Abstract
Background:
During skin expansion, subcutaneous adipose tissue undergoes the greatest change. The adipose layer appears to gradually thin or even disappear in long-term expansion. The response and contribution of adipose tissue to skin expansion remain to be elucidated.
Methods:
The authors established a novel expansion model by transplanting luciferase-transgenic adipose tissue into the rat dorsum, followed by integrated expansion, to trace the dynamic changes in subcutaneous adipose tissue during expansion and the migration of adipose tissue–derived cells. In vivo luminescent imaging was performed to continuously track the adipose tissue changes. Histologic analysis and immunohistochemical staining evaluated the regeneration and vascularization of the expanded skin. Growth factor expression in expanded skin with or without adipose tissue was determined to evaluate the paracrine effect of adipose tissue. Adipose tissue–derived cells were traced in vitro by anti-luciferase staining, and their fate was determined by costaining for PDGFRα, DLK1, and CD31.
Results:
In vivo bioimaging showed that cells in adipose tissue were alive during expansion. After expansion, the adipose tissue exhibited fibrotic-like structures, with more DLK1+ preadipocytes. Skin expanded with adipose tissue was significantly thicker than that without adipose tissue, with more blood vessels and cell proliferation. Vascular endothelial growth factor, epidermal growth factor, and basic fibroblast growth factor expression was higher in adipose tissue than in skin, indicating paracrine support from adipose tissue. Luciferase-positive adipose tissue-derived cells were observed in expanded skin, indicating direct participation in skin regeneration.
Conclusion:
Adipose tissue transplantation can effectively promote long-term skin expansion by contributing to vascularization and cell proliferation by means of various mechanisms.
Clinical Relevance Statement:
The authors’ findings suggest that it would be better if the expander pocket is dissected over the superficial fascia to preserve a layer of adipose tissue with skin. In addition, their findings support the treatment of fat grafting when expanded skin presents with thinning.
Harvesting large amounts of skin tissue for reconstruction has always been a challenge for surgeons.1 Skin expansion is a procedure that induces skin regeneration by the stretch provided by an inflating silicone expander buried underneath.2 Because regenerated skin has normal texture, structure, and appendages, it has been largely used for skin defect recovery.3–6 However, there are many unknown mechanisms of tissue change during expansion. One of the most changed structures is the subcutaneous adipose layer. The thinning of the adipose layer is the earliest presenting change during expansion.7–9 In long-term expansion, the adipose layer may even disappear. Moreover, the thinned adipose layer is usually accompanied by deteriorated skin texture and impaired vascularization.10–12 The fate of adipose tissue under long-term expansion and how adipose tissue participates in expanding skin regeneration remain to be elucidated.
To date, there is no animal model that can trace the fate of subcutaneous adipose tissue during expansion. The most commonly used model in which a tissue expander is buried underneath rodent dorsal skin is not suitable for studying the change in adipose tissue, as there is no subcutaneous adipose layer under rodent dorsal skin.13,14 Other studies transplanted the inguinal fat pad to the dorsal area before expansion or directly buried the expander underneath the inguinal fat pad to achieve the simultaneous expansion of adipose tissue and skin.15 However, these models have limitations in accurately tracing the migration and differentiation of cells from adipose tissue.
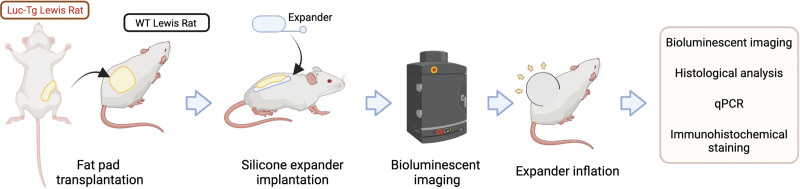
In this article, we report a new model using luciferase-transgenic (Luc-Tg) fat pad transplantation that can stably trace the change in subcutaneous fat tissue during expansion. In our protocol, we first transplanted the inguinal fat pad of Luc-Tg rats to the dorsal area of wild-type (WT) rats. After 4 weeks, the transplanted fat pad was stable, with an integral structure similar to that of normal subcutaneous adipose tissue. All live cells derived from Luc-Tg tissue expressed luciferase and ejected photons in the presence of luciferin. Through this procedure, we assessed a composite tissue of WT skin and luciferase with or without subcutaneous adipose tissue. Using this model, we continuously traced the subcutaneous fat tissue during expansion and investigated the contribution of fat tissue to the regeneration of expanded skin.
METHODS
Animals
This study was approved by the institutional review board of the Shanghai Ninth People’s Hospital. All rats were maintained in a pathogen-free environment, and all experiments were performed under laminar flow hoods.
Luc+ Adipose Tissue Transplantation and Skin Expansion Model
We used ROSA/Luciferase-LEW rats16 (Luc-Tg rats, Luc+ or Luc−, bred by Shanghai Experimental Animal Center, Shanghai, People’s Republic of China) as fat pad donors and WT Lewis rats as recipients. We harvested both sides of the inguinal fat pad from 12-week-old male Luc-Tg Lewis rats. After transplantation, all live cells from Luc-Tg rats expressed luciferase and could be traced by in vivo bioluminescent imaging and in vitro immunohistochemical staining.17 WT Lewis rats (6 weeks old, 78 to 85 g) were randomized into two groups, with eight rats in each group. For the adipose tissue–transplanted group (AT group), the Luc+ fat pads of Luc-Tg Lewis rats were implanted into the WT rats’ dorsal area. For the control group, animals underwent sham surgery (only pocket dissection, with no fat pad transplantation). After 4 weeks of fat pad transplantation, a skin expansion model was established according to a previously reported protocol,18 with a 10-mL silicone expander implanted underneath the fat pad in the AT group and underneath the skin in the control group (Fig. 1). [See Figure, Supplemental Digital Content 1, which demonstrates the surgical procedure. (left and second from left) The transplanted adipose tissue successfully survived. (Second from right) Skin expansion reached 100 mL in volume. (Right) The majority of the adipose tissue layer disappeared, and only some dense tissue was observed (arrow), http://links.lww.com/PRS/G488.] The infusion of 5 mL of saline was performed every 3 days until the total volume reached 100 mL.
Fig. 1.
Illustration of the workflow. (Created with BioRender.com.)
In Vivo Observation of Transplanted Adipose Tissue
In vivo bioluminescence imaging was performed with a noninvasive IVIS bioimaging system (PerkinElmer, Valencia, CA) to continuously track the survival of transplanted fat as described previously.19 To detect luminescence from luciferase-expressing cells, D-luciferin (potassium salt; Synchem, Altenburg, Germany) was injected intraperitoneally into rats anesthetized with isoflurane (30 mg/kg body weight). Viable luciferase-expressing fat tissue was analyzed using the IVIS Living Image software package. Signal intensity was quantified as photon flux in units of photons per second in the region of interest. In vivo bioimaging was performed before expansion and when the total volume of saline reached 100 mL.
Harvesting of Expanded Skin and Adipose Tissue
Expanded skin samples and transplanted adipose tissue were harvested after 100 mL of expansion, according to a previously described protocol.18 Briefly, longitudinal full-thickness skin specimens with underlying adipose tissue covering an area of approximately 2 × 2 cm to 5 × 5 cm (approximately 10% of the expanded skin surface area) were harvested at the highest tension.
Histologic Analysis
After fixation, skin and adipose tissue were cut into 5-μm sections for hematoxylin and eosin staining and Masson trichrome staining. The epidermal and dermal thickness of each skin sample was measured using Image-Pro Plus 6 software (Media Cybernetics, Rockville, MD).
Real-Time Quantitative Polymerase Chain Reaction Analysis
Total RNA was extracted from expanded skin and fat tissue separately using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan) was used for cDNA synthesis. Gene expression was evaluated by real-time quantitative polymerase chain reaction performed on a real-time 7500 HT system (Applied Biosystems, Foster City, CA) using the SYBR Green PCR Master Mix Kit (Takara Bio, Shiga, Japan). The expression of target genes was measured using the primers shown. (See Figure, Supplemental Digital Content 2, which shows primer information, http://links.lww.com/PRS/G489.) β-Actin was used as an internal control. Relative gene expression was calculated using the 2-ΔΔCt method and normalized to the expression in the control group to obtain the fold change in expression.
Immunofluorescence and Immunohistochemical Staining
To detect the vessels and proliferating cells (early G1 and S phases) in expanded skin, rabbit anti-rat CD31 (BM2966; Boster, Wuhan, People’s Republic of China) and mouse anti-rat proliferating cell nuclear antigen (PCNA) (BM0104; Boster) antibodies were applied. To determine the cell type change in expanded adipose tissue, mouse anti-DLK1 antibody (sc-376755; Santa Cruz Biotechnology, Dallas, TX) was used as the primary antibody. To trace in vitro cells from the transplanted adipose tissue, we used anti-luciferase primary antibody (ab185924; Abcam, Cambridge, UK) and antibodies against platelet-derived growth factor receptor alpha (PDGFRα) (sc-21789; Santa Cruz), DLK1, and CD31 to detect the differentiation of migrated cells. For immunofluorescence staining, secondary antibodies conjugated to 488-nm or 555-nm absorbing fluorophores (Invitrogen, Carlsbad, CA) were applied to visualize primary antibodies. We used 4′,6-diamidino-2-phenylindole (Roche, Mannheim, Germany) to counterstain the nuclei. Sections subjected to immunohistochemical staining were visualized using diaminobenzidine and counterstained with Mayer hematoxylin. The number of stained cells was counted manually for five random high-power fields (200×) from microscopy images (DS-Ri2; Nikon, Tokyo, Japan).
Western Blot Analysis
Protein extracts were obtained by lysis in ice-cold radioimmunoprecipitation assay lysis buffer containing a protease inhibitor cocktail (Roche, Indianapolis, IN). A total of 40 mg of each protein sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. After incubation in 5% bovine serum albumin in Tris-buffered saline with Tween, the polyvinylidene difluoride membranes were incubated in dilution buffer (2% bovine serum albumin in Tris-buffered saline with Tween) containing primary antibodies overnight at 4°C and then incubated with horseradish peroxidase–conjugated secondary antibodies diluted in dilution buffer. Protein expression was assessed using the following primary antibodies: rabbit anti-rat vascular endothelial growth factor (VEGF) (19003-1-AP; Proteintech, Rosemont, IL), rabbit anti-rat type epidermal growth factor (EGF) (AF5148; Affinity, Changzhou, People’s Republic of China), and rabbit anti-rat basic fibroblast growth factor (bFGF) (DF6038; Affinity).
Statistical Analysis
The results are expressed as mean (SD). A paired t test was used to assess the changes in variables repeatedly measured before and after expansion (bioluminescence imaging results). A two-tailed t test was used to evaluate the significance of the difference in independent variables. Comparison of the expression of growth factors among multiple groups (control skin, AT skin, and AT adipose tissue) was performed using one-way analysis of variance and Tukey post hoc test. Values of P < 0.05 indicated statistical significance.
RESULTS
In Vivo Tracing of Subcutaneous Adipose Tissue during Expansion
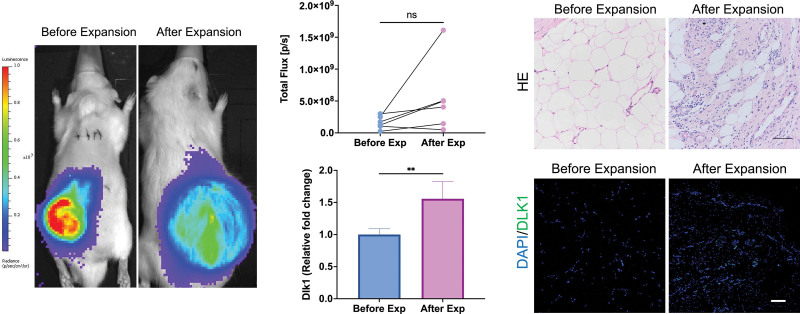
By the transplantation of Luc+ inguinal fat pads, we traced the change in subcutaneous adipose tissue during skin expansion in vivo. The transplanted adipose tissue had a stable bioluminescent signal, allowing for imaging. Photons emitted from subcutaneous adipose tissue showed no significant difference before and after expansion (Fig. 2). The results of bioluminescent imaging indicated that the number of live cells derived from adipose tissue did not significantly change.
Fig. 2.
Change in transplanted adipose tissue during expansion. (Left) Luminescent images of transplanted adipose tissue in vivo. The adipose tissue was continuously observed during expansion. (Above, center) Quantified result of luminescent imaging. (Above, right) Histologic results of adipose tissue. The tissue exhibited a dense fibrotic-like appearance with a sharp decrease in adipocytes. (Below, center) mRNA expression of Dlk1 in adipose tissue before and after expansion. (Below, right) Immunohistochemical staining for Dlk1 in adipose tissue before and after expansion. Scale bar = 100 µm; **P < 0.01; ns, no significance.
Changes in Structure and Cell Type in Adipose Tissue before and after Expansion
Adipose tissues were harvested at the end of expansion. Compared with those that did not undergo expansion, expanded tissues lost the characteristics of adipose tissue. The number of adipocytes was sharply decreased, and the tissues showed a dense fibrotic-like appearance with abundant fibroblast-like cells. The structural changes of adipose tissue provide substantial evidence that grafted fat responds similarly to native fat. The expression of Dlk1, a marker of preadipocytes, was increased after expansion. Immunohistochemical staining also confirmed that more Dlk1 cells were observed in adipose tissues harvested after expansion (Fig. 2). These results indicated that the change in adipose tissue structure may be attributable to the dedifferentiation of mature adipocytes to preadipocytes.
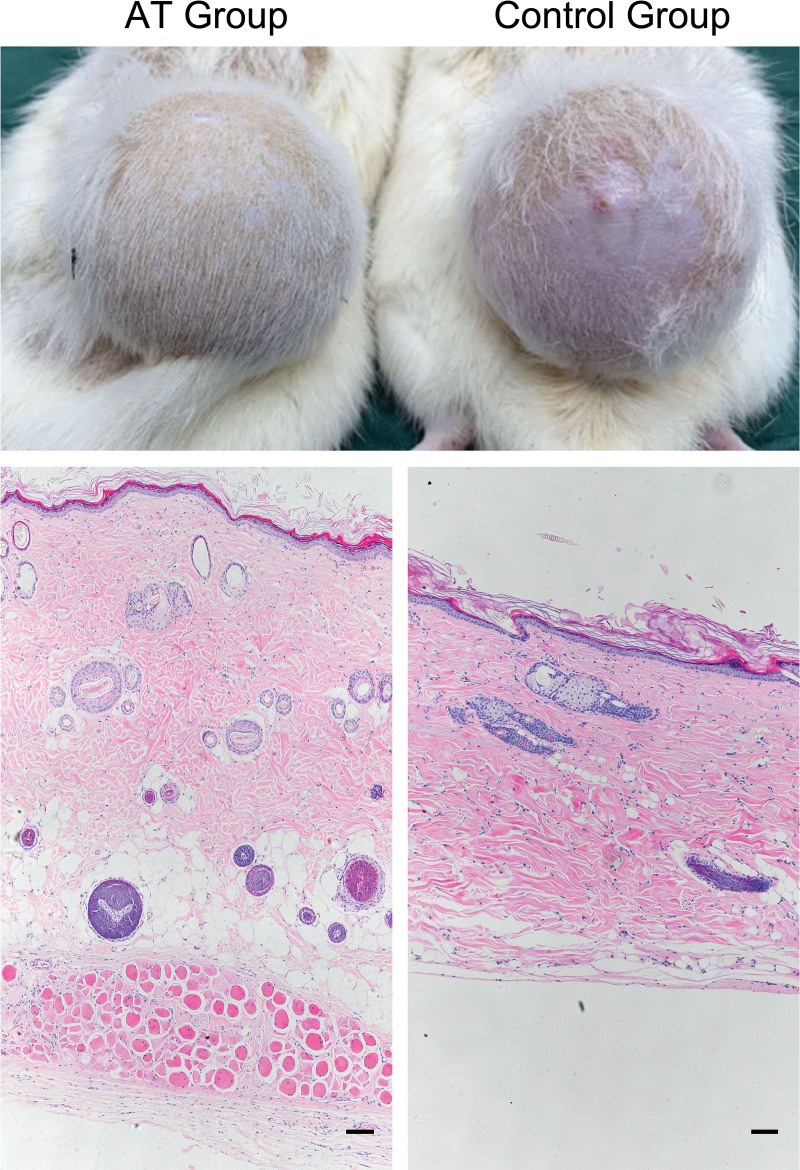
Skin Expanded with the Existence of Adipose Tissue Exhibited Better Regeneration
By transplantation of the fat pad, a model was prepared in which dorsal skin with subcutaneous adipose tissue could be expanded. Compared with the control group with no subcutaneous adipose tissue, expanded skin in the AT group exhibited better regeneration. The skin in the control group presented clear signs of deterioration, including thinning and hair loss (Fig. 3). [See Figure, Supplemental Digital Content 3, which shows (left) measurement of the skin thickness of samples from the AT group and the control group. (Center and right) Masson staining of samples from these two groups. Scale bar = 100 µm; ***P < 0.001, http://links.lww.com/PRS/G490.] The histologic results showed that the skin specimens from the AT group were significantly thicker than those from the control group. The layer of dermal white adipose tissue was diminished or even nonexistent in the control group, whereas it was still clear and well-structured in the AT group. In addition, more hair follicles in the anagen phase were observed in the AT group.
Fig. 3.
Comparison of skin expanded with or without adipose tissue. (Above) Expanded skin in the AT group exhibited better regenerative conditions. (Below) Hematoxylin and eosin staining of skin samples from the AT group and the control group. Scale bar = 100 µm.
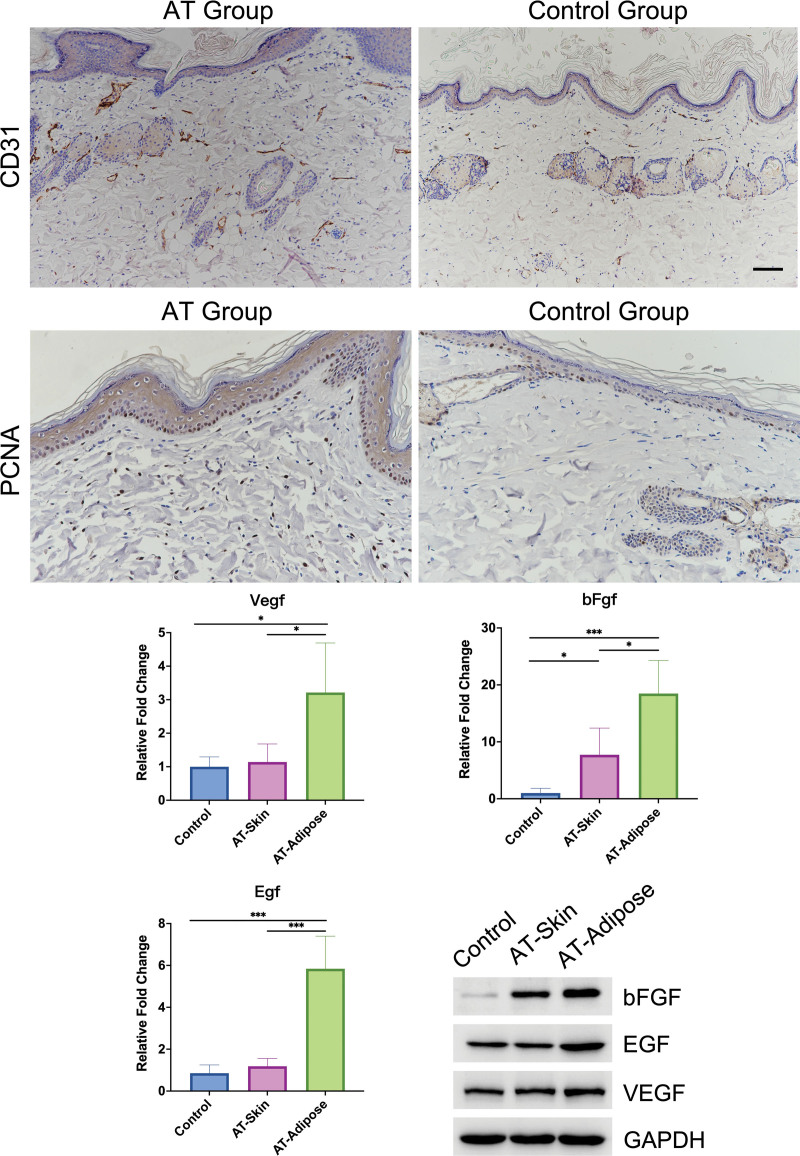
Skin Expanded with the Existence of Adipose Tissue Had Better Vascularization and More Proliferating Cells
We used anti-CD31 staining to mark the vessels. The results showed that more CD31+ cells were found in skin from the AT group (P < 0.05), indicating that improved vascularization and blood supply were achieved (Fig. 4). [See Figure, Supplemental Digital Content 4, which shows analysis of anti-CD31 (above, left) and anti-PCNA (above, right) staining results. (Center and below) Staining of CD31, podoplanin, and luciferase. The results showed that CD31+ cells in expanded skin seldom colocalized with podoplanin, indicating that these cells were vessel endothelial cells. Scale bar = 100 μm, http://links.lww.com/PRS/G491.] We applied anti-PCNA antibodies to observe cell proliferation in expanded skin. Compared with the control group, the number of PCNA+ cells in the epidermis was significantly increased in the adipose tissue transplanted group (Fig. 4; see Figure, Supplemental Digital Content 4, http://links.lww.com/PRS/G491) (P < 0.01). Collectively, these results indicated that the existence of subcutaneous adipose tissue supported the vascularization and regeneration of overlying skin during expansion.
Fig. 4.
Subcutaneous adipose tissue promoted vascularization and cell proliferation in expanded skin by means of paracrine growth factors. (Above) Immunohistochemical staining for CD31 and PCNA to mark vessels and proliferating cells. (Center) mRNA expression levels of EGF, bFGF, and VEGF in skin samples from the control group and the AT group (AT-Skin), and adipose tissue from the AT group (AT-Adipose). (Below, right) Immunoblot analysis of the expression of bFGF, EGF, and VEGF in skin samples from the control group and the AT group, and adipose tissue from the AT group. Scale bar = 100 µm; *P < 0.05; ***P < 0.001.
Adipose Tissue Supports Skin Regeneration through Paracrine Growth Factors
Real-time quantitative polymerase chain reaction analysis was performed to compare the mRNA expression of VEGF, EGF, and bFGF in skin expanded without adipose tissue, skin expanded with adipose tissue, and skin expanded adipose tissue. The expression of VEGF and EGF did not change in expanded skin with or without adipose tissue. However, the expression levels of these growth factors were significantly higher in expanded adipose tissue. The level of bFGF mRNA expression in expanded skin was increased with the presence of adipose tissue but was still significantly lower than that in adipose tissue alone. Similar differences were also found in the protein levels of VEGF, EGF, and bFGF (Fig. 4). Thus, these results indicated that, during expansion, subcutaneous adipose tissue promoted skin regeneration through the paracrine effects of growth factors.
Cells from Subcutaneous Adipose Tissue Migrated into Expanded Skin
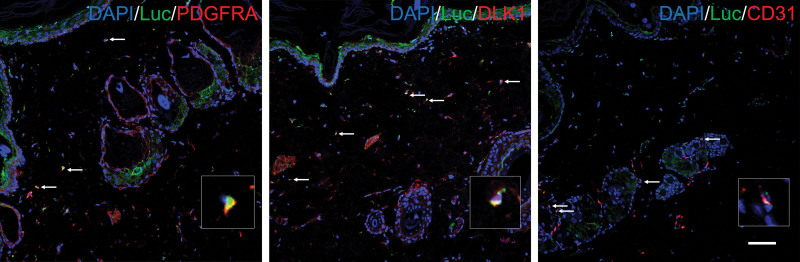
To trace the cells derived from subcutaneous adipose tissue, we used an anti-luciferase antibody to mark live cells originating from Luc+-transplanted adipose tissue. Luc+ cells were found in skin after expansion (see Figure, Supplemental Digital Content 5, which shows anti-luciferase staining of sections from expanded skin and relative subcutaneous adipose tissue; scale bar = 100 µm, http://links.lww.com/PRS/G492), indicating that cells derived from subcutaneous adipose tissue migrated to overlying skin during expansion. Antibodies against PDGFRα, DLK1, and CD31 were applied to determine the fate of migrated cells. Cells expressing luciferase/PDGFRα and luciferase/DLK1 were found in expanded skin. These cells could be either preadipocytes existing in adipose tissue before expansion or dedifferentiated adipocytes.20,21 These migrated cells could support skin regeneration. Moreover, some Luc+ cells were colocalized with CD31 (Fig. 5), indicating that cells from adipose tissue directly contributed to vascularization.
Fig. 5.
Tracing of cells derived from adipose tissue. Cells derived from subcutaneous adipose tissue were labeled with an anti-luciferase antibody (green). (Left) Cells labeled with both luciferase and PDGFRα represent subcutaneous adipose tissue-derived stem cells, and (center) those labeled with both luciferase and DLK1 represent adipose tissue-derived preadipocytes. (Right) Cells derived from adipose tissue also differentiated into endothelial cells, as evidenced by Luc+/CD31 cells located in blood vessels. Scale bar = 100 µm.
DISCUSSION
It has been known for a long time that the thickness of subcutaneous adipose tissue decreases during skin expansion. We have previously reported the continuous thinning of the subcutaneous adipose layer, as determined by ultrasonic scanning.7,9 However, little is known regarding the destination of cells from adipose tissue during thinning. One major obstacle was the lack of suitable animal models. The most commonly used expansion model is the rodent dorsal skin expansion model.22 However, rodents do not have subcutaneous adipose tissue, which limits the practicality of this model. Some studies transplanted the inguinal fat pad to the dorsal area before starting expansion.15 Although this model mimics the process in humans, in which the fat tissue and skin are expanded simultaneously, it is unable to trace the change and fate of cells of adipose tissue. In this study, we reported an innovative model that can trace cells from subcutaneous tissue during skin expansion both in vivo and in vitro.
In this model, we used adipose tissue from Luc-Tg rats.16 With the Luc-Tg gene, subcutaneous adipose tissue can be continuously traced in vivo, and there is no need to kill animals. Moreover, with the Luc-Tg gene, cells from adipose tissue could be easily distinguished from those from skin that were luciferase-negative.18 We transplanted Luc+ inguinal fat pads from Luc-Tg rats to the dorsum of WT rats. Bioluminescence imaging showed that the transplanted fat tissue had a stable signal after transplantation, enabling dynamic observation of subcutaneous fat tissue. Through this procedure, we established a composite tissue flap with Luc+ adipose and Luc− skin. When inflating the expander buried under this flap, the overlying adipose tissue and skin could be stretched simultaneously.
Recently, the transition between adipocytes and preadipocytes has been reported in some physical processes. In the breast, adipocytes can dedifferentiate into preadipocytes during lactation and redifferentiate during the involution period.23 In the periodic growth of hair follicles, the white fat in the dermis also has a periodic process of differentiation and dedifferentiation with the cycle of hair follicles and has a supporting role in their growth and development.24 For the first time, we report the dynamic change in subcutaneous adipose tissue during expansion. When the expansion inflation reached 100 mL, the transplanted subcutaneous fat tissue lost the original adipose tissue structure, which was replaced with a dense fibrotic-like structure. Meanwhile, the bioluminescent images showed continuous signals from living cells of adipose tissue. The results indicated that the decrease in adipose tissue volume may be attributable to cell-type transition, not apoptosis. By immunohistochemical staining, we found Luc+/DLK1+ preadipocytes in fibrotic-like tissue, and foam cells, which are macrophages that endocytose free lipids, indicating that adipocytes release lipid drops and dedifferentiate into preadipocytes.15,25 The dedifferentiated preadipocytes had an improved ability to migrate26 and could migrate toward the overlying layer of skin.
In clinical observation, we found that skin expanded with an adipose layer exhibited better skin regeneration. The thinning adipose layer is usually accompanied by a vulnerably deteriorating skin texture. Consistent with clinical observation, skin expanded with transplanted subcutaneous fat pads exhibited a clear improvement in texture. Previously, we reported that adding fat grafts to thinning expanded skin can help promote skin regeneration.7 In this study, we confirmed that with the existence of an adipose layer, the expanded skin exhibited more PCNA+ cells. Skin in the presence of adipose tissue presented heathier properties. Hair follicles in the control group entered the telogen phase, whereas those in the adipose transplanted group were in the anagen phase.
Here, we helped explain the mechanism by which adipose tissue supports skin regeneration. By secreting growth factors of the paracrine pathway, adipose tissue can promote cell proliferation and vascularization.27 Higher levels of VEGF, EGF, and bFGF expression were found in expanded skin with adipose tissue. In addition, adipocytes can promote tissue regeneration in a paracrine manner.27 Adiponectin secreted by adipocytes can promote the proliferation of keratinocytes through the ERK signaling pathway.28 Leptin from adipocytes can induce hair follicle activation.29
By in vitro staining with anti-luciferase antibodies, we observed cells derived from transplanted adipose tissue in expanded skin, indicating that cells from adipose tissue migrated into the skin layer. The cells from subcutaneous adipose tissue may be recruited by the chemokine SDF1a released by expanded skin.18 Engrafted Luc+ cells were found to colocalize with DLK1 or PDGFRα, indicating that these cells were preadipocytes. These engrafted cells may be either preadipocytes or dedifferentiated cells from adipocytes. The dedifferentiation of adipocytes into preadipocytes during expansion may provide them an enhanced migration ability and help them participate directly in skin regeneration.26,30,31
The findings of this research showed the important support of subcutaneous adipose tissue for expanded skin regeneration. Our findings suggest that a composite flap containing subcutaneous adipose tissue would achieve better outcomes and that it would be better if the expander pocket is dissected over the superficial fascia to preserve a layer of adipose tissue with skin. In addition, our findings support the treatment of fat grafting when expanded skin presents thinning.32
There are still limitations of our study. Although we observed changes in the structure and cell types of the subcutaneous fat layer after expansion, we did not identify the source of increased preadipocytes. Further lineage studies are needed to confirm whether adipocytes dedifferentiated and to reveal the underlying mechanism. Moreover, a cohort study is planned to assess the relationship between the subcutaneous fat thickness preserved with a skin flap and the expansion outcome, which we believe would provide more guidance for clinical practice.
CONCLUSIONS
In this work, we established a novel expansion model by transplanting Luc-Tg adipose tissue into the rat dorsal back followed by integrated expansion. Using this model, we traced the dynamic changes in subcutaneous adipose tissue during expansion and the migration of cells originating from adipose tissue. We reported the dedifferentiation of adipocytes instead of apoptosis during expansion. Subcutaneous adipose tissue promoted skin regeneration through both the paracrine pathway and direct migration. In this research, we revealed the temporal dedifferentiation change of subcutaneous adipose tissue during skin expansion and the contribution of subcutaneous adipose tissue to skin regeneration.
DISCLOSURE
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
The project was supported by the National Natural Science Foundation of China (81971848 to S.Z. and 81620108019 to Q.L.), the Shanghai Municipal Key Clinical Specialty (shslczdzk00901), the Clinical Research Plan of SHDC (SHDC2020CR1019B to Q.L. and SHC2020CR402 to S.Z.), and the Innovative Research Team of High-Level Local Universities in Shanghai (SSMU-ZDCX20180700). The authors gratefully acknowledge technological support from the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine. They thank American Journal Experts for providing English-language editing of the manuscript.
Supplementary Material
Footnotes
The first three authors contributed equally to this article.
Disclosure statements are at the end of this article, following the correspondence information.
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
REFERENCES
- 1.Bozkurt A, Groger A, O’Dey D, et al. Retrospective analysis of tissue expansion nn reconstructive burn surgery: evaluation of complication rates. Burns 2008;34:1113–1118. [DOI] [PubMed] [Google Scholar]
- 2.De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109:2450–2462. [DOI] [PubMed] [Google Scholar]
- 3.Mangubat EA. Scalp repair using tissue expanders. Facial Plast Surg Clin North Am. 2013;21:487–496. [DOI] [PubMed] [Google Scholar]
- 4.Inbal A, Lemelman BT, Millet E, Greensmith A. Tissue expansion using hyaluronic acid filler for single-stage ear reconstruction: a novel concept for difficult areas. Aesthet Surg J. 2017;37:1085–1097. [DOI] [PubMed] [Google Scholar]
- 5.Mostafapour SP, Murakami CS. Tissue expansion and serial excision in scar revision. Facial Plast Surg. 2001;17:245–252. [DOI] [PubMed] [Google Scholar]
- 6.Zhou SB, Gao BW, Tan PC, Zhang HZ, Li QF, Xie F. A strategy for integrative reconstruction of midface defects using an extended forehead flap. Facial Plast Surg Aesthet Med. 2021;23:430–436. [DOI] [PubMed] [Google Scholar]
- 7.Jiang T, Zhao P, Cheng C, Liu K, Xie Y, Li Q. Preventing the complications of tissue expansion using fat grafting under expanded skin. J Plast Reconstr Aesthet Surg. 2017;70:1151–1153. [DOI] [PubMed] [Google Scholar]
- 8.Tan PC, Chao PC, Cheng C, et al. A randomized, controlled clinical trial of autologous stromal vascular fraction cells transplantation to promote mechanical stretch-induced skin regeneration. Stem Cell Res Ther. 2021;12:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou SB, Zhang GY, Xie Y, et al. Autologous stem cell transplantation promotes mechanical stretch induced skin regeneration: a randomized phase I/II clinical trial. EBioMedicine 2016;13:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu X, Fang L, Li H, Li X, Cheng B, Sheng Z. Adipose tissue extract enhances skin wound healing. Wound Repair Regen. 2007;15:540–548. [DOI] [PubMed] [Google Scholar]
- 11.Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(Suppl):108S–120S. [DOI] [PubMed] [Google Scholar]
- 12.Pezeshk RA, Stark RY, Small KH, Unger JG, Rohrich RJ. Role of autologous fat transfer to the superficial fat compartments for perioral rejuvenation. Plast Reconstr Surg. 2015;136:301e–309e. [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Lei L, Liu S, et al. Macrophages are necessary for skin regeneration during tissue expansion. J Transl Med. 2019;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou SB, Chiang CA, Liu K, Li QF. Intravenous transplantation of bone marrow mesenchymal stem cells could effectively promote vascularization and skin regeneration in mechanically stretched skin. Br J Dermatol. 2015;172:1278–1285. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Xia J, Gao J, Lu F, Liao Y. Mechanical signals induce dedifferentiation of mature adipocytes and increase the retention rate of fat grafts. Plast Reconstr Surg. 2019;144:1323–1333. [DOI] [PubMed] [Google Scholar]
- 16.Hakamata Y, Murakami T, Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 2006;81:1179–1184. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu H, Kobayashi E, Gonzalez N, et al. Critical considerations in bioluminescence imaging of transplanted islets: dynamic signal change in early posttransplant phase and signal absorption by tissues. Pancreas 2022;51:234–242. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF-1alpha in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells 2013;31:2703–2713. [DOI] [PubMed] [Google Scholar]
- 19.Zhou SB, Chiang CA, Xie Y, et al. In vivo bioimaging analysis of stromal vascular fraction-assisted fat grafting: the interaction and mutualism of cells and grafted fat. Transplantation 2014;98:1048–1055. [DOI] [PubMed] [Google Scholar]
- 20.Marcelin G, Cunha C, Gamblin C, et al. Autophagy inhibition blunts PDGFRA adipose progenitors’ cell-autonomous fibrogenic response to high-fat diet. Autophagy 2020;16:2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Games LG, Cunha-Silva M, Crespo RP, et al. DLK1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab. 2019;104:2112–2120. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Li Q, Sheng L, Li H, Weng R, Zan T. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Ann Surg. 2011;253:202–209. [DOI] [PubMed] [Google Scholar]
- 23.Wang QA, Song A, Chen W, et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 2018;28:282–288.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Shao M, Hepler C, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest. 2019;129:5327–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Yao Y, Wang J, et al. Dedifferentiated adipocytes promote adipose tissue generation within an external suspension device. Plast Reconstr Surg. 2017;140:525–536. [DOI] [PubMed] [Google Scholar]
- 26.Abuhattum S, Gefen A, Weihs D. Ratio of total traction force to projected cell area is preserved in differentiating adipocytes. Integr Biol. 2015;7:1212–1217. [DOI] [PubMed] [Google Scholar]
- 27.Shook B, Gonzalez GR, Ebmeier S, Grisotti G, Zwick R, Horsley V. The role of adipocytes in tissue regeneration and stem cell niches. Annu Rev Cell Dev Biol. 2016;32:609–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata S, Tada Y, Asano Y, et al. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189:3231–3241. [DOI] [PubMed] [Google Scholar]
- 29.Watabe R, Yamaguchi T, Kabashima-Kubo R, Yoshioka M, Nishio D, Nakamura M. Leptin controls hair follicle cycling. Exp Dermatol. 2014;23:228–229. [DOI] [PubMed] [Google Scholar]
- 30.Sheng L, Yang M, Liang Y, Li Q. Adipose tissue-derived stem cells (ADSCs) transplantation promotes regeneration of expanded skin using a tissue expansion model. Wound Repair Regen. 2013;21:746–754. [DOI] [PubMed] [Google Scholar]
- 31.Aoki S, Toda S, Ando T, Sugihara H. Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell 2004;15:4647–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng C, Fang B, Xie Y, et al. Autologous fat transfer rescues expanded skin from expansion failure: a retrospective cohort study in Asians. J Plast Reconstr Aesthet Surg. 2022;75:1094–1099. [DOI] [PubMed] [Google Scholar]