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA.. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol 1986;154:39–43. [DOI] [PubMed] [Google Scholar]
- Sangi-Haghpeykar H, Poindexter AN.. Epidemiology of endometriosis among parous women. Obstet Gynecol 1995;85:983–992. [DOI] [PubMed] [Google Scholar]
- Sarria-Santamera A, Orazumbekova B, Terzic M, Issanov A, Chaowen C, Asúnsolo-Del-Barco A.. Systematic review and meta-analysis of incidence and prevalence of endometriosis. Healthcare 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto N, Farland LV, Vitonis AF, Harris HR, DiVasta AD, Laufer MR, Terry KL, Missmer SA.. In utero and early life exposures in relation to endometriosis in adolescents and young adults. Eur J Obstet Gynecol Reprod Biol 2020;252:393–398. [DOI] [PubMed] [Google Scholar]
- Schüler S, Ponnath M, Engel J, Ortmann O.. Ovarian epithelial tumors and reproductive factors: a systematic review. Arch Gynecol Obstet 2013;287:1187–1204. [DOI] [PubMed] [Google Scholar]
- Seidman JD. The presence of mucosal iron in the fallopian tube supports the "incessant menstruation hypothesis" for ovarian carcinoma. Int J Gynecol Pathol 2013;32:454–458. [DOI] [PubMed] [Google Scholar]
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA.. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- Shafrir AL, Mu F, Eliassen AH, Thombre Kulkarni M, Terry KL, Hankinson SE, Missmer SA.. Endogenous steroid hormone concentrations and risk of endometriosis in nurses' health study II. Am J Epidemiol 2023;192:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Liu X, Zhang H, Guo SW.. Transforming growth factor β1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod 2016;31:355–369. [DOI] [PubMed] [Google Scholar]
- Short RV. Human reproduction in an evolutionary context. Ann N Y Acad Sci 1994;709:416–425. [DOI] [PubMed] [Google Scholar]
- Short RV. The evolution of human reproduction. Proc R Soc Lond B Biol Sci 1976;195:3–24. [DOI] [PubMed] [Google Scholar]
- Sirohi D, Al Ramadhani R, Knibbs LD.. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: a systematic literature review. Rev Environ Health 2021;36:101–115. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Benaglia L, Crovetto F, Vercellini P, Fedele L.. Endometriosis in a rural remote setting: a cross-sectional study. Gynecol Endocrinol 2012;28:979–982. [DOI] [PubMed] [Google Scholar]
- Strassmann BI. Menstrual cycling and breast cancer: an evolutionary perspective. J Womens Health 1999;8:193–202. [DOI] [PubMed] [Google Scholar]
- Strassmann BI. The biology of menstruation in homo sapiens: total lifetime menses, fecundity, and nonsynchrony in a natural-fertility population. Curr Anthropol 1997;38:123–129. [Google Scholar]
- Strassmann BI. The evolution of endometrial cycles and menstruation. Q Rev Biol 1996;71:181–220. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- Tang J, Wang Z, Lu D, Xie Y, Zhang D.. Value of early laparoscopic exploration for primary infertile patients with patent fallopian tubes complicated with pelvic effusion. Med Sci Monit 2022;28:e938637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Kotlyar AM, Flores VA.. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839–852. [DOI] [PubMed] [Google Scholar]
- Thomas EJ. Endometriosis: should not be treated just because it's there. Br Med J 1993;306:6871. [Google Scholar]
- Thomas SL, Ellertson C.. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000;355:922–924. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC.. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol 2010;202:534.e1–534.e6. [DOI] [PubMed] [Google Scholar]
- Uberti F, Morsanuto V, Lattuada D, Colciaghi B, Cochis A, Bulfoni A, Colombo P, Bolis G, Molinari C.. Protective effects of vitamin D3 on fimbrial cells exposed to catalytic iron damage. J Ovarian Res 2016;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs, Population Division. World Fertility Data. 2019. https://www.un.org/development/desa/pd/data/world-fertility-data (13 October 2023, date last accessed).
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022 Demographic Indicators by Region, Subregion and Country Annually for 1950-2100. 2022. https://web.archive.org/web/20220809032514/https://population.un.org/wpp/Download/Files/1_Indicators%20(Standard)/EXCEL_FILES/1_General/WPP2022_GEN_F01_DEMOGRAPHIC_INDICATORS_REV1.xlsx (13 October 2023, date last accessed).
- Upson K, Missmer SA.. Epidemiology of adenomyosis. Semin Reprod Med 2020;38:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée A, Ceccaldi PF, Carbonnel M, Feki A, Ayoubi JM.. Pollution and endometriosis: a deep dive into the environmental impacts on women's health. BJOG 2023. doi: 10.1111/1471-0528.17687. [DOI] [PubMed] [Google Scholar]
- van der Linden PJ, Dunselman GA, de Goeij AF, van der Linden EP, Evers JL, Ramaekers FC.. Epithelial cells in peritoneal fluid—of endometrial origin? Am J Obstet Gynecol 1995;173:566–570. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Dolmans MM, Donnez J.. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil Steril 2002a;77:561–570. [DOI] [PubMed] [Google Scholar]
- Van Langendonckt A, Casanas-Roux F, Donnez J.. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil Steril 2002b;78:712–718. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Abbiati A, Viganò P, Somigliana ED, Daguati R, Meroni F, Crosignani PG.. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum Reprod 2007;22:2359–2367. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Aimi G, De Giorgi O, Maddalena S, Carinelli S, Crosignani PG.. Is cystic ovarian endometriosis an asymmetric disease? Br J Obstet Gynaecol 1998;105:1018–1021. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Crosignani P, Somigliana E, Viganò P, Buggio L, Bolis G, Fedele L.. The ‘incessant menstruation’ hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod 2011;26:2262–2273. [DOI] [PubMed] [Google Scholar]
- Vercellini P, De Giorgi O, Aimi G, Panazza S, Uglietti A, Crosignani PG.. Menstrual characteristics in women with and without endometriosis. Obstet Gynecol 1997;90:264–268. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Bandini V, Buggio L, Berlanda N, Somigliana E.. Association of endometriosis and adenomyosis with pregnancy and infertility. Fertil Steril 2023;119:727–740. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Somigliana E, Daguati R, Abbiati A, Fedele L.. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol 2006;20:465–477. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Viganò P, Somigliana E, Fedele L.. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- Viganò P, Parazzini F, Somigliana E, Vercellini P.. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004;18:177–200. [DOI] [PubMed] [Google Scholar]
- Vihko R, Apter D.. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem 1984;20:231–236. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nicholes K, Shih IM.. The origin and pathogenesis of endometriosis. Annu Rev Pathol 2020;15:71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Long AJ, Noga H, Allaire C, Bedaiwy MA, Lisonkova S, Yong PJ.. East and South East Asian ethnicity and moderate-to-severe endometriosis. J Minim Invasive Gynecol 2019;26:507–515. [DOI] [PubMed] [Google Scholar]
- Weiss EA, Gandhi M.. Preferential cyclooxygenase 2 inhibitors as a nonhormonal method of emergency contraception: a look at the evidence. J Pharm Pract 2016;29:160–164. [DOI] [PubMed] [Google Scholar]
- Westwood S, Fannin M, Ali F, Thigpen J, Tatro R, Hernandez A, Peltzer C, Hildebrand M, Fernandez-Pacheco A, Raymond-Lezman JR. et al. Disparities in women with endometriosis regarding access to care, diagnosis, treatment, and management in the United States: a scoping review. Cureus 2023;15:e38765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Liang Z, Jiang J, Feng X, Liu J, Zhang Z, Wang H, Wang N, Gou Y, Li Z. et al. Macrophages originated IL-33/ST2 inhibits ferroptosis in endometriosis via the ATF3/SLC7A11 axis. Cell Death Dis 2023;14:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J, Fernando SM, Powell SG, Hill CJ, Arshad I, Probert C, Ahmed S, Hapangama DK.. The role of iron in the pathogenesis of endometriosis: a systematic review. Hum Reprod Open 2023;2023:hoad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE.. Evidence for a secular trend in age of menarche. N Engl J Med 1982;306:1033–1035. [DOI] [PubMed] [Google Scholar]
- Xholli A, Molinari F, Oppedisano F, Scovazzi U, Vacca I, Schiaffino MG, Cavalli E, Cagnacci A.. Relation between adenomyosis and elastographic characteristics of the cervix. Hum Reprod 2023;38:621–628. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang Y, Cai H, Zhou Q, Zeng L, Li S, Du H, Wei W, Zhang W, Dai W. et al. Translocation of vaginal and cervical low-abundance non-Lactobacillus bacteria notably associate with endometriosis: a pilot study. Microb Pathog 2023;183:106309. doi: 10.1016/j.micpath.2023.106309. [DOI] [PubMed] [Google Scholar]
- Yovich JL, Rowlands PK, Lingham S, Sillender M, Srinivasan S.. Pathogenesis of endometriosis: look no further than John Sampson. Reprod Biomed Online 2020;40:7–11. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu X, Guo SW.. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod Biomed Online 2017;34:124–136. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P.. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data included in this article were extracted as published in the available original articles. No new data were generated or analysed to support this article.