Abstract
We examined the antibacterial activities against Staphylococcus aureus of three diterpenes, namely, geranylgeraniol, teprenone, and phytol, by using a broth dilution with shaking method to identify the effects of diterpenes with long aliphatic carbon chains. We also performed time-kill assays and measured the leakage of K+ ions from bacterial cells in response to these diterpenes. The diterpenes used inhibited the growth of S. aureus at concentrations of 0.15 μg/ml, as determined by damage to the cell membranes, and had clear bactericidal activity. However, the inhibitory effects of the diterpenes decreased when the concentration of each was raised above a certain level. The diterpenes tested in this study appeared to have both growth-inhibitory and growth-accelerating effects, and the net effect of each depended on its concentration.
The essential oils that are extracted from certain plants and the constituents of these oils have attracted considerable attention as antibacterial agents (9, 11). It is becoming increasingly necessary to identify and develop novel antibacterial agents since nosocomial infections have become a serious problem and various common pathogens have become resistant to antibiotics. Many essential oils and their constituents have been used as folk remedies, but their antibacterial activities and modes of action remain to be clarified.
It is difficult to estimate the antibacterial activities of many plant-derived compounds because of their low solubility in water. Solubilizers, such as surfactants and alcohols, have been used to overcome this problem, but it can be difficult to distinguish the contribution to the antibacterial activity of the solubilizer from the contribution of the compound under investigation. Arai et al. established the broth dilution with shaking (BDS) method to solve this problem (2), and this method has been used to investigate the antibacterial activities of essential oils and their constituents against Staphylococcus aureus, which is one of the major pathogens responsible for nosocomial infections (1, 10). Studies using the BDS method revealed, for example, that farnesol, nerolidol, and plaunotol can inhibit the growth of S. aureus (5, 6). Farnesol and nerolidol are sesquiterpenes, and plaunotol is a diterpene, and a common feature of these compounds is a long aliphatic carbon chain. Monoterpenes do not effectively inhibit the growth of S. aureus (5), and the antibacterial activities of diterpenes remain to be clarified. Therefore, to investigate the antibacterial activities of diterpene alcohols in detail, we decided to examine diterpenes with one oxygen atom in their structure.
In this study, we estimated the antibacterial activities of geranylgeraniol, teprenone, and phytol against S. aureus by the BDS method to clarify the antibacterial activities of diterpenes with long aliphatic carbon chains. We also performed time-kill assays and measured the leakage of K+ ions from bacterial cells to confirm the validity of the BDS method.
MATERIALS AND METHODS
Reagents and microorganism.
Geranylgeraniol and phytol were purchased from Sigma Chemical Co. (St. Louis, Mo.) and Wako Pure Chemical Industries, Ltd. (Osaka, Japan), respectively. Teprenone (6,10,14,18-tetramethyl-5,9,13,17-nonadecatetraen-2-one) was a gift from Eisai Co., Ltd. (Tokyo, Japan).
S. aureus FDA209P was used as the standard strain (2).
BDS method.
The BDS method was described previously (10), and we applied it as follows. The compound to be tested was added, at the indicated concentrations, to 10-ml aliquots of brain heart infusion (BHI) broth (Difco, Detroit, Mich.) in L-tubes (internal diameter, 17 mm; length of arms, 180 mm and 70 mm) without any solubilizing agent or surfactant. An aliquot of an overnight culture of S. aureus was added to each sample to give approximately 105 CFU of S. aureus per ml. Each culture was incubated, with shaking at 40 rpm, in air for 24 h at 37°C. The inhibitory activity of each tested compound was monitored turbidimetrically. The optical density at 660 nm (OD660) was determined with a biophotorecorder (TN-1520; Advantec, Tokyo, Japan).
The delay in proliferation was calculated from a comparison with the growth curve generated from a control culture.
Quantitation of leakage of K+ ions.
The concentration of K+ ions in 4.0 ml of a suspension of cells (initially, 109 CFU/ml) was determined with K+-selective and reference electrodes. A silver/silver chloride electrode was used as the reference electrode. The K+-selective electrode was prepared using valinomycin as described by Katsu et al. (8). The system for quantitation of K+ ions was constructed as reported previously (7). Compounds were added to 100-μl aliquots of cell suspensions. Each assay was performed at 37°C. Data were recorded digitally. Amounts and initial rates of efflux of K+ ions that leaked from cells were expressed in terms of bacterial mass. For estimations of the bacterial mass in each test suspension, bacterial cells were disrupted by sonication and centrifuged (30,000 rpm, 1 h, 4°C), and then the concentration of protein in the supernatant was determined as described by Bradford (3), with bovine serum albumin as the standard protein.
Time-kill assay.
Cells from an overnight culture of S. aureus were washed twice with phosphate-buffered saline (PBS; pH 7.0). The cell pellet was resuspended in 1 ml of PBS at 7 × 106 CFU/ml. An aliquot of 100 μl of this suspension was added to 10 ml of buffer that contained geranylgeraniol, teprenone, or phytol (Fig. 1) at the indicated concentrations, and the mixture was incubated at 37°C. The buffer did not contain any solubilizer. Samples were removed for determination of viable cell counts after 0, 1, 2, 4, 6, 8, 10, and 24 h. Serial 10-fold dilutions of the samples of bacterial suspensions (10−1 to 10−4) were prepared in PBS. Aliquots of 50 μl of each serially diluted sample were plated on agar-solidified Mueller Hinton broth (Difco, Detroit, Mich.) with a spiral plater (Autoplate 3000; Spiral Biotech, Bethesda, Md.). The plates were incubated at 37°C for 24 h, and then colonies were counted. Killing curves were constructed by plotting numbers of viable cells against time. All assays were performed in triplicate.
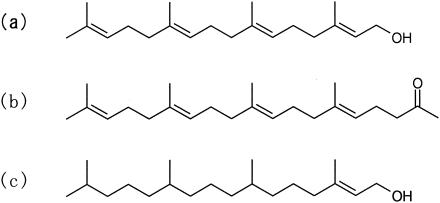
FIG. 1.
Structures of diterpenes used in the present study: geranylgeraniol (a), teprenone (b), and phytol (c).
RESULTS
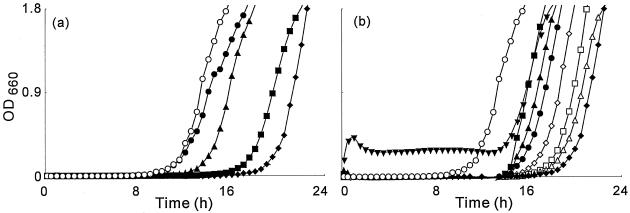
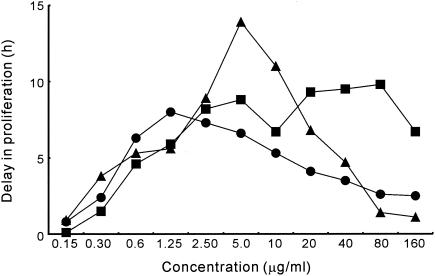
Figure 2 shows typical growth curves for S. aureus FDA209P cultured in broth medium that contained geranylgeraniol. The concentration of geranylgeraniol was varied from 0.15 to 160 μg/ml. Growth inhibition by geranylgeraniol increased with increases in the concentration of geranylgeraniol from 0.15 to 1.25 μg/ml. The delay in proliferation (DP) at 1.25 μg/ml was 8.0 h. When the concentration of geranylgeraniol was higher than 1.25 μg/ml, its inhibitory effect on growth decreased. At 80 μg/ml, DP decreased to 2.6 h. Teprenone and phytol gave results similar to those obtained with geranylgeraniol. In the case of teprenone, increases in growth inhibition were observed as the concentration of teprenone was raised from 0.15 to 5.0 μg/ml (Fig. 3). At 5.0 μg/ml, the DP was 13.9 h. At concentrations above 5.0 μg/ml, the inhibitory effect on growth decreased.
FIG. 2.
Inhibitory effects of geranylgeraniol on the growth of S. aureus FDA209P. S. aureus FDA209P was inoculated into BHI broth medium that contained no geranylgeraniol (○) or into broth that contained geranylgeraniol at 0.15 (•), 0.3 (▴), 0.6 (▪), or 1.25 (♦) μg/ml (a) or 1.25 (♦), 2.5 (▵), 5.0 (□), 10 (⋄), 20 (•), 40 (▴), 80 (▪), or 160 (▾) μg/ml (b), and then cell growth was monitored in terms of the OD660.
FIG. 3.
Effects of diterpenes on the growth of S. aureus FDA209P. Each delay in proliferation was calculated from the growth curve of a control culture and that of a culture that contained the indicated diterpene at the indicated concentration. Symbols: •, geranylgeraniol; ▴, teprenone; and ▪, phytol.
The inhibitory activity of phytol increased until the concentration of phytol reached 10 μg/ml (Fig. 3). The activity barely increased at concentrations above 10 μg/ml and below 160 μg/ml, and at the latter concentration, the inhibitory activity began to decrease. It was difficult to define the most effective concentration of phytol, even though the most effective concentrations of geranylgeraniol and teprenone were clearly apparent. We confirmed that the inhibitory effect of phytol on the growth of bacteria in suspension did not increase with increases in concentration above 20 μg/ml.
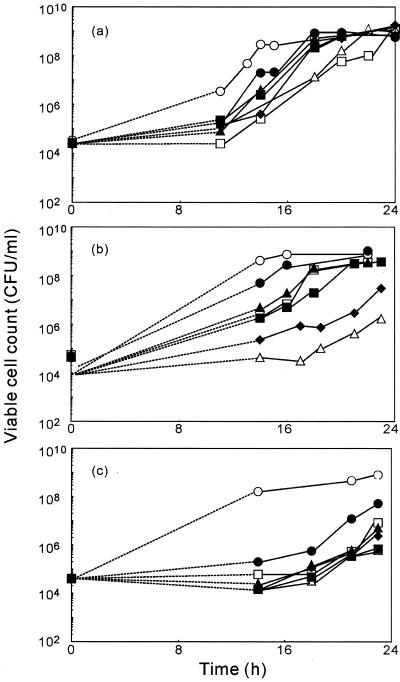
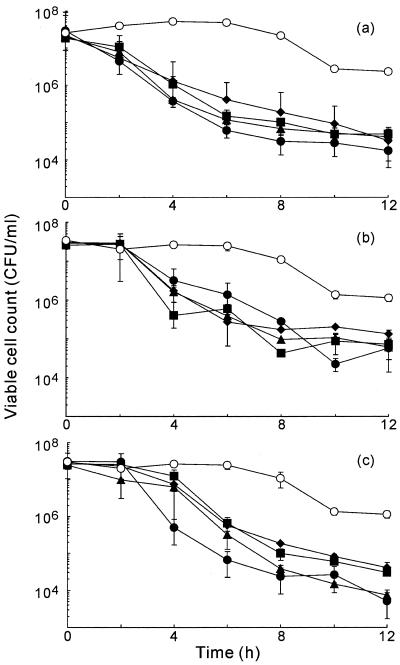
Figure 4 shows the changes in viable cell counts in suspensions of bacteria that contained individual diterpenes at various concentrations. Bacterial growth was observed at all concentrations tested.
FIG. 4.
Changes in the viable cell count of S. aureus FDA209P in medium that contained the diterpenes geranylgeraniol (a), teprenone (b), and phytol (c). S. aureus FDA209P was inoculated into BHI medium that contained no terpene (○) or that contained a diterpene at 2.5 (□), 5 (▵), 10 (♦), 20 (▪), 40 (▴), or 80 (•) μg/ml.
Teprenone and geranylgeraniol suppressed the growth of S. aureus most effectively at 5 and 1.25 μg/ml, respectively (Fig. 3). Phytol inhibited growth over a range of concentrations from 1.25 to 40 μg/ml. These results are supported by the results deduced from the growth curves.
We next investigated the bactericidal activities of geranylgeraniol, teprenone, and phytol (Fig. 5). Geranylgeraniol decreased the viable cell count by two orders of magnitude within 2 h. The viable cell count decreased with the duration of exposure to this diterpene and fell to approximately 105 CFU/ml after 12 h. No significant differences in bactericidal activity were observed among the various concentrations investigated, which ranged from 10 to 80 μg/ml. We also confirmed the bactericidal activities of teprenone and phytol. The trends in changes in viable cell counts were similar to the results obtained with geranylgeraniol. No significant differences in bactericidal activity were observed among concentrations from 10 to 80 μg/ml.
FIG. 5.
Time-kill assays of the effect of geranylgeraniol (a), teprenone (b), and phytol (c) on S. aureus FDA209P. S. aureus FDA209P was suspended in PBS that contained no terpenes (○) or in PBS that contained a diterpene at 10 (♦), 20 (▪), 40 (▴), or 80 (•) μg/ml.
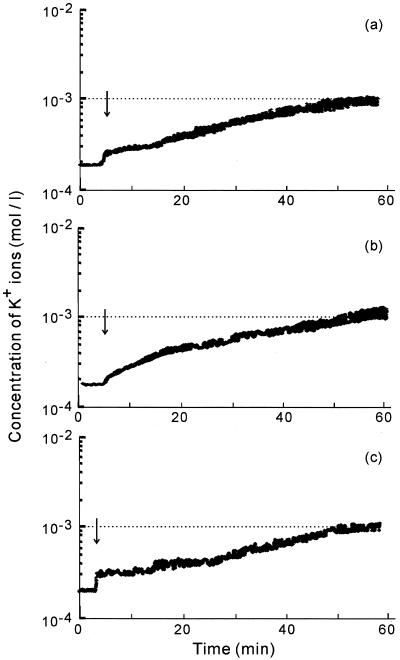
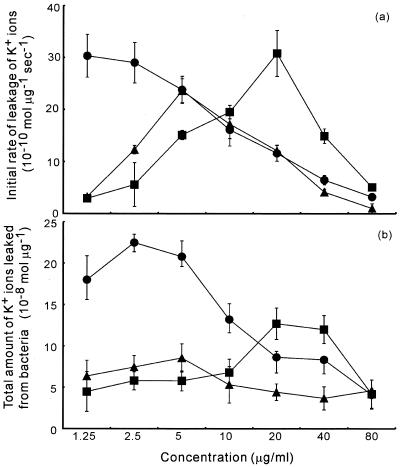
The concentration of K+ ions in the medium started to increase dramatically immediately after the addition of geranylgeraniol to bacterial suspensions (Fig. 6). The rate of leakage of K+ ions decreased, and the level of K+ ions reached a plateau value within an hour. We calculated the total amounts of K+ ions that had leaked from bacterial cells and the initial rates of leakage of K+ ions. The results plotted in Fig. 7 show rates of leakage and total leakage of K+ ions for each diterpene at each concentration examined. The total amount of K+ ions released increased with increases in the concentration of geranylgeraniol up to 2.5 μg/ml. At concentrations above 2.5 μg/ml, the total amount of K+ ions decreased. The initial rate of leakage of K+ ions was highest at 1.25 to 2.5 μg/ml geranylgeraniol, as was the total amount of K+ ions released. Teprenone and phytol induced leakage of K+ ions most effectively at 5 and 20 μg/ml, respectively.
FIG. 6.
Changes in the concentration of K+ ions in suspensions of S. aureus FDA209P in response to 80 μg/ml concentrations of geranylgeraniol (a), teprenone (b), and phytol (c).
FIG. 7.
Leakage of K+ ions from S. aureus FDA209P in response to geranylgeraniol, teprenone, and phytol. Panel a shows the initial rates of leakage of K+ ions, and panel b shows the total amounts of K+ ions leaked from bacteria. Symbols: •, geranylgeraniol; ▴, teprenone; and ▪, phytol.
DISCUSSION
We used the BDS method to investigate the inhibitory effects of geranylgeraniol, teprenone, and phytol on the growth of S. aureus. Figure 2 shows that, in comparison to controls, higher OD660 values were recorded during the initial 10 h of culture of S. aureus when the concentration of geranylgeraniol in the suspension was 160 μg/ml. Figure 4 shows that the viable cell count under control conditions at 10 h was higher than that at any concentration of geranylgeraniol during that period. Similarly, at 80 and 160 μg/ml teprenone, the OD660 was higher than in the control culture for the initial 10 h (data not shown), and the viable cell counts at 80 and 160 μg/ml teprenone were lower than under control conditions. When geranylgeraniol or teprenone was added to BHI broth and no bacteria were added to the medium, similar changes in the OD660 were observed. It is likely that the higher OD660 values, compared to the values for control cultures, during the initial 10 h were not due to viable cells. They might have been due to the turbidity of the diterpenes themselves at elevated concentrations. Figure 4 confirms that the OD660 reflected cell viability accurately. All three compounds exhibited antibacterial activity against S. aureus at 0.15 μg/ml, which was the lowest concentration observing the activity, and the inhibitory effect of each was reversed at an elevated concentration.
The time-kill assay demonstrated that the diterpenes also had bactericidal activity, which was not dependent on concentration over a given range. The diterpenes had similar activities at the concentrations above which their growth-inhibitory activities decreased.
The leakage of K+ ions started just after the addition of each diterpene (Fig. 6), as observed previously for farnesol, nerolidol, and plaunotol (data not shown). These compounds are also terpenes with long aliphatic carbon chains. Hada et al. and Cox et al. reported that the initial rate of K+ efflux and total leakage of K+ ions provide estimates of antibacterial activity against S. aureus (4, 7). The growth curves and leakage of K+ ions indicate that the diterpenes used in this study damaged cell membranes to allow leakage of K+ ions from cells, and their inhibitory effects on the growth of S. aureus corresponded to their abilities to damage cell membranes. The extent of leakage of K+ ions after membrane damage varied with the final concentration of each diterpene.
Results of time-kill assays suggested that constant bactericidal effects were achieved independently of final concentrations between 10 and 80 μg/ml. Inhibitory effects on cell growth decreased at elevated concentrations, even though a certain fraction of the bacteria in each bacterial suspension was killed. Thus, surviving bacteria grew faster than did cells under control conditions. It appears that the diterpenes used in this study have both growth-inhibitory and growth-accelerating activities. The net effects depended on the final concentration, with inhibitory activity predominating at lower concentrations.
At lower concentrations, the inhibitory effects of the tested diterpenes were reflected by the leakage of K+ ions, with higher rates of leakage and larger amounts of leaked K+ ions as the concentrations of the diterpenes increased. Beyond a certain concentration, however, the growth-accelerating effect was predominant, and surviving bacteria took up K+ ions faster than those in the control medium without diterpenes.
Our results indicate that it is possible to reverse the effects of diterpenes on the growth of S. aureus. They also suggest the importance of investigating antibacterial activity in detail by dynamic assay methods since it is impossible to detect biphasic activity by static assay methods, such as the microbroth dilution method.
REFERENCES
- 1.Akimoto, Y., H. Hamashima, M. Sasatsu, T. Arai, F. Murakami, and M. Mizoguchi. 1998. The inhibitory effect of camellia oil on the growth of Staphylococcus aureus isolated from patients with atopic dermatitis. Jpn. J. Chemother. 46:12-16. [Google Scholar]
- 2.Arai, T., H. Hamashima, and M. Sasatsu. 1996. Inhibitory effects of fatty acids, purified camellia oil and olive oil on the growth of Staphylococcus aureus. Jpn. J. Chemother. 44:786-791. [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Cox, S. D., C. M. Mann, J. L. Markham, J. E. Gustafson, J. R. Warmington, and S. G. Wyllie. 2001. Determination the antimicrobial actions of tea tree oil. Molecules 6:87-91. [Google Scholar]
- 5.Hada, T., A. Shiraishi, S. Furuse, Y. Inoue, H. Hamashima, Y. Matsumoto, K. Masuda, K. Shiojima, and J. Shimada. 2003. Inhibitory effects of terpenes on the growth of Staphylococcus aureus. Nat. Med. 57:64-67. [Google Scholar]
- 6.Hada, T., S. Furuse, Y. Matsumoto, H. Hamashima, K. Masuda, K. Shiojima, T. Arai, and M. Sasatsu. 2001. Comparison of the effects in vitro of tea tree oil and plaunotol on methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. Microbios 106:133-141. [PubMed] [Google Scholar]
- 7.Hada, T., Y. Inoue, A. Shiraishi, and H. Hamashima. 2003. Leakage of K+ ions from Staphylococcus aureus in response to tea tree oil. J. Microbiol. Methods 53:309-312. [DOI] [PubMed] [Google Scholar]
- 8.Katsu, T., H. Kobayashi, and Y. Fujita. 1986. Mode of action of gramicidin S on Escherichia coli membrane. Biochim. Biophys. Acta 860:608-619. [DOI] [PubMed] [Google Scholar]
- 9.Knobloch, K., A. Pauli, B. Iberl, N. Weis, and H. Weigand. 1988. Mode of action of essential oil components on whole cells of bacteria and fungi in plate tests, p. 287-299. In P. Schreier (ed.), Bioflavour '87. Walter de Gruyter, Berlin, Germany.
- 10.Matsumoto, Y., H. Hamashima, K. Masuda, K. Shiojima, M. Sasatsu, and T. Arai. 1998. The antibacterial activity of plaunotol against Staphylococcus aureus isolated from the skin of patients with atopic dermatitis. Microbios 96:149-155. [PubMed] [Google Scholar]
- 11.Pattnaik, S., V. R. Suvramanyam, M. Bapaji, and C. R. Kole. 1997. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 89:39-46. [PubMed] [Google Scholar]