Abstract
Cells exposed to Helicobacter pylori toxin VacA develop large vacuoles which originate from massive swelling of membranous compartments at late stages of the endocytic pathway. When expressed in the cytosol, VacA induces vacuolization as it does when added from outside. This and other evidence indicate that VacA is a toxin capable of entering the cell cytosol, where it displays its activity. In this study, we have used cytosolic expression to identify the portion of the toxin molecule responsible for the vacuolating activity. VacA mutants with deletions at the C and N termini were generated, and their activity was analyzed upon expression in HeLa cells. We found that the vacuolating activity of VacA resides in the amino-terminal region, the whole of which is required for its intracellular activity.
The vacuolating toxin VacA, produced by pathogenic strains of Helicobacter pylori, is a major virulence factor in the pathogenesis of gastroduodenal ulcers (4, 5, 19). Within a few hours from addition of VacA to culture medium, cells develop intracellular vacuoles, which eventually fill the entire cytosol and cause cell sufferance and necrosis (7, 9). Vacuoles develop in cells of the stomach mucosae of mice (11) and dogs (17) infected orally with VacA. Epithelial and mucosal necrosis is usually followed by cell proliferation and tissue regeneration, but necrotic factors released from the inflamed stomach mucosa are believed to contribute to the establishment of chronic inflammation (18). Vacuoles are acidic and, as such, they take up membrane-permeative weak bases such as neutral red, which provides a rapid and quantitative assay of the extent of vacuolization (2, 3). Vacuoles originate from late stages of the endocytic route and contain protein markers of late endosomes and lysosomes (12, 15, 16).
In the growth medium of H. pylori, VacA is present as a 95-kDa protein as well as 37- and 58-kDa fragments associated by noncovalent interactions (18). Recently, it was shown that VacA can be expressed in the cytosol of HeLa cells, where it causes the formation of vacuoles indistinguishable from those induced by VacA added to the medium and endocytosed by cultured cells (6, 8). Taken together, these results indicate that VacA is a toxin capable of binding to cells and entering the cytosol, where it expresses its toxic activity, as all A-B-type toxins do. These toxins consist of a B domain involved in cell surface binding and in the entry in to the cell of the catalytically active A protomer (5, 6, 14). Presently, no information is available on the location of domain A of VacA within its 95-kDa polypeptide chain and the size of this domain.
To answer these questions, we have progressively shortened the gene encoding VacA in such a way that smaller and smaller fragments are produced in the cytosol of HeLa cells transfected with the vacA gene constructs. In most dichain A-B-type toxins, the A chain is amino terminal. Thus, we began with progressive deletions at the C terminus of VacA and then analyzed the effect of N-terminal deletions. The transfected cells were assayed for vacuole formation both by visual inspection and by quantitative measurement of neutral red uptake 20 h after transfection.
The mutants with deletions at the C terminus listed in the left panel of Fig. 1 were obtained by PCR amplification with plasmid ptox140 (10) by using appropriate 5′ and 3′ oligonucleotides containing SphI and BamHI restriction sites, respectively. PCR amplifications were performed by mixing 10 ng of the plasmid ptox140 and 50 pmol of each primer. The reaction mixtures were preincubated for 5 min at 94°C, and 30 amplification cycles were performed with the following scheme: denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 1.5 min. The last PCR step was performed at 72°C for 5 min. DNA fragments were purified from agarose gels before the ligation reaction. The vector pGEM7Zf(+) (Promega) was digested with SphI and BamHI and mixed in a ligation reaction with the fragments. The ligation mixture was introduced into Escherichia coli XL-1 Blue by the CaCl2 transformation procedure. The nucleotide sequence was checked by the dideoxy sequencing method. The N-terminus deletion mutants were prepared by using the shortest C-terminus deletion VacA mutant displaying full activity in the cytosol: the plasmid pGEM containing the VacA gene from positions 316 to 2152 was digested with SphI and EcoRI (this restriction site is naturally present in the sequence) and ligated with the PCR products obtained by PCR amplification with the plasmid ptox140 by using appropriate 5′ and 3′ oligonucleotides containing the SphI and EcoRI restriction sites, respectively. PCR amplifications were performed by mixing 10 ng of the plasmid ptox140 and 50 pmol of each primer. The reaction mixtures were preincubated for 2 min at 94°C, and 27 amplification cycles were performed with the following scheme: denaturation at 94°C for 1 min, annealing at 52°C for 2 min, and extension at 72°C for 2 min. The last PCR step was performed at 72°C for 5 min. Plasmid DNA, prepared from ampicillin-resistant colonies, was used to transfect HeLa cells. The transfection was performed as described before (6): briefly, HeLa cells were incubated with recombinant vaccinia virus vT7 in Dulbecco’s modified Eagle medium (DMEM) supplemented with 20 mM HEPES (pH 7.2) for 30 min and then transfected in DMEM containing 3.7 g of NaHCO3 liter−1, 10 mM HEPES (pH 7.2), 10 mM hydroxyurea, DNA (9 ng μl−1), and DOTAP (Boheringer) (27 ng μl−1) for 2 h at 37°C.
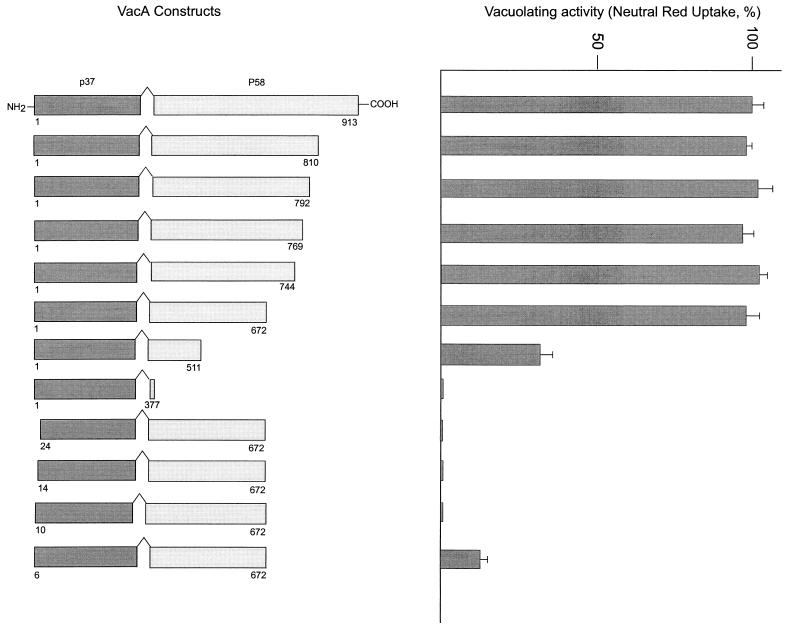
FIG. 1.
Vacuolating activities of cytosol-expressed VacA deletion mutants. The left panel lists the VacA fragments generated inside the cytosol of HeLa cells in the present study. The right panel reports the extent of cell vacuolation, assayed quantitatively as the amount of neutral red taken up by transfected cells (20 h after transfection). Data are shown as percentages of the maximal value. Values reported are the averages for at least three different experiments, and bars represent standard deviations. The first 33 residues, corresponding to the signal sequence, not present in the mature toxin molecule, are not included here (4).
The results obtained after this series of transfection experiments are summarized in Fig. 1. More than 250 residues can be removed from the C terminus of the 95-kDa toxin without any loss of vacuolating activity. Moreover, deletion of an additional 160 residues (VacA1-511) still leaves a toxin with considerable cytosolic activity. The HeLa cells transfected with fragment 1-511 showed smaller vacuoles with a similar round appearance, mainly localized in the perinuclear area. Similar levels of expression of different VacA fragments (fragments 1-913, 1-511, and 1-377) were detected, as assessed by immunoblots of transfected-cell extracts subjected to electrophoresis and stained with anti-VacA antibodies (data not shown). Hence, it appears that the vacuolating activity of VacA is confined to the amino-terminal portion of the polypeptide chain. The role of the residual amino-terminal part of p58, present in the construct 1-672, which is required for activity, is most likely that of assisting in the correct folding of the p37 domain. This conclusion is reinforced by the analysis of the results of N-terminal deletion, performed with the VacA1-672 construct, which is the shortest C-terminally deleted VacA displaying full activity in the cytosol. The N-terminally deleted VacA lacking 24 residues is totally inactive (lower part of Fig. 1). Progressively shorter deletions were then considered, but even the VacA6-672 construct, lacking only five N-terminal residues, manifests a strongly reduced activity.
The results presented here show clearly that the cytosolic activity of VacA resides in the amino-terminal region of the toxin and that its entire N terminus is required for the induction of vacuoles by acting in the cell cytosol. The explanation of these results is not straightforward. The amino-terminal 24-residue-long segment has an overall hydrophobic character (4), and it could be involved in mediating the binding of the toxin to the cytosolic face of the cell membrane or in directing the toxin to a selected cytosolic substrate. Another possibility is that the removal of a few N-terminal residues affects the stability of the protein, as was found to be the case with human interleukin 1 beta (1). However, this does not appear to be the case, since a control experiment performed by immunoblotting indicated that similar levels of the different fragments are present in the transfected cells at the time of assay of vacuolization (not shown).
It is also evident from the present work that a large portion of the 58-kDa fragment is not necessary for the cytosolic activity of VacA. This finding is in agreement with the fact that p58 is membrane active and increases the permeability of liposomes to K+ (13), a property shared by the B protomers of several A-B toxins. Together, these data provide strong evidence that VacA is an A-B-type toxin.
Using the same experimental approach, we are currently testing the roles of different toxin segments, as well as those of single amino acid residues of the p37 domain, even though extensive sequence comparisons run with different methods have not revealed possible biological activities of VacA, which could pinpoint selected residues to be mutagenized. Such information will be highly valuable in the design of VacA mutants to be considered as possible components of a therapeutic vaccine against the gastroduodenal diseases caused by H. pylori.
Acknowledgments
We thank Stefano Censini for the synthesis of oligonucleotides and for gene sequencing.
This work was supported by grants from the European Community (TMR FMRX CT96 0004 and Biomed-2 BMH4 CT97 2410), from the Armenise-Harvard Medical School Foundation, and from CNR Progetto Finalizzato Biotecnologie (97.01168.PF 49) and has been carried out in part under a research contract with Consorzio Autoimmunità Tardiva C.A.U.T., Pomezia, Italy, within the “Programma Nazionale Farmaci-seconda fase” of the Italian Ministry of the University Scientific and Technological Research.
REFERENCES
- 1.Baldari C, Massone A, Macchia G, Telford J L. Differential stability of human interleukin 1 beta fragments expressed in yeast. Protein Eng. 1987;1:433–437. doi: 10.1093/protein/1.5.433. [DOI] [PubMed] [Google Scholar]
- 2.Catrenich C E, Chestnut M H. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- 3.Cover T L, Puryear W, Perez-Perez G I, Blaser M J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cover T L, Blaser M J. Purification and characterisation of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 5.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1997;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 6.de Bernard M, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R, Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 7.Figura N, Guglielmetti P, Rossolini A, Barberi A, Cusi G, Musmanno R A, Russi M, Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner J A, Cover T L. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;46:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxin activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 10.Manetti R, Massari P, Burroni D, de Bernard M, Marchini A, Olivieri R, Papini E, Montecucco C, Rappuoli R, Telford J L. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect Immun. 1995;63:4476–4480. doi: 10.1128/iai.63.11.4476-4480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchetti M, Aricò B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 12.Molinari M, Galli C, Norais N, Telford J L, Rappuoli R, Luzio J P, Montecucco C. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J Biol Chem. 1997;272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 13.Moll G, Papini E, Colonna R, Burroni D, Telford J L, Rappuoli R, Montecucco C. Lipid interaction of the 37-kDa and 58-kDa fragments of the Helicobacter pylori cytotoxin. Eur J Biochem. 1995;234:947–952. doi: 10.1111/j.1432-1033.1995.947_a.x. [DOI] [PubMed] [Google Scholar]
- 14.Montecucco C, Papini E, Schiavo G. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett. 1994;346:92–98. doi: 10.1016/0014-5793(94)00449-8. [DOI] [PubMed] [Google Scholar]
- 15.Papini E, de Bernard M, Milia E, Bugnoli M, Zerial M, Rappuoli R, Montecucco C. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc Natl Acad Sci USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papini E, Satin B, Bucci C, de Bernard M, Telford J L, Manetti R, Rappuoli R, Zerial M, Montecucco C. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 1997;16:15–24. doi: 10.1093/emboj/16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappuoli, R., and G. Del Giudice. Unpublished results.
- 18.Telford J L, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Purification and characterisation of the vacuolating toxin from Helicobacter pylori. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telford J L, Covacci A, Rappuoli R, Ghiara P. Immunobiology of Helicobacter pylori infection. Curr Opin Immunol. 1997;9:498–503. doi: 10.1016/s0952-7915(97)80101-x. [DOI] [PubMed] [Google Scholar]