Combination antiretroviral therapy (ART) has dramatically decreased morbidity and mortality among human immunodeficiency virus (HIV) type 1 (HIV-1)-infected patients through the durable suppression of viral replication to undetectable levels (22). Although ART has changed significantly in the decades since the advent of monotherapy, nucleoside and nucleotide reverse transcriptase (RT) inhibitors (NRTIs and NtRTIs, respectively) remain important foundations of therapy. The current guidelines for the treatment of HIV infection in the United States and the United Kingdom recommend dual NRTI and NtRTI therapy in combination with a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) for most patients (1, 3). New NRTI and NtRTI options for HIV therapy include two new fixed-dose once-daily combinations, abacavir (ABC) plus lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) plus emtricitabine (FTC).
As combination ART has continued to evolve, the nature of RT resistance mutations encountered in patients has also changed. Previously uncommon mutations such as K65R have become more prevalent among patients experiencing virologic treatment failure (11, 34, 36; L. Bacheler, H. Vermeiren, P. McKenna, and M. VanHoutte, Abstr 43rd Intersci. Conf. Antimicrob. Agents Chemother, abstr. H-917, 2003; R. D. MacArthur, L. R. Crane, D. Alvarez, M. Fairfax, D. Richmond, and G. Curtis, Int. AIDS Soc. 2nd Conf. Pathogen. Treatment, abstr. 835, 2003). The increasing prevalence of resistance-associated HIV-1 mutations also increases the risk of transmission of drug-resistant variants of HIV-1, which are now considered responsible for between 10% and 20% of new infections in North America and Western Europe (P. Cane, G. Dean, M. Fisher, D. Pao, S. Drake, and D. Pillay, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 684, 2004). Some RT resistance mutations are highly stable and can persist for years, despite changes in therapy and ongoing treatment with antiretroviral agents (Cane et al., Abstr. 11th Conf. Retrovir. Opportunistic Infect.). As mutations such as K65R and L74V become more prevalent among treatment-experienced patients, their prevalence in newly infected individuals may also increase.
Integration of current knowledge about the epidemiology of drug resistance and mechanisms and interactions among RT resistance mutations is necessary to achieve optimal success with ART regimens and to preserve future treatment options. This minireview summarizes the changing patterns in the selection of viral mutations among patients receiving NRTI-NtRTI combinations and addresses the clinical implications of these changes.
ZDV AND 3TC: M184V AND TAMs
The combination of 3TC and zidovudine (ZDV) has served as a cornerstone of ART for many years and has a well-characterized resistance mutation profile. Among patients who experience virologic rebound or treatment failure with a 3TC-ZDV regimen, M184V is usually the first RT mutation to emerge and often is found in the absence of mutations to other NRTI, NNRTI, or PI components of the regimen (13, 14, 19). In recent years, the prevalence of M184V and thymidine analog mutations (TAMs) among treatment-experienced patients has remained stable or even decreased. (H. Faruki, J. Sebastian, J. Scott, J. Stamp, and E. R. Lanier, Abstr. XIII Int. HIV Drug Resist. Workshop, abstr. 80, 2004). What are referred to here as TAMs are substitutions at codons 41, 67, 70, 210, 215, and 219. These have also been referred to as ZDV resistance mutations or nucleoside analog mutations (NAMs), but they have been shown to be selected by stavudine (d4T) as well and in different combinations confer various degrees of resistance to other NRTIs (32). A less common set of mutations also appears to be selected during ZDV therapy. These include 44D/A, 118I, 207D/E, and 208Y. Resistance to the entire nucleoside class of drugs may require a combination of TAMs and additional multinucleoside resistance mutations, such as those listed above and others such as the Q151 complex of mutations (A62V, V75I, F77L, F116Y, and Q151M) and T69 insertions (16).
The resistance mutational profile seen with FTC is similar to that seen with 3TC, and M184V is also the predominant RT mutation seen in patients receiving FTC together with other drugs (K. Borroto-Esoda, J. Waters, J. B. Quinn, A. Shaw, J. Hinkle, and F. Rousseau, Abstr XII Int. HIV Drug Resist. Workshop, abstr 7, 2003; P. Cahn, F. Raffi, M. Saag, M. Wolff, D. Pearce, J. M. Molina, and K. E. Borroto-Esoda, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 606, 2003; I. Sanne, J. Anderson, D. Kargl, J. Sorbel, and C. Wakeford, Abstr. 43rd Intersci. Conf. Antimicrob. Agents. Chemother., abstr. H-686, 2003). In most patients, the presence of the M184V mutation is not followed by the emergence of TAMs, provided that the regimen maintains virologic suppression. A 48-week randomized, double-blind trial compared the efficacy and safety of 3TC-ZDV plus efavirenz (EFV) versus those of ABC-3TC-EFV, each administered twice daily to 649 ART-naive patients (D. Irlbeck, E. Rouse, S. Castillo, J. Scott, W. Spreen, H. Zhao, B. Hernandez, and E. R. Lanier, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 661, 2004). Among 33 patients who experienced virologic treatment failure, longitudinal genotyping showed similar mutation patterns in the groups treated with ABC and ZDV. M184V was present in the majority of patients who developed mutations during therapy and remained the only mutation detected in most individuals up to week 48. No treatment-emergent K65R or TAMs were detected, and only a single patient developed L74V (12, 26). The combination of 3TC and ZDV together with indinavir (IDV) has been shown to maintain efficacy in the presence of M184V (7, 13). The durability of 3TC-ZDV when it is combined with a PI or NNRTI is thought to be attributable, at least in part, to the fact that M184V can partially restore susceptibility to ZDV and prevent or delay the development of TAMs (17; T. Melby, S. Tortell, D. Thorborn, G. Pearce, W. Spreen, J. Scott, S. Madison, S. Lafon, and E. R. Lanier, Abstr. 8th Conf. Retrov. Opportunistic Infect., abstr. 448, 2001). Several clinical trials have shown that TAMs are absent or rare among a majority of patients receiving 3TC-ZDV plus an NNRTI or PI who had the M184V mutation at the time of on-therapy viral rebound (9; R. M. Gulick, E. J. Eron, Jr., K. Squires, R. Murphy, A. T. Pavia, E. M. Dale, N. Hellman, H. Huang, N. Parkin, R. A. Grosso, and R. Stevens, Abstr. 37th Infect. Dis. Soc. Am., abstr. 442, 1999; C. Vavro, C. McCarty, D. Shortino, and S. Hetherington, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. H-2052, 2002). There is evidence that selection of the M184V mutation does not preclude the use of didanosine in subsequent therapy (35). Table 1 presents a summary of the different mutations and mutational patterns associated with resistance to NRTIs and NtRTIs.
TABLE 1.
Characteristics of mutations associated with NRTI and NtRTI therapy
| Mutation | Effect on antiretrovirals |
|---|---|
| M184V | Primarily selected by 3TC and FTC in the clinical setting |
| Increases susceptibility to ZDV, d4T, and TDF | |
| Causes phenotypic resistance to 3TC and FTC | |
| ABC and ddl remain active in the presence of M184V alone although there are increases in the 50% inhibitory concentration these agents of compared to those for wild-type virus | |
| Delays or prevents the emergence of TAMs | |
| Partially reverses loss of susceptibility due to selection of TAMs | |
| K65R | Primarily selected by TDF in the clinical setting, although it may be selected with ABC-containing regimens |
| Associated with loss of susceptibility to TDF, ABC, and ddl | |
| Increases susceptibility to ZDV and d4T | |
| May be prevented by the presence of TAMs or ZDV | |
| L74V | Primarily selected by ddl and ABC in the clinical setting |
| Reduces susceptibility to ddl and ABC | |
| Does not significantly reduce the activity of TDF | |
| Suppresses ZDV resistance resulting from the T215Y mutation | |
| Prevented by the presence of ZDV in the regimen | |
| 41, 210, 215 (TAM pathway) | The most common TAM pathway |
| Primarily selected by ZDV and d4T in the clinical setting, although ZDV more efficiently selects for TAMs than d4T | |
| Presence of multiple mutations in this pathway causes variable degrees of resistance to all NRTIs and NtRTIs | |
| 67, 70, 215 (TAM pathway) | A less common TAM pathway |
| Primarily selected by ZDV and d4T in the clinical setting, although ZDV more efficiently selects for TAMs than d4T | |
| Presence of multiple mutations in this pathway causes variable resistance to all NRTIs and NtRTIs but is considered to cause less nucleoside and nucleotide class resistance than the TAM pathway consisting of mutations at codons 41, 210, and 215 | |
| Multinucleoside resistance mutations (Q151M, T69 insertions) | The Q151M complex includes mutations at codons 75, 77, and 116, and is primarily selected by suboptimal regimens containing ddl with ZDV or d4T |
| The Q151M complex demonstrates significant resistance to most NRTIs but not TDF | |
| The T69 insertion mutations occur primarily in patients with preexisting resistance mutations and significant treatment experience with NRTIs | |
| In combination with TAMs, T69 insertions confer resistance to ZDV, TDF, d4T, and ddl |
K65R: AN EMERGING MUTATION THAT CONFERS CLASS RESISTANCE
K65R prevalence.
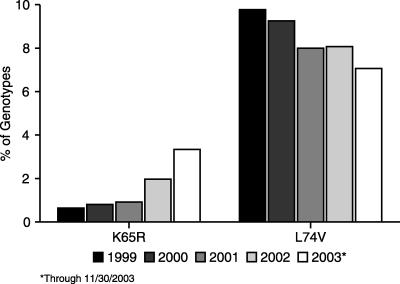
K65R is selected by TDF, ABC, didanosine (ddI), zalcitabine (ddC), and, in rare cases, d4T. The mutation confers variable phenotypic resistance to all NRTIs, excluding ZDV and, interestingly, d4T (11, 28, 30). Several large studies of HIV-1 isolates submitted for genotypic resistance testing revealed a three- to fourfold increase in the prevalence of K65R in the past several years. In one study, analysis of more than 80,000 genotypes showed that K65R had increased in prevalence from 0.6% in 1999 to 3.3% in 2003 (Fig. 1) (Faruki et al., Abstr. XIII Int. HIV Drug Resist. Workshop). Another study, in which approximately 60,000 samples were examined, found that K65R increased in frequency from 0.8% in 1998 to 3.8% in 2003 (34; U. Parikh, D. Koontz, J. Hammond, L. Bacheler, R. Schinazi, P. Meyer, W. Scott, and J. Mellors, Abstr. XII Int. HIV Drug Resist. Workshop, abstr. 136, 2003). Finally, an analysis of 900 genotypes determined at two large U.S. academic hospitals found a doubling in the prevalence of K65R between 2001 and 2003 (MacArthur et al., Int. AIDS Soc. 2nd Conf. Pathogen. Treatment). While K65R remains a relatively uncommon mutation, the risk of multinucleoside resistance associated with this substitution is substantial, and clinical vigilance is needed to prevent its emergence.
FIG. 1.
Prevalence of K65R and L74V mutations among genotypes of more than 80,000 isolates submitted to a commercial laboratory for resistance testing. Reprinted with permission (H. Faruki, J. Sebastian, J. Scott, J. Stamp, and E. R. Lanier, unpublished data).
K65R interaction with M184V.
The K65R mutation has been shown to decrease the susceptibility of HIV-1 to ABC (3.0-fold) and TDF (2.1-fold) compared to that of the wild-type virus, but the clinical implications of these reductions are unclear (15). When both K65R and M184V are present, the susceptibility of HIV-1 to ABC is further decreased, while susceptibility to TDF is increased relative to that for virus that contains K65R alone (15, 30, 33). When K65R is present alone in recombinant HIV-1, it results in a lower decrease in the level of binding to RT by the active moiety of ABC (2-fold), whereas binding by the active form of TDF is diminished by 6.7-fold (P < 0.001 compared with the results for the wild type). In contrast, when the M184V and K65R mutations are both present, the binding of the active phosphorylated forms of both ABC and TDF are diminished by 4.5- and 5.3-fold, respectively (30, 33). Hence, the M184V mutation appears to moderate some of the effects of K65R on TDF susceptibility. In the presence of both the K65R and the M184V mutations, as opposed to K65R alone, there is less efficient enzyme binding of natural nucleotide substrates, which possibly allows TDF to compete more effectively for incorporation into elongating chains of viral DNA.
In addition to inhibiting drug binding and incorporation, RT mutations may also confer resistance by increasing the rate of chain terminator excision, which is the primary mechanism of resistance by TAMs. The degree of ATP-mediated removal of TDF or ddI chain-terminated primers with K65R was found to be minimal, while a moderate degree of ATP-mediated excision occurred in the case of ABC (33). The velocity of chain terminator removal with ABC in the presence of K65R was observed to be less than half that observed for TAM-mediated excision of ZDV. However, the clinical significance of these findings is not clear because of both the modest levels of excision and the fact that the velocity of excision is substantially influenced by experimental conditions. Nevertheless, these findings suggest that K65R may confer resistance to different drugs through distinct mechanisms.
K65R is antagonized by TAMs and is suppressed by the use of thymidine analogs.
Although the K65R mutation arises most commonly following treatment with ABC, TDF, and ddI, there is increasing evidence to suggest that its presence may be antagonized by the earlier presence of TAMs. Viral isolates containing K65R remain susceptible to ZDV, and enhanced susceptibility to ZDV can be contributed to additively by both the K65R and the M184V mutations (33). A strong negative correlation between ZDV resistance and the presence of K65R was observed in a study of clinical isolates submitted for resistance testing, which also showed that HIV-1 variants containing K65R exhibited 2.5- to 10-fold reduced susceptibilities to all commonly used NRTIs, with the exception of those possessing a 3′-azido moiety, such as ZDV (Parikh et al., Abstr. XII Int. HIV Drug Resist. Workshop). Among clinical isolates with TAMs that were resistant to ZDV, the simultaneous presence of K65R reduced the resistance to ZDV by about 10-fold. Experiments have been performed to analyze the effects of both ZDV resistance mutations as well as mutations that suppress ZDV resistance while a patient is receiving TDF. The results demonstrate that the ATP-mediated excision of the TDF-blocked primer template is reduced in the presence of the ZDV-suppressive M184V, Y181C, and L100I mutations (N. H. Espil, A. Pavlova, T. Bergroth, A. Sonnergorg, and J. Lennerstrand, Abstr. 7th Int. Cong. Drug Ther. HIV Infect., abstr PL5.4, 2004). Further studies are needed to determine the likely resistance pathway of TDF resistance among patients with multiple ZDV mutations.
In a prospective study in the United Kingdom, the genotypes of isolates from 705 patients experiencing virologic treatment failure were determined between 2000 and 2002 (34). Patients receiving thymidine analogs at the time of virologic treatment failure were 76% less likely to have the K65R mutation than patients who were failing treatment and who were not receiving such drugs. Although this study did not specify the proportion of patients receiving d4T versus the proportion receiving ZDV, a retrospective study of nearly 1,000 genotypes from U.S. medical centers found that 70% of patients with K65R on an ABC-containing regimen were also taking d4T, whereas no one on the fixed-dose formulation of ABC-3TC-ZDV had developed K65R (MacArthur et al., Int. AIDS Soc. 2nd Conf. Pathogen. Treatment).
The suppressive effect of ZDV on K65R was also shown in an open-label study that examined the efficacy and safety of once-daily ABC-3TC-ZDV plus TDF treatment in 88 treatment-naive patients (R. Elion, C. Cohen, E. De Jesus, R. Redfield, J. Gathe, R. Hsu, L. Yau, L. Ross, B. Ha, R. Lanier, and J. Scott, Abstr 11th Conf. Retrovir. Opportunistic Infect., abstr. 53, 2004). At week 24, the genotypes of isolates from eight patients with HIV-1 RNA levels greater than 400 copies/ml were determined and revealed K65R in only one case. The inclusion of ZDV in a once-daily dosing regimen that also contained ABC, 3TC, and TDF did not lead to high rates of virologic treatment failure in the presence of K65R, but five of eight patients had TAMs with or without M184V. ZDV also appears to adequately suppress K65R when it is given once daily instead of at its Food and Drug Administration-approved dosage of twice daily, although further study of the effects of taking ZDV once daily within a quadruple regimen are needed to determine the risks and benefits of this strategy. It should be noted, in contrast, that a triple-NRTI regimen consisting of ABC, 3TC, and TDF without ZDV is contraindicated, owing to the high rates of early virologic nonresponse and the selection of M184V and K65R (C. Farthing, H. Khanlou V. Yeh, G. Harris, Abstr. 2nd Int. AIDS Soc. Conf. HIV Pathogen., abstr. 43, 2003; J. E. Gallant, A. E. Rodriguez, W. Weinberg, B. Young, D. Berger, M. L. Lim, Q. Liao, L. Ross, J. Johnson, and M. S. Shafer, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother, abstr. H-1722a, 2003; R. Landman, G. Peytavin, D. Descamps, F. Brun Vezinet, H. Benech, A. Benaslisherif, A. Trylesinski, C. Katlama, P. M. Girard, F. Raffi, M. Yeni, M. Bentata, B. Jarrousse, C. Mishelet, and P. Flandre, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 52, 2004).
Incidence of K65R associated with TDF or ABC plus 3TC.
The overall rates of virologic treatment failure in patients receiving TDF or ABC with 3TC or FTC and a potent NNRTI are low. In contrast, there is a high rate of early virologic treatment failure in the presence of M184V and K65R among patients taking TDF with either ABC or ddI and a third NRTI. In tissue culture studies, exposure of HIV-1 to escalating doses of ABC alone selected for M184V, followed by mixtures of K65R, L74V, and Y115, whereas exposure to TDF alone or in combination with ABC or 3TC selected only K65R (28). The latter study also showed that TDF deselected the M184V mutation in the absence of 3TC or ABC.
In accordance with observations from tissue culture studies, the combination of TDF and 3TC with either ABC or ddI represents a low genetic barrier for the selection of drug resistance in the clinic. A retrospective analysis of more than 1,700 isolates submitted for resistance testing found that the use of ddI-TDF was significantly more common among patients who failed therapy and who developed K65R than among those in whom therapy failed without K65R (14.3% and 3.8%, respectively; P = 0.007) (34). A high rate of virologic treatment failure was also noted in a pilot study of a triple-NRTI regimen containing TDF, ddI, and 3TC (J. Jemsek, P. Hutcherson, and E. Harper, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 51, 2004). In the latter study, the genotypes of isolates from 12 patients with virologic treatment failure at week 12 revealed that 95% had M184V and 50% had K65R. A 48-week trial comparing once-daily ABC-3TC plus EFV versus once-daily ABC-3TC plus TDF was stopped when an interim analysis performed with samples collected after 16 weeks revealed that 49% of patients in the TDF arm failed to achieve virologic suppression (viral load, <50 copies/ml), whereas only 5% in the EFV arm failed to achieve virologic suppression (Gallant et al., 43rd ICAAC). All 36 patients in the TDF arm with early treatment failure had developed M184V by 16 weeks, while 23 of the 36 also had K65R at that time.
In a pivotal double-blind study, 600 treatment-naive patients were randomized to receive TDF or d4T with 3TC plus EFV. After 144 weeks, 47 patients receiving TDF and 49 patients receiving d4T had experienced virologic treatment failure, defined as plasma HIV-1 RNA levels exceeding 400 copies/ml (10). With regard to the mutational profiles, 2.7% and 0.6% of patients in the TDF and d4T arms, respectively, developed K65R (P = 0.06). The genotypes of those experiencing virologic treatment failure with TDF-3TC-EFV showed that 26 of 47 (55%) had developed resistance to EFV. Of these same 47 patients, 8 (17%) developed K65R (all by week 96); phenotypic susceptibility to TDF was decreased by 0.9- to 2.2-fold among these individuals. All eight patients in the TDF-3TC-EFV arm who developed K65R also manifested resistance to EFV, while five of the eight had M184V.
L74V: A COMMON MUTATION SELECTED BY DDI AND ABC
L74V is the mutation that is the most frequently selected by ddI monotherapy, but it remains rare after combination ART. L74V results in reduced susceptibility to ddI and ABC, but its effect on virologic suppression is dependent on what other mutations may also be present (27). L74V does not confer resistance to either ZDV, TDF, or d4T. As with K65R, M184V, and Q151M, the primary mechanism of resistance with L74V is discrimination against incoming nucleotide analogs (B. Selmi, J. Deval, J. Courcambeck, J. Feng, L. Rimsky, J. Boretto, S. R. Sarfati, C. Guerreiro, L. Mulard, and B. Canard, Abstr. HIV Conf. Drug Development Antiretrovir. Therapies, abstr. 15, 2002). The prevalence of L74V in genotyping reports has declined in recent years, most likely due to changes in standards of care from monotherapy and dual therapy to triple-combination regimens as well as the death of many patients who had been treated with substandard regimens (Fig. 1) (Faruki et al., Abstr. XIII Int. HIV Drug Resist. Workshop).
L74V diminishes viral replication capacity.
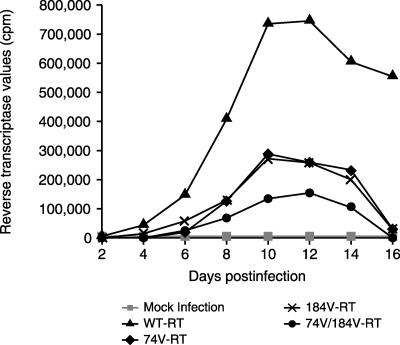
M184V and L74V each diminish viral fitness and increase susceptibility to ZDV. The M184V and L74V mutations individually impair DNA synthesis by RT, and these effects are additive when the two mutations occur together (Fig. 2) (8). The ability of L74V to compromise viral replication was also illustrated in a study of 76 patients who discontinued ddI and hydroxyurea after 1 year of maintenance therapy. During viral rebound, viruses containing the L74V mutation were overgrown by non-L74V viruses within 8 weeks, whereas patients who also possessed TAMs continued to display detectable viruses with L74V over long periods (6). Both L74V and K65R can impair viral replication, which may explain why this combination of mutations is not commonly seen in patients with HIV-1 infection (J. Deval, J.-M. Navarro, B. Selmi, J. Courcambeck, J. Boretto, P. Halfon, J. Sire, and B. Canard, Abstr. XII Int. HIV Drug Resist. Workshop, abstr. 35, 2003).
FIG. 2.
HIV-1 replication in viral clones after introduction of the L74V and M184V mutations. (Adapted from reference 8.)
Incidence of L74V in association with ABC.
A placebo-controlled clinical trial studied EFV together with ABC-3TC given either once or twice daily in 770 treatment-naive patients (B. Gazzard, E. DeJesus, P. Cahn, S. Castillo, H. Zhao, D. Gordon, W. Spreen, and T. Scott, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr H-1722b, 2003). Among 31 patients experiencing virologic treatment failure (10% and 8% of individuals in the once- and twice-daily ABC-3TC arms, respectively, after 48 weeks), less than half had enough HIV RNA for genotyping, i.e., 500 copies/ml. Among the patients whose isolates could be genotyped, M184V and K103N were the most commonly detected treatment-emergent mutations (48% and 45% of patients, respectively), and L74V was seen in 26% of individuals. Isolates from all patients whose therapy failed retained susceptibility to TDF, ZDV, and d4T; and isolates from 75% to 85% of patients retained susceptibility to both ABC and ddI. NNRTI-associated resistance was common among those experiencing virologic treatment failure (C. Craig, C. Stone, T. Bonny, H. Zhao, D. Gordon, S. Castillo, and D. Paes, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 551, 2004).
In an attempt to predict resistance to ABC in patients with mutations conferring resistance to ZDV and 3TC, one group examined the genotypic and phenotypic characteristics of 307 samples from patients with viral rebound (31). Low-level resistance to ABC was defined as a 2.5- to 5.5-fold increase in the 50% inhibitory concentration relative to that for the wild-type virus, and high-level resistance was defined as greater than 5.5-fold-reduced susceptibility to ABC. ABC has some clinical effect in the face of low-level resistance and very limited or no clinical effect in the face of high-level resistance. Twenty-four percent of isolates with low- and high-level resistance to ABC contained the L74V mutation, most in addition to M184V. TAMs were two to three times more likely to be present in isolates that were susceptible to ABC or that had low-level resistance to ABC. The best predictor of modest phenotypic resistance to ABC was the simultaneous presence of any two mutations at codons 41, 210, or 215 and 74 or the presence of the Q151M mutation and/or 69S insertions. High-level ABC resistance was best predicted by any three mutations at codons 41, 184, 210, and 215 and the presence of L74V or Q151M and/or 69S insertions. A slight decrease in susceptibility to ABC was seen in viral clones containing the E44D mutation, but neither E44D/A nor V118A was predictive of phenotypic resistance to ABC, and both of the last two mutations are probably selected in the context of ZDV resistance.
In one trial, analysis of the phenotypes of isolates from 180 patients exposed to ABC was used to predict the genotypic correlates of clinical resistance to ABC. Selection of any of four TAMs (M41L, D67N, L210W, or T215Y/F), as well as E44D or V118I, was associated with reduced responsiveness to ABC; and each of these mutations was present in at least 10% of patients. The virologic responses in patients with less than four mutations, four mutations, and more than four mutations were −1.64 log10 copies/ml, −0.69 log10 copies/ml, and −0.19 log10 copies/ml, respectively. The presence of two TAMs, K70R and K219Q/E, was not associated with a reduced response to ABC. The L74V and M184V mutations improved the predictive value of the genotype but were not associated with a decreased virologic response in a univariate analysis. The K65R and Y115F mutations, which are selected by ABC in vitro, were present in few patients (4). These findings suggest that L74V, M184V, and TAMs may contribute to ABC resistance and provide an important context within which to assess the potential roles of these mutations in the presence of resistance to ABC (23).
Thymidine analogs suppress L74V.
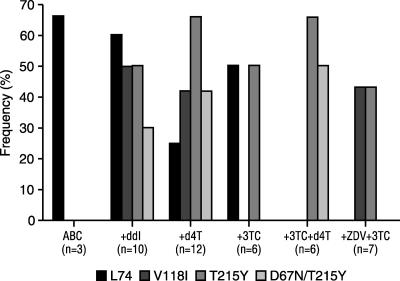
L74V is associated with increased sensitivity to ZDV and decreased sensitivity to ddI and ABC (14, 20, 27). As is the case for K65R, the selective pressure of thymidine analogs seems to reduce or prevent the occurrence of the L74V mutation (18). In one study, genotypes were examined in 44 patients in whom highly active ART failed with either ABC as the sole NRTI or ABC administered together with two or three additional NRTIs. The RT genotype profiles of the isolates from these patients varied considerably, depending on which other NRTIs these patients had received (M. Daeumer, N. Beerenwinkel, S. Sierra, J. Klein, J. Selbig, M. Oette, G. Faetkenheuer, J. Rockstroh, D. Hoffman, H. Pfeifer, and R. Kaiser, Abstr. 1st Euro. HIV Drug Resist. Workshop, abstr 1.15, 2003). Notably, the addition of d4T partially suppressed the emergence of L74V, while either d4T or ZDV together with 3TC seemed to protect completely against the development of L74V (Fig. 3). In another analysis, pooled data from several clinical trials were used to examine the incidence of L74V following the use of ABC as the sole NRTI or in combination with other NRTIs, PIs, or an NNRTI (M. Ait Khaled, E. R. Lanier, N. Richards, C. Stone, P. Griffin, D. M. Gibb, A. S. Walker, C. Craig, A. E. Loeliger, and M. Tisdale, Abstr XI Int. HIV Drug Resist. Workshop, abstr. 129, 2002). The results showed that L74V rarely developed in patients experiencing virologic treatment failure while they were receiving ABC-containing regimens that also included ZDV compared with the rate of development while they were receiving regimens that lacked ZDV (2% and 38%, respectively; P < 0.001). These data suggest that susceptibility to ZDV, and possibly to d4T as well, may be enhanced by L74V, resulting in the diminished selection of variants containing this mutation (27). An explanation for this result may be that the L74V mutation causes diminished excision of incorporated AZT monophosphate, even in the presence of TAMs (F. Frankel, D. Turner, B. Brenner, Y. Quan, and M. A. Wainberg, Abstr. XIII Int. HIV Drug Resist. Workshop, abstr. 27, 2004). This characteristic of diminished excision is shared together with M184V and K65R, which also hypersensitize HIV-1 to ZDV.
FIG. 3.
Frequencies of L74V, V118I, T215Y, and D67N/T215Y in patients with viral rebound on ABC-containing highly active ART regimens. Reprinted with permission (M. Daeumer, N. Beerenwinkel, S. Sierra, J. Klein, J. Selbig, M. Oette, G. Faetkenheuer, J. Rockstroh, D. Hoffman, H. Pfeifer, and R. Kaiser, unpublished data).
L74V AND K65R ARE RARE WITH POTENT TRIPLE REGIMENS
When TDF or ABC is used with 3TC or FTC and a potent PI, neither the K65R nor the L74V mutation is likely to emerge. In a pilot study of TDF-FTC in 190 patients also receiving lopinavir/ritonavir either once or twice daily, zero of five patients with virologic treatment failure at week 48 had either of these mutations (J. Gathe, D. Podzamczer, M. Johnson, R. Schwartz, V. Yeh, N. Travers, K. Luff, and S. Brun, Abstr. 11th Conf. Retrovir. Opportunistic Infect., abstr. 570, 2004). Another study examined the pooled results of two 48-week trials comparing ritonavir-boosted and unboosted fosamprenavir with nelfinavir, both of which were administered with ABC-3TC (S. MacManus, P. J. Yates, S. White, N. Richards, and W. Snowden, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 598, 2003). Of 898 patients exposed to ABC-3TC in both trials, 2 patients with virologic treatment failure had K65R and 3 patients with virologic treatment failure had L74V.
DISCUSSION: CLINICAL IMPLICATIONS OF EMERGING RESISTANCE PROFILES
The genotypic resistance profiles encountered in the clinic will continue to evolve in complexity as new antiretroviral agents are integrated into therapeutic strategies for the treatment of HIV-1 infection. Clinicians must be conscious of how new drugs and novel combinations might change the resistance landscape in terms of the prevalence of certain mutations, the interactions among mutations, and the consequences of mutations on second-line treatment options.
K65R causes some level of phenotypic resistance to both ABC and TDF, but any clinical difference in the relative effect of this mutation on ABC and TDF resistance remains unclear. Recent findings indicate that patients experiencing virologic treatment failure while they are receiving TDF-containing regimens select for K65R at higher rates than those experiencing virologic treatment failure while they are receiving ABC-containing regimens. The mutation diminishes TDF binding and incorporation into viral DNA, causing significant drug resistance. It is theorized that K65R may confer less resistance to ABC because of increased chain terminator excision activity that does not modify the affinity of the drug to RT. The presence of M184V may moderate K65R-mediated resistance to TDF, but M184V increases the level of resistance to ABC by diminishing the affinity of the active form of ABC for RT.
The ability of ZDV to suppress K65R and L74V has been documented in tissue culture and clinical studies (2, 27). K65R can destabilize the alignment of incoming phosphates, and L74V constrains nucleotides through displacement of the DNA template (Selmi et al., Abstr. HIV DART). Despite these subtle differences in the effects of K65R and L74V, both can alter important sites that are involved in RT catalysis and may interfere with primer unblocking, the predominant mechanism of ZDV and d4T resistance. K65R and L74V have been shown to impede ATP-dependent excision of AZT monophosphate from the growing DNA chain (Frankel et al., Abstr. XIII Int. HIV Drug Resist. Workshop; Parikh et al., Abstr. XII Int. HIV Drug Resist. Workshop). The potential clinical benefit of exploiting these antagonisms needs to be studied in controlled clinical trials.
Long-term success with ART depends, in part, on the use of drug combinations that circumvent the likelihood of multiclass resistance. Accumulating resistance data provide insights for making decisions about which drugs to use in initial versus later therapeutic regimens and the avoidance of the use of combinations of NRTIs that may present an unacceptably low barrier for the emergence of resistance. Maintaining a high level of potency with an NNRTI or PI may help prevent the occurrence of the multinucleoside resistance patterns associated with TAMs, K65R, or L74V. A patient who developed K65R in the aftermath of a failed TDF-based regimen would also likely be resistant to ABC and ddI. A patient who developed L74V in the aftermath of a failed ABC-based regimen would likely be resistant to ddI. The coadministration of ZDV with ABC or TDF may prevent the emergence of both K65R and L74V. Further studies are needed in this area.
Several explanations regarding the early treatment failure observed with regimens containing TDF together with either ABC or ddI have been explored. Drug-drug interactions have been identified for TDF and ddI at the plasma level that requires a ddI dose reduction from 400 mg once a day to 250 mg once a day (250 mg to 200 mg in patients weighing <60 kg), but this interaction has not been linked to higher rates of treatment failure (J. Flaherty, B. Kearney, and J. Wolf, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-1729, 2001). In one study, TDF was shown to decrease the cellular degradation of ddI (24), although this was not confirmed in a second study (J. Rodman, G. Robbins, and A. Fridland, Abstr. 6th Int. Cong. Drug Ther. HIV Infect., abstr. P108, 2002). The effects of this potential intracellular interaction have not been fully explored. Studies to date have found no plasma or intracellular interaction between TDF and ABC (T. Hawkins, W. Veikley, R. St. Claire, A. Hey, B. Guyer, and B. P. Kearney, Abstr. 5th Int. Workshop Clin. Pharm. HIV Ther., abstr 2.4, 2004; B. Kearney, E. Isaacson, J. Sayre, R. Ebrahimi, and A. Cheng, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr A-1615, 2003; A. Ray, J. Vela, L. Olson, G. Eisenberg, and A. Fridland, Abstr. 5th Int. Workshop Clin. Pharm. HIV Ther., abstr 2.3, 2004).
The inclusion of ZDV with ABC, 3TC, and TDF as quadruple therapy appears to result in a greater degree of viral suppression than that seen with the suboptimal triple combination of ABC, 3TC, and TDF, although these regimens have not been directly compared. It is conceivable that the inclusion of ZDV with ABC, 3TC, and TDF provided sufficient antiviral coverage to prevent selection of the K65R substitution. A possible reason for the early virologic nonresponsiveness with certain of these triple-NRTI regimens is a low genetic barrier to resistance on the part of the combination that allows the rapid emergence of M184V with or without K65R. The combination of TDF and ddI or TDF and ABC, either in a triple-NRTI regimen or as the dual-NRTI backbone in combination with a PI or NNRTI, are currently not recommended. In contrast, the 3TC and ABC combination and the 3TC and TDF combination seem to be effective NRTI backbones when they are used together with an NNRTI or a PI. Triple-NRTI regimens containing ZDV continue to play an important role in ART as an alternative to PI- and NNRTI-containing regimens as initial therapy, as simplified therapy, and in some quadruple regimens (5, 21, 25, 29; J. A. Bartlett, J. Johnson, G. Herrera, N. Sosa, A. E. Rodriguez, and M. S. Shaefer, Abstr. XIV Int. AIDS Conf., abstr. TuOrB1189, 2002; M. Markowitz, J. M. Lang, E. DeJesus, C. Hill-Zabala, E. R. Lanier, Q. Liao, K. Pappa, and M. Shaefer, Abstr. Int. AIDS Soc. 2nd Conf. Pathogen. Treatment, abstr. 42, 2003). Evidence also suggests that a triple-NRTI regimen that includes ZDV remains a rational treatment approach for many patients, especially those who initiate therapy with low viral loads (Ait Khaled et al., Abstr. XI Int. HIV Drug Res. Workshop; Melby et al., Abstr. 8th Conf. Retrov. Opportunistic Infect.). Several studies have suggested that the triple combination of ZDV, ABC, and 3TC is less potent than two NRTIs plus either a PI or an NNRTI (12, 25, 29). Although the difference in potency between PIs and triple NRTIs may often be overcome by an adherence advantage among patients with lower baseline viral loads, this may not be the case when regimens consisting of triple NRTIs are compared with those containing EFV and two NRTIs (12, 25, 29).
Because NRTI-NtRTI combinations are the backbone of virtually all therapeutic regimens, knowledge of mutational profiles and their impacts is essential to the formulation of durably suppressive regimens that preserve future therapeutic options. These issues, in addition to individual patient factors, tolerability, convenience, and pill burden, will remain vital considerations in the treatment of HIV-infected individuals.
Acknowledgments
Research in our laboratory is supported by grants from the Canadian Institutes of Health Research (CIHR) and the Canadian Foundation for AIDS Research and by a generous donation from Diane and Aldo Bensadoun.
REFERENCES
- 1.Anonymous. 29 October 2004. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/default_db2.asp?id=50. Accessed 26 November 2004. U.S. Department of Health and Human Services, Washington, D.C.
- 2.Bazmi, H. Z., J. L. Hammond, S. C. Cavalcanti, C. K. Chu, R. F. Schinazi, and J. W. Mellors. 2000. In vitro selection of mutations in the human immunodeficiency virus type 1 reverse transcriptase that decrease susceptibility to (−)-β-d-dioxolane-guanosine and suppress resistance to 3′-azido-3′-deoxythymidine. Antimicrob. Agents Chemother. 44:1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British HIV Association. 2003. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 4(Suppl. 1):1-41. [PubMed] [Google Scholar]
- 4.Brun-Vezinet, F., D. Descamps, A. Ruffault, B. Masquelier, V. Calvez, G. Peytavin, F. Telles, L. Morand-Joubert, J. L. Meynard, M. Vray, and D. Costagliola. 2003. Clinically relevant interpretation of genotype for resistance to abacavir. AIDS 17:1795-1802. [DOI] [PubMed] [Google Scholar]
- 5.Clumeck, N., F. Goebel, W. Rozenbaum, J. Gerstoft, S. Staszewski, J. Montaner, M. Johnson, B. Gazzard, C. Stone, R. Athisegaran, and S. Moore. 2001. Simplification with abacavir-based triple nucleoside therapy versus continued protease inhibitor-based highly active antiretroviral therapy in HIV-1-infected patients with undetectable plasma HIV-1 RNA. AIDS 15:1517-1526. [DOI] [PubMed] [Google Scholar]
- 6.de Mendoza, C., E. Paxinos, P. Barreiro, N. Camino, M. Nunez, and V. Soriano. 2004. Different viral rebound following discontinuation of antiretroviral therapy in cases of infection with viruses carrying L74V or thymidine-associated mutations. J. Clin. Microbiol. 42:862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Descamps, D., P. Flandre, V. Calvez, G. Peytavin, V. Meiffredy, G. Collin, C. Delaugerre, S. Robert-Delmas, B. Bazin, J. P. Aboulker, G. Pialoux, F. Raffi, F. Brun-Vezinet, et al. 2000. Mechanisms of virologic failure in previously untreated HIV-infected patients from a trial of induction-maintenance therapy. JAMA 283:205-211. [DOI] [PubMed] [Google Scholar]
- 8.Diallo, K., B. Marchand, X. Wei, L. Cellai, M. Gotte, and M. A. Wainberg. 2003. Diminished RNA primer usage associated with the L74V and M184V mutations in the reverse transcriptase of human immunodeficiency virus type 1 provides a possible mechanism for diminished viral replication capacity. J. Virol. 77:8621-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer, E., D. Podzamczer, M. Arnedo, E. Fumero, P. McKenna, A. Rinehart, J. L. Perez, M. J. Barbera, T. Pumarola, J. M. Gatell, and F. Gudiol. 2003. Genotype and phenotype at baseline and at failure in human immunodeficiency virus-infected antiretroviral-naive patients in a randomized trial comparing zidovudine and lamivudine plus nelfinavir or nevirapine. J. Infect. Dis. 187:687-690. [DOI] [PubMed] [Google Scholar]
- 10.Gallant, J. E., S. Staszewski, A. L. Pozniak, E. DeJesus, J. M. Suleiman, M. D. Miller, D. F. Coakley, B. Lu, J. J. Toole, and A. K. Cheng. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191-201. [DOI] [PubMed] [Google Scholar]
- 11.Gu, Z., E. J. Arts, M. A. Parniak, and M. A. Wainberg. 1995. Mutated K65R recombinant reverse transcriptase of human immunodeficiency virus type 1 shows diminished chain termination in the presence of 2′,3′-dideoxycytidine 5′-triphosphate and other drugs. Proc. Natl. Acad. Sci. USA 92:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulick, R. M., H. J. Ribaudo, C. M. Shikuma, S. Lustgarten, K. E. Squires, W. A. Meyer, E. P. Acosta, B. R. Schackman, C. D. Pilcher, R. L. Murphy, W. E. Maher, M. D. Witt, R. C. Reichman, S. Snyder, K. Klingman, and D. R. Kuritzkes. 2004. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N. Engl. J. Med. 350:1850-1861. [DOI] [PubMed] [Google Scholar]
- 13.Havlir, D. V., N. S. Hellmann, C. J. Petropoulos, J. M. Whitcomb, A. C. Collier, M. S. Hirsch, P. Tebas, J. P. Sommadossi, and D. D. Richman. 2000. Drug susceptibility in HIV infection after viral rebound in patients receiving indinavir-containing regimens. JAMA 283:229-234. [DOI] [PubMed] [Google Scholar]
- 14.Kuritzkes, D. R., J. B. Quinn, S. L. Benoit, D. L. Shugarts, A. Griffin, M. Bakhtiari, D. Poticha, J. J. Eron, M. A. Fallon, and M. Rubin. 1996. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS 10:975-981. [DOI] [PubMed] [Google Scholar]
- 15.Lanier, E., N. Givens, C. Stone, P. Griffin, D. Gibb, S. Walker, M. Tisdale, D. Irlbeck, M. Underwood, M. St. Clair, and M. Ait-Khaled. 2004. Effect of concurrent zidovudine use on the resistance pathway selected by abacavir-containing regimens. HIV Med. 5:394-399. [DOI] [PubMed] [Google Scholar]
- 16.Larder, B. A., S. Bloor, S. D. Kemp, K. Hertogs, R. L. Desmet, V. Miller, M. Sturmer, S. Staszewski, J. Ren, D. K. Stammers, D. I. Stuart, and R. Pauwels. 1999. A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance. Antimicrob. Agents Chemother. 43:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 18.Larder, B. A., A. Kohli, S. Bloor, S. D. Kemp, P. R. Harrigan, R. T. Schooley, J. M. Lange, K. N. Pennington, M. H. St. Clair, and the Protocol 34,225-02 Collaborative Group. 1996. Human immunodeficiency virus type 1 drug susceptibility during zidovudine (AZT) monotherapy compared with AZT plus 2′,3′-dideoxyinosine or AZT plus 2′,3′-dideoxycytidine combination therapy. J. Virol. 70:5922-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire, M., M. Gartland, S. Moore, A. Hill, M. Tisdale, R. Harrigan, and J. P. Kleim. 2000. Absence of zidovudine resistance in antiretroviral-naive patients following zidovudine/lamivudine/protease inhibitor combination therapy: virological evaluation of the AVANTI 2 and AVANTI 3 studies. AIDS 14:1195-1201. [DOI] [PubMed] [Google Scholar]
- 20.Miller, V., M. Ait-Khaled, C. Stone, P. Griffin, D. Mesogiti, A. Cutrell, R. Harrigan, S. Staszewski, C. Katlama, G. Pearce, and M. Tisdale. 2000. HIV-1 reverse transcriptase (RT) genotype and susceptibility to RT inhibitors during abacavir monotherapy and combination therapy. AIDS 14:163-171. [DOI] [PubMed] [Google Scholar]
- 21.Opravil, M., B. Hirschel, A. Lazzarin, H. Furrer, J. P. Chave, S. Yerly, L. R. Bisset, M. Fischer, P. Vernazza, E. Bernasconi, M. Battegay, B. Ledergerber, H. Gunthard, C. Howe, R. Weber, and L. Perrin. 2002. A randomized trial of simplified maintenance therapy with abacavir, lamivudine, and zidovudine in human immunodeficiency virus infection. J. Infect. Dis. 185:1251-1260. [DOI] [PubMed] [Google Scholar]
- 22.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 23.Petrella, M., and M. A. Wainberg. 2002. Might the M184V mutation in HIV-1 RT confer clinical benefit? AIDS Rev. 4:224-233. [PubMed] [Google Scholar]
- 24.Ray, A. S., L. Olson, and A. Fridland. 2004. Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob. Agents Chemother. 48:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staszewski, S., P. Keiser, J. Montaner, F. Raffi, J. Gathe, V. Brotas, C. Hicks, S. M. Hammer, D. Cooper, M. Johnson, S. Tortell, A. Cutrell, D. Thorborn, R. Isaacs, S. Hetherington, H. Steel, and W. Spreen. 2001. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: a randomized equivalence trial. JAMA 285:1155-1163. [DOI] [PubMed] [Google Scholar]
- 26.Staszewski, S., J. Morales-Ramirez, K. T. Tashima, A. Rachlis, D. Skiest, J. Stanford, R. Stryker, P. Johnson, D. F. Labriola, D. Farina, D. J. Manion, N. M. Ruiz, et al. 1999. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N. Engl. J. Med. 341:1865-1873. [DOI] [PubMed] [Google Scholar]
- 27.St. Clair, M. H., J. L. Martin, G. Tudor-Williams, M. C. Bach, C. L. Vavro, D. M. King, P. Kellam, S. D. Kemp, and B. A. Larder. 1991. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science 253:1557-1559. [DOI] [PubMed] [Google Scholar]
- 28.Stone, C., M. Ait-Khaled, C. Craig, P. Griffin, and M. Tisdale. 2004. Human immunodeficiency virus type 1 reverse transcriptase mutation selection during in vitro exposure to tenofovir alone or combined with abacavir or lamivudine. Antimicrob. Agents Chemother. 48:1413-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vibhagool, A., P. Cahn, M. Schechter, et al. 2004. Triple nucleoside treatment with abacavir plus the lamivudine/zidovudine combination tablet (COM) compared to indinavir/COM in antiretroviral therapy-naive adults: results of a 48-week open-label, equivalence trial (CNA3014). Curr. Med. Res. Opin. 20:1103-1114. [DOI] [PubMed] [Google Scholar]
- 30.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 31.Walter, H., B. Schmidt, M. Werwein, E. Schwingel, and K. Korn. 2002. Prediction of abacavir resistance from genotypic data: impact of zidovudine and lamivudine resistance in vitro and in vivo. Antimicrob. Agents Chemother. 46:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitcomb, J. M., N. T. Parkin, C. Chappey, N. S. Hellmann, and C. J. Petropoulos. 2003. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J. Infect. Dis. 188:992-1000. [DOI] [PubMed] [Google Scholar]
- 33.White, K. L., N. A. Margot, T. Wrin, C. J. Petropoulos, M. D. Miller, and L. K. Naeger. 2002. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R + M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 46:3437-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winston, A., A. Pozniak, S. Mandalia, B. Gazzard, D. Pillay, and M. Nelson. 2004. Which nucleoside and nucleotide backbone combinations select for the K65R mutation in HIV-1 reverse transcriptase? AIDS 18:949-951. [DOI] [PubMed] [Google Scholar]
- 35.Winters, M. A., R. J. Bosch, M. A. Albrecht, and D. A. Katzenstein. 2003. Clinical impact of the M184V mutation on switching to didanosine or maintaining lamivudine treatment in nucleoside reverse-transcriptase inhibitor-experienced patients. J. Infect. Dis. 188:537-540. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, D., A. M. Caliendo, J. J. Eron, K. M. DeVore, J. C. Kaplan, M. S. Hirsch, and R. T. D'Aquila. 1994. Resistance to 2′,3′-dideoxycytidine conferred by a mutation in codon 65 of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 38:282-287. [DOI] [PMC free article] [PubMed] [Google Scholar]