Abstract
OBJECTIVES:
Extracorporeal cardiopulmonary resuscitation (ECPR) is the implementation of venoarterial extracorporeal membrane oxygenation (VA-ECMO) during refractory cardiac arrest. The role of left-ventricular (LV) unloading with Impella in addition to VA-ECMO (“ECMELLA”) remains unclear during ECPR. This is the first systematic review and meta-analysis to characterize patients with ECPR receiving LV unloading and to compare in-hospital mortality between ECMELLA and VA-ECMO during ECPR.
DATA SOURCES:
Medline, Cochrane Central Register of Controlled Trials, Embase, and abstract websites of the three largest cardiology societies (American Heart Association, American College of Cardiology, and European Society of Cardiology).
STUDY SELECTION:
Observational studies with adult patients with refractory cardiac arrest receiving ECPR with ECMELLA or VA-ECMO until July 2023 according to the Preferred Reported Items for Systematic Reviews and Meta-Analysis checklist.
DATA EXTRACTION:
Patient and treatment characteristics and in-hospital mortality from 13 study records at 32 hospitals with a total of 1014 ECPR patients. Odds ratios (ORs) and 95% CI were computed with the Mantel-Haenszel test using a random-effects model.
DATA SYNTHESIS:
Seven hundred sixty-two patients (75.1%) received VA-ECMO and 252 (24.9%) ECMELLA. Compared with VA-ECMO, the ECMELLA group was comprised of more patients with initial shockable electrocardiogram rhythms (58.6% vs. 49.3%), acute myocardial infarctions (79.7% vs. 51.5%), and percutaneous coronary interventions (79.0% vs. 47.5%). VA-ECMO alone was more frequently used in pulmonary embolism (9.5% vs. 0.7%). Age, rate of out-of-hospital cardiac arrest, and low-flow times were similar between both groups. ECMELLA support was associated with reduced odds of mortality (OR, 0.53 [95% CI, 0.30–0.91]) and higher odds of good neurologic outcome (OR, 2.22 [95% CI, 1.17–4.22]) compared with VA-ECMO support alone. ECMELLA therapy was associated with numerically increased but not significantly higher complication rates. Primary results remained robust in multiple sensitivity analyses.
CONCLUSIONS:
ECMELLA support was predominantly used in patients with acute myocardial infarction and VA-ECMO for pulmonary embolism. ECMELLA support during ECPR might be associated with improved survival and neurologic outcome despite higher complication rates. However, indications and frequency of ECMELLA support varied strongly between institutions. Further scientific evidence is urgently required to elaborate standardized guidelines for the use of LV unloading during ECPR.
Keywords: cardiac arrest, extracorporeal cardiopulmonary resuscitation, extracorporeal membrane oxygenation, Impella, left ventricular unloading
KEY POINTS.
Question: How are patients receiving venoarterial extracorporeal membrane oxygenation (VA-ECMO) plus Impella (ECMELLA) during extracorporeal cardiopulmonary resuscitation (ECPR) characterized, and how is the in-hospital mortality rate between patients receiving ECMELLA and VA-ECMO alone during ECPR?
Findings: In this systematic review and meta-analysis, patients with ECMELLA had more frequently shockable electrocardiogram rhythms, acute myocardial infarctions, and percutaneous coronary interventions than patients with VA-ECMO. ECMELLA support might be associated with reduced odds of mortality compared with VA-ECMO alone. Results were heterogeneous across studies.
Meaning: ECMELLA support might be considered in patients with acute myocardial infarction during ECPR.
Extracorporeal cardiopulmonary resuscitation (ECPR) is the implementation of venoarterial extracorporeal membrane oxygenation (VA-ECMO) during refractory cardiac arrest when return of spontaneous circulation is not achieved. The use of ECPR has shown conflicting results with one recent smaller, randomized controlled trial (RCT) showing a survival benefit, whereas two other RCTs did not (1–3). It has been shown in a recent systematic review and meta-analysis that ECPR was associated with improved outcomes compared with conventional cardiopulmonary resuscitation (CPR) (4). Given its immediate provision of full circulatory support, ECPR can be considered in selected patients with regards to important considerations, such as procedural complexity and post-procedural complications according to international guidelines (5–7).
Although VA-ECMO allows for full circulatory support, its retrograde aortic flow may result in increased left ventricular (LV) afterload, leading to increased wall stress and oxygen demand, blood stasis, thrombus formation, pulmonary edema, and ventricular arrhythmias impairing myocardial recovery (8–10). In the field of cardiogenic shock, additional LV unloading with an Impella microaxial flow pump (“ECMELLA”; Abiomed, Danvers, MA) has been shown to be a feasible and effective percutaneous strategy to reduce LV wall stress and oxygen demand (“LV unloading”), to improve cardiac perfusion, and to attenuate cardiac injury in preclinical models (11, 12). Additionally, associations with improved survival have been shown in retrospective cohort studies, although with a relatively high risk of bias due to confounding (13–18). In the field of refractory cardiac arrest, single-cohort studies suggested that an ECMELLA approach might be associated with better survival; however, RCTs targeting this question are lacking (19–21).
To our best knowledge, there is no systematic literature review and meta-analysis on LV unloading in ECPR. Therefore, the primary aim of this systematic literature review and meta-analysis was to describe the landscape of LV unloading during ECPR, that is, characteristics of patients undergoing treatment with ECMELLA or VA-ECMO and to investigate treatment effects of ECMELLA compared with VA-ECMO alone, both used as part of ECPR, on in-hospital mortality in adult patients with refractory cardiac arrest.
MATERIALS AND METHODS
The protocol of this study was registered at the PROSPERO registry (identifier: CRD42022339499). The systematic review and meta-analyses were conducted according to the Preferred Reported Items for Systematic Reviews and Meta-Analysis checklist (Supplemental Table 1, http://links.lww.com/CCM/H470) (22).
Information Sources, Search Strategy, Study Selection, and Data Collection
The systematic literature review was performed by three independent and blinded reviewers with clinical and scientific experience (T.T., L.F., M.F.) on Medline, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and abstract websites of the three largest cardiology societies (i.e., American Heart Association, American College of Cardiology, and European Society of Cardiology). Additionally, all references from included studies were reviewed. Studies were screened if they were published until July 30, 2023, in English or German language on adult patients with refractory cardiac arrest treated either with ECMELLA or with VA-ECMO as part of ECPR. Search strings included: “extracorporeal membrane oxygenation,” “Impella,” “microaxial pump,” “shock, cardiogenic,” and/or “heart arrest” (see Supplemental Material: “Search strategy,” http://links.lww.com/CCM/H470). Although this study was aimed to include records with refractory cardiac arrest, “cardiogenic shock” was also considered as a search term since certain studies on cardiogenic shock included subgroup analyses on patients with refractory cardiac arrest and ECPR treatment.
The search results were retrieved and imported into “Rayyan,” an application for managing systematic reviews (23). Each of the following steps was performed upon agreement of all three reviewers: First, duplicate studies were removed manually. Second, animal studies, case reports, comments, letters to the editor, editorials, reviews, and study protocols were excluded by screening the title and abstract of each study. Third, the full-text articles of all remaining studies were reviewed. Studies were included if ECMELLA or VA-ECMO were used as part of ECPR during refractory cardiac arrest. ECPR cases with another method of LV unloading added to VA-ECMO different than Impella (e.g., intra-aortic balloon pump or atrial septostomy) were excluded. Studies on refractory cardiac arrest due to trauma or surgical cases were excluded. Fourth, of the remaining studies, information was extracted using a standardized data extraction form (Supplemental Table 2, http://links.lww.com/CCM/H470). If data were not provided or unclear in the publication, the study’s corresponding author was consulted via email. If the corresponding author did not respond within 21 days, coauthors were contacted via email. If there was no email response within 12 weeks, studies were excluded from the meta-analysis.
Quality Assessment
The risk of bias of each included study was assessed independently by two reviewers (T.T., L.F.) using the “The Risk Of Bias In Non-randomized Studies of Interventions” (ROBINS-I) tool (24). The level of certainty was assessed with the “Grading of Recommendations, Assessment, Development, and Evaluation” (GRADE) approach (25). The ROBINS-I tool and GRADE approach are commonly used methods in Cochrane reviews.
Study Outcome
The a priori defined primary outcome was risk of in-hospital or 30-day mortality after treatment with ECMELLA compared with VA-ECMO alone in the overall patient population. As the majority of included studies reported in-hospital mortality as study outcomes, the primary outcome was further specified to in-hospital mortality to allow for better inter-study comparability. With an exploratory intent, neurologic outcomes and complication rates after ECPR with ECMELLA compared with VA-ECMO were analyzed. A good neurologic outcome was defined by a Cerebral Performance Category (CPC) scale of 1 or 2. Complications were defined by the Extracorporeal Life Support Organization (ELSO) code (26).
Statistical Analyses
The authors (T.T., L.F.) had full access to all the data in the study and take responsibility for its integrity and the data analysis. Patient and treatment characteristics of each study were tabulated and average total values across all included studies were calculated. Odds ratios (ORs) for mortality outcomes were calculated. The pooled OR and 95% CI were computed with the Mantel-Haenszel test using a random-effects model (27). Results for each study and pooled results for all studies were visualized with a forest plot. Statistical significance was defined as a p value of less than 0.05 (two-sided).
Heterogeneity among the included studies was assessed by using the Cochrane Q test to compute I2 (28). Publication bias was estimated by visual inspection of asymmetry in funnel plots as well as the Egger’s regression test (29). All data were processed using R Studio (R Core Team, Vienna, Austria).
Sensitivity Analyses
The primary analysis was repeated in sensitivity analyses stratified by type of statistical method, centers, cardiac arrest characteristics of patients, and study outcomes. In one sensitivity analysis, only peer-reviewed articles were analyzed excluding “gray literature” with no affiliation to a peer-reviewed publication in a scientific journal at the time of the meta-analysis (e.g., conference abstracts).
RESULTS
Search Results
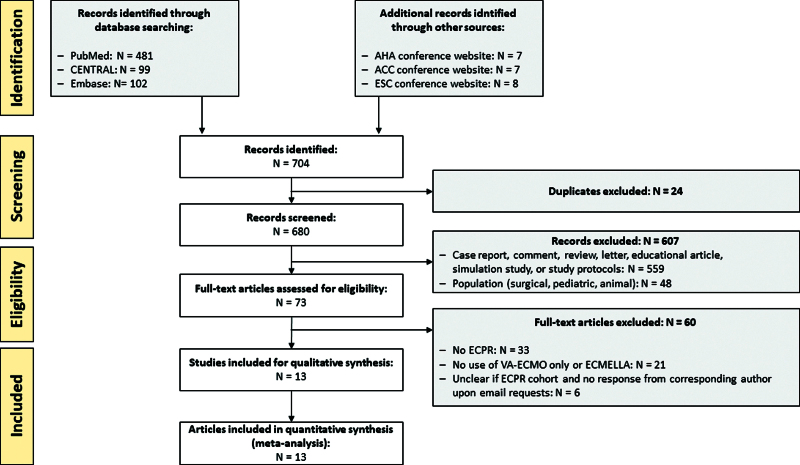
A total of 704 records were identified in the initial literature review (PubMed: n = 481, CENTRAL: n = 99, Embase: n = 102, conference websites: n = 22), out of which 607 records were excluded based on a priori defined exclusion criteria (e.g., no original research article, no ECPR, or no human subjects; Fig. 1). Thirteen records contained unclear information as to whether patients with ECPR were included, out of which corresponding authors of seven records provided numbers upon email requests, whereas the remaining six records were excluded due to missing author response. Nine of the included studies had patients with ECPR as subgroups while the main study was on cardiogenic shock. Overall, 13 records were included in the final meta-analysis, out of which one study was considered to be gray literature (30).
Figure 1.
Preferred Reported Items for Systematic Reviews and Meta-Analysis flow chart of study literature retrieval. ACC = American College of Cardiology, AHA = American Heart Association, CENTRAL = Cochrane Central Register of Controlled Trials, ECMELLA = concomitant treatment with venoarterial extracorporeal membrane oxygenation and Impella microaxial pump, ECPR = extracorporeal cardiopulmonary resuscitation, ESC = European Society of Cardiology, VA-ECMO = venoarterial extracorporeal membrane oxygenation.
The 13 studies were performed at 32 tertiary care centers over an average study period of 7.0 years (± 3.1 yr), out of which 23 centers (71.9%) were located in European countries, eight centers (25.0%) in the United States, and one center (3.1%) in Asia (Supplemental Fig. 1, http://links.lww.com/CCM/H470; and Table 1). All records, except for one, included studies that were performed after the year 2010. Three records were designed as multicenter studies, 11 records had a retrospective observational design while two records were prospectively conducted. No RCT on LV unloading during ECPR was published. The study size varied from seven to 256 patients with ECPR across study centers. Differences across included studies can explain 38.7% of the heterogeneity (I2 = 38.7%).
TABLE 1.
Characteristics of Included Studies
| References | Country | Study Center(s) | Study Duration | Study Design | Mortalitya |
|---|---|---|---|---|---|
| Lim (31) | United Kingdom | 1 | January 2012 to January 2016 | Retrospective, cohort | 30-d mortality |
| Akanni et al (32) | United States | 1 | January 2010 to October 2014 | Retrospective, cohort | Hospital mortality |
| Garan et al (33) | United States | 1 | April 2015 to March 2017 | Prospective, cohort | Hospital mortality |
| Gonçalves-Teixeira (30) | Portugal | 1 | 2011–2018 | Retrospective, cohort | Hospital mortality |
| Axtell et al (34) | United States | 1 | January 2009 to January 2019 | Retrospective, cohort | Hospital mortality |
| Schrage et al (16) | Germany, United States, France, Italy | 16 | 2005–2019 | Retrospective, cohort | 30-d mortality |
| Mørk et al (35) | Denmark | 4 | July 2011 to December 2020 | Retrospective, cohort | 30-d mortality |
| Vakil et al (36) | United States | 1 | January 2018 to June 2020 | Prospective, cohort | Hospital mortality |
| Thevathasan et al (20) | Germany | 3 | January 2016 to June 2021 | Retrospective, cohort | 30-d mortality |
| Alonso-Fernandez-Gatta et al (37) | Spain | 1 | 2013 to October 2020 | Retrospective, cohort | Hospital mortality |
| Gaisendrees et al (38) | Germany | 1 | January 2016 to December 2020 | Retrospective, cohort | Hospital mortality |
| Mørk et al (39) | Denmark | 1 | January 2015 to December 2019 | Retrospective, cohort | Hospital mortality |
| Unoki et al (21) | Japan | 1 | January 2012 to January 2020 | Retrospective, cohort | Hospital mortality |
In-hospital mortality was requested for meta-analysis from corresponding authors of all included studies.
Study Population
A total of 1,014 adult patients with ECPR were included in the meta-analysis, out of whom 762 (75.1%) were treated with VA-ECMO and 252 (24.9%) with ECMELLA (Supplemental Table 3, http://links.lww.com/CCM/H470). Impella was on average implanted 56 minutes ([interquartile range (IQR) 35–97 min) after VA-ECMO initiation. ECMELLA and VA-ECMO groups had a similar patient age (55.5 yr [± 13.4 yr] vs. 56.4 yr [± 13.7 yr]), rate of out-of-hospital cardiac arrests (OHCAs) (132 [55.7%] vs. 366 [60.8%] patients), low-flow times (53 min [IQR 37–78 min] vs. 54 min [IQR 36–69 min]), pre-ECPR pH (7.07 [0.26] vs. 7.11 [± 0.27]), and lactate levels (10.9 mmol/L [± 4.19 mmol/L] vs. 10.4 mmol/L [± 5.69 mmol/L]). Patients with ECMELLA had more frequently shockable electrocardiogram rhythms (139 [58.6%] vs. 297 [49.3%] patients) as well as an acute myocardial infarction (AMI) as the cause of cardiac arrest and received a percutaneous coronary intervention (PCI), compared with VA-ECMO: 189 (79.7%) vs. 312 (51.8%) patients with AMI and 181 (79.0%) vs. 265 (47.5%) patients with PCI, respectively. In contrast, VA-ECMO support was more frequently used for pulmonary embolism (PE) and miscellaneous causes of cardiac arrest: 49 (9.5%) vs. 1 (0.7%) and 145 (28.2%) vs. 11 (7.2%), respectively.
Study Outcomes
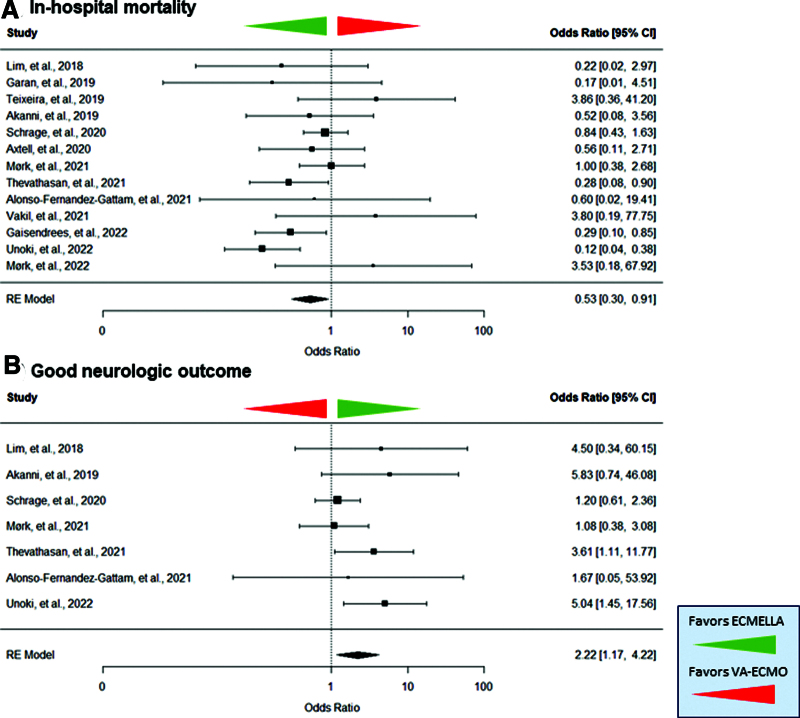
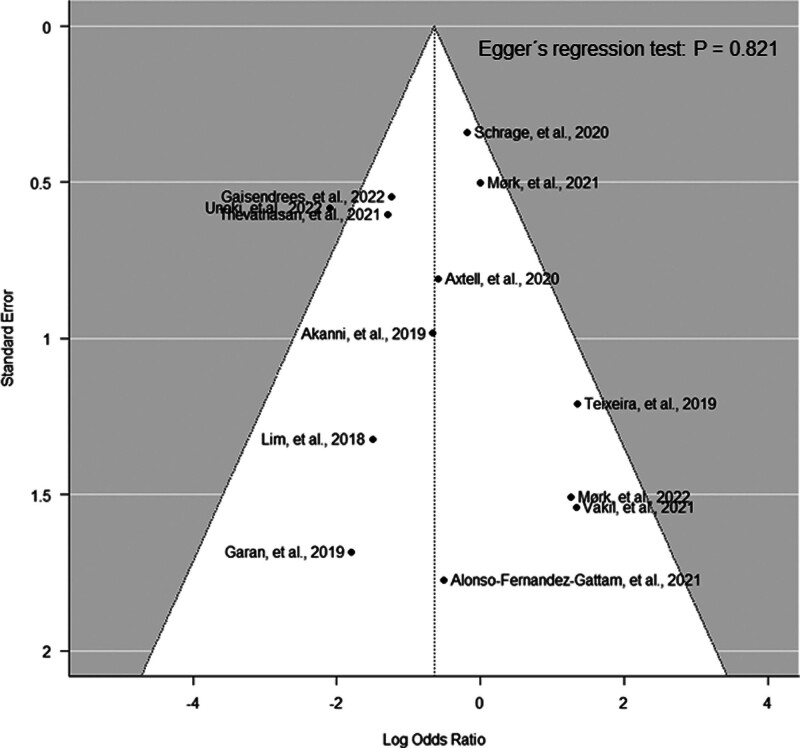
ECMELLA support was associated with reduced odds of mortality compared with VA-ECMO support alone (OR, 0.53 [95% CI, 0.30–0.91]; p = 0.023 and absolute risk difference, –0.09 [95% CI, –0.21 to –0.02; and Fig. 2). Patients with ECMELLA had a longer hospital length of stay (18.7 d [IQR 6.7–39.7 d] vs. 10.4 d [IQR 3.4–24.3 d]). The average treatment effect for in-hospital mortality was in favor of ECMELLA therapy across all studies except for three smaller studies. The Funnel plot showed a symmetric distribution (Egger’s regression test: p = 0.821; Fig. 3). Trim-and-fill analysis resulted in imputation of two potentially missing studies and the overall effect estimate was in favor of ECMELLA therapy (Supplemental Fig. 2, http://links.lww.com/CCM/H470). The average risk of bias was serious to critical according to ROBINS-I tool, mostly caused by confounding given the observational study design (Supplemental Table 4, http://links.lww.com/CCM/H470). Assessment of the certainty of included studies with the GRADE approach showed a very low level of certainty (Supplemental Table 5, http://links.lww.com/CCM/H470).
Figure 2.
Forest plot on in-hospital mortality (A) and good neurologic outcome (B) in patients treated with venoarterial extracorporeal membrane oxygenation (VA-ECMO) and Impella microaxial pump (ECMELLA) or VA-ECMO during extracorporeal cardiopulmonary resuscitation. The green arrow indicates a favor of ECMELLA therapy. The red arrow indicates a favor of VA-ECMO therapy. RE = random effect.
Figure 3.
Funnel plot analysis.
Patients with ECMELLA support showed a similar average CPC scale at hospital discharge compared with patients treated with VA-ECMO (2 [IQR 1.3–2.4] vs. 2 [IQR 1–2]; and Supplemental Table 3, http://links.lww.com/CCM/H470). Notably, ECMELLA patients had a significantly higher rate of good neurologic outcomes (CPC 1 or 2) than VA-ECMO patients at hospital discharge: 27.9% vs. 15.3% (p = 0.015; Fig. 2).
There were no statistically significant differences between both treatment groups across all complications (Supplemental Table 6, http://links.lww.com/CCM/H470). ECMELLA therapy showed numerically more bleedings (62.8% vs. 28.1%), more hemolysis (26.0% vs. 5.6%), more strokes (14.2% vs. 8.0%), more access-site–related ischemia (21.9% vs. 10.4%), higher need for renal replacement therapies (48.5% vs. 27.7%), and more sepsis (25.4% vs. 15.1%).
Sensitivity Analyses
Sensitivity analyses revealed that ECMELLA therapy was beneficial in peer-reviewed publications (without gray literature), studies without critical risk of bias, adjusted analyses (including propensity score [PS] matching), centers with greater than 50 ECPR cases, as well as in studies with higher rates of in-hospital cardiac arrest (IHCA), shorter low-flow times (≤ 60 min), better metabolic conditions (pre-ECPR pH ≥ 7.0 and lactate levels < 11.1 mmol/L), and higher proportion of AMI as the cause of cardiac arrest (Supplemental Table 7, http://links.lww.com/CCM/H470 and Supplemental Fig. 3, http://links.lww.com/CCM/H470). In an additional analysis, ECMELLA therapy showed a 41% lower mortality risk than VA-ECMO in patients with AMI only (Supplemental Fig. 4, http://links.lww.com/CCM/H470; OR, 0.58 [95% CI, 0.35–0.98]; p = 0.041).
DISCUSSION
ECMELLA support was predominantly used in patients with AMI and VA-ECMO for PE. ECMELLA support during ECPR might be associated with improved survival and neurologic outcome despite numerically higher complication rates. However, indications and frequency of ECMELLA support varied strongly between institutions. There are multiple recent meta-analyses on VA-ECMO and ECMELLA support during cardiogenic shock (40–44), but, to our best knowledge, this is the first systematic review and meta-analysis on patient and treatment characteristics as well as mortality outcomes after LV unloading during ECPR.
ECPR Patient Selection
The characteristics of the pooled study cohort in this meta-analysis fulfilled the present international ECPR guideline criteria across institutions (5, 45); younger age (56.0 yr), reversible cause of cardiac arrest (e.g., AMI in 49.4% or PE in 4.9% of ECPR cases), average low-flow time of 54 minutes as well as average pre-ECPR pH of 7.09, and lactate level of 10.7 mmol/L. Hence, the included study centers appeared to screen patients diligently and performed ECPR in well-selected patients in accordance with international recommendations.
Variations in ECMELLA Use Between Institutions
While ECPR with VA-ECMO is advocated in recent international guidelines and has gained wider application with a ten-fold increase in use (46), the indication, patient criteria, and clinical benefits of ECMELLA treatment in the setting of ECPR is still unclear: the number of facilities providing ECPR with ECMELLA therapy appears to be limited to few specialized tertiary care centers with broad experience in mechanical circulatory support (MCS) and the distribution in ECPR case volumes was asymmetric: eight study records included less than 10 ECMELLA cases each, whereas the remaining five study records contributed 82.1% of all included ECMELLA cases. Additionally, VA-ECMO was used more than three times as often as ECMELLA (75.1% vs. 24.9%) during ECPR. Mortality rates varied widely across institutions from 22.7% to 89.6% after VA-ECMO treatment and from 14.3% to 100% after ECMELLA treatment. Furthermore, certain study records included cases in which ECPR was provided exclusively for patients with AMI as cause of cardiac arrest, in contrast to other centers, where patients with AMI comprised only 15.4% of all ECPR cases.
These significant interinstitutional variations in ECMELLA use and ECPR patient characteristics might be explained by the circumstance that the decision for LV unloading is presently left to the discretion of the treating clinicians based on clinical, echocardiographic, radiographic, and invasive hemodynamic signs. The ELSO published indicators for LV overload in VA-ECMO therapy but standardized, evidence-based guideline recommendations have not yet been published on indications and timing of LV unloading with Impella concomitant to VA-ECMO during ECPR. Given the aforementioned variations between institutions, standardized operating procedures are urgently required.
Mortality After ECPR
In this study cohort, 523 patients (68.6%) died in the VA-ECMO and 123 (48.8%) in the ECMELLA group. The average treatment effects were in favor of ECMELLA therapy in almost all included studies. All sensitivity analyses remained robust for the main findings. The overall mortality in this meta-analysis was considerably high with 63.7% (absolute risk difference between both groups, –0.09), which however was similar to other published ECPR studies (8).
Survival was superior in the ECMELLA group although pre-ECPR pH and lactate levels were similar to the VA-ECMO group. Survivors of ECMELLA therapy also more frequently exhibited a better neurologic outcome at hospital discharge after ECPR. First, one potential explanation for the survival benefit in favor of ECMELLA might be the differential use of ECMELLA and VA-ECMO during ECPR: most of the ECMELLA cases had an AMI as the cause of cardiac arrest (79.7%) while VA-ECMO alone was less frequently used in these patients (51.8%). Instead, VA-ECMO was employed for indications such as PE (9.5%) or miscellaneous causes of cardiac arrest (28.2%), which have worse prognosis: PE has been reported in 2–5% of OHCA cases with high-mortality rates between 65% and 95% (47, 48). The current use of ECMELLA and VA-ECMO for different clinical indications across institutions highlights the urgent need for further research informing guideline recommendations on MCS during ECPR. Additionally, studies investigating homogenous cohorts (i.e., a single etiology of cardiac arrest) are required, as, for example, the multicenter International Registry on Unloading during Cardiac Arrest registry (NCT05175898) studying patients with cardiac arrest and ECPR due to AMI exclusively. Second, the percentage of patients with initial shockable electrocardiogram rhythms was higher in the ECMELLA group, which could have influenced its better outcome. Third, another explanation for the diminished survival outcomes in the VA-ECMO group might be the retrograde aortic perfusion leading to increased LV afterload resulting in higher LV end-diastolic volume and pressure, elevated cardiac wall stress, and stroke work associated with increased myocardial oxygen consumption and mitochondrial dysfunction (49), as being previously reported (50). LV overload may result in pulmonary edema, LV blood stasis, and hampered myocardial recovery. Thus, mechanical LV unloading with Impella should be considered, which has become an increasingly used and effective solution in recent years (17, 18). Impella causes three main effects: increase in cardiac power output, increase in oxygen supply, and decrease in oxygen demand, which may explain the better survival rate compared with VA-ECMO alone during refractory cardiac arrest. Indeed, survival benefits with ECMELLA compared with VA-ECMO alone have recently been shown in observational studies and meta-analyses in patients with AMI-induced cardiogenic shock, while research on LV unloading during refractory cardiac arrest has only been emerging recently (19–21). As of now, there is a lack of RCTs showing a benefit of ECMELLA therapy in refractory cardiac arrest. This meta-analysis included sensitivity analyses across studies with PS matching and confounder adjustment, in which the pooled results showed stronger benefits toward ECMELLA therapy. The stronger benefits might be explained due to a higher proportion of patients with AMI in the PS-matched studies and exclusion of extremely critically ill patients based on covariate balancing as a consequence of PS matching. However, the results of the aforementioned sensitivity analyses were not statistically significant due to smaller study sizes and, thus, lower study power. Although ECPR criteria have been defined by international committees (5), there are currently no established consensus criteria when LV unloading should be considered during ECPR. Hence, there is an urgent need for further research to better define indications and timeframes of LV unloading on VA-ECMO therapy in ECPR as reinforced by the data presented in this meta-analysis.
Of note, clinicians should consider complications caused by MCS during ECPR, such as increased risk of bleeding, hemolysis, or renal replacement therapy that can have a significant impact on mortality. Therefore, close monitoring of ECPR patients is necessary. However, the analysis of complications in this study clearly indicated that the survival advantage of ECMELLA therapy might not be due to an increased complication rate with VA-ECMO therapy.
LV unloading during ECPR is an emerging treatment strategy with an increasing number of studies being published in recent years from a limited number of ECPR centers. While there are three smaller and conflicting RCTs comparing ECPR to conventional CPR (1–3), there has not yet been a single RCT investigating LV unloading during ECPR. Therefore, all studies included into this meta-analysis were observational, either based on retrospective or prospective data collection. Hence, the quality assessment for risk of bias showed an overall serious to critical risk of bias and the evidence was very uncertain. However, as ECPR represents a challenging field for clinicians with time constraints and limited patient information before ECPR initiation, as well as ethical restrictions (given no patient consent during cardiac arrest), the collection of preliminary study data is crucial to inform best clinical practice and eventually the conception of a future RCT on LV unloading during ECPR.
Study data revealed interinstitutional heterogeneity. Possible reasons might be a limited number of studies on LV unloading during ECPR, clinician-based variations in decisions on LV unloading and a strong imbalance in ECPR use across institutions. Additionally, variations in the etiology of cardiac arrest between different studies (e.g., different rates of AMI) may increase study heterogeneity. Furthermore, the type of Impella being used and the time of implantation were not recorded in this meta-analysis. The relatively strict approach by clinicians to consider patients for ECPR treatment may explain publication bias due to a lack of larger ECPR study cohorts. To increase the number of included studies, we included gray literature and contacted study authors to provide missing data. Additionally, ECPR subgroups from primary cardiogenic shock studies were included. The main results on mortality remained robust in all sensitivity analyses.
CONCLUSIONS
Indications, frequency, and mortality for ECMELLA support varied strongly between institutions because heterogeneous causes of cardiac arrest cases were treated with ECMELLA. ECMELLA support during ECPR might be associated with improved survival and good neurologic outcomes across studies. In particular, ECMELLA therapy showed a significant survival benefit in study populations with higher proportions of AMI, IHCA, shorter low-flow times, and benign pre-ECPR metabolic conditions as well as in centers with greater ECPR volumes. Despite better survival, ECMELLA therapy was associated with higher complication rates than VA-ECMO therapy. Therefore, ECMELLA therapy might be considered in well-selected patients with refractory cardiac arrest in close consideration of potential post-procedural complications. Given considerable risk of bias and a very uncertain evidence of the included studies, further scientific evidence is urgently required to formulate best clinical practice and to inform standardized guidelines on the use of LV unloading during ECPR.
Supplementary Material
Footnotes
Drs. Thevathasan and Füreder made equal contributions.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Schrage and Garan received funding from Abiomed. Dr. Schrage received funding from Abbott and AstraZeneca. Dr. Westermann received grants for honorary talks from AstraZeneca, Abiomed, Bayer, Boehringer Ingelheim, Edwards, Novartis, and Medtronic. Dr. Gregers received funding from the Odense University Hospital, Rigshospitalet’s Common Research Foundation, Novo Nordisk Foundation, and Beckett Foundation. Dr. Alonso-Fernandez-Gatta received funding from Instituto de Salud Carlos III (CM19/00055). Dr. Garan received funding from NuPulseCV and Maquet; he received support for article research from Abbott. The remaining authors have disclosed that they do not have any potential conflicts of interest.
*See also p. 512.
REFERENCES
- 1.Yannopoulos D, Bartos J, Raveendran G, et al. : Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020; 396:1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belohlavek J, Smalcova J, Rob D, et al. ; Prague OHCA Study Group: Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: A randomized clinical trial. JAMA. 2022; 327:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suverein MM, Delnoij TSR, Lorusso R, et al. : Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med. 2023; 388:299–309 [DOI] [PubMed] [Google Scholar]
- 4.Low CJW, Ramanathan K, Ling RR, et al. : Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with cardiac arrest: A comparative meta-analysis and trial sequential analysis. Lancet Respir Med. 2023; 11:883–893 [DOI] [PubMed] [Google Scholar]
- 5.Richardson AC, Tonna JE, Nanjayya V, et al. : Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. 2021; 67:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panchal AR, Berg KM, Hirsch KG, et al. : 2019 American Heart Association focused update on advanced cardiovascular life support: Use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: An update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019; 140:e881–e894 [DOI] [PubMed] [Google Scholar]
- 7.Perkins GD, Graesner JT, Semeraro F, et al. ; European Resuscitation Council Guideline Collaborators: European resuscitation council guidelines 2021: Executive summary. Resuscitation. 2021; 161:1–60 [DOI] [PubMed] [Google Scholar]
- 8.Abrams D, Maclaren G, Lorusso R, et al. : Extracorporeal cardiopulmonary resuscitation in adults: Evidence and implications. Intensive Care Med. 2022; 48:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller P, Vikholm P, Hellgren L: Experimental venoarterial extracorporeal membrane oxygenation induces left ventricular dysfunction. ASAIO J. 2016; 62:518–524 [DOI] [PubMed] [Google Scholar]
- 10.Truby LK, Takeda K, Mauro C, et al. : Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017; 63:257–265 [DOI] [PubMed] [Google Scholar]
- 11.Xie A, Forrest P, Loforte A: Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann Cardiothorac Surg. 2019; 8:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito ML, Zhang Y, Qiao X, et al. : Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol. 2018; 72:501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappalardo F, Schulte C, Pieri M, et al. : Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017; 19:404–412 [DOI] [PubMed] [Google Scholar]
- 14.Russo JJ, Aleksova N, Pitcher I, et al. : Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019; 73:654–662 [DOI] [PubMed] [Google Scholar]
- 15.Patel SM, Lipinski J, Al-Kindi SG, et al. : Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019; 65:21–28 [DOI] [PubMed] [Google Scholar]
- 16.Schrage B, Becher PM, Bernhardt A, et al. : Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: Results from an international, multicenter cohort study. Circulation. 2020; 142:2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrage B, Sundermeyer J, Beer BN, et al. : Use of mechanical circulatory support in patients with non-ischemic cardiogenic shock. Eur J Heart Fail. 2023; 25:562–572 [DOI] [PubMed] [Google Scholar]
- 18.Schrage B, Sundermeyer J, Blankenberg S, et al. : Timing of active left ventricular unloading in patients on venoarterial extracorporeal membrane oxygenation therapy. JACC Heart Fail. 2023; 11:321–330 [DOI] [PubMed] [Google Scholar]
- 19.Gaisendrees C, Djordjevic I, Sabashnikov A, et al. : Impact of left ventricular unloading using a peripheral Impella®-pump in eCPR patients. Artif Organs. 2022; 46:451–459 [DOI] [PubMed] [Google Scholar]
- 20.Thevathasan T, Megan K, Boie S, et al.: Abstract 12405: Treatment with impella and veno-arterial extracorporeal membrane oxygenation during extracorporeal cardiopulmonary resuscitation improves survival—a multicenter cohort study. Circulation. 2021; 144:A12405 [Google Scholar]
- 21.Unoki T, Kamentani M, Nakayama T, et al. : Impact of extracorporeal CPR with transcatheter heart pump support (ECPELLA) on improvement of short-term survival and neurological outcome in patients with refractory cardiac arrest—a single-site retrospective cohort study. Resusc Plus. 2022; 10:100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouzzani M, Hammady H, Fedorowicz Z, et al. : Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016; 5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochrane Training: Chapter 14: Completing “Summary of Findings” Tables and Grading the Certainty of the Evidence. 2023. Available at: https://training.cochrane.org/handbook/current/chapter-14. Accessed September 9, 2023
- 26.Extracorporeal Life Support Organization: Codes for Extracorporeal Life Support (ECLS) Complications. Available at: https://testingdnn.elso.org/registry/supportdocuments/eclscomplicationscode.aspx. Accessed September 9, 2023.
- 27.Deeks JJ, Higgins JPT, Altman DG; the Cochrane Statistical Methods Group: 9.4.4.3 Random-Effects Method. 2011. Available at: https://handbook-5-1.cochrane.org/chapter_9/9_4_4_3_random_effects_method.htm. Accessed September 9, 2023
- 28.Deeks JJ, Higgins JPT, Altman DG; the Cochrane Statistical Methods Group: 9.5.2 Identifying and Measuring Heterogeneity. 2011. Available at: https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm. Accessed September 10, 2023
- 29.Sterne JAC, Egger M, Moher D; Cochrane Bias Methods Group: 10.4.3.1 Recommendations on Testing for Funnel Plot Asymmetry. 2011. Available at: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Accessed September 10, 2023
- 30.Gonçalves-Teixeira P: Venoarterial extracorporeal membrane oxygenation in cardiogenic shock: exploring prognostic variables and risk prediction tools. ESC Congress Presentation; 2019. Available at: https://esc365.escardio.org/presentation/196616. Accessed September 10, 2023 [Google Scholar]
- 31.Lim HS: The effect of Impella CP on cardiopulmonary physiology during venoarterial extracorporeal membrane oxygenation support. Artif Organs. 2017; 41:1109–1112 [DOI] [PubMed] [Google Scholar]
- 32.Akanni OJ, Takeda K, Truby LK, et al. : EC-VAD: Combined use of extracorporeal membrane oxygenation and percutaneous microaxial pump left ventricular assist device. ASAIO J. 2019; 65:219–226 [DOI] [PubMed] [Google Scholar]
- 33.Garan AR, Takeda K, Salna M, et al. : Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J Am Heart Assoc. 2019; 8:e012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Axtell AL, Funamoto M, Legassey AG, et al. : Predictors of neurologic recovery in patients who undergo extracorporeal membrane oxygenation for refractory cardiac arrest. J Cardiothorac Vasc Anesth. 2020; 34:356–362 [DOI] [PubMed] [Google Scholar]
- 35.Mørk SR, Stengaard C, Linde L, et al. : Mechanical circulatory support for refractory out-of-hospital cardiac arrest: a Danish nationwide multicenter study. Crit Care. 2021; 25:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vakil D, Soto C, D’Costa Z, et al. : Short-term and intermediate outcomes of cardiogenic shock and cardiac arrest patients supported by venoarterial extracorporeal membrane oxygenation. J Cardiothorac Surg. 2021; 16:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso-Fernandez-Gatta M, Merchan-Gomez S, Toranzo-Nieto I, et al. : Short-term mechanical circulatory support in elderly patients. Artif Organs. 2022; 46:867–877 [DOI] [PubMed] [Google Scholar]
- 38.Gaisendrees C, Djordjevic I, Sabashnikov A, et al. : Impact of left ventricular unloading using a peripheral Impella®-pump in eCPR patients. Artif Organs. 2022; 46:451–459 [DOI] [PubMed] [Google Scholar]
- 39.Mørk SR, Bøtker MT, Christensen S, et al. : Survival and neurological outcome after out-of-hospital cardiac arrest treated with and without mechanical circulatory support. Resusc Plus. 2022; 100:100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iannaccone M, Venuti G, di Simone E, et al. : Comparison of ECMO vs ECpella in patients with non-post-pericardiotomy cardiogenic shock: An updated meta-analysis. Cardiovasc Revasc Med. 2022; 40:134–141 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Han Y, Sun S, et al. : Mortality in cardiogenic shock patients receiving mechanical circulatory support: A network meta-analysis. BMC Cardiovasc Disord. 2022; 22:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorelli F, Panoulas V: Impella as unloading strategy during VA-ECMO: Systematic review and meta-analysis. Rev Cardiovasc Med. 2021; 22:1503–1511 [DOI] [PubMed] [Google Scholar]
- 43.Baldetti L, Gramegna M, Beneduce A, et al. : Strategies of left ventricular unloading during VA-ECMO support: A network meta-analysis. Int J Cardiol. 2020; 312:16–21 [DOI] [PubMed] [Google Scholar]
- 44.Grajeda Silvestri ER, Pino JE, Donath E, et al. : Impella to unload the left ventricle in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock: A systematic review and meta-analysis. J Card Surg. 2020; 35:1237–1242 [DOI] [PubMed] [Google Scholar]
- 45.Michels G, Wengenmayer T, Hagl C, et al. : Recommendations for extracorporeal cardiopulmonary resuscitation (eCPR): Consensus statement of DGIIN, DGK, DGTHG, DGfK, DGNI, DGAI, DIVI and GRC. Kardiologe. 2018; 12:332–341 [DOI] [PubMed] [Google Scholar]
- 46.Richardson ASC, Schmidt M, Bailey M, et al. : ECMO cardio-pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation. 2017; 112:34–40 [DOI] [PubMed] [Google Scholar]
- 47.Javaudin F, Lascarrou JB, Le Bastard Q, et al. ; Research Group of the French National Out-of-Hospital Cardiac Arrest Registry (GR-RéAC): Thrombolysis during resuscitation for out-of-hospital cardiac arrest caused by pulmonary embolism increases 30-day survival: Findings from the French National Cardiac Arrest Registry. Chest. 2019; 156:1167–1175 [DOI] [PubMed] [Google Scholar]
- 48.Bailén MR, Cuadra JAR, Aguayo de Hoyos E: Thrombolysis during cardiopulmonary resuscitation in fulminant pulmonary embolism: A review. Crit Care Med. 2001; 29:2211–2219 [DOI] [PubMed] [Google Scholar]
- 49.Swain L, Reyelt L, Bhave S, et al. : Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol. 2020; 76:684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thevathasan T, Füreder L, Donker DW, et al. : Case report: Refractory cardiac arrest supported with veno-arterial-venous extracorporeal membrane oxygenation and left-ventricular Impella CP®–Physiological insights and pitfalls of ECMELLA. Front Cardiovasc Med. 2022; 9:3220. [DOI] [PMC free article] [PubMed] [Google Scholar]