Abstract
The combination of two candidate microbicides, cellulose acetate 1,2-benzenedicarboxylate (CAP), a polymer that blocks human immunodeficiency virus type 1 (HIV-1) entry by targeting gp120 and gp41, and UC781, a tight-binding HIV-1 reverse transcriptase inhibitor (RTI), resulted in effective synergy for inhibition of MT-2 cell infection by HIV-1IIIB, a laboratory-adapted virus strain. The 95% effective concentration values for the combination were reduced about 15- to 20-fold compared with those corresponding to the single compounds. The combination of CAP and UC781 is also synergistic in inhibiting infection of peripheral blood mononuclear cells by a primary HIV-1 isolate, 92US657. Combinations of CAP with other RTIs, such as efavirenz or zidovudine, also had significant synergistic effects on the inhibition of HIV-1 infection. In addition, CAP and UC781 had complementary effects against HIV-1 infection since (i) CAP inhibited infection by the UC781-resistant strain HIV-1IIIB A17 and (ii) pretreatment of MT-2 cells with UC781, but not CAP, abolished subsequent infection after removal of the compound. This suggests that the combination of CAP and UC781 represents a promising candidate microbicide for prevention of sexual transmission of HIV-1.
By the end of 2003, more than 60 million people worldwide had been infected by the human immunodeficiency virus (HIV), and over one-third of them died of AIDS (40). Due to the unavailability of anti-HIV vaccines, development of topically applied microbicides is urgently needed since sexual transmission is the major cause of HIV infection (9, 19, 36, 37, 39).
There are three major categories of candidate microbicides with different mechanisms of action: (i) inactivating HIV-1 and other sexually transmitted disease (STD) pathogens, including surfactants (e.g., nonoxynol-9 [21] and C31G [3]) and acidifying agents (e.g., BufferGel [23]); (ii) blocking HIV-1 attachment/fusion/entry, such as long-chain anionic polymers (e.g., Carraguard [9] and PRO 2000 [24]) and fusion inhibitors (e.g., T20 [25]); and (iii) disrupting intracellular HIV replication, such as reverse transcriptase inhibitors (RTIs) (38). Recently, in the first completed microbicide phase II/III clinical trial, nonoxynol-9 failed to protect against HIV-1 infection, presumably due to inflammatory lesions associated with frequent nonoxynol-9 use (41). Therefore, more effective microbicides or microbicide combinations are needed urgently. Cellulose acetate 1,2-benzenedicarboxylate (CAP), a mixture of polymers with a mean molecular mass of 45 to 60 kDa, is a pharmaceutical excipient with a long history of safe use in humans as an enteric film coating polymer for capsules and tablets (14). It has potent anti-HIV-1 activity (18, 31, 34, 43) through multiple mechanisms of action, i.e., inactivating viruses directly, inhibiting HIV-1 entry by blocking the coreceptor (CXCR4 or CCR5) binding sites on gp120, and eliciting “dead-end” gp41 six-helix bundle formation on the virus envelope (28, 30). It can also block the infection in rhesus macaque and pig-tailed macaque monkeys (4, 33). In addition, CAP also has microbicidal activity against a broad spectrum of STD pathogens, including herpesviruses HSV-1, HSV-2, cytomegalovirus, Neisseria gonorrhoeae, Trichomonas vaginalis, Haemophilus ducreyi, Chlamydia trachomatis, Treponema pallidum, and bacteria associated with bacterial vaginosis (31). CAP has low cytotoxicity and no immunoinflammatory side effects as determined in a human in vitro model of vaginal inflammation (11, 13). This suggests that CAP is an ideal candidate microbicide for preventing sexual transmission of HIV-1 and other STD pathogens (22, 29). The phase I clinical trial of CAP is ongoing in multicenters (35).
In order to develop more effective anti-HIV-1 microbicides, we intend to design combinations of CAP with other candidate microbicides that have mechanisms of action different from the mechanism of CAP and that may be synergistic with CAP in inhibiting HIV-1 infection. We are especially interested in candidate microbicides in the third category described above, i.e., RTIs. Currently, two nonnucleoside RTIs (NNRTIs), UC781 (Biosyn, Huntingdon Valley, PA) (6, 45) and TMC120 (Tibotec-Virco, Mechelen, Belgium) (12, 42), and one nucleotide RTI (NRTI), tenofovir (Gilead, Foster City, CA), have shown great potential to be developed as microbicides (38). Since UC781, a thiocaboxanilide derivative (Fig. 1), binds with high affinity to reverse transcriptase and has high potency in blocking in vitro and ex vivo transmission of cell-free and cell-associated HIV-1 strains as determined in cell culture and cervical tissue organ culture systems (1, 45), we selected it for combination with CAP and investigated whether the two compounds inhibit HIV-1 infection synergistically and complementarily.
FIG. 1.
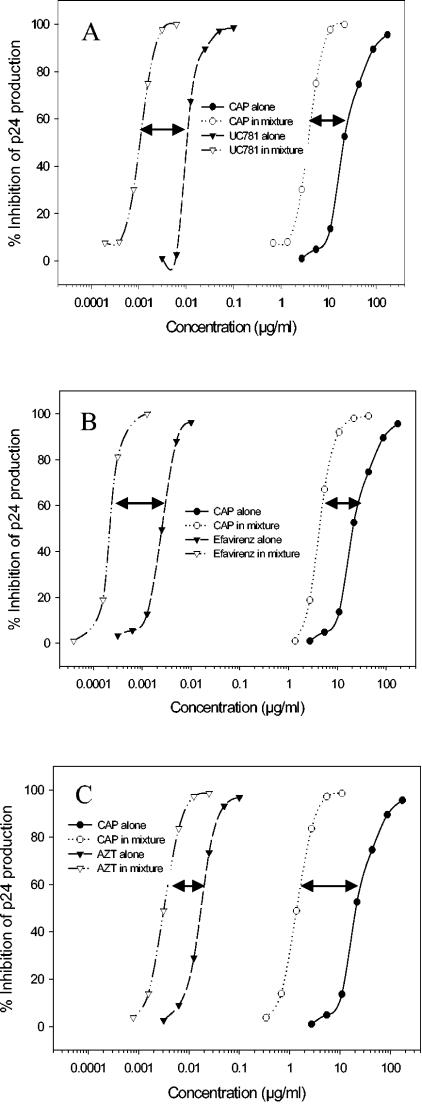
Synergistic effect of CAP in combinations with UC781 (A), efavirenz (B), and AZT (C) against HIV-1IIIB infection of MT-2 cells. The effective concentrations for inhibition of HIV-1 replication by a compound alone and in combination with another compound are plotted in two curves. The length of a line with two arrows between two curves represents the dose reduction (n-fold) of a compound when it was tested alone and in combination with another compound. Data are the means of three independent assays performed in triplicate.
MATERIALS AND METHODS
Reagents.
MT-2 cells, HIV-1IIIB, HIV-1IIIB A17, anti-p24 monoclonal antibody (183-12H-5C), zidovudine (AZT), and efavirenz were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, contributed by D. Richman, R. Gallo, E. Emini, B. Chesebro, and H. Chen, respectively. CAP was a gift from Eastman Chemical Company (Kingsport, TN). A soluble form of CAP was prepared every 6 weeks as a 30 mg/ml stock solution in 30 mM sodium acetate buffer (pH ≈ 5.8). Working solutions of CAP and acetate buffer control were prepared fresh for each experiment in appropriate culture medium. The stability of CAP in stock solution and culture medium was confirmed by the ruthenium red method (26). UC781 was kindly provided by D. Ho at the Aaron Diamond AIDS Research Center, The Rockefeller University (New York, NY).
Detection of HIV-1 replication as measured by p24 antigen production.
The inhibitory activity of compounds on HIV-1 infection was determined as previously described (28, 44). In brief, 104 MT-2 cells were infected with HIV-1IIIB or HIV-1IIIB A17 (100 times the 50% tissue culture infective dose [TCID50]) in 200 μl of RPMI 1640 medium containing 10% fetal bovine serum (FBS) in the presence or absence of testing compounds at graded concentrations overnight. Then the culture supernatants were removed and fresh medium containing no testing compounds was added. On the fourth day postinfection, 100 μl of culture supernatant was collected from each well, mixed with equal volumes of 5% Triton X-100, and assayed for p24 antigen, which was quantitated by enzyme-linked immunosorbent assay (ELISA). Briefly, wells of polystyrene plates (Immulon 1B; Dynex Technology, Chantilly, VA) were coated with HIV immunoglobulin, which was prepared from plasma of HIV-seropositive donors with high neutralizing titers against HIV-1IIIB, in 0.085 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight, followed by washes with washing buffer (0.01 M phosphate-buffered saline containing 0.05% Tween-20) and blocking with phosphate-buffered saline containing 1% dry fat-free milk (Bio-Rad Inc., Hercules, CA). Virus lysates were added to the wells and incubated at 37°C for 1 h. After extensive washes, anti-p24 monoclonal antibody (183-12H-5C), biotin-labeled anti-mouse immunoglobulin G1 (Santa Cruz Biotech., Santa Cruz, CA), streptavidin-labeled horseradish peroxidase (Zymed, South San Francisco, CA), and the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, MO) were added sequentially. Reactions were terminated by the addition of 1N H2SO4. Absorbance at 450 nm was recorded in an ELISA reader (Ultra 386; TECAN, Research Triangle Park, NC). Recombinant protein p24 purchased from US Biological (Swampscott, MA) was included for establishing standard dose-response curves. Each sample was tested in triplicate. The percentage of inhibition of p24 production was calculated as previously described (28). The effective concentrations for 50, 70, 90 and 95% inhibition (EC50, EC70, EC90, and EC95, respectively) were calculated using a computer program, designated CalcuSyn (8), kindly provided by T. C. Chou (Sloan-Kettering Cancer Center, New York, N.Y.).
Inhibitory activity of compounds on infection of peripheral blood mononuclear cells (PBMC) by a primary HIV-1 isolate was determined as previously described (17). Briefly, PBMC were isolated from the blood of healthy donors at the New York Blood Center by standard density gradient centrifugation using Histopaque-1077 (Sigma). The cells were cultured in 75-cm2 plastic flasks at 37°C for 2 h. The nonadherent cells were collected and resuspended at 5 × 106 cells in 10 ml of RPMI 1640 medium containing 10% FBS, 5 μg/ml phytohemagglutinin and 100 U/ml interleukin-2 (IL-2; Sigma), followed by incubation at 37°C for 3 days. The phytohemagglutinin-stimulated cells were infected with a primary HIV-1 isolate 92US657 (clade B) at a 0.01 multiplicity of infection in the presence or absence of compounds. Culture media were changed on the second day and then every 3 days. The supernatants were collected 7 days postinfection and tested for p24 antigen by ELISA as described above. The percent inhibition of p24 production and EC50 values were calculated as described above.
Assessment of in vitro cytotoxicity.
The in vitro cytotoxicity of compounds on MT-2 cells was measured by the XTT [2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-(phenylamino) carbonyl-2H-tetrazolium hydroxide] assay (27). Briefly, 100 μl of a compound at graded concentrations was added to equal volumes of cells (5 × 105 cells/ml) in wells of 96-well plates. After incubation at 37°C for 4 days, 50 μl of XTT solution (1 mg/ml) containing 0.02 μM of phenazine methosulphate was added. After 4 h, the absorbance at 450 nm was measured with an ELISA reader. The 50% cytotoxicity concentration (CC50) values were calculated using the computer program CalcuSyn (7).
Compounds washout assays.
MT-2 cells (104 cells/well) in 100 μl of RPMI 1640 medium containing 10% FBS were incubated with 100 μl of a testing compound at indicated concentrations at 37°C for 1 h. The cells were washed three times with 300 μl of RPMI 1640 medium to remove unbound compounds, while the cells in the wells of controls were not washed. Then, the MT-2 cells were infected with HIV-1IIIB (100 TCID50) in 200 μl of RPMI 1640 medium containing 10% FBS at 37°C overnight. The culture supernatants were removed and fresh medium containing no testing compounds was added. On the fourth day postinfection, 100 μl of culture supernatants was collected from each well, mixed with equal volumes of 5% Triton X-100, and assayed for p24 antigen using the ELISA described above.
To exclude the possibility that the “memory” effect of UC781 (2, 5, 20) is due to the nonspecific binding of the compound to surface of wells of the culture plates, Falcon 5-ml polystyrene round-bottom tubes (Becton Dickinson Labware, Franklin Lakes, NJ) with a low binding property were used for repeating the washout experiment. In brief, MT-2 cells (105 cells/ml) in RPMI 1640 medium containing 10% FBS were incubated with UC781 at graded concentrations in polystyrene tubes at 37°C for 1 h, followed by three washes with 4 ml of RPMI 1640 medium or no wash (for the controls). Then, the MT-2 cells (104 cells/well) were transferred to wells of culture plates and infected with HIV-1IIIB (100 TCID50). Virus replication in MT-2 cells was determined by measuring p24 production as described above.
Synergy analysis.
Inhibition data from three independent assays were averaged and analyzed for cooperative effects by using the CalcuSyn program for calculating the combination index (CI) as described (8). In all analyses, CAP and the RTIs were assumed to act noncompetitively, which leads to a more conservative estimate of synergy. CI values of <1 and >1 indicate synergy and antagonism, respectively. Dose reductions were calculated as the compound concentrations required for inhibition of HIV-1 replication when the compound was used alone and in combination (8). Statistical analysis was performed by a one-way analysis of variance method using Origin version 6.1 software (OriginLab Corp., Northampton, MA).
RESULTS
Combination of CAP with UC781 is synergistic against HIV-1 infection.
The ratio of compounds in a combination (about 1:1) was determined based on their respective EC50 values. The synergistic effect can be calculated as long as the compounds in the combination are mixed at concentrations having equal or similar potencies. In preliminary studies, the average EC50 ratio for CAP and UC781 for inhibiting the laboratory-adapted and primary HIV-1 strains was about 2,000:1 (wt/wt; ranging from 1,258:1 to 3,860:1). Based on the molar concentrations, the EC50 ratio for CAP and UC781 is about 10:1 to 18:1 since the molecular mass of CAP (∼45 to 60 kDa) is much larger than that of UC781 (335.9 Da). We prefer to use the weight concentrations, rather than the molar concentrations, since CAP is a mixture of polymers with different molecular sizes. Therefore, the combination of CAP and UC781 at a weight ratio of 2,000:1 was tested for possible synergistic effects on the inhibition of HIV-1IIIB infection as measured by ELISA for p24 antigen. The results are shown in Fig. 1A and Table 1. When CAP and UC781 were used in combination, their EC50, EC70, EC90, and EC95 values for inhibition of HIV-1 replication decreased significantly. Approximately 15-fold less CAP and 20-fold less UC781 were needed to inhibit HIV-1 infection by 95% compared with the respective compounds used alone. The CI values ranged from 0.12 to 0.24, suggesting that the combination of the candidate microbicides CAP and UC781 is potently synergistic in inhibiting HIV-1 infection.
TABLE 1.
Combination index and dose reduction values for inhibition of HIV-1IIIB infection on MT-2 cells by combinations of CAP with HIV-1 RTIsa
| % Inhibition for CAP with RTIs (weight ratio) | CI | CAP
|
RTI
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Concn (μg/ml)
|
Dose reduction | P | Concn (μg/ml)
|
Dose reduction | P | ||||
| Alone | Mix | Alone | Mix | ||||||
| CAP:UC781 (2,000:1) | |||||||||
| 50 | 0.2364 | 15.176 | 2.685 | 5.652 | 0.00111 | 0.01218 | 0.00062 | 19.731 | 0.00083 |
| 70 | 0.1904 | 27.310 | 3.640 | 7.503 | 0.00047 | 0.01660 | 0.00084 | 19.827 | 0.00098 |
| 90 | 0.1392 | 69.646 | 5.910 | 11.784 | 0.00124 | 0.02716 | 0.00136 | 19.982 | 0.00153 |
| 95 | 0.1192 | 116.933 | 7.729 | 15.133 | 0.00258 | 0.03568 | 0.00178 | 20.068 | 0.00211 |
| CAP:efavirenz (40,000:1) | |||||||||
| 50 | 0.3291 | 15.176 | 3.365 | 4.510 | 0.00153 | 0.00225 | 0.00019 | 11.592 | 0.00038 |
| 70 | 0.2529 | 27.310 | 4.498 | 6.072 | 0.00008 | 0.00342 | 0.00026 | 13.212 | 0.00029 |
| 90 | 0.1696 | 69.646 | 7.099 | 9.811 | 0.00065 | 0.00664 | 0.00041 | 16.274 | 0.00021 |
| 95 | 0.1372 | 116.933 | 9.139 | 12.795 | 0.00201 | 0.00960 | 0.00053 | 18.264 | 0.00019 |
| CAP:AZT (1,000:1) | |||||||||
| 50 | 0.2950 | 15.176 | 1.407 | 10.786 | 0.00067 | 0.0175 | 0.00324 | 5.401 | 0.00194 |
| 70 | 0.2696 | 27.310 | 2.047 | 13.341 | 0.00011 | 0.0260 | 0.00471 | 5.522 | 0.00159 |
| 90 | 0.2376 | 69.646 | 3.720 | 18.722 | 0.00032 | 0.0489 | 0.00856 | 5.720 | 0.00144 |
| 95 | 0.2233 | 116.933 | 5.179 | 22.578 | 0.00114 | 0.0695 | 0.01191 | 5.833 | 0.00146 |
Data are the means of three independent assays performed in triplicate.
CAP and UC781 used alone and in combination are also effective for inhibiting infection of PBMC by a primary HIV-1 isolate, 92US657 (clade B). The CI values for 50 to 95% inhibition by combinations of CAP and UC781 ranged between 0.38 and 0.52, suggesting potent synergy for CAP and UC781 at a weight ratio of 2,000:1 (Table 2).
TABLE 2.
Combination index and dose reduction values for inhibition of infection by a primary HIV-1 isolate 92US657 of PBMCa
| % Inhibition | CI | CAP
|
UC781
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Concn (μg/ml)
|
Dose reduction | P | Concn (μg/ml)
|
Dose reduction | P | ||||
| Alone | Mix | Alone | Mix | ||||||
| 50 | 0.3878 | 29.823 | 5.480 | 5.442 | 0.0016 | 0.00772 | 0.00274 | 2.821 | 0.0034 |
| 70 | 0.4161 | 45.119 | 7.565 | 5.964 | 0.00036 | 0.00921 | 0.00378 | 2.435 | 0.0043 |
| 90 | 0.4761 | 87.260 | 12.648 | 6.899 | 0.00001 | 0.01219 | 0.00633 | 1.927 | 0.0069 |
| 95 | 0.5182 | 125.712 | 16.809 | 7.479 | 0.00017 | 0.01422 | 0.00845 | 1.692 | 0.0108 |
Combinations of CAP with UC781 (2,000:1 [wt/wt]) were used. Data are the means of triplicate experiments.
The combination of CAP and UC781 did not show synergistic effects on cytotoxicity (CI, 1.02). The CC50 of CAP was 2.342 ± 0.052 and 2.480 ± 0.076 mg/ml (P > 0.05) when tested alone and in combination with UC781, respectively. This suggests that the cytotoxicity of CAP does not increase when it is used in combination with UC781. UC781 has a selectivity index (selectivity index = CC50/EC50) of about 23,550 (CC50, 94.4 ± 13.2 μg/ml; EC50, 0.004 ± 0.001 μg/ml), indicating that UC781 has very low cytotoxicity compared to its highly potent anti-HIV-1 activity.
Combination of CAP with other RTIs also has synergistic effect on inhibition of HIV-1 infection.
To determine whether combinations of CAP with other RTIs with lower reverse transcriptase (RT)-binding affinity also result in synergistic anti-HIV-1 activity, we selected efavirenz, another NNTRI, and AZT, an NTRI, for parallel testing since both drugs are widely used for treatment of HIV-1 infection. As shown in Fig. 1B and C and Table 1, combinations of CAP with either efavirenz or AZT showed potent synergistic effects in inhibiting HIV-1 infection, with CI values ranging from 0.14 to 0.33 and about 3- to 21-fold dose reductions. These results indicate that CAP may be combined with not only UC781 but also other RTIs to design combination microbicides for prevention of mucosal HIV-1 transmission.
CAP is effective in inhibiting infection by the UC781-resistant strain HIV-1IIIB A17.
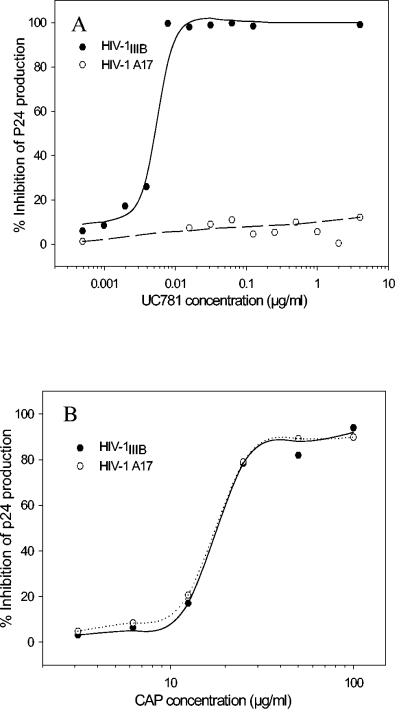
The HIV-1 strain A17, a variant of HIV-1IIIB, is highly resistant to inhibition by NNRTIs, including pyridinone derivatives, BI-RG-587, and TIBO (tetrahydro-imidazo[4,5,1-jk][1,4]-benzodiazepin-2-one) compounds (15, 32). Resistance results from mutations at amino acids 103 (K→N) and 181 (Y→C) in the viral RT. Here we demonstrate that HIV-1IIIB A17 was also highly resistant to UC781 with a dose increase of more than 1,000-fold (Fig. 2A and Table 3). The HIV-1IIIB A17 strain is also resistant to efavirenz but not to the NRTI AZT (Table 3). CAP is equally effective in inhibiting infection by HIV-1IIIB A17 and wild-type HIV-1IIIB (Fig. 2B). This suggests that the combination of CAP and UC781 has complementary effects against HIV-1 infection since it can prevent infection by HIV-1 strains resistant to NNRTIs, including UC781.
FIG. 2.
Inhibitory activity of UC781 (A) and CAP (B) on infection by HIV-1IIIB and its variant HIV-1 A17 strain with a resistance to NNRTIs because of the site mutation of amino acids 103 (K→N) and 181 (Y→C) in the viral RT domain. Data are the means of two independent assays performed in triplicate.
TABLE 3.
Inhibitory activity of CAP and RTIs on infection by HIV-1IIIB and its variant strain A17 that is resistant to NNRTIs
| Agent | EC50 (μg/ml) for inhibition of p24 productiona
|
Dose increase (n-fold) | P | |
|---|---|---|---|---|
| HIV-1IIIB | HIV-1IIIB A17 | |||
| CAP | 19.01000 ± 4.08 | 20.17000 ± 4.46 | 1.1 ± 0.1 | 0.75626 |
| UC781 | 0.00405 ± 0.00112 | >4.0 | >1036.3 ± 266.3 | 0.00001 |
| Efavirenz | 0.00051 ± 0.00004 | 0.02686 ± 0.01004 | 52.2 ± 16.8 | 0.01045 |
| AZT | 0.04202 ± 0.00320 | 0.03309 ± 0.00370 | 0.8 ± 0.1 | 0.03393 |
Data are the means of two independent assays performed in triplicate.
Pretreatment of cells with UC781 alone or in combination with CAP treatment blocks HIV-1 infection.
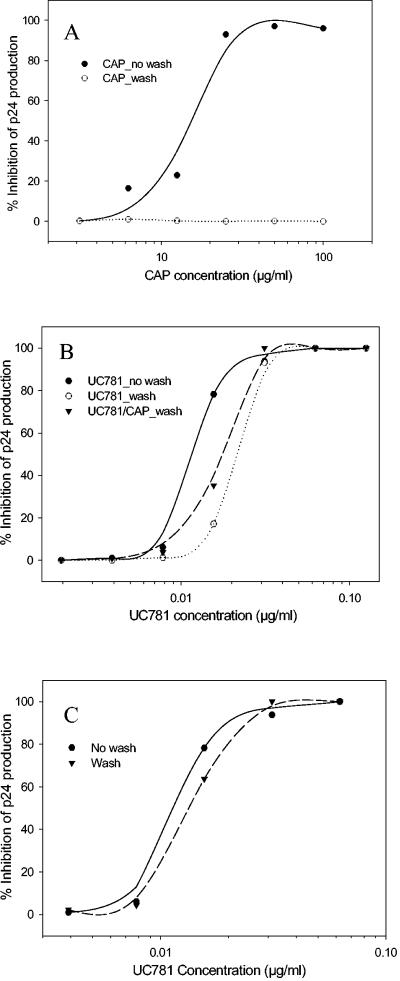
Borkow et al. reported that short exposure of uninfected lymphocytes to UC781 can render these cells refractory to subsequent HIV infection in the absence of exogenous drug (5). Recently, Kiselyeva et al. confirmed this point by briefly treating ex vivo human lymphoid tissue with UC781 (20). This so-called memory effect would make UC781 an ideal candidate microbicide. In this study, MT-2 cells were pretreated with UC781 or CAP for 1 h, and then the unbound compounds were removed by washing the cells three times. In the controls, the cells were not washed, with the result that the unbound compounds were retained. HIV-1 replication in the cells with and without washes was compared. Without washes, CAP effectively inhibited HIV-1 infection of MT-2 cells. However, after washes to remove unbound CAP, the cells were not protected from HIV-1 infection (Fig. 3A). In contrast, pretreatment of MT-2 cells with UC781 and removal of the unbound compound by washes reduced subsequent HIV-1 infection (Fig. 3B). The cells pretreated by the UC781 and CAP combination were also resistant to HIV-1 infection (Fig. 3B).
FIG. 3.
Protection of MT-2 cells pretreated with CAP (A) and UC781 (B) from subsequent HIV-1 infection. MT-2 cells were pretreated with UC781 in polystyrene tubes before the cells were transferred to culture plates for HIV-1 inoculation (C). Data are the means of two independent assays performed in triplicate.
One may argue that the so-called memory effect of UC781 may be due to its nonspecific binding to the surface of wells of culture plates (39, 42). To exclude this possibility, we repeated the washout experiment using polystyrene tubes that have low binding properties. MT-2 cells were pretreated with UC781 in the polystyrene tubes. After extensive washing, the pretreated cells were transferred to wells of culture plates and infected with HIV-1. HIV-1 replication in MT-2 cells pretreated by UC781 was determined by measuring p24 production. As shown in Fig. 3C, there was no significant difference in HIV-1 replication in UC781-pretreated cells with or without washes (P = 0.7091). This suggests that the memory effect of UC781 is not due to its nonspecific binding to the surfaces of wells of culture plates.
DISCUSSION
Clinical applications of antiretroviral drugs with different targets in combinations (i.e., cocktail regimens) have shown significant synergism in inhibiting HIV-1 infection, reducing adverse effects, and delaying the emergence of drug resistance (16). It is expected that combinations of topical microbicides with distinct mechanisms of action may also have synergistic effects on the prevention of sexual transmission of HIV-1 (36).
Although CAP has potent anti-HIV-1 activity and broad-spectrum microbicidal activity against other STD pathogens (31), it may be more effective for preventing sexual transmission of HIV-1 if it is combined with other candidate microbicides with mechanisms of action different from the mechanism of CAP. We previously demonstrated that there is synergism between CAP and soluble CD4 for inhibiting HIV-1 infection since these two molecules bind to the different regions on gp120 (28). Soluble CD4 is not an ideal anti-HIV-1 microbicide since it has only moderate antiviral activity against primary HIV-1 strains and is too expensive to be used topically. Therefore, it is necessary to search for other candidate microbicides suitable for combination with CAP.
In the present study, we demonstrated that the combination of CAP with another candidate microbicide, UC781, a tight-binding NNRTI, results in significant synergy for inhibiting infection by both laboratory-adapted and primary HIV-1 strains. Other RTIs with lower RT-binding affinity than UC781, such as efavirenz and AZT, when combined with CAP also had synergistic anti-HIV-1 activity. The RTIs could not have enhanced CAP-mediated inhibition on HIV-1 entry since there was no synergy when a combination of CAP and UC781 was tested in a cell-cell fusion assay (data not shown). We believe that the synergistic effect of the CAP/UC781 combination is due to the fact that CAP is targeted to earlier stages of the HIV-1 replication cycle, virus fusion and entry, while UC781 acts on later stages of virus infection, reverse transcription. Therefore, a combination of CAP with UC781 for prevention of sexual transmission of HIV-1 may have the following advantages: (i) maximization of anti-HIV-1 activity because of synergistic effects, (ii) minimization of potential toxic effects due to dose reduction, and (iii) complementary or cooperative microbicidal activity.
Although CAP used alone is a virucidal agent and/or blocks HIV-1 entry, some residual virus particles might escape from CAP-mediated antiviral activity and enter into cells. If UC781 is present, it will work as a secondary inhibitor against residual virus. Especially if these residual virions pass through the multilayered epithelium and enter into draining lymph nodes, where the large-molecule CAP is unlikely to reach, the small-molecule compound UC781 may enter these locations and block infection by these virions. In addition, UC781 pretreatment of cells renders them refractory to HIV-1 infection (6, 20), while CAP does not have such properties.
UC781 is potent in inhibiting in vitro HIV-1 infection (1). However, it may not be efficient in vivo against some primary HIV-1 isolates with preexisting resistance to NNRTIs. For example, HIV-1 group O strains are de novo resistant to current NNRTIs (10), but these viruses are susceptible to CAP-mediated antiviral activity (18). In the present study, we have demonstrated that CAP is effective in inhibiting infection by HIV-1 strain A17, which is highly resistant to UC781 and other NNRTIs (15, 32), suggesting that CAP can be used for preventing sexual transmission of NNRTI-resistant variants. Furthermore, UC781 has no documented activity against other STD pathogens. This shortcoming can be overcome by combining UC781 with CAP, since CAP has potent microbicidal activity against a broad spectrum of STD pathogens.
In summary, the combination of CAP and UC781 resulted in significant synergistic and complementary effects against HIV-1 infection. This was translated into meaningful dose reductions for each compound. These findings provide a strong rationale for developing combinations of microbicides with distinct mechanisms of action for the prevention of sexual transmission of HIV-1 and other STD pathogens.
Acknowledgments
We thank David Ho at Aaron Diamond AIDS Research Center, The Rockefeller University, for providing UC781 and Nathan Strick for preparing CAP solutions.
This work was supported by grants from the National Institutes of Health (HD41761 and HD48957).
REFERENCES
- 1.Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. Van Damme, M. Parniak, L. Van Mellaert, J. Anne, and E. De Clercq. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 12:1129-1138. [DOI] [PubMed] [Google Scholar]
- 2.Barnard, J., G. Borkow, and M. A. Parniak. 1997. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36:7786-7792. [DOI] [PubMed] [Google Scholar]
- 3.Bax, R., K. Douville, D. McCormick, M. Rosenberg, J. Higgins, and M. Bowden. 2002. Microbicides: evaluating multiple formulations of C31G. Contraception 66:365-368. [DOI] [PubMed] [Google Scholar]
- 4.Boadi, T., M. Ratterree, A. Gettie, A. R. Neurath, J. Blanchard, and C. Cheng-Mayer. 2004. Safety and efficacy of cellulose acetate phthalate (CAP) against vaginal transmission of simian/human immunodeficiency viruses in rhesus macaques, abstr. 02414. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 5.Borkow, G., J. Barnard, T. M. Nguyen, A. Belmonte, M. A. Wainberg, and M. A. Parniak. 1997. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckheit, R. W., Jr., M. Hollingshead, S. Stinson, V. Fliakas-Boltz, L. A. Pallansch, J. Roberson, W. Decker, C. Elder, S. Borgel, C. Bonomi, R. Shores, T. Siford, L. Malspeis, and J. P. Bader. 1997. Efficacy, pharmacokinetics, and in vivo antiviral activity of UC781, a highly potent, orally bioavailable nonnucleoside reverse transcriptase inhibitor of HIV type 1. AIDS Res. Hum. Retrovir. 13:789-796. [DOI] [PubMed] [Google Scholar]
- 7.Chou, T.-C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 8.Chou, T.-C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In T.-C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, San Diego, Calif.
- 9.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315-336. [DOI] [PubMed] [Google Scholar]
- 10.de Baar, M. P., W. Janssens, A. de Ronde, K. Fransen, R. Colebunders, L. Kestens, G. G. Van der, and J. Goudsmit. 2000. Natural residues versus antiretroviral drug-selected mutations in HIV type 1 group O reverse transcriptase and protease related to virological drug failure in vivo. AIDS Res. Hum. Retrovir. 16:1385-1394. [DOI] [PubMed] [Google Scholar]
- 11.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Fabio, S., J. Van Roey, G. Giannini, M. G. Van den, M. Spada, A. Binelli, M. F. Pirillo, E. Germinario, F. Belardelli, M. P. de Bethune, and S. Vella. 2003. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS 17:1597-1604. [DOI] [PubMed] [Google Scholar]
- 13.Fichorova, R. N., F. Zhou, V. Ratnam, V. Atanassova, S. Jiang, N. Strick, and A. R. Neurath. 2005. Anti-human immunodeficiency virus type 1 microbicide cellulose acetate 1,2-benzenedicarboxylate in a human in vitro model of vaginal inflammation. Antimicrob. Agents Chemother. 49:323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyotoku, T., L. Aurelian, and A. R. Neurath. 1999. Cellulose acetate phthalate (CAP): an “inactive” pharmaceutical excipient with antiviral activity in the mouse model of genital herpesvirus infection. Antivir. Chem. Chemother. 10:327-332. [DOI] [PubMed] [Google Scholar]
- 15.Hara, H., T. Fujihashi, T. Sakata, A. Kaji, and H. Kaji. 1997. Tetrahydronaphthalene lignan compounds as potent anti-HIV type 1 agents. AIDS Res. Hum. Retrovir. 13:695-705. [DOI] [PubMed] [Google Scholar]
- 16.Hogg, R. S., S. A. Rhone, B. Yip, C. Sherlock, B. Conway, M. T. Schechter, M. V. O'Shaughnessy, and J. S. Montaner. 1998. Antiviral effect of double and triple drug combinations amongst HIV-infected adults: lessons from the implementation of viral load-driven antiretroviral therapy. AIDS 12:279-284. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, S., H. Lu, S. Liu, Q. Zhao, Y. He, and A. K. Debnath. 2004. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob. Agents Chemother. 48:4349-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, S., H. Lu, Q. Zhao, G. Wallace, R. J. Shattock, and A. R. Neurath. 2004. Cellulose acetate phthalate inhibits infection by cell-free and cell-associated primary HIV-1 strains, abstr. 02183. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 19.Keller, M. J., M. E. Klotman, and B. C. Herold. 2003. Development of topical microbicides for prevention of human immunodeficiency virus and herpes simplex virus. Am. J. Reprod. Immunol. 49:279-284. [DOI] [PubMed] [Google Scholar]
- 20.Kiselyeva, Y., P. Watts, L. Margolis, and R. J. Shattock. 2004. UC781 protects ex vivo lymphoid tissue from HIV-1 infection, abstr. 02460. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 21.Krebs, F. C., S. R. Miller, D. Malamud, M. K. Howett, and B. Wigdahl. 1999. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir. Res. 43:157-173. [DOI] [PubMed] [Google Scholar]
- 22.Manson, K. H., M. S. Wyand, C. Miller, and A. R. Neurath. 2000. Effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 44:3199-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer, K. H., J. Peipert, T. Fleming, A. Fullem, T. Moench, S. Cu-Uvin, M. Bentley, M. Chesney, and Z. Rosenberg. 2001. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin. Infect. Dis. 32:476-482. [DOI] [PubMed] [Google Scholar]
- 24.Morrow, K., R. Rosen, L. Richter, A. Emans, A. Forbes, J. Day, N. Morar, L. Maslankowski, A. T. Profy, C. Kelly, S. S. Abdool Karim, and K. H. Mayer. 2003. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J. Womens Health 12:655-666. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima, K. A., D. A. Thompson, S. I. Rosenfield, P. J. Maddon, T. Dragic, and W. C. Olson. 2001. Human immunodeficiency virus type 1 entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J. Infect. Dis. 183:1121-1125. [DOI] [PubMed] [Google Scholar]
- 26.Neurath, A. R., and N. Strick. 2001. Quantitation of cellulose acetate phthalate in biological fluids as a complex with ruthenium red. Anal. Biochem. 288:102-104. [DOI] [PubMed] [Google Scholar]
- 27.Neurath, A. R., N. Strick, P. Haberfield, and S. Jiang. 1992. Rapid prescreening for antiviral agents against HIV-1 based on their inhibitory activity in site-directed immunoassays. II. Porphyrins reacting with the V3 loop of gp120. Antiv. Chem. Chemother. 31:55-63. [Google Scholar]
- 28.Neurath, A. R., N. Strick, S. Jiang, Y. Y. Li, and A. K. Debnath. 2002. Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of “dead-end” gp41 six-helix bundles. BMC Infect. Dis. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neurath, A. R., N. Strick, and Y. Y. Li. 2002. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect. Dis. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neurath, A. R., N. Strick, Y. Y. Li, and A. K. Debnath. 2001. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect. Dis. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neurath, A. R., N. Strick, Y.-Y. Li, K. Lin, and S. Jiang. 1999. Design of a “microbicide” for prevention of sexually transmitted disease using “inactive” pharmaceutical excipients. Biologicals 27:11-21. [DOI] [PubMed] [Google Scholar]
- 32.Nunberg, J. H., W. A. Schleif, E. J. Boots, J. A. O'Brien, J. C. Quintero, J. M. Hoffman, E. A. Emini, and M. E. Goldman. 1991. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J. Virol. 65:4887-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otten, R., D. Adams, C. Kim, E. Jackson, K. Lee, L. Grohskopf, M. Monsour, S. Butera, and T. Folks. 2004. Novel low-dose vaginal exposure strategy to study HIV microbicide interventions in non-human primate, abstr. MT-01. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 34.Ramos, A., C. Dezzutti, L. Paxton, L. Grohskopf, C. Hart, and S. Subbarao. 2004. In vitro anti-HIV-1 activity of microbicide formulations against non-subtype B primary strains, abstr. 02686. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 35.Sangeeta, S., M. Cowen, N. Kaganson, A. Nubb, S. McCormack, A. R. Neurath, and C. Lacey. 2004. An open phase I trial of 13% cellulose acetate phthalate (CAP) vaginal microbicide, abstr. 02092_1. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 36.Shattock, R. 2003. The rationale for combination microbicides: viral and cellular targets. Microb. Q. 1:1-5. [Google Scholar]
- 37.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 38.Stone, A. 2002. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discov. 1:977-985. [DOI] [PubMed] [Google Scholar]
- 39.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 40. UNAIDS/WHO 2003. AIDS epidemic update. Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization, Geneva, Switzerland.
- 41.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 42.Van Herrewege, Y., J. Michiels, J. Van Roey, K. Fransen, L. Kestens, J. Balzarini, P. Lewi, G. Vanham, and P. Janssen. 2004. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob. Agents Chemother. 48:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace, G., S. Jiang, P. GWatts, N. Strick, G. Griffin, A. R. Neurath, and R. J. Shattock. 2004. Cellulose acetate phthalate inhibits HIV-1 infection via different clades in cellular, dendritic cell and human cervical explant models, abstr. 02508. Abstr. Microbicides 2004 Conf., London, United Kingdom.
- 44.Zhao, Q., J. T. Ernst, A. D. Hamilton, A. K. Debnath, and S. Jiang. 2002. XTT formazan widely used to detect cell viability inhibits HIV type 1 infection in vitro by targeting gp41. AIDS Res. Hum. Retrovir. 18:989-997. [DOI] [PubMed] [Google Scholar]
- 45.Zussman, A., L. Lara, H. H. Lara, Z. Bentwich, and G. Borkow. 2003. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS 17:653-661. [DOI] [PubMed] [Google Scholar]