Abstract
Samples of effluent and soil were collected from a reed bed system used to remediate liquid waste from a wool finishing mill with a high use of quaternary ammonium compounds (QACs) and were compared with samples of agricultural soils. Resistance quotients of aerobic gram-negative and gram-positive bacteria to ditallowdimethylammomium chloride (DTDMAC) and cetyltrimethylammonium bromide (CTAB) were established by plating onto nutrient agar containing 5 μg/ml or 50 μg/ml DTDMAC or CTAB. Approximately 500 isolates were obtained and screened for the presence of the intI1 (class 1 integrase), qacE (multidrug efflux), and qacEΔ1 (attenuated qacE) genes. QAC resistance was higher in isolates from reed bed samples, and class 1 integron incidence was significantly higher for populations that were preexposed to QACs. This is the first study to demonstrate that QAC selection in the natural environment has the potential to coselect for antibiotic resistance, as class 1 integrons are well-established vectors for cassette genes encoding antibiotic resistance.
Integrons are recombination and expression systems that capture genes as part of a genetic element known as a gene cassette (33). Most cassettes with known functions confer antibiotic or quaternary ammonium compound (QAC) resistance.
QAC resistance genes fall into two families. The qacA/B genes belong to the major facilitator superfamily and are only found in staphylococci on multiresistance plasmids (30). Other QAC resistance genes belong to the small multidrug resistance family and include qacC/D, now known as smr, qacE, qacEΔ1, qacF, qacG, qacH, and qacJ (3, 7, 15-17, 21, 23, 31, 32). qacE, qacEΔ1, qacF, and qacG have been identified on integrons, and the remaining genes have been identified on multiresistance plasmids in staphylococci (qacG is unusual in that it has been reported to be carried by staphylococcal multiresistance plasmids and class 1 integrons in gram-negative bacteria).
Class 1 integrons consist of a 5′ conserved region consisting of an integrase gene encoding a site-specific recombinase (42), an attI site (28) where cassettes are integrated, and a promoter, Pant, that regulates the expression of gene cassettes (10). Gene cassettes contain a protein coding region and a recombination site known as a 59-be site which is responsible for the orientation of integration (9). The variable regions of class 1 integrons contain the cassette genes, to the right of which lies the 3′ conserved region, which may have one of three different backbone structures (27). The first backbone type consists of a Tn402 (In16)-like arrangement consisting of a tni module containing three transposition genes and a resolvase gene. The second, In5 type consists of qacEΔ1, sul1, orf5, orf6, and a partial tni module, tniΔ, consisting of two transposition genes. The third, In4 type carries just qacEΔ1, sul1, orf5, and orf6. Integrons carrying the complete tni module are able to undergo self-transposition, and it is thought that the In5 and In4 types may also be able to move if the tni gene products are supplied in trans (27).
The role of class 1 integrons in conferring antibiotic resistance to clinical isolates of many bacterial strains is well documented (5, 14, 22, 38, 44). Studies of the incidence of class 1 integrons in bacterial pathogens associated with agriculture and fish farming, such as Escherichia coli and Aeromonas salmonicida, have also shown a link between integrons and antibiotic resistance (2, 41). Studies of the incidence of integrons in environmental bacteria have been undertaken by Rosser and Young (34), who studied the incidence of class 1 integrons in isolates from the Tay estuary, where 3.6% of isolates carried class 1 integrons. Nield and coworkers (25) identified three new integron classes in total community DNAs extracted from Australian soils by using primers for a conserved region of the integrase gene and the 59-be site. A similar approach using degenerate primers targeting the conserved regions of 59-be sites identified 164 gene cassettes, with the majority showing no relationship to known sequences.
Antibiotic and QAC resistance genes, e.g., qacE, are both carried on class 1 integrons, so selection for QAC resistance may cause coselection for antibiotic resistance. This provides a potential reservoir of antibiotic-resistant bacteria in QAC-polluted environments. In staphylococci, the qacA/B genes carried on multiresistance plasmids confer a low-level resistance to chlorhexidine and QACs, and it has been suggested that the introduction of chlorhexidine into clinical environments has resulted in the selection of staphylococci containing qacA carried on multiresistance plasmids (35).
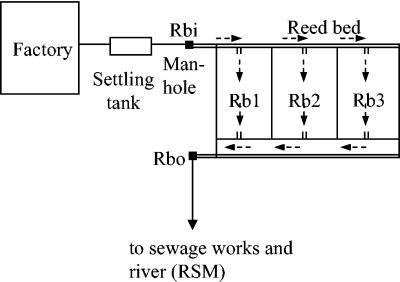
For this study, resistance to ditallowdimethylammomium chloride (DTDMAC) and cetyltrimethylammonium bromide (CTAB) was assessed in environmental bacteria. Bacteria were isolated from a reed bed system (Fig. 1) used to remediate effluent from a textile mill with high QAC usage (wool finishing effluent only) and also from control sites with low QAC exposure. The incidence of class 1 integrons was also investigated to test the hypothesis that QAC exposure coselects for antibiotic resistance by selecting for class 1 integrons.
FIG. 1.
Diagram of mill and reed bed system. Rbi, reed bed inlet; RB1 to RB3, reed beds 1 to 3; Rbo, reed bed outlet.
MATERIALS AND METHODS
Sample collection.
Samples were taken from a textile mill in Yorkshire, including effluent leaving the mill, core samples from reed beds used for remediation, reed bed outflow, and river sediment downstream of the sewage farm outfall to which the effluent was discharged (Fig. 1). The workload at the mill was highly variable, as was the type of cloth treated; consequently, the nature of the effluent was highly variable. A detailed breakdown of the compounds used is not available, although a variety of QACs were used as scouring agents, antistatic agents (used at 0.1 to 0.2% of the fabric weight), dyeing retardants, softeners, finishers, moth-proofers, etc. Control soil samples were taken from agricultural land in Warwick, the Cotswolds, and Droitwich.
Isolation.
Viable counts were carried out in triplicate on nutrient agar (NA) containing 5 μg/ml or 50 μg/ml CTAB or DTDMAC or with no selection; all plates contained 50 μg/ml cycloheximide to reduce fungal growth. Plates were incubated at 26°C. Forty to 50 colonies encompassing the range of colony morphologies observed were picked for each sample and streaked onto NA plates.
PCR.
Isolates were grown overnight in nutrient broth at 26°C, and phenol-chloroform extraction was performed. DNA concentrations were determined by measuring the absorbance of the sample at 260 nm on a spectrophotometer and were standardized to 100 ng/μl. intA- and intB-specific primers (34) were used to amplify the class 1 integrase gene, and specific primers were used to amplify the qacE and qacEΔ1 genes (19). Integron-positive isolates and approximately 20 additional isolates from both contaminated and control sites were identified by PCR amplification and sequencing of 16S rRNAs by the use of pA-pH primers (11). Standard PCR conditions were used, as described by Rosser and Young (34) and Kazama et al. (19). Confirmation of the presence of qacE on a class 1 integron was achieved by using CASS1/CASS2 primers (34), which amplify the variable region of integrons priming the intI1 and qacE or qacEΔ1 genes. PCR products were detected by electrophoresis in a 1.5% agarose gel, sequenced by the use of Big Dye Terminator, version 3.1, chemistry (Applied Biosystems), and run on a 3100 genetic analyzer. To aid in the identification of some isolates, we used an API 20NE test kit (bioMerieux) according to the manufacturer's instructions, incubating the bacteria at 30°C. The identification of strains was carried out according to the instructions in the API 20NE identification manual.
Statistical analysis.
Plate counts were expressed as resistance quotients (RQs), for which the counts with selection were expressed as percentages of the counts with no selection. Differences in class 1 integrase gene frequencies between the polluted and control isolates were tested for significance by use of a chi-square test for comparisons of two proportions (from independent samples).
RESULTS
Plate counts.
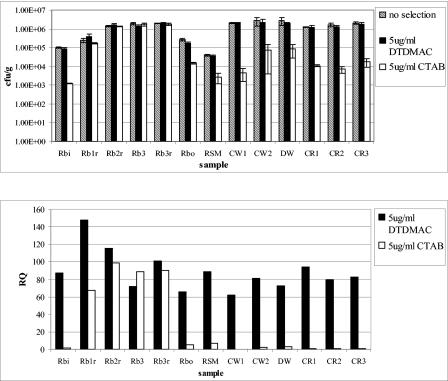
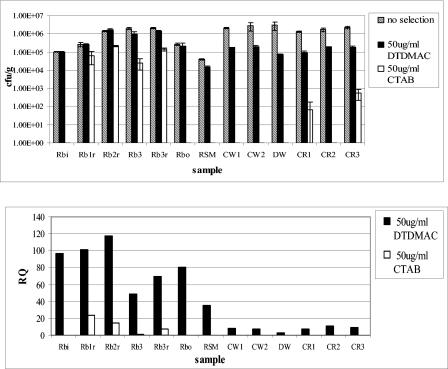
Viable plate counts and RQs for all samples inoculated onto QAC-selective plates at 5 μg and 50 μg/ml are shown in Fig. 2 and 3, respectively. Most culturable bacteria appeared resistant to 5 μg/ml DTDMAC, with RQs varying from 62% for Cotswold soil sample 1 (CW1) to 147% for reed bed sample 1 (RB1). RQs were marginally higher for the mill samples overall, with three reed bed samples yielding RQs of >100%. For selection with 50 μg/ml DTDMAC, bacteria from the control samples were much more sensitive than those selected with 5 μg/ml DTDMAC, with RQs between 2.5 and 11%, and the mill samples had RQs of 49 to 117%, which were slightly lower than those at 5 μg/ml DTDMAC. The RQ for the river sediment sample (RSM) taken downstream of the sewage outfall into which the mill effluent discharges was intermediate between those of contaminated and control samples. Isolates from the river sample showed less resistance than mill samples but more resistance than isolates from agricultural soils at 50 μg/ml DTDMAC.
FIG. 2.
Relative resistance to DTDMAC and CTAB at 5 μg/ml. (Top) Viable plate counts, with error bars giving standard deviations. (Bottom) RQs showing percentages of resistant bacteria in each sample. Rbi, reed bed inlet; RB1 to RB3, reed beds 1 to 3; r, root-associated sample material; Rbo, reed bed outlet; RSM, river sediment downstream of outfall; CW1 and CW2, Cotswold farmland soil; DW, Droitwich farmland soil; CR1 to CR3, Warwickshire farmland soil.
FIG. 3.
Relative resistance to DTDMAC and CTAB at 50 μg/ml. (Top) Viable plate counts, with error bars giving standard deviations. (Bottom) RQs showing percentages of resistant bacteria in each sample. Rbi, reed bed inlet; RB1 to RB3, reed beds 1 to 3; r, root-associated sample material; Rbo, reed bed outlet; RSM, river sediment downstream of outfall; CW1 and CW2, Cotswold farmland soil; DW, Droitwich farmland soil; CR1 to CR3, Warwickshire farmland soil (Cryfield).
Resistance to CTAB at 5 μg/ml was low, with high RQs limited to the reed bed samples, ranging from 67% to 99%. Control samples all had RQs of <3%. At the higher concentration for CTAB selection, virtually all culturable bacteria were sensitive, with the exception of reed bed samples, which had RQs of 1.3 to 23%.
PCR screening.
The results of screening for intI1, the integrase gene carried on class 1 integrons, are shown in Table 1. For isolates from contaminated samples (including river sediment), 7.98% were integron positive, in contrast to 0% of control samples, which was a statistically significant difference, with a chi-square value of 19.604 and a P value of <0.0001 when a comparison of proportions test was applied. When restricted to the reed bed samples, the incidence increased to 14.9%, which was also significantly different from that of the control samples.
TABLE 1.
Incidence of intI1, qacE, and qacEΔ1
| Sample | Value for polluted site
|
Total for polluted sites | Value for control site
|
Total for control sites | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rbi | RB1r | RB2r | RB3 | RB3r | Rbo | RSM | CW1 | CW2 | DW | CR1 | CR2 | CR3 | |||
| Total no. of isolates | 38 | 25 | 30 | 40 | 32 | 43 | 30 | 238 | 49 | 50 | 42 | 40 | 41 | 40 | 262 |
| No. of isolates with intI1 | 0 | 13 | 5 | 1 | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. of isolates with qacE | 0 | 13 | 5 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. of isolates with qacEΔ1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The 19 class 1 integron-positive isolates were screened by the use of specific primers for the qacE and qacEΔ1 genes, with 18 isolates testing positive for qacE and 0 testing positive for qacEΔ1 (Table 1). Putative qacE PCR products were sequenced and illustrated 100% similarity to known qacE genes. Amplification of the integron variable region by use of the CASS1/CASS2 primers produced an 1,800-bp product, indicating that 18/19 integrons carried the qacE gene. 16S rRNA PCRs and subsequent sequencing of a 700-bp fragment revealed that the 18 isolates carrying qacE showed the highest similarities to Pseudomonas sp. (3 isolates), Serratia sp. (2 isolates), and Aeromonas hydrophila (13 isolates) and that the isolate lacking qacE showed the highest similarity to Enterobacteriaceae. A full list of the species identified is given in Table 2. In many cases, the 16S rRNA sequences showed high similarities to those of Aeromonas hydrophila, Aeromonas salmonicida, and Vibrio parahaemolyticus. The taxonomy of the genus Aeromonas is not straightforward, with the so-called “A. hydrophila complex” also including the closely related A. salmonicida and Aeromonas bestiarum (40). A. hydrophila is motile, whereas A. salmonicida strains are generally nonmotile. The API 20NE test kit gave an identification of A. hydrophila with a 99.3% certainty. Microscopic observation revealed the cells to be highly motile rods, strengthening the identification of A. hydrophila.
TABLE 2.
Identification of isolates
| Sample | Isolate (% similarity to 16S sequences, no. of isolates)a |
|---|---|
| Reed bed inlet | Bacillus licheniformis (99, 1) |
| Brevundimonas vesicularis (99, 1) | |
| Rhodococcus erythropolis (99, 1) | |
| Reed beds | Acinetobacter venetianus (98, 1) |
| Acinetobacter sp. (99, 1) | |
| Acinetobacter sp. (92, 1) | |
| Bacillus weihenstephanensis (99, 1) | |
| Enterobacter sp. (98, 1) (pathogen) | |
| Pseudomonas sp. (99, 1) | |
| Pseudomonas aurantiaca (97, 1) | |
| Stenotrophomonas sp. (98, 2) | |
| Aeromonas hydrophila (99, 13)*b | |
| Enterobacteriaceae (97, 1)* | |
| Pseudomonas fluorescens (98, 1)* | |
| Pseudomonas sp. (97, 2)* | |
| Serratia proteamaculans (98, 1)* | |
| Serratia sp. (95, 1)* | |
| Reed bed outlet | Citrobacter sp. (99, 2) |
| Rhodococcus erythropolis (99, 1) | |
| River sediment | Acinetobacter sp. (98, 2) |
| Bacillus sp. (98, 2) | |
| Exiguobacterium sp. (98, 2) | |
| Pseudomonas sp. (98, 3) | |
| Cryfield soil | Bacillus weihenstephanensis (99, 3) |
| Paenibacillus sp. (96, 2) | |
| Pseudomonas sp. (99, 3) | |
| Pseudomonas veronii (99, 2) | |
| Cotswold soil | Bacillus sp. (98, 2) |
| Paenibacillus sp. (99, 1) | |
| Pseudomonas sp. (98, 1) | |
| Droitwich soil | Bacillus sp. (99, 2) |
| Paenibacillus sp. (97, 1) | |
| Pseudomonas sp. (98, 1) |
16S sequences were compared to those in the National Center for Biotechnology Information database. *, isolates carrying class 1 integrons.
99.3% by the API NE20 test.
DISCUSSION
The majority of isolates were resistant to DTDMAC at 5 μg/ml, suggesting that this concentration only affects the growth of a few sensitive strains. Among the reed bed samples, three of four had RQs of>100% due to the fact that DTDMAC may act as a carbon source. Pseudomonas fluorescens has been reported to utilize a dialkyl-QAC, didecyldimethylammonium chloride, as a sole carbon source (26). Pseudomonads have also been reported to utilize dodecyldimethylamine (20) and hexadecyltrimethylammonium chloride (43). In addition, Acinetobacter sp., Serratia marcescens, and Aeromonas hydrophila have also been reported to degrade QACs (1, 29, 36). It is probable that the Pseudomonas sp., Serratia sp., and Aeromonas hydrophila isolates identified in this study as carrying class 1 integrons are involved in QAC degradation. With 50 μg/ml DTDMAC, there was a much larger distinction between the RQs observed for the polluted and control sites, illustrating that culturable bacteria were much more highly resistant in populations that were preexposed to QACs. This differential in resistance was likely the result of QAC selective pressure within the factory effluent and reed bed system and correlates with a significantly higher class 1 integron frequency in QAC-polluted than control samples. RQs of >100% were still apparent for two reed bed samples, suggesting that some strains are still able to metabolize DTDMAC at 50 μg/ml.
Resistance to CTAB at both concentrations showed a clear linkage with the reed bed habitat. Isolates from the mill effluent flowing into the reed bed and from the effluent discharged from the reed bed illustrated much less resistance than other isolates, for the former because there was little time for selection to occur upstream of the reed beds and for the latter because resistant bacteria may be associated with root systems or biofilms. Bacteria are known to be less susceptible to QACs when growing in biofilms (6), and the low levels of resistant bacteria in the reed bed outlet effluent confirmed that resistant strains reside within the reed bed sediment. It is also possible that low counts in the reed bed outlet effluent can be accounted for by imperfect operation of the system, i.e., some effluent flowed over the surface of the reed bed, diluting that passing through the beds. Very little resistance was seen for control samples at 5 μg/ml CTAB, and almost none was seen at 50 μg/ml. Again, the integron frequency was generally higher for samples with populations with high RQs. Integron-positive isolates were screened for qacE and qacEΔ1, and 18/19 isolates from the reed bed samples carried qacE, a fully functional multidrug efflux gene carried on a class 1 integron that has been shown to confer increased resistance to a variety of QACs, including CTAB (31). The integron frequency was also highest in RB1, which had a higher effluent flow rate than RB2 and RB3. Rosser and Young (34) reported a 3.6% incidence of class 1 integrons in environmental coliforms, i.e., Pseudomonas spp. and Vibrio spp. collected from the River Tay estuary water, which was similar to the overall incidence of 3.8% seen for this study. However, it is clear that class 1 integrons were not evenly distributed among samples but were only found in reed bed samples exposed to QACs (14.9% of bacterial isolates from reed bed samples) and not in isolates from agricultural soil. The high incidence of qacE observed (approximately 95% of integrons) is higher than those observed in other studies of environmental bacteria, which showed incidences of 46% (34) and 0% (19). qacE confers resistance to QACs, and its prevalence probably represents selection by QACs contained in the mill effluent. Integrons from clinical isolates are characterized by possessing a 3′ conserved region, usually containing sul1 and qacEΔ1, but this arrangement seems far less usual in environmental isolates, possibly due to less exposure to sulfonamides, synthetic antimicrobials that have historically been used extensively in medicine (34).
In addition to acquired resistance conferred by multidrug efflux genes such as qacE, selection for intrinsically resistant strains and innate resistance mechanisms is also likely to occur. Bacteria such as Acinetobacter calcoaceticus (Acinetobacter sp. isolates were isolated from reed bed samples) are known to produce emulsan, a polyanionic heteropolysaccharide bioemulsifier that provides the cell with a protective barrier against CTAB (39). Rhodococcus erythropolis, which was isolated from reed bed inlet and outlet samples, is also highly resistant to disinfectants (4). E. coli mutants produced spontaneously by repeated exposure to CTAB have been reported to develop multidrug resistance (18), and it was suggested that the mechanism of resistance is a combination of an altered lipopolysaccharide profile and a change in outer membrane porin expression, which may reduce permeability.
The isolates identified indicated that there were differences between the contaminated and control-site populations. This was expected, bearing in mind the fact that QACs select for resistant bacteria. QAC disinfectants are inefficient against gram-negative bacteria (1), and this was borne out by the large proportion of gram-negative strains isolated from the reed bed samples and the small number of Bacillus sp. strains isolated compared to those from the agricultural soils. Mesophilic, motile Aeromonas sp. such as A. hydrophila (13 integron-positive isolates) are normal inhabitants of soil and freshwater (13). A study of the effects of QAC-based disinfectants on bacterial community dynamics found an increase in Aeromonas sp. isolates after QAC exposure (24), which agrees with the large number of isolates identified from reed bed samples; aeromonads have also previously been reported to carry class 1 integrons on R plasmids (37). Pseudomonads were common at both sample sites, and as shown in this study, commonly carry class 1 integrons in environments that select for biocide resistance. It is also interesting that many of the genera identified from the contaminated site contain pathogenic species.
It is evident that there are a variety of mechanisms to explain the observed resistance of cultured isolates from the QAC-contaminated reed bed system, but this study is unique in linking increased class 1 integron frequencies with increased QAC resistance. Ninety-five percent of the class 1 integrons carried qacE, reinforcing the hypothesis that QAC pollution selects for integrons. Since class 1 integrons constitute a well-known mechanism for the horizontal transfer of antibiotic resistance genes, it is clear that QAC pollution significantly increases the chances for coselection of antibiotic resistance within environmental bacteria. Recent studies concentrating on investigations of household biocide use and antibacterial resistance (8) failed to establish a link due to the fact that samples were taken from areas where lethal exposures to biocides occur. A comprehensive review of the literature (12) also concluded that the risk of reduced susceptibility to antimicrobial agents associated with exposure to sublethal concentrations of biocides was small. However, these last authors speculated that although biocides at the point of use are rapidly bactericidal and do not therefore pose a risk of increasing antibiotic resistance, downstream of their application a gradient may occur by which sublethal concentrations may provide selective pressure. This hypothesis appears to concur with the data presented in this paper showing that at certain concentrations downstream of application, such as occurs in the reed bed system described here, selection can and does occur for mobile genetic elements carrying biocide resistance genes that are also capable of carrying antibiotic resistance determinants.
Acknowledgments
This work was supported by Natural and Environmental Research Council (NERC) grant NER/A/S/2000/01253. N. Abdouslam thanks the Libyan government for funding.
We give many thanks to Ronald Skurray for providing reference strains. We also thank Oceans-ESU Ltd. for information regarding the reed bed system.
REFERENCES
- 1.Al-Ahmad, A., M. Wiedmann-Al-Ahmad, G. Schon, F. D. Daschner, and K. Kummerer. 2000. Role of Acinetobacter for biodegradability of quaternary ammonium compounds. Bull. Environ. Contam. Toxicol. 64:764-770. [DOI] [PubMed] [Google Scholar]
- 2.Bass, L., C. A. Liebert, M. D. Lee, A. O. Summers, D. G. White, S. G.Thayer, and J. J. Maurer. 1999. Incidence and characterization of integrons, genetic elements mediating multiple-drug resistance, in avian Escherichia coli. Antimicrob. Agents Chemother. 43:2925-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorland, J., T. Steinum, M. Sunde, S. Waage, and E. Heir. 2003. Novel plasmid-borne gene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus intermedius. Antimicrob. Agents Chemother. 47:3046-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bore, E., and S. Langsrud. 2005. Characterization of micro-organisms isolated from dairy industry after cleaning and fogging disinfection with alkyl amine and peracetic acid. J. Appl. Microbiol. 98:96-105. [DOI] [PubMed] [Google Scholar]
- 5.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campanac, C., L. Pineau, A. Payard, G. Baziard-Mouysset, and C. Roques. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 46:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, Y. W., M. Afzal-Shah, E. T. Houang, M. I. Palepou, D. J. Lyon, N. Woodford, and D. M. Livermore. 2001. IMP-4, a novel metallo-beta-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, E. C., R. M. Addison, J. R. Rubino, K. E. Leese, P. D. Dulaney, M. S. Newell, J. Wilkins, D. J. Gaber, T. Wineinger, and D. A. Criger. 2003. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J. Appl. Microbiol. 95:664-676. [DOI] [PubMed] [Google Scholar]
- 9.Collis, C. M., G. Grammaticopoulos, J. Briton, H. W. Stokes, and R. M. Hall. 1993. Site-specific insertion of gene cassettes into integrons. Mol. Microbiol. 9:41-52. [DOI] [PubMed] [Google Scholar]
- 10.Collis, C. M., and R. M. Hall. 1995. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob. Agents Chemother. 39:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, P., and A. J. McBain. 2003. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 16:189-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goni-Urriza, M., L. Pineau, M. Capdepuy, C. Roques, P. Caumette, and C. Quentin. 2000. Antimicrobial resistance of mesophilic Aeromonas spp. isolated from two European rivers. J. Antimicrob. Chemother. 46:297-301. [DOI] [PubMed] [Google Scholar]
- 14.Kucken, D., H. Feucht, and P. Kaulfers. 2000. Association of qacE and qacEDelta1 with multiple resistance to antibiotics and antiseptics in clinical isolates of gram-negative bacteria. FEMS Microbiol. Lett. 183:95-98. [DOI] [PubMed] [Google Scholar]
- 15.Hall, R. M., H. J. Brown, D. E. Brookes, and H. W. Stokes. 1994. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J. Bacteriol. 176:6286-6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heir, E., G. Sundheim, and A. L. Holck. 1998. The Staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol. Lett. 163:49-56. [DOI] [PubMed] [Google Scholar]
- 17.Heir, E., G. Sundheim, and A. L. Holck. 1999. The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J. Appl. Microbiol. 86:378-388. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa, S., Y. Matsumura, F. Yoshizako, and T. Tsuchido. 2002. Characterization of a cationic surfactant-resistant mutant isolated spontaneously from Escherichia coli. J. Appl. Microbiol. 92:261-268. [DOI] [PubMed] [Google Scholar]
- 19.Kazama, H., H. Hamashima, M. Sasatsu, and T. Arai. 1998. Distribution of the antiseptic-resistance genes qacE and qacE delta 1 in gram-negative bacteria. FEMS Microbiol. Lett. 159:173-178. [DOI] [PubMed] [Google Scholar]
- 20.Kroon, A. G., M. A. Pomper, and C. G. van Ginkel. 1994. Metabolism of dodecyldimethylamine by Pseudomonas MA3. Appl. Microbiol. Biotechnol. 42:134-139. [DOI] [PubMed] [Google Scholar]
- 21.L'Abee-Lund, T. M., and H. Sorum. 2001. Class 1 integrons mediate antibiotic resistance in the fish pathogen Aeromonas salmonicida worldwide. Microb. Drug Resist. 7:263-272. [DOI] [PubMed] [Google Scholar]
- 22.Leverstein-van Hall, M. A., H. E. M. Blok, A. R. T. Donders, A. Paauw, A. C. Fluit, and J. Verhoef. 2003. Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J. Infect. Dis. 187:251-259. [DOI] [PubMed] [Google Scholar]
- 23.Mayer, S., M. Boos, A. Beyer, A. C. Fluit, and F. J. Schmitz. 2001. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible European isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 47:896-897. [DOI] [PubMed] [Google Scholar]
- 24.McBain, A. J., R. G. Ledder, L. E. Moore, C. E. Catrenich, and P. Gilbert. 2004. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 70:3449-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nield, B. S., A. J. Holmes, M. R. Gillings, G. D. Recchia, B. C. Mabbutt, K. M. Nevalainen, and H. W. Stokes. 2001. Recovery of new integron classes from environmental DNA. FEMS Microbiol. Lett. 195:59-65. [DOI] [PubMed] [Google Scholar]
- 26.Nishihara, T., T. Okamoto, and N. Nishiyama. 2000. Biodegradation of didecyldimethylammonium chloride by Pseudomonas fluorescens TN4 isolated from activated sludge. J. Appl. Microbiol. 88:641-647. [DOI] [PubMed] [Google Scholar]
- 27.Partridge, S. R., H. J. Brown, and R. M. Hall. 2002. Characterization and movement of the class 1 integron known as Tn2521 and Tn1405. Antimicrob. Agents Chemother. 46:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partridge, S. R., G. D. Recchia, C. Scaramuzzi, C. M. Collis, H. W. Stokes, and R. M. Hall. 2000. Definition of the attI1 site of class 1 integrons. Microbiology 146:2855-2864. [DOI] [PubMed] [Google Scholar]
- 29.Patrauchan, M. A., and P. J. Oriel. 2003. Degradation of benzyldimethylalkylammonium chloride by Aeromonas hydrophila sp. K. J. Appl. Microbiol. 94:266-272. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen, I. T., T. G. Littlejohn, P. Radstrom, L. Sundstrom, O. Skold, G. Swedberg, and R. A. Skurray. 1993. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob. Agents Chemother. 37:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ploy, M. C., P. Courvalin, and T. Lambert. 1998. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 42:2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 34.Rosser, S. J., and H. K. Young. 1999. Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J. Antimicrob. Chemother. 44:11-18. [DOI] [PubMed] [Google Scholar]
- 35.Russell, A. D. 2000. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 52:227-233. [DOI] [PubMed] [Google Scholar]
- 36.Sautter, R. L., L. H. Mattman, and R. C. Legaspi. 1984. Serratia marcescens meningitis associated with a contaminated benzalkonium chloride solution. Infect. Control 5:223-225. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, A. S., M. S. Bruun, J. L. Larsen, and I. Dalsgaard. 2001. Characterization of class 1 integrons associated with R-plasmids in clinical Aeromonas salmonicida isolates from various geographical areas. J. Antimicrob. Chemother. 47:735-743. [DOI] [PubMed] [Google Scholar]
- 38.Segal, H., R. Thomas, and B. Gay Elisha. 2003. Characterization of class 1 integron resistance gene cassettes and the identification of a novel IS-like element in Acinetobacter baumannii. Plasmid 49:169-178. [DOI] [PubMed] [Google Scholar]
- 39.Shabtai, Y., and D. L. Gutnick. 1985. Tolerance of Acinetobacter calcoaceticus RAG-1 to the cationic surfactant cetyltrimethylammonium bromide: role of the bioemulsifier emulsan. Appl. Environ. Microbiol. 49:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soler, L., M. A. Yanez, M. R. Chacon, M. G. Aguilera-Arreola, V. Catalan, M. J. Figueras, and A. J. Martinez-Murcia. 2004. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 54:1511-1519. [DOI] [PubMed] [Google Scholar]
- 41.Sorum, H., T. M. L'Abee-Lund, A. Solberg, and A. Wold. 2003. Integron-containing IncU R plasmids pRAS1 and pAr-32 from the fish pathogen Aeromonas salmonicida. Antimicrob. Agents Chemother. 47:1285-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokes, H. W., and R. M. Hall. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3:1669-1683. [DOI] [PubMed] [Google Scholar]
- 43.van Ginkel, C. G., J. B. van Dijk, and A. G. Kroon. 1992. Metabolism of hexadecyltrimethylammonium chloride in Pseudomonas strain B1. Appl. Environ. Microbiol. 58:3083-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White, P. A., and W. D. Rawlinson. 2001. Current status of the aadA and dfr gene cassette families. J. Antimicrob. Chemother. 47:495-502. [DOI] [PubMed] [Google Scholar]