Abstract
Context
With age, the prevalence of subclinical hypothyroidism rises. However, incidence and determinants of spontaneous normalization remain largely unknown.
Objective
To investigate incidence and determinants of spontaneous normalization of TSH levels in older adults with subclinical hypothyroidism.
Design
Pooled data were used from the (1) pretrial population and (2) in-trial placebo group from 2 randomized, double-blind, placebo-controlled trials (Thyroid Hormone Replacement for Untreated Older Adults With Subclinical Hypothyroidism Trial and Institute for Evidence-Based Medicine in Old Age thyroid 80-plus thyroid trial).
Setting
Community-dwelling 65+ adults with subclinical hypothyroidism from the Netherlands, Switzerland, Ireland, and the United Kingdom.
Participants
The pretrial population (N = 2335) consisted of older adults with biochemical subclinical hypothyroidism, defined as ≥1 elevated TSH measurement (≥4.60 mIU/L) and a free T4 within the laboratory-specific reference range. Individuals with persistent subclinical hypothyroidism, defined as ≥2 elevated TSH measurements ≥3 months apart, were randomized to levothyroxine/placebo, of which the in-trial placebo group (N = 361) was included.
Main Outcome Measures
Incidence of spontaneous normalization of TSH levels and associations between participant characteristics and normalization.
Results
In the pretrial phase, TSH levels normalized in 60.8% of participants in a median follow-up of 1 year. In the in-trial phase, levels normalized in 39.9% of participants after 1 year of follow-up. Younger age, female sex, lower initial TSH level, higher initial free T4 level, absence of thyroid peroxidase antibodies, and a follow-up measurement in summer were independent determinants for normalization.
Conclusion
Because TSH levels spontaneously normalized in a large proportion of older adults with subclinical hypothyroidism (also after confirmation by repeat measurement), a third measurement may be recommended before considering treatment.
Trial registration
ClinicalTrials.gov, NCT01660126 and Netherlands Trial Register, NTR3851.
Keywords: thyrotropin, subclinical hypothyroidism, older adults, follow-up studies
With increasing age, circulating levels of TSH generally rise, accompanied by a higher prevalence of subclinical hypothyroidism (1, 2). Subclinical hypothyroidism is defined as an elevated TSH level while the serum free T4 (fT4) concentration is within the normal range (3). Several randomized controlled trials have shown that treatment of mild subclinical hypothyroidism in older adults does not improve clinical outcomes (4-9). Therefore, it has been suggested to reevaluate the reference range for TSH in older adults (10). To this end, we need to further explore the natural course of subclinical hypothyroidism in older adults. Interestingly, several studies in adults have shown that subclinical hypothyroidism can spontaneously normalize (11-18). However, it is not known what the most important determinants are for spontaneous TSH normalization in older adults. Enhanced understanding of the natural history and factors contributing to normalization may help clinical decision making on the follow-up strategy.
In this longitudinal study, we aimed to investigate the incidence of spontaneous normalization of TSH levels and identify determinants of normalization in a large group of adults aged 65 years and older with (persistent) subclinical hypothyroidism. We combined individual participant level data from 2 randomized trials investigating the effect of levothyroxine treatment in older adults with subclinical hypothyroidism: the Thyroid Hormone Replacement for Untreated Older Adults With Subclinical Hypothyroidism Trial (TRUST) and Institute for Evidence-Based Medicine in Old Age (IEMO) trials (4, 6, 19, 20). Because we were interested in spontaneous normalization, we only included the pretrial screening populations and the in-trial placebo groups of the 2 clinical trials.
Materials and Methods
Study Design
The present study pooled data from 2 randomized, double-blind, placebo-controlled parallel-group clinical trials investigating the effect of levothyroxine treatment for older adults with subclinical hypothyroidism: the TRUST and the IEMO 80-plus thyroid trial (4, 6). TRUST included community-dwelling participants aged 65 years and older in the Netherlands, Switzerland, Ireland, and the United Kingdom recruited between April 2013 and May 2015 (4, 19). Participants for the IEMO 80-plus thyroid trial were aged 80 years and older and recruited in the Netherlands and Switzerland between May 2014 and May 2017 (6, 20). Both trials shared a near-identical design and recruitment strategy. Trial protocols were approved by the relevant ethics committees and regulatory authorities in all the countries involved in the trials. The trials were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and participants provided written informed consent.
Inclusion of Study Participants
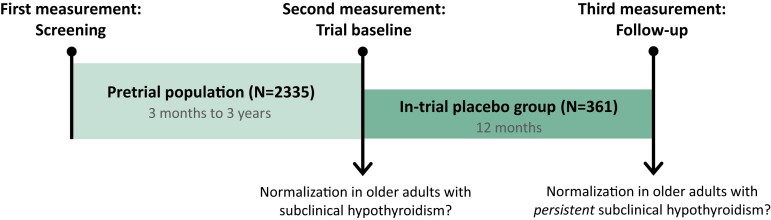
The timeline and the 2 populations of the present study are presented in Fig. 1 (Fig 1).
Figure 1.
Timeline of the study. The pretrial population consisted of older adults with ≥1 elevated TSH measurement (≥4.60 mIU/L) and an fT4 level within the laboratory-specific reference range, during the previous 3 months to 3 years (screening). The second measurement was at the trial baseline to assess whether they had persistent subclinical hypothyroidism or had normalized their TSH levels. The in-trial placebo group consisted of older adults with ≥2 elevated TSH measurements, both at the screening and trial baseline, receiving placebo in the clinical trials. After 12 months, at the (third) follow-up measurement, it was determined whether the in-trial placebo group had normalized their TSH levels.
Inclusion of pretrial population
Participants were identified from clinical laboratory databases and general practice records. Older adults with biochemical subclinical hypothyroidism, defined as an elevated TSH level (4.60-19.99 mIU/L) and an fT4 level within the laboratory-specific reference range, 3 months to 3 years before the trial baseline were invited for a repeated measurement. This repeated measurement was taken to assess whether they had persistent subclinical hypothyroidism and were eligible for trial inclusion. Individuals with 2 TSH measurements (the pretrial screening and the trial baseline) were included in the pretrial population. Participants recruited during the screening period in Switzerland were excluded because Swiss demographic data were only registered centrally for those who were randomized.
Inclusion of in-trial placebo group
Persistent subclinical hypothyroidism was defined as an elevated TSH level (4.60-19.99 mIU/L) and an fT4 level within the laboratory-specific reference range at both the pretrial screening (first measurement) and at the trial baseline (second measurement). Participants with persistent subclinical hypothyroidism who fulfilled the inclusion criteria were randomized to receive levothyroxine treatment or placebo in the clinical trials. For the present study, we only included the in-trial participants assigned to placebo treatment with mock titration. TSH normalization was checked at the follow-up visit after 300 to 400 days (third measurement).
Thyroid Status Measurements
For nearly all participants, TSH and fT4 measurements were performed at the same clinical laboratory or general practice where the initial measurement for identification of subclinical hypothyroidism was performed. A subsample (80.1%) of the in-trial placebo group donated blood for biobanking at randomization (a few weeks after the trial baseline), which was stored centrally at −80° C to be analyzed later. In these serum samples, thyroid peroxidase antibodies (anti-TPO) were measured using the Cobas E602 module from Roche, Almere, the Netherlands, performed in a single batch at the Department of Clinical Chemistry and Laboratory Medicine of the Leiden University Medical Center. The threshold for elevated anti-TPO is > 34 kU/L (detection range, 10-599.99 kU/L), the coefficient of variation was 11.3% to 15.6%.
Participant Characteristics
Pretrial population
Determinants of interest were age, sex, TSH level at first measurement, fT4 level at first measurement, and the interval between the first and second measurements (divided into <6 months, 6-12 months, > 12 months). To investigate whether season of follow-up testing has an influence on TSH normalization, the season of the second measurement (divided into meteorological summer [1 June-31 August], autumn [1 September-30 November], winter [1 December-28/29 February], and spring [1 March-31 May]) was assessed as a determinant.
In-trial placebo group
Determinants of interest for the in-trial placebo group were age, sex, TSH level at trial baseline, fT4 level at trial baseline, and anti-TPO positivity. Because the interval between the trial baseline and follow-up measurements for all participants in the in-trial placebo group was between 300 to 400 days, the interval and season of testing were not included as determinants.
Statistical Analyses
Summary statistics were estimated using median and interquartile range for continuous variables and number and percentage for categorical variables. Logistic regression was performed with TSH normalization as the outcome using the glm function in R. Univariable models were created for the determinants of interest separately and multivariable models were created with all determinants combined. All models were adjusted for country as fixed effect. To visualize the probability of normalization for each unit in TSH level, probability plots were created using logistic regression models with initial TSH level as independent determinant and TSH normalization as outcome, adjusted for country (United Kingdom as reference). For the pretrial phase, a sensitivity analysis was performed excluding the participants who had both measurements in the same season (N = 20%) to investigate the true effect of a follow-up measurement in a certain season compared to another season. Analyses were conducted in R version 4.1.2 (21) and figures were produced using GraphPad, Microsoft Word, and Microsoft PowerPoint.
Results
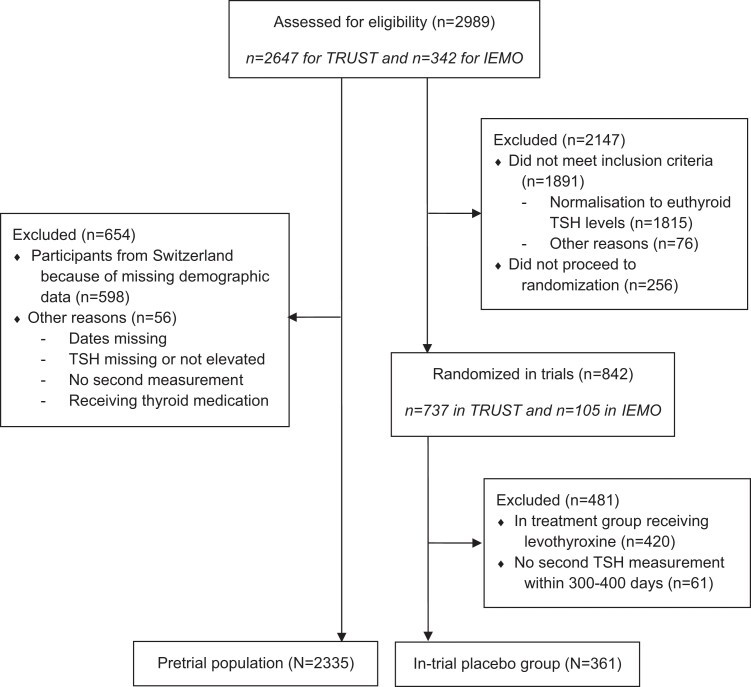
As presented in Fig. 2 (Fig 2), a total of 2989 older adults were identified from clinical laboratory databases and general practice records and assessed for eligibility for the TRUST or IEMO trials. Of these, 2335 participants were included in the pretrial population to investigate the incidence and determinants of spontaneous TSH normalization in older adults with at least 1 measurement of biochemical subclinical hypothyroidism. For the in-trial placebo group, we included participants who were randomized to placebo in 1 of the 2 trials and had a follow-up in-trial TSH measurement in 300 to 400 days. In total, 361 participants were included in the in-trial placebo group to investigate the incidence and determinants of spontaneous TSH normalization in older adults with persistent subclinical hypothyroidism (defined as at least 2 measurement of elevated TSH levels with normal fT4 levels more than 3 months apart).
Figure 2.
Flow diagram of study populations.
Pretrial Population: Older Adults With Biochemical Subclinical Hypothyroidism (N = 2335)
Characteristics of study population
The median (interquartile range[IQR]) age of the pretrial population was 72.9 (68.0-79.3) years and 60.7% of the participants were female (Table 1). Median (IQR) levels of TSH and fT4 at the first measurement (screening) were 5.40 (4.91-6.31) mIU/L and 13.6 (12.3-15.0) pmol/L, respectively. Most participants had their second measurement (trial baseline) in summer (32.7%) or autumn (31.5%) and were included in The Netherlands (50.3%) cohort.
Table 1.
Characteristics of older adults with subclinical hypothyroidism
| Characteristic | Pretrial population (N = 2335) |
|---|---|
| Age, y | 72.9 (68.0-79.3) |
| Age ≥80 y, n (%) | 535 (22.9) |
| Female, n (%) | 1418 (60.7) |
| TSH, mIU/L | |
| First measurement (screening) | 5.40 (4.91-6.31) |
| >7 mIU/L, n (%) | 368 (15.8) |
| >10 mIU/L, n (%) | 57 (2.4) |
| Second measurement (trial baseline) | 4.02 (3.01-5.42) |
| fT4, pmol/L | |
| First measurement (screening)a | 13.6 (12.3-15.0) |
| Second measurement (trial baseline) | 13.0 (12.0-14.6) |
| Interval between first and second measurements | 344 (207-594) |
| <6 mo, n (%) | 465 (19.9) |
| 6-12 mo, n (%) | 778 (33.3) |
| >12 mo, n (%) | 1092 (46.8) |
| Season of second measurement (trial baseline), n (%) | |
| Summer | 764 (32.7) |
| Autumn | 735 (31.5) |
| Winter | 318 (13.6) |
| Spring | 518 (22.2) |
| Country, n (%) | |
| United Kingdom | 494 (21.2) |
| Ireland | 667 (28.6) |
| The Netherlands | 1174 (50.3) |
Values shown are median (interquartile range) unless indicated otherwise.
Abbreviation: fT4, free T4.
a First fT4 measurement was missing for n = 10.
Normalization of subclinical hypothyroidism
From a total of 2335 participants, TSH levels normalized in 1419 (60.8%) participants in a median follow-up of 344 (IQR, 207-594) days. Lower age (odds ratio [OR], 0.98 [95% CI, .97-.99], P = .007), female sex (OR, 1.39 [95% CI, 1.15-1.69], P < .001), lower screening TSH level (OR, 0.57 [95% CI, .52-.62], P < .001), higher normal screening fT4 level (OR, 1.06 [95% CI, 1.01-1.11], P = .03), and a second measurement in summer (compared with winter: OR, 0.59 [95% CI, .44-.79], P < .001) were independently associated with a higher chance of TSH normalization (Table 2). An interval between measurements of more than 12 months was associated with TSH normalization (OR, 1.40 [95% CI, 1.07-1.82], P = .01), but this was not statistically significant in the multivariable model (Table 2). When restricted to participants having their second measurement in another season than the first (N = 1866), having the second measurement in summer was still independently associated with a higher chance of normalization (compared with winter: OR, 0.55 [95% CI, .39-.78], P < .001), data not shown.
Table 2.
Association between TSH normalization and characteristics in older adults with subclinical hypothyroidism from the pretrial population (N = 2335)
| Characteristic | Univariable model | Multivariable model | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (per year) | .98 (.97-.99) | <.001 | .98 (.97-.99) | .007 |
| Female sex | 1.39 (1.16-1.66) | <.001 | 1.39 (1.15-1.69) | <.001 |
| TSH level (per unit mIU/L) | .56 (.52-.61) | <.001 | .57 (.52-.62) | <.001 |
| fT4 level (per unit pmol/L) | 1.08 (1.04-1.13) | <.001 | 1.06 (1.01-1.11) | .03 |
| Interval between measurements | ||||
| <6 mo | 1 (reference) | 1 (reference) | ||
| 6-12 mo | 1.05 (.81-1.35) | .73 | 1.02 (.78-1.34) | .87 |
| >12 mo | 1.40 (1.07-1.82) | .01 | 1.30 (.97-1.72) | .07 |
| Season second measurement | ||||
| Summer | 1 (reference) | 1 (reference) | ||
| Autumn | .74 (.59-.93) | .008 | .72 (.57-.92) | .008 |
| Winter | .62 (.47-.82) | <.001 | .59 (.44-.79) | <.001 |
| Spring | .73 (.57-.92) | .009 | .73 (.56-.95) | .02 |
Odds ratios (OR) with 95% CIs resulting from logistic regression analyses. Univariable models were created for each characteristic separately and multivariable models were created with all characteristics combined. All analyses were adjusted for country.
Abbreviation: OR, odds ratio.
In-trial Placebo Group: Older Adults With Persistent Subclinical Hypothyroidism (N = 361)
Characteristics of study population
The median (IQR) age of the in-trial placebo group was 75.1 (69.6-81.4) years and 51.8% of the participants were female (Table 3). Median (IQR) levels of TSH and fT4 at the trial baseline measurement were 5.75 (5.10-6.86) mIU/L and 13.4 (12.1-14.7) pmol/L, respectively. One quarter (25.3%) of the participants had antibodies to TPO.
Table 3.
Characteristics of older adults with persistent subclinical hypothyroidism
| Characteristic | In-trial placebo group (N = 361) |
|---|---|
| Age, y | 75.1 (69.6-81.4) |
| Age ≥ 80 y, n (%) | 113 (31.3) |
| Female, n (%) | 187 (51.8) |
| TSH, mIU/L | |
| First measurement (screening) | 5.84 (5.20-7.17) |
| Second measurement (trial baseline) | 5.75 (5.10-6.86) |
| >7 mIU/L, n (%) | 77 (21.3) |
| >10 mIU/L, n (%) | 16 (4.4) |
| Third measurement (follow-up) | 4.91 (3.96-6.49) |
| fT4 at second measurement (trial baseline), pmol/L | 13.4 (12.1-14.7) |
| Anti-TPO positive, n (%)a | 73 (25.3) |
| Interval between second and third measurements | 362 (345-370) |
| Country, n (%) | |
| United Kingdom | 64 (17.8) |
| Ireland | 50 (13.9) |
| The Netherlands | 149 (41.3) |
| Switzerland | 98 (27.1) |
Values shown are median (interquartile range) unless indicated otherwise.
Abbreviations: fT4, free T4; anti-TPO, thyroid peroxidase antibodies.
a Information on TPO antibodies was missing for n = 72.
Normalization of persistent subclinical hypothyroidism
From a total of 361 participants, in 144 (39.9%) participants, TSH levels normalized in a median follow-up of 362 (IQR, 345-370) days. Lower age (OR, 0.96 [95% CI, .92-1.00], P = .05), female sex (OR, 1.80 [95% CI, 1.01-3.23], P = .05), lower trial baseline TSH level (OR, 0.52 [95% CI, .38-.67], P < .001), higher normal trial baseline fT4 levels (OR, 1.22 [95% CI, 1.05-1.44], P = .01), and the absence of TPO antibodies (OR, 0.36 [95% CI, .17-.77], P = .007) were independently associated with a higher chance of normalization (Table 4).
Table 4.
Association between TSH normalization and characteristics in older adults with persistent subclinical hypothyroidism from the in-trial placebo group (N = 361)
| Characteristic | Univariable model | Multivariable model | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (per year) | .99 (.96-1.02) | .59 | .96 (.92-1.00) | .05 |
| Female sex | 1.23 (.79-1.92) | .36 | 1.80 (1.01-3.23) | .05 |
| TSH level at trial baseline (per unit mIU/L) | .51 (.40-.63) | <.001 | .52 (.38-.67) | <.001 |
| fT4 level at trial baseline (per unit pmol/L) | 1.32 (1.17-1.50) | <.001 | 1.22 (1.05-1.44) | .01 |
| Anti-TPO positivity× | .38 (.19-.72) | .004 | .36 (.17-.77) | .007 |
Odds ratios (OR) with 95% CIs resulting from logistic regression analyses. Univariable models were created for each characteristic separately and multivariable models were created with all characteristics combined. All analyses were adjusted for country. ×Information on TPO antibodies was missing for n = 72.
Abbreviation: anti-TPO, thyroid peroxidase antibodies.
TSH Level as Determinant of Normalization of Subclinical Hypothyroidism
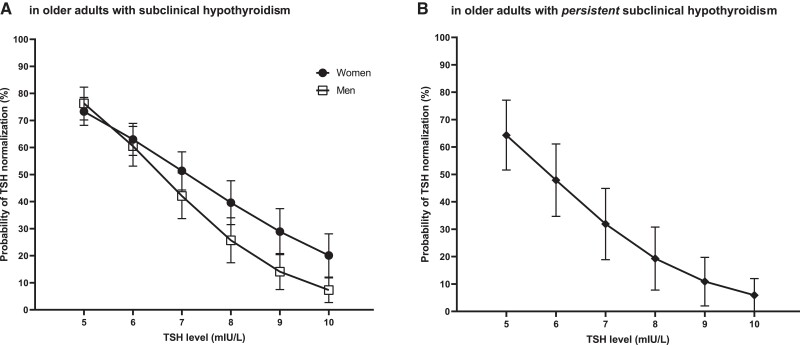
Figure 3 visualizes that the probability of TSH normalization decreases for each unit in TSH level from 5 to 10 mIU/L in both populations. In the pretrial population, there was an interaction between TSH screening level and sex (P = .004) showing that the probability of normalization tended to decrease more for men than for women (Fig 3A). As an example, older men with biochemical subclinical hypothyroidism with a screening TSH level of 5 mIU/L have a 76.3% (95% CI, 70.2-82.3) chance of normalization, whereas the chance becomes 7.3% (95% CI, 2.7-11.8) when having a screening TSH level of 10 mIU/L. For older women, the chance of TSH normalization decreases from 73.3% (95% CI, 68.2-78.5) at 5 mIU/L to 20.1% (95% CI, 12.1-28.1) at 10 mIU/L. Older adults who already had 2 elevated TSH measurements more than 3 months apart still have a 64.3% (95% CI, 51.6-77.1) chance to normalize when their second (trial baseline) TSH level is 5 mIU/L (Fig 3B). However, when the second (trial baseline) TSH level is 10 mIU/L in older adults with persistent subclinical hypothyroidism, then the chance of normalization is only 5.9% (95% CI, .0-12.0).
Figure 3.
Probability of TSH normalization (95% CI) based on initial TSH level in older adults with (persistent) subclinical hypothyroidism. (A) Probability of normalization stratified for sex in the pretrial population (N = 2335), which includes older adults with subclinical hypothyroidism. (B) Probability of normalization in the in-trial placebo group (N = 361), which includes older adults with persistent subclinical hypothyroidism, defined as having at least 2 measurements of elevated TSH levels ≥3 months apart.
Discussion
In this study, we aimed to investigate the incidence and determinants of spontaneous TSH normalization in a large group of adults aged 65 years and older with (persistent) subclinical hypothyroidism. In 60.8% of the older adults with biochemical subclinical hypothyroidism based on at least 1 elevated TSH measurement, TSH levels had returned to the normal range without intervention after a median follow-up of 1 year. Subsequently, TSH levels had still normalized after 1 year in 39.9% of older adults with persistent subclinical hypothyroidism, defined as at least 2 elevated TSH measurements more than 3 months apart. Younger age, female sex, lower initial TSH level, higher normal initial fT4 level, the absence of TPO antibodies, and a second measurement in summer were independent determinants for TSH normalization.
The large proportions of normalization of TSH levels in older adults with subclinical hypothyroidism after approximately 1 year in the present study are in line with percentages of 35% to 70% during a follow-up of 0.5 to 5 years found in studies in adults aged 55 years and older (11-15, 17). In 3 of those studies, a lower initial TSH levels was found to be the strongest determinant for normalization (11, 13, 15). A lower initial TSH level as a determinant for TSH normalization can be statistically explained by regression to the mean, but it can also be reasoned that mildly elevated TSH levels normalize easier than higher levels, especially considering normal fluctuations in TSH levels (22). These fluctuations within an individual over time are caused by both internal and external factors, such as pulsatile secretion, the biological clock, illness, and medication use (22-24). The association between high normal fT4 levels and TSH normalization was also found by Díez and Iglesias (11), and was expected given the negative feedback of thyroid hormones on TSH secretion. TPO antibodies generally associate with higher TSH levels (25), which might explain anti-TPO negativity as a determinant for normalization as confirmed by 2 studies (11, 15), but not by others (13). Although older women are generally more affected by (subclinical) hypothyroidism and tend to have higher TSH levels than older men (26, 27), we found in our study that women have a higher probability for normalization than men, especially at a higher TSH level. TSH levels generally rise with age (1), so older age coincides with higher TSH levels, which might explain the association found between older age and a lower chance of TSH normalization. However, in 3 studies with older adults with subclinical hypothyroidism without known previous thyroid disease, no relation was observed between sex, age, and TSH normalization (11, 13, 15). A second measurement in summer was associated with normalization, which is in line with a study in individuals aged 18 to 90 years (28). TSH levels are subject to change of season with highest levels in winter (22), which is probably caused by changes in environmental temperature (29).
The present study is a large, multicenter study in which adults aged 65 years and older, enriched for adults aged 80+, with mild subclinical hypothyroidism were included. Strengths of this study are that changes in TSH levels were observed over a long follow-up time ranging from 3 months to 4 years, without intervention. Another strength of this study is that we compared TSH normalization after at least 1 elevated TSH measurement and after at least 2 elevated TSH measurements. Measurements follow real-world practice because all thyroid measurements were performed in routine clinical care, thus preventing potential degradation of TSH by long-term sample storage and/or freeze/thaw cycles. Although biochemical assays differed between clinical centers, baseline and follow-up measurements were performed in the same clinical centers for nearly all participants. Unfortunately, time of blood sampling was not recorded. Selection bias could play a role in the pretrial screening population because individuals who became overt hypothyroid or started with thyroid medication for other reasons were not enrolled in the study. Therefore, we were not able to investigate the incidence and determinants of the progression of subclinical hypothyroidism to overt hypothyroidism in the pretrial screening population. Almost all older subjects had mild subclinical hypothyroidism (TSH < 10 mIU/L) in our study, which limits the generalizability to higher TSH levels. With age, the TSH distribution shift toward a higher level, and the 97.5th percentile of the TSH distribution was shown to be 7.5 mU/L in subjects aged 80 years and older (30).
In this study, we have demonstrated that in a large proportion of older adults with mild subclinical hypothyroidism, TSH levels spontaneously normalized in a median follow-up of 1 year, even after 2 consecutive measurements (≥3 months apart) of elevated TSH levels. These results have clinical relevance. Current guidelines recommend that a single measurement of elevated serum TSH, with fT4 within reference range, should be confirmed by a repeat measurement of both serum TSH and fT4, along with thyroid peroxidase antibodies, after a 2- to 3-month interval (31). However, international guidelines differ in their recommendations on the TSH threshold at which treatment of subclinical hypothyroidism should be considered in older adults (32). Once levothyroxine treatment is initiated, it is often continued lifelong (33). Based on the observation that with age, the TSH distribution shifts toward a higher level, it has been proposed to extend the upper limit of the TSH reference to 7 mU/L for people aged 80 years or older (34). Although increasing the upper reference limit will likely reduce unnecessary levothyroxine prescribing for older people, instead of a binary approach, our results support applying more continuous age-related reference ranges, which may be modified by other factors such as sex and season. Moreover, based on the high incidence of spontaneous normalization of TSH levels in a large proportion of older adults with subclinical hypothyroidism (also after confirmation by repeat measurement), a third measurement may be recommended before considering initiation of treatment. Based on such an approach, the frequency of the diagnosis subclinical hypothyroidism can likely be reduced in older adults. This could potentially contribute to a reduction in health care costs, treatment burden, and risk of overtreatment. These results may also have implications for follow-up studies. Levothyroxine is among the most frequently prescribed drugs. Although general practitioner prescription practices vary widely between countries (35), many older adults have initiated levothyroxine treatment based on a mildly elevated TSH. Moreover, in clinical practice, treatment has often been started after a single measurement of TSH (33). A recent meta-analysis indicated that deprescribing levothyroxine could be successful for carefully selected patients, although the quality of available evidence was considered low (36). This underscores the need for future high-quality studies to assess the effects of safely withholding levothyroxine treatment in older adults who initiated levothyroxine treatment based on a diagnosis of mild subclinical hypothyroidism on relevant patient outcomes (36).
Abbreviations
- fT4
free T4
- IEMO
Institute for Evidence-Based Medicine in Old Age
- IQR
interquartile range
- OR
odds ratio
- TPO
thyroid peroxidase
- TRUST
Thyroid Hormone Replacement for Untreated Older Adults With Subclinical Hypothyroidism Trial
Contributor Information
Evie van der Spoel, Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Nicolien A van Vliet, Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Rosalinde K E Poortvliet, Department of Public Health and Primary Care, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Robert S Du Puy, Department of Public Health and Primary Care, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Wendy P J den Elzen, Amsterdam UMC, University of Amsterdam, Department of Clinical Chemistry, Amsterdam Public Health Research Institute, 1081 HV Amsterdam, The Netherlands.
Terence J Quinn, Department of Geriatric Medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow G4 0SF, UK.
David J Stott, Department of Geriatric Medicine, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow G4 0SF, UK.
Naveed Sattar, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow G12 8TA, UK.
Patricia M Kearney, School of Public Health, University College Cork, Cork T12 K8AF, Ireland.
Manuel R Blum, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern, 3008 Bern, Switzerland; Institute of Primary Health Care (BIHAM), University of Bern, 3012 Bern, Switzerland.
Heba Alwan, Institute of Primary Health Care (BIHAM), University of Bern, 3012 Bern, Switzerland; Graduate School for Health Sciences, University of Bern, Mittelstrasse 43, 3012 Bern, Switzerland.
Nicolas Rodondi, Institute of Primary Health Care (BIHAM), University of Bern, 3012 Bern, Switzerland; Graduate School for Health Sciences, University of Bern, Mittelstrasse 43, 3012 Bern, Switzerland.
Tinh-Hai Collet, Service of Endocrinology, Diabetes, Nutrition and Therapeutic Education, Department of Medicine, Geneva University Hospitals, 1205 Geneva, Switzerland; Diabetes Centre, Faculty of Medicine, University of Geneva, 1206 Geneva, Switzerland.
Rudi G J Westendorp, Department of Public Health, University of Copenhagen, 1353 Copenhagen, Denmark; Center for Healthy Aging, University of Copenhagen, 2200 Copenhagen, Denmark.
Bart E Ballieux, Department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
J Wouter Jukema, Department of Cardiology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Netherlands Heart Institute, 3511 EP Utrecht, The Netherlands.
Olaf M Dekkers, Department of Internal Medicine, Section of Endocrinology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Department Clinical Epidemiology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Jacobijn Gussekloo, Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Department of Public Health and Primary Care, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Simon P Mooijaart, Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Diana van Heemst, Department of Internal Medicine, Section of Gerontology and Geriatrics, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Funding
The TRUST trial was supported by research grant (278148) from the European Commission FP7-HEALTH-2011 program and by grants from the Swiss National Science Foundation (SNSF 320030-150025 to N.D.) and the Swiss Heart Foundation and Velux Stiftung (grant 974a to N.D.). The IEMO trial was supported by research grant (627001001) from ZonMw and by grants from the Swiss National Science Foundation (SNSF 320030-150025 and 320030-172676 to N.D.). N.D.’s research on thyroid is funded by a grant from the Swiss National Science Foundation (32003B_200606). M.R.B.'s work is funded by grants from the Swiss National Science Foundation (P2BEP3-175289, 32003B_205067) and the Swiss Heart Foundation (FF21106). T.-H.C.'s research was funded by a grant from the Swiss National Science Foundation (PZ00P3-167826). Study medication (levothyroxine and matching placebo) was supplied free of charge by Merck KGaA. The research of E.v.d.S., N.A.v.V., and D.v.H. was funded by the European Commission project THYRAGE (Horizon 2020, 666869). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors have contributed substantially to the conception or design of the work, or the acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; and final approval of the version published.
Disclosures
All authors have declared that they have no conflicts of interest to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554‐1562. [DOI] [PubMed] [Google Scholar]
- 2. Calsolaro V, Niccolai F, Pasqualetti G, et al. Overt and subclinical hypothyroidism in the elderly: when to treat? Front Endocrinol (Lausanne). 2019;10:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: A review. JAMA. 2019;322(2):153‐160. [DOI] [PubMed] [Google Scholar]
- 4. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376(26):2534‐2544. [DOI] [PubMed] [Google Scholar]
- 5. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham elderly thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623‐3632. [DOI] [PubMed] [Google Scholar]
- 6. Mooijaart SP, Du Puy RS, Stott DJ, et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA. 2019;322(20):1977‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villar HC, Saconato H, Valente O, et al. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev. 2007;2007(3):Cd003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: A systematic review and meta-analysis. Jama. 2018;320(13):1349‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross DS. Treating hypothyroidism is not always easy: when to treat subclinical hypothyroidism, TSH goals in the elderly, and alternatives to levothyroxine monotherapy. J Intern Med. 2022;291(2):128‐140. [DOI] [PubMed] [Google Scholar]
- 10. Walsh JP. Thyroid function across the lifespan: do age-related changes matter? Endocrinol Metab (Seoul). 2022;37(2):208‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Díez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metabol 2004; 89(10):4890‐4897. [DOI] [PubMed] [Google Scholar]
- 12. Gussekloo J, van Exel E, de Craen AJ, et al. Thyroid status, disability and cognitive function, and survival in old age. Jama. 2004;292(21):2591‐2599. [DOI] [PubMed] [Google Scholar]
- 13. Imaizumi M, Sera N, Ueki I, et al. Risk for progression to overt hypothyroidism in an elderly Japanese population with subclinical hypothyroidism. Thyroid. 2011;21(11):1177‐1182. [DOI] [PubMed] [Google Scholar]
- 14. Roberts L, McCahon D, Johnson O, et al. Stability of thyroid function in older adults: the Birmingham Elderly Thyroid Study. Br J Gen Pract. 2018;68(675):e718‐e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Somwaru LL, Rariy CM, Arnold AM, et al. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012;97(6):1962‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: A review of the literature. J Am Geriatr Soc. 2015;63(8):1663‐1673. [DOI] [PubMed] [Google Scholar]
- 17. Jaeschke R, Guyatt G, Gerstein H, et al. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? J Gen Intern Med. 1996;11(12):744‐749. [DOI] [PubMed] [Google Scholar]
- 18. Meyerovitch J, Rotman-Pikielny P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167(14):1533‐1538. [DOI] [PubMed] [Google Scholar]
- 19. Stott DJ, Gussekloo J, Kearney PM, et al. Study protocol; Thyroid hormone Replacement for Untreated older adults with Subclinical hypothyroidism—a randomised placebo controlled trial (TRUST). BMC Endocr Disord. 2017;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du Puy RS, Postmus I, Stott DJ, et al. Study protocol: a randomised controlled trial on the clinical effects of levothyroxine treatment for subclinical hypothyroidism in people aged 80 years and over. BMC Endocr Disord. 2018;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 22. van der Spoel E, Roelfsema F, van Heemst D. Within-Person variation in Serum thyrotropin concentrations: main sources, potential underlying biological mechanisms, and clinical implications. Front Endocrinol (Lausanne) 2021; 12:619568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23(6):793‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weeke J, Gundersen HJ. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol (Copenh) 1978;89(4):659‐672. [DOI] [PubMed] [Google Scholar]
- 25. Roos A, Links TP, de Jong-van den Berg LT, et al. Thyroid peroxidase antibodies, levels of thyroid stimulating hormone and development of hypothyroidism in euthyroid subjects. Eur J Intern Med. 2010;21(6):555‐559. [DOI] [PubMed] [Google Scholar]
- 26. Wang D, Yu S, Ma C, et al. Reference intervals for thyroid-stimulating hormone, free thyroxine, and free triiodothyronine in elderly Chinese persons. Clin Chem Lab Med. 2019;57(7):1044‐1052. [DOI] [PubMed] [Google Scholar]
- 27. Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363(9411):793‐803. [DOI] [PubMed] [Google Scholar]
- 28. Kim TH, Kim KW, Ahn HY, et al. Effect of seasonal changes on the transition between subclinical hypothyroid and euthyroid status. J Clin Endocrinol Metab. 2013;98(8):3420‐3429. [DOI] [PubMed] [Google Scholar]
- 29. Kuzmenko NV, Tsyrlin VA, Pliss MG, et al. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: a meta-analysis. Chronobiol Int. 2021;38(3):301‐317. [DOI] [PubMed] [Google Scholar]
- 30. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575‐4582. [DOI] [PubMed] [Google Scholar]
- 31. Pearce SH, Brabant G, Duntas LH, et al. ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biondi B, Cappola AR. Subclinical hypothyroidism in older individuals. Lancet Diabetes Endocrinol. 2022;10(2):129‐141. [DOI] [PubMed] [Google Scholar]
- 33. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 34. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322(20):1961‐1962. [DOI] [PubMed] [Google Scholar]
- 35. den Elzen WP, Lefebre-van de Fliert AA, Virgini V, et al. International variation in GP treatment strategies for subclinical hypothyroidism in older adults: a case-based survey. Br J Gen Pract. 2015;65(631):e121‐e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burgos N, Toloza FJK, Singh Ospina NM, et al. Clinical outcomes after discontinuation of thyroid hormone replacement: A systematic review and meta-analysis. Thyroid. 2021;31(5):740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.