Abstract
l-Nucleoside analogs are a new class of antiviral and anticancer agents, several of which are currently used in the clinic. The phosphorylation of these agents to the triphosphate form is thought to be important for exertion of their pharmacological activities. 1-(2′-Deoxy-2′-fluoro-β-l-arabinofuranosyl)-5-methyluracil (l-FMAU; Clevudine) is a thymidine analog that is currently under phase III clinical trials as an anti-human hepatitis B virus agent. We examined the behavior of its monophosphate metabolite with human recombinant thymidylate kinase (TMPK) and showed that l-FMAU monophosphate (l-FMAUMP) is a poorer substrate than its d-configuration anomer (d-FMAUMP). The phosphorylation efficiency of l-FMAUMP is similar to that of the monophosphate of 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T), an anti-human immunodeficiency virus analog, both of which are approximately 1% TMP. To clarify the role of human TMPK in the phosphorylation of l-FMAUMP to the diphosphate metabolite in cells, a Tet-On inducible human TMPK cell line system was established. In this system, the expression of TMPK is closely regulated in response to various concentrations of doxycycline. When the cells were treated with l-FMAU or d4T, the amounts of the diphosphate and triphosphate metabolites of these analogs were increased, in accordance with an increase in human TMPK activity in cells. In conclusion, this is the first demonstration of the behavior of TMPK toward l-FMAUMP. This study indicates that human TMPK can phosphorylate l-FMAUMP and play a critical role in l-FMAU metabolism in cells.
l-Nucleosides, the mirror image of naturally occurring nucleosides which are in the d conformation, are a novel class of compounds shown to have antiviral and anticancer activities (3, 9). These potent antiviral compounds may be useful not only for the treatment of viral diseases but also for the prevention or the delayed onset of those cancers that have a strong association with viruses (4).
Several pyrimidine analogs, such as β-l(−)-2′,3′-dideoxy-3′-thiacytidine [l(−)SddC, 3TC, or lamivudine], β-l-nucleosides (telbivudine and valtorcitabine), and 1-(2′-deoxy-2′-fluoro-β-l-arabinofuranosyl)-5-methyluracil (l-FMAU; Clevudine), have been recognized as potent and selective inhibitors of the replication of hepatitis B virus (HBV). These anti-HBV pyrimidine analogs are assumed to target the HBV DNA polymerase (1, 3, 5, 6, 8, 18).
Most nucleoside analogs require stepwise phosphorylation to the respective triphosphate metabolites to exert their pharmacological activities. The phosphorylation has been shown for 3TC and 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine (l-Fd4C) in HepG2 cells; telbivudine and valtorcitabine in HepG2 cells (6, 11); and l-FMAU in H1, L8, and 2.2.15 cells (1, 19). During the phosphorylation processes of l-nucleoside analogs, the most important intracellular or viral enzymes involved are deoxycytidine kinase (dCK) and/or thymidine kinase (TK), pyrimidine nucleoside monophosphate kinase (cytidine-UMP kinase) or thymidylate kinase (TMPK), and nucleoside diphosphate kinase or 3-phosphoglycerate kinase (6). However, the respective nucleoside kinases for phosphorylation in each step may differ considerably from one compound to another. The first step of l-FMAU phosphorylation could be catalyzed by thymidine kinase 1 (TK1), dCK, or mitochondrial deoxypyrimidine nucleoside kinase (TK2) (7, 10); and the phosphorylation of l-FMAU from the diphosphate form (l-FMAUDP) to the triphosphate form (l-FMAUTP) is carried out by 3-phosphoglycerate kinase (12). While our previous data (data not shown) have shown that the recombinant human cytidine-UMP kinase was not the enzyme responsible for the formation of l-FMAUDP, it is unclear whether human TMPK is the enzyme responsible for phosphosylation from the monophosphate form (l-FMAUMP) to the diphosphate form and how critical the role of TMPK is in the metabolism of l-FMAU in cells.
2′,3′-Didehydro-2′,3′-dideoxythymidine (d4T), a d-configuration nucleoside analog, is an important anti-human immunodeficiency virus drug and can be phosphorylated by TK to d4T monophosphate (d4TMP), but with a relatively poor efficiency (2) in comparison with that by thymidine (dThd). How critical the role of TMPK is in the phosphorylation of d4TMP to d4T diphosphate (d4TDP) and how the increased amount of d4TDP subsequently affects its triphosphate form (d4TTP) are not clear. We examined whether the increase in the amounts of d4TMP metabolites could be dependent on TMPK activity.
l-Nucleoside analogs have some factors that limit their efficacies, just like other antiviral analogs. One of the factors that causes inefficient metabolic activation could determine the antiviral activities of those deoxynucleoside analogs. In an attempt to gain better insight into the mechanism of thymidine analog action for anti-HBV, anti-Epstein-Barr virus, and anti-human immunodeficiency virus activity, we established a stable Tet-On inducible TMPK cell line. This cell line, which expresses human TMPK, is closely regulated in response to various concentrations of doxycycline and would therefore seem to be useful for investigation of the role of TMPK in the metabolism of l-FMAU and d4T.
MATERIALS AND METHODS
Compounds.
l-FMAUMP, d-FMAUMP, and D4TMP were synthesized by previously described procedures (17). dTMP (TMP) was purchased from Sigma Chemical Co. St. Louis, MO. [methyl-3H]l-FMAU and [methyl-3H]d4T were purchased from Moravek Biochemicals, Inc., Brea, CA. The chemical structures are shown in Fig. 1.
FIG. 1.
Chemical structures of the dThd analogs used in this study.
Cloning, expression, and purification of human TMPK.
The human TMPK-coding sequence was amplified from HeLa cells by reverse transcription-PCR and cloned into the pCR2.1AT vector (Invitrogen Co., Carlsbad, CA). The primers (5′-GGAATTCCATATGATGGCGGCCCGG and 5′-CGCGGATCCTCACTTCCATAGCTC) were engineered to create an NdeI site at the 5′end of the amplified DNA and a BamHI site at the 3′end of the amplified DNA. The coding sequence of human TMPK was inserted as an NdeI-BamHI fragment into the pET-28a(+) vector (Novagen Inc., Stamford, CT) to encode the TMPK bearing a His tag at the N terminus with a thrombin cleavage site before the His tag. The purified enzyme was aliquoted and stored at −70°C.
Antibody production and immunoblotting.
The purified recombinant TMPK protein (0.5 mg) mixed with Freund's complete adjuvant (Sigma Chemical Co.) was used as an antigen for immunization of rabbits. Rabbit serum (5 ml) was collected as a serum control before the first immunization. The rabbits were then boosted twice with the antigen mixed with Freund's incomplete adjuvant (Sigma Chemical Co.). Two weeks after the second boost, the serum was taken and tested for specific antibody production. The antibody was tested for specificity and titer by using Western blotting analysis with the recombinant human TMPK protein.
TMPK activity assays.
A high-pressure liquid chromatography (HPLC) assay was used to determine the enzyme activities, as described previously (15), with the following assay buffer: 60 mM Tris-HCl (pH 7.4), 2.5 mM dithiothreitol, 4 mM MgCl2, and 0.15% bovine serum albumin. ATP was chosen as the phosphate donor at a fixed concentration 4 mM or at various concentrations, as indicated. The kinetic properties of dThd analog monophosphates and TMP were measured. TMP concentrations ranging from 6.25 to 100 μM were used, and dThd analog monophosphates were used at different concentrations (6.25 to 250 μM). All the reactions were performed at 37°C. The data were analyzed with the Michaelis-Menten equation. One unit of TMPK was defined as the amount of enzyme required to convert 1 nmol of TMP to TDP in 1 min at 37°C under the standard assay conditions. The protein concentration of the TMPK was determined by using a protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA).
Establishment of inducible Tet-On TMPK cell line.
The inducible Tet-On TMPK cell line system was established by using the Tet-On Gene expression system (Clonetech Laboratories, Inc., Palo Alto, CA). The procedures presented in the user manual of Tet-On Gene expression system were followed. The Tet-On RKO cell line, which was derived from a human colorectal carcinoma with a regulator plasmid, was a gift from Edward Chu (Yale University School of Medicine). We cloned the coding sequence of TMPK into the pTRE vector with a tetracycline (Tet)-responsive element at the EcoRI site (Clonetech Laboratories, Inc.). To isolate the cell lines that stably expressed TMPK, the transfected cells were passaged at a 1:60 dilution into fresh medium at 37°C 48 h after transfection; on the next day, the selection medium was added. At all times, the cells were grown in the presence of 0.4 mg/ml G418 and 0.5 mg/ml hygromycine for selection. To induce the expression of TMPK, the cells were grown to 60% confluence in tissue culture medium, to which various concentrations of doxycycline were then added for 48 h. Standard TMPK assays and Western blotting assays were used for evaluation of the TMPK activation and protein expression levels. The data were analyzed with a densitometer (Amersham Biosciences Co. Piscataway, NJ), using personal densitometer SI software (Molecular Dynamics Co.) and Microsoft Excel software.
Confocal microscopy.
The localization of TMPK in cells was determined by confocal microscopy, as described previously (13).
Metabolism of l-FMAU in Tet-On TMPK RKO cells.
The impact of intracellular TMPK activity on the metabolism of l-FMAU was examined by inducing TMPK expression to various levels in cells. Tet-On TMPK RKO cells were seeded at 5 × 105 cells per culture dish in the presence of increasing concentrations of doxycycline (0, 0.05, 0.3 and 1.0 μg/ml) for 48 h, which induced increasing amounts of TMPK. Then, 2 μM [methyl-3H]l-FMAU (1.7 Ci/mmol) was added to the induced cells for different periods of time, as indicated below. Parallel cultures were incubated with nonradiolabeled compounds at the same concentrations for calculation of the cell number. At the end of the incubation with the radiolabeled compounds, the cells were washed twice with ice-cold phosphate-buffered saline and scraped into 60% methanol. The pellets were washed two additional times with 60% methanol and then mixed with SafeScint scintillation fluid (American Bioanalytical, Natick, MA) and quantitated in a Beckman scintillation counter (Beckman Instruments, Fullerton, CA). The methanol-soluble fractions were evaporated to dryness, resuspended in water, and analyzed on an anion-exchange Partisil-SAX column (Whatman, Inc., Clifton, NJ) by using a Shimadzu Class-VP high-pressure liquid chromatograph (Shimadzu, Braintree, MA) with a linear gradient of water to 300 mM potassium phosphate buffer. The HPLC eluate was assayed for radioactivity by mixing it with 3 ml/min of mono Flow 5 scintillation fluid (National Diagnostics, Atlanta, GA) in tandem with a 150TR Radiomatic flow scintillation analyzer, as described previously (7).
RESULTS
General properties of recombinant human TMPK.
The kinetic properties of both the recombinant TMPK and the TMPK partially purified from CEM cells (14) were compared. The apparent Km values of TMP and ATP for the recombinant TMPK were 12.3 ± 2.2 μM and 51.2 ± 4.3 μM, respectively, and the Kcat value was 2.40 ± 0.36 s−1. No significant difference in Km values for TMP and ATP was observed between the recombinant TMPK and the partially purified TMPK from CEM cells. Enzyme stability studies showed that the recombinant TMPK was stabilized by its substrates. This was consistent with a previous report of a study from this laboratory (14) performed with partially purified human TMPK from leukemia patients.
Phosphorylation of thymidine analog monophosphates by recombinant TMPK.
The monophosphates of the l-dThd analogs shown in Fig. 1. were assessed as substrates; and the d-dThd analogs, d-FMAU, and d4T were used for comparison. The Km, Vmax, and Kcat values of the dThd analog monophosphates for recombinant TMPK are shown in Table 1. The kinetic properties of different analogs toward TMPK exhibited different characteristics. The Km values of the dThd analog monophosphates examined were in the following order: d-FMAUMP < TMP < l-FMAUMP < d4TMP. The Vmax or Kcat values were in the order TMP > d-FMAUMP > d4TMP > l-FMAUMP. The relative efficiencies of l-FMAUMP and d-FMAUMP compared with that of TMP were 1.2% and 70%, respectively. The relative efficiency of l-FMAUMP was about 60-fold lower than that of d-FMAUMP. The d4TMP Kcat value was 0.09 s−1 in these studies, which is consistent with the value reported by others (16) and about the same as that of l-FMAUMP.
TABLE 1.
Kinetic characteristics of TMP and dThd analog monophosphates as substrates for recombinant TMPKa
| dThd analog | Km (μM) | Vmax (μmol/min/mg) | Kcat (s−1) | Relative efficiencyb (%) 1 |
|---|---|---|---|---|
| TMP | 12.3 ± 2.2 | 6 ± 0.9 | 2.4 ± 0.36 | 100 |
| l-FMAUMP | 21.7 ± 2.9 | 0.12 ± 0.02 | 0.05 ± 0.008 | 1.17 |
| d-FMAUMP | 7.0 ± 0.2 | 2.6 ± 1.7 | 1 ± 0.07 | 73 |
| d4TMP | 28.0 ± 1.7 | 0.23 ± 0.03 | 0.09 ± 0.01 | 1.6 |
The Km and Kcat values of the dThd analog monophosphates were evaluated by using 4.5 mM ATP as the phosphate donor. The reactions were carried out with 0.01 U of TMPK. Values are means ± standard deviations from three independent experiments.
A 100% relative efficiency of TMP corresponds to the rate of Kcat/Km.
Expression of TMPK protein and activity in Tet-On TMPK RKO cells induced by doxycycline.
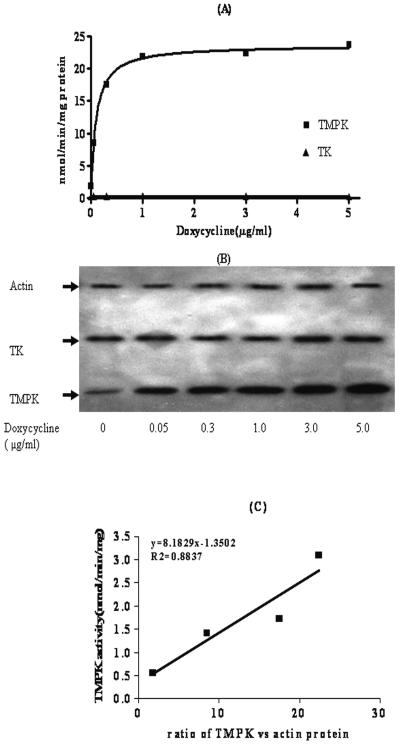
Stable Tet-On TMPK RKO cells were evaluated for their regulation of TMPK in response to various concentrations of doxycycline. Tet-On TMPK RKO cells were cultured for 48 h in the presence of doxycycline at concentrations ranging from 0 to 5 μg/ml. Cellular extracts were prepared and examined for TMPK activity. TMPK protein was analyzed by Western blotting with rabbit polyclonal TMPK antibody. Actin and TK1 were used as the internal controls. The data depicted in Fig. 2A demonstrate that the TMPK activity increased in a doxycycline concentration-dependent manner up to 1 μg/ml. There was no change in TK activity among samples treated with different concentrations of doxycycline ranging 0 to 5 μg/ml. The activities and protein amounts of deoxycytidine kinase, thymidylate synthase, nucleoside diphosphate kinase, and urdylate-cytidylate kinase were also evaluated after the increase in the amount of TMPK was achieved; and the results showed that none of them had any significant change in either activity or protein amount (data not shown). The maximal level of induction of TMPK activity, which could be achieved in the Tet-On-TMPK RKO cells by doxycycline induction, was approximately 12-fold higher than the basal level in noninduced Tet-On TMPK RKO cells. A Western blotting assay was used to analyze the corresponding protein amounts of TMPK. As shown in Fig. 2B, the amounts of TMPK protein in cellular extracts depended on the doxycycline concentration in the culture medium and correlated with the TMPK activity, as shown in Fig. 2C. There was no change in either the cell growth rate or the intracellular TTP level (data not shown) upon induction of TMPK by doxycycline at concentrations ranging from 0 to 3 μg/ml.
FIG. 2.
(A) TMPK activity in cell extracts of doxycycline-induced Tet-On-TMPK RKO cells. The activity of the enzyme TK was used as a control. (B) Expression of TMPK in Tet-On-TMPK RKO cells, as detected by Western blotting. Actin was used as the internal control. (C) Correlation of TMPK activity and its protein amount quantitated with a densitometer.
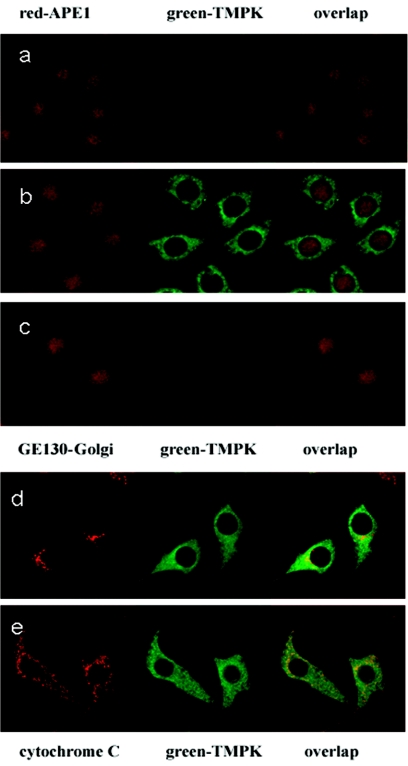
To assess the intracellular localization of the human TMPK protein, we analyzed Tet-On-TMPK RKO cell cultures by confocal microscopy using rabbit polyclonal anti-TMPK antibody that was produced by immunizing rabbits with recombinant purified TMPK. Markers for the visualization of the nuclei (anti-APE antibody), the Golgi apparatus (GE130), and the mitochondria (cytochrome c) were included as controls. These data clearly point to the predominant localization of TMPK in the cytosol of TMPK gene-transfected cells, with less TMPK detected in the Golgi apparatus, mitochondria, and nuclei, as shown in Fig. 3.
FIG. 3.
Confocal microscopy of noninduced or doxycycline-induced Tet-On-TMPK RKO cells using polyclonal rabbit anti-TMPK antibody. (A) Noninduced TMPK; (B) doxycycline-induced TMPK; (C) blockage with purified TMPK; (D) induced TMPK plus GE30-Golgi antibody; (E) induced TMPK plus cytochrome c antibody.
Phosphorylation of l-FMAU in cells with different TMPK activities.
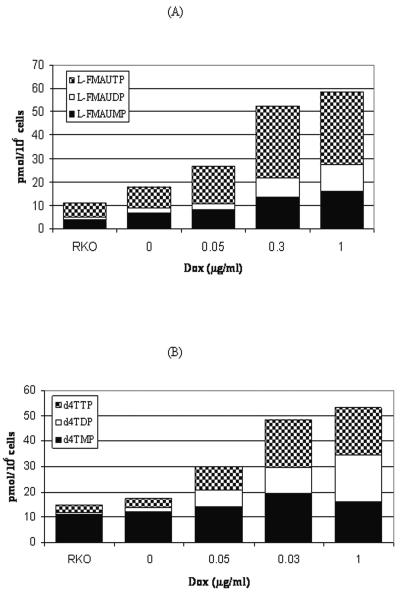
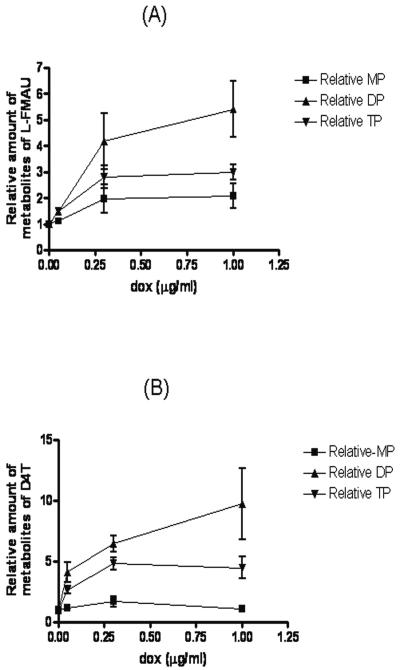
The metabolism of l-FMAU and d4T were examined in Tet-On TMPK RKO cells that were induced with various concentrations (0, 0.05, 0.3 and 1.0 μg/ml) of doxycycline for 48 h, since they have similar efficiencies of phosphorylation as the substrates of TMPK. The intracellular phosphorylation of methyl-3H-labeled l-FMAU and d4T by cells was examined upon incubation of the cells with those compounds for 2 h and 12 h, respectively (Fig. 4). Upon incubation with 1 μM l-FMAU for 2 h, the relative amounts of intracellular l-FMAU metabolites were measured by using the HPLC assay. The results are shown in Fig. 4A. l-FMAUTP was the major metabolite in RKO cells. In cells with induced TMPK, l-FMAUDP and l-FMAUTP gradually increased from 1.9 to 6.8 pmol/106 cells and from 10.0 to 34.2 pmol/106 cells, respectively, depending on the activity of TMPK in the cells. The amounts of diphosphate and triphosphate metabolites of l-FMAU increased in total to 28.8 pmol/106 cells after an increase in the amount of TMPK in the cells by about 12-fold. TMPK phosphorylated d4TMP and l-FMAUMP at similar efficiencies, as depicted as in Table 1. The d4T metabolites were analyzed in TMPK-induced cells treated with 2 μM d4T for 12 h, and the d4T metabolites were analyzed. The longer incubation was chosen for the cellular metabolism studies due to the slower rate of formation of d4TMP from d4T in cells (2) relative to the rate of formation of l-FMAUMP from l-FMAU, which is catalyzed by TK and dCK (7). The results are presented in Fig. 4B. The increases in the amounts of d4TDP and d4TTP ranged from 0.9 to 18.6 mol/106 cells and from 2.7 to 18.3 pmol/106 cells, respectively, with the increases in intracellular TMPK levels. As shown in Fig. 5, the induction of TMPK by different concentrations of doxycycline in cells did not influence the levels of l-FMAUMP or d4TMP (P = 0.151 and P = 0.235, respectively, from one-way analysis of variance [ANOVA]). The increases in the amounts of the diphosphate and triphosphate metabolites of l-FMAU in cells was dependent on the levels of TMPK induced by doxycycline (P = 0.0101 and P = 0.002, respectively, from one-way ANOVA). The amounts of TMPK induced by different concentrations of doxycycline had a more significant effect on l-FMAUDP formation than on l-FMAUTP formation (P = 0.0017 from two-way ANOVA). Similar results were obtained for the metabolism of d4T in cells; the increase in the amount of the diphosphate and triphosphate metabolites of d4T in the cells was dependent on the levels of TMPK induced by doxycycline (P = 0.0228 and P = 0.0032, respectively from one-way ANOVA), and the amounts of TMPK induced by different concentrations of doxycycline had an effect on d4TDP formation more significant than its effect on d4TTP formation (P value < 0.0001 from two-way ANOVA). The slight increase in the amounts of the metabolites of l-FMAU and d4T in Tet-On TMPK RKO cells with doxycycline at 0 μg/ml compared with that in the control RKO cells without plasmids could be due to the slight leakage of TMPK, or the medium and serum obtained commercially contained a small amount of doxycycline. Additionally, the recombinant “repressor” proteins used in this system might have played a role as transcriptional activators and therefore may possibly have led to an up-regulatory effect at a minimal promoter region, as described in the product manual of Invitrogen.
FIG. 4.
Typical metabolism of l-FMAU and d4T in doxycycline (Dox)-induced Tet-On-TMPK RKO cells. (A) Phosphorylation of 1 μM l-FMAU in doxycycline-induced Tet-On-TMPK RKO cells (6 × 106) after 2 h of incubation; (B) phosphorylation of 2 μM d4T in doxycycline-induced Tet-On-TMPK RKO cells (6 × 106) after 12 h of incubation.
FIG. 5.
Relative amounts of metabolites of l-FMAU and d4T in the doxycycline-induced Tet-On-TMPK RKO cells (data are presented as the means of three independent experiments). (A) Relative amounts of the metabolites of l-FMAU in Tet-On-TMPK RKO cells treated with different doses of doxycycline (dox); (B) relative amounts of the metabolites of d4T in Tet-On-TMPK RKO cells treated with different doses of doxycycline. The relative amount of metabolites of l-FMAU or d4T in cells without doxycycline treatment was equal to 1.
DISCUSSION
l-FMAU is phosphorylated stepwise to the mono-, di-, and triphosphate metabolites in both Epstein-Barr virus-producing and nonproducing cells (19) as well as human hepatoma cells carrying HBV (i.e., designated 2.2.15 cells) (1). The phosphorylation of l-FMAU to its monophosphate form is catalyzed by TK1 and the mitochondrial TK2, in addition to dCK (7). Our results showed that human TMPK can further phosphorylate l-FMAUMP to l-FMAUDP, but the efficiency is much less than that for its d-configuration analog, d-FMAUMP. Marked differences in Km, Vmax, and Kcat between l-FMAUMP and d-FMAUMP were observed. The differences could be explained by the preferential chiral specificity of the enzyme at the active site toward its d-dThd analog in terms of the binding and catalytic processes.
Although l-FMAUMP and d4TMP are substrates of human TMPK, the efficiency of their phosphorylation is only 1% of that of TMP, as shown in Table 1. However, l-FMAU could be phosphoryated to its DP form by multiple enzymes (TK1, TK2, and dCK) (7). In contrast, d4T is phosphoryated only by TK1, but with a poor efficiency (2). Thus, the incubation times for l-FMAU and d4T were chosen to be 2 h and 12 h, respectively. The total intracellular levels of phosphorylated metabolites under these conditions were almost the same (Fig. 4). We demonstrated that increased TMPK activity in cells could increase the total amounts of the di- and triphosphate metabolites of l-FMAU and d4T in cells. No statistical difference in the amounts of the monophosphate metabolites was shown upon the induction of intracellular TMPK. The lack of the depleted monophosphate metabolite as the result of the rapid conversion to the diphosphate metabolite by TMPK could be due to the formation of more l-FMAUMP that was catalyzed by the nucleoside kinases involved or more d4TMP that was caused by the longer incubation time; thus, the steady-state level of the monophosphates did not change.
Thus, TMPK is a critical enzyme for the formation of those active metabolites of l-FMAU and d4T. It is known that the activities of TMPK in different tissues are different; the role of TMPK activity in the formation of the active metabolites of these analogs suggests that there might be different levels of metabolism of l-FMAU and d4T in different tissues or cells. Furthermore, it also suggests that the different responses to dThd analogs in different individuals could be related to their different TMPK enzyme activities in target cells.
In conclusion, this study suggests that TMPK can phosphorylate l-FMAUMP with lesser efficiencies than it does d-FMAUMP. The increased amount of TMPK in cells could increase the amounts of the di- and triphosphate metabolites of l-FMAU and d4T formed.
Acknowledgments
We thank Chuan-Jen Wang and Chih-Hung Hsu for critical reading of the manuscript.
This work was supported by grant AI-38204 from NIAID, National Institutes of Health, and grant CA-63477 from NCI, National Institutes of Health. Y.-C.C. is a fellow of the National Foundation for Cancer Research. B.D. is supported by grants from the Fulbright and the Belgian American Educational Foundations.
REFERENCES
- 1.Balakrishna Pai, S., S. H. Liu, Y. L. Zhu, C. K. Chu, and Y. C. Cheng. 1996. Inhibition of hepatitis B virus by a novel l-nucleoside, 2′-fluoro-5-methyl-β-l-arabinofuranosyl uracil. Antimicrob. Agents Chemother. 40:380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., P. Herdewijn, and E. De Clercq. 1989. Differential patterns of intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J. Biol. Chem. 264:6127-6133. [PubMed] [Google Scholar]
- 3.Buti, M., and R. Esteban. 2003. Entecavir, FTC, l-FMAU, LdT and others. J. Hepatol. 39:S139-S142. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Y. C. 2001. l-Nucleoside analogues against cancer-causing viruses have potential in the prevention, delayed onset and treatment of viral associated cancers. Antivir. Chem. Chemother. 12:5-11. [PubMed] [Google Scholar]
- 5.Chu, C. K., F. D. Boudinot, S. F. Peek, J. H. Hong, Y. Choi, B. E. Korba, J. L. Gerin, P. J. Cote, B. C. Tennant, and Y. C. Cheng. 1998. Preclinical investigation of l-FMAU as an anti-hepatitis B virus agent. Antivir. Ther. 3:113-121. [PubMed] [Google Scholar]
- 6.De Clercq, E. 1999. Perspectives for the treatment of hepatitis B virus infections. Int. J. Antimicrob. Agents 12:81-95. [DOI] [PubMed] [Google Scholar]
- 7.Dutschman, G. E., E. G. Bridges, S. H. Liu, E. Gullen, X. Guo, M. Kukhanova, and Y. C. Cheng. 1998. Metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus β-d(+) nucleoside analogs in vitro. Antimicrob. Agents Chemother. 42:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu, L., S. H. Liu, and Y. C. Cheng. 1999. Sensitivity of l-(−)2,3-dideoxythiacytidine resistant hepatitis B virus to other antiviral nucleoside analogues. Biochem. Pharmacol. 57:1351-1359. [DOI] [PubMed] [Google Scholar]
- 9.Giles, F. J., G. Garcia-Manero, J. E. Cortes, S. D. Baker, C. B. Miller, S. M. O'Brien, D. A. Thomas, M. Andreeff, C. Bivins, J. Jolivet, and H. M. Kantarjian. 2002. Phase II study of troxacitabine, a novel dioxolane nucleoside analog, in patients with refractory leukemia. J. Clin. Oncol. 20:656-664. [DOI] [PubMed] [Google Scholar]
- 10.Gumina, G., Y. Chong, H. Choo, G. Y. Song, and C. K. Chu. 2002. l-Nucleosides: antiviral activity and molecular mechanism. Curr. Top. Med. Chem. 2:1065-1086. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Santiago, B., L. Placidi, E. Cretton-Scott, A. Faraj, E. G. Bridges, M. L. Bryant, J. Rodriguez-Orengo, J. L. Imbach, G. Gosselin, C. Pierra, D. Dukhan, and J. P. Sommadossi. 2002. Pharmacology of β-l-thymidine and β-l-2′-deoxycytidine in HepG2 cells and primary human hepatocytes: relevance to chemotherapeutic efficacy against hepatitis B virus. Antimicrob. Agents Chemother. 46:1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan, P., E. A. Gullen, W. Lam, G. E. Dutschman, S. P. Grill, and Y. C. Cheng. 2003. Novel role of 3-phosphoglycerate kinase, a glycolytic enzyme, in the activation of l-nucleoside analogs, a new class of anticancer and antiviral agents. J. Biol. Chem. 278:36726-36732. [DOI] [PubMed] [Google Scholar]
- 13.Lam, W., C. Chen, S. Ruan, C. H. Leung, and Y. C. Cheng. 2004. Expression of deoxynucleotide carrier is not associated with the mitochondrial DNA depletion caused by anti-HIV dideoxynucleoside analogs and mitochondrial dNTP uptake. Mol. Pharmacol. 11:11. [DOI] [PubMed] [Google Scholar]
- 14.Lee, L. S., and Y. Cheng. 1977. Human thymidylate kinase. Purification, characterization, and kinetic behavior of the thymidylate kinase derived from chronic myelocytic leukemia. J. Biol. Chem. 252:5686-5691. [PubMed] [Google Scholar]
- 15.Liou, J. Y., G. E. Dutschman, W. Lam, Z. Jiang, and Y. C. Cheng. 2002. Characterization of human UMP/CMP kinase and its phosphorylation of d- and l-form deoxycytidine analogue monophosphates. Cancer Res. 62:1624-1631. [PubMed] [Google Scholar]
- 16.Ostermann, N., D. Segura-Pena, C. Meier, T. Veit, C. Monnerjahn, M. Konrad, and A. Lavie. 2003. Structures of human thymidylate kinase in complex with prodrugs: implications for the structure-based design of novel compounds. Biochemistry 42:2568-2577. [DOI] [PubMed] [Google Scholar]
- 17.Ruth, J. L., and Y. C. Cheng. 1981. Nucleoside analogues with clinical potential in antivirus chemotherapy. The effect of several thymidine and 2′-deoxycytidine analogue 5′-triphosphates on purified human (alpha, beta) and herpes simplex virus (types 1, 2) DNA polymerases. Mol. Pharmacol. 20:415-422. [PubMed] [Google Scholar]
- 18.Seigneres, B., P. Martin, B. Werle, O. Schorr, C. Jamard, L. Rimsky, C. Trepo, and F. Zoulim. 2003. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 47:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao, G. Q., S. H. Liu, E. Chou, M. Kukhanova, C. K. Chu, and Y. C. Cheng. 1996. Inhibition of Epstein-Barr virus replication by a novel l-nucleoside, 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. Biochem. Pharmacol. 51:941-947. [DOI] [PubMed] [Google Scholar]