Abstract
Background
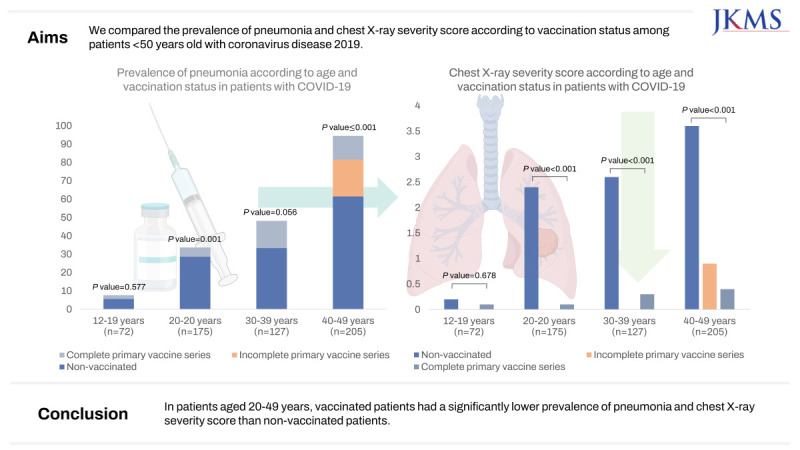
Coronavirus disease 2019 (COVID-19) vaccination is effective in preventing the disease transmission and progression. However, the relatively mild disease course of the omicron variant and the decrease in antibodies over time after vaccination raise questions about the effectiveness of vaccination, especially in young people. We compared the prevalence of pneumonia and chest X-ray severity score according to vaccination status among patients < 50 years old with COVID-19.
Methods
From January 17 to March 17, 2022, 579 patients with COVID-19, who were < 50 years old and had a known vaccination history in our institution, were all included in this study. All patients underwent initial chest radiography, and follow-up chest radiographs were obtained every two days until discharge. Pneumonia was scored from the radiographs using the Brixia scoring system. The scores of the six lung zones were added for a total score ranging from 0 to 18. Patients were divided into four groups according to 10-year age intervals. Differences between groups were analyzed using the χ2 or Fisher’s exact tests for categorical variables and the Kruskal–Wallis test or analysis of variance for continuous variables.
Results
Among patients aged 12–19 years, the prevalence of pneumonia did not differ depending on vaccination status (non-vaccinated vs. vaccinated, 1/47 [2.1%] vs. 1/18 [5.6%]; P = 0.577). Among patients in their 20s, the prevalence of pneumonia was significantly higher among non-vaccinated patients than among vaccinated patients (8/28, 28.6% vs. 7/138, 5.1%, P < 0.001), similar to patients in their 40s (32/52 [61.5%] vs. 18/138 [13.0%]; P < 0.001). The chest X-ray severity score was also significantly higher in non-vaccinated patients than that in vaccinated patients in their 20s to their 40s (P < 0.001), but not among patients aged 12–19 years (P = 0.678).
Conclusion
In patients aged 20–49 years, vaccinated patients had a significantly lower prevalence of pneumonia and chest X-ray severity score than non-vaccinated patients.
Keywords: COVID-19, Vaccination, Pneumonia Frequency, Chest X-Ray Severity
Graphical Abstract
INTRODUCTION
Since the start of the coronavirus disease 2019 (COVID-19) pandemic, several mutant variants have emerged, including the alpha, delta, and omicron variants.1 In South Korea, the omicron variant spread rapidly in December 2021 and became dominant.2 The omicron variant has a relatively milder disease course and is more contagious than other variants.3
COVID-19 vaccines effectively prevent infection and improve the prognosis, especially in older adults and individuals with underlying diseases.4,5,6 A vaccine booster is also effective against the omicron variant.7 The US Centers for Disease Control and Prevention (CDC) recommended COVID-19 vaccines for individuals aged 6 years and older and boosters for individuals aged 65 years and older.8 However, the recommendation of COVID-19 vaccination of children and young adults was met with considerable resistance anxiety in the early days of vaccine administration owing to reported serious side effects, such as myocarditis.9
Data concerning the prognosis and prevalence of pneumonia according to vaccination status in young patients with the omicron variant are lacking. Recent studies have suggested that vaccination is less effective for the omicron variant than for the delta variant; however, data on the effectiveness of the vaccine for the omicron variant, specifically for younger patients, are lacking.10,11
Therefore, we aimed to investigate the prevalence and chest X-ray severity score of individuals < 50 years old with the omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) according to vaccination status. In addition, we investigated the progression to severe disease course as a secondary outcome.
METHODS
Patients
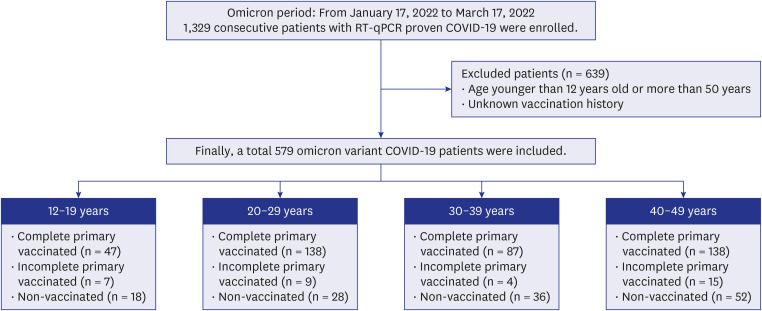
In South Korea, the omicron variant became the dominant SARS-CoV-2 variant in January 2022.12 From January 17, 2022 to March 17, 2022, 1,329 patients diagnosed with COVID-19 via real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) of sputum or nasal secretions were admitted to our hospital. We excluded 639 patients > 50 years or < 12 years and 111 patients with an unknown vaccination history. Children < 12 years old were excluded as the vaccination rate in that population was approximately 1%. In March 2022, at the start of the study period, vaccination was allowed for individuals > 12 years; hence, the teen group comprised patients aged 12–19 years.
Finally, 579 patients were included in this study (Fig. 1). In our institution, patients who progressed to severe disease and required high-flow oxygen or mechanical ventilation were transferred to tertiary hospitals.
Fig. 1. Flow diagram of patients with COVID-19 (omicron variant) in the four age groups.
COVID-19 = coronavirus disease 2019, RT-qPCR = reverse transcriptase-real time polymerase chain reaction.
Vaccination status
The patient’s vaccination history was obtained through an interview upon admission, and it was further verified through the COVID-19 vaccination verification system on the patient’s mobile phone. Patients who could not recall their vaccination history accurately checked it on the Korea Disease Control and Prevention Agency (KDCA)’s COVID-19 information management system.
Because booster vaccinations were not widely administered in the general population during the study period, only a few patients had received booster shots, and we did not include booster vaccination status in the study. Patients were classified as not vaccinated, incomplete primary vaccine series, or complete primary vaccine series. According to a previous study,13 patients with a positive SARS-CoV-2 RT-qPCR test result at ≥ 14 days after receipt of the first vaccine dose and before receipt of the second dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca), the BNT162b2 vaccine (Pfizer–BioNTech), or the mRNA-1273 vaccine (Moderna) were classified as incomplete primary vaccine series. Patients with positive test results at ≥ 14 days after receipt of the second vaccine dose or ≥ 14 days after receipt of the first dose of the Ad26.COV2.S vaccine (Johnson & Johnson–Janssen) were classified as complete primary vaccine series.
Clinical data extraction
Patients’ demographic data, underlying diseases, and initial laboratory results were reviewed. We divided patients into four age groups: 12–19, 20–29, 30–39, and 40–49 years. We also investigated whether patients improved and were discharged, or their condition worsened and they were transferred to a tertiary hospital.
Chest radiograph scoring
A digital radiography system (INNOVISION; DK Medical Systems Co., Ltd., Seoul, Korea) was used, and most chest radiographs were obtained with the patients in the erect position (posterior-anterior projection) except for patients who could not stand. All patients underwent initial chest radiography on the day of admission or the next day. Follow-up chest radiographs were obtained every two or three days until discharge. Pneumonia was diagnosed when ground glass opacities or consolidation compatible with pneumonia was observed on a chest radiograph during hospitalization. A single expert chest radiologist (12 years of experience), blinded to the patient’s clinical information, reviewed and scored the chest radiographs.
Pneumonia was scored from the chest radiographs (the highest pneumonia score for each patient’s stay) by an expert radiologist (11 years of experience with chest radiographs), blinded to the patient’s clinical information, using the Brixia scoring system. The radiograph fields were divided into six zones: upper, middle, and lower lung zones on each side. The lung abnormalities in each zone were scored from 0 to 3 (0: no lung abnormalities, 1: interstitial infiltrates, 2: interstitial and alveolar infiltrates [interstitial predominance], 3: interstitial and alveolar infiltrates [alveolar predominance]). The scores of the six lung zones were added together for a total score ranging from 0 to 18.14
Risk factors of pneumonia occurrence
Patients with risk factors were defined as those who had one or more of the following conditions, based on previous studies15,16: body mass index of ≥ 30, diabetes, chronic respiratory diseases, chronic liver disease, cerebrovascular disease, chronic heart disease, chronic kidney disease, cancer, or a history of transplant. Age was not considered in this analysis because our research targeted patients < 50 years old. Multivariate analysis was performed with various factors, including risk factors, age, and vaccination status.
Statistical analysis
Categorical variables are presented as frequencies with percentages, and continuous variables as means and standard deviations. Differences between groups were analyzed using the χ2 or Fisher’s exact tests for categorical variables and the Kruskal–Wallis test or analysis of variance for continuous variables. Odds ratio (OR) and multivariable regression analysis were employed to investigate factors associated with pneumonia. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Two-sided P values < 0.05 were considered statistically significant.
Ethics statement
This single-center, retrospective cohort study was approved by our Institutional Review Board (IRB No. 2023-07-040), which waived the requirement to obtain informed patient consent.
RESULTS
Patient characteristics
A total of 579 patients (mean age: 33 years, 272 men and 307 women) were included in this study. Seventy-two patients were aged 12–19 years, 175 were aged 20–29 years, 127 were aged 30–39 years, and 205 were aged 40–49 years. Underlying diseases, laboratory test results, duration from symptom onset to hospitalization, use of antiviral agents, antibiotics, oxygen usage, and the vaccination status of patients in each age group are summarized in Table 1. We also represented clinical data based on vaccine status (Supplementary Table 1).
Table 1. Clinical data of COVID-19 patients according to the age groups.
| Clinical data | 12–19 years (n = 72) | 20–29 years (n = 175) | 30–39 years (n = 127) | 40–49 years (n = 205) | P value | |
|---|---|---|---|---|---|---|
| Sex, male | 35 (48.6) | 82 (46.9) | 60 (47.2) | 95 (46.3) | 0.990 | |
| Underlying disease | ||||||
| Hypertension | 1 (1.4) | 4 (2.3) | 13 (10.2) | 26 (12.7) | < 0.001 | |
| Diabetes | 0 (0.0) | 1 (0.6) | 3 (2.4) | 10 (4.9) | 0.031 | |
| Hyperlipidemia | 0 (0.0) | 2 (1.1) | 7 (5.6) | 19 (9.3) | 0.001 | |
| Chronic lung disease | 4 (5.6) | 11 (6.4) | 6 (4.8) | 8 (3.9) | 0.728 | |
| Cardiovascular disease | 0 (0.0) | 2 (1.1) | 0 (0.0) | 5 (2.4) | 0.243 | |
| Neurovascular disease | 1 (1.4) | 0 (0.0) | 3 (2.4) | 11 (5.4) | 0.005 | |
| Chronic liver disease | 0 (0.0) | 0 (0.0) | 1 (0.8) | 4 (2.0) | 0.236 | |
| Chronic kidney disease | 1 (1.4) | 3 (1.7) | 1 (0.8) | 3 (1.5) | 0.959 | |
| Autoimmune disease | 0 (0.0) | 3 (1.7) | 7 (5.6) | 6 (2.9) | 0.113 | |
| Malignancy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (5.4) | < 0.001 | |
| Laboratory findings | ||||||
| WBC count, ×103 cells/µL | 4.460 (3.748–5.710) | 4.430 (3.717–5.618) | 4.720 (3.650–5.940) | 4.440 (3.465–5.465) | 0.354 | |
| Hemoglobin, g/dL | 13.6 (12.6–14.8) | 13.6 (12.58–14.7) | 13.7 (12.6–14.8) | 13.35 (12.6–14.4) | 0.586 | |
| Platelet, ×103 cells/µL | 214.5 (179.3–252.8) | 211.5 (177.0–245.0) | 208.0 (169.0–260.0) | 206.0 (170.0–243.0) | 0.247 | |
| Absolute neutrophil count, cells/µL | 2,465.2 (1,860–3,573.1) | 2,597.8 (1,880.9–3,516.2) | 2,730.5 (1,872.1–3,749.8) | 2,569.7 (1,820.5–3,531.0) | 0.901 | |
| Absolute lymphocyte count, cells/µL | 1,353.0 (1,069.2–1,684.3) | 1,311.0 (993.8–1,637.7) | 1,343.2 (1,038.2–1,720.6) | 1,228.9 (936.9–1,517.2) | 0.018 | |
| BUN, mg/dL | 12 (10–14) | 12 (10–14) | 12 (10–14) | 12 (10–15.8) | 0.172 | |
| Creatinine, mg/dL | 0.72 (0.59–0.83) | 0.76 (0.61–0.88) | 0.76 (0.64–0.91) | 0.77 (0.63–0.90) | 0.108 | |
| CRP, mg/dL | 0.51 (0.2–1.28) | 0.64 (0.14–1.49) | 0.68 (0.19–1.76) | 0.87 (0.22–1.83) | 0.239 | |
| LDH, U/L | 335.3 (286.9–379.4) | 314.7 (285.1–367.9) | 327.9 (293.9–411.4) | 337.1 (298.2–396.9) | 0.048 | |
| Time duration from last vaccination date to diagnosis, daysa | 70 (38.5–126.0) | 93 (51–127) | 94.5 (44.5–118.5) | 100 (69.3–126) | 0.328 | |
| Duration from symptom onset to hospitalization, daysb | 2.7 (2.3–3.0) | 3.2 (2.9–3.5) | 3.7 (3.3–4.2) | 3.6 (3.2–3.9) | 0.006 | |
| Use of antiviral agents | 1 (1.4) | 10 (5.7) | 15 (11.8) | 26 (12.7) | 0.002 | |
| Use of antibiotics | 25 (34.7) | 27 (15.4) | 20 (15.7) | 42 (20.5) | 0.006 | |
| Oxygen usage | 0 (0.0) | 1 (0.6) | 3 (2.4) | 4 (2.0) | 0.123 | |
| Vaccination status | 0.044 | |||||
| Non-vaccinated | 18 (25.0) | 28 (16.0) | 36 (28.3) | 52 (25.4) | ||
| Incomplete primary vaccine series | 7 (9.7) | 9 (5.1) | 4 (3.1) | 15 (7.3) | ||
| Complete primary vaccine series | 47 (65.3) | 138 (78.9) | 87 (68.5) | 138 (67.3) | ||
Data are presented as the number of patients (%) or median (25th-75th percentiles).
COVID-19 = coronavirus disease 2019, WBC = white blood count, BUN = blood urea nitrogen, CRP = C-reactive protein, LDH = lactate dehydrogenase.
aAmong the 445 vaccinated patients, information about the date of the last vaccination was available for 379.
bPatients with no symptoms (6 patients in their 20s, and 2 patients each in their 30s and 40s) were excluded from the analysis.
Hypertension, diabetes, hyperlipidemia, neurovascular disease, and malignancy were significantly more prevalent in older age groups (all P < 0.05). The vaccination rate significantly differed according to age group (P = 0.044). Vaccination was most common in the 20s age group (78.9%), and non-vaccination in the 30s age group (28.3%); the teenage group (9.7%) had the highest rate of incomplete primary vaccine series than others (the time from the last vaccination to diagnosis did not significantly differ between age groups (70–100 days; P = 0.328).
Prevalence of pneumonia according to age and vaccination status
Among the 579 patients, 274 were diagnosed with pneumonia upon chest radiography. The prevalence of pneumonia according to age and vaccination status are summarized in Table 2. The prevalence of pneumonia was significantly higher in older patients (12–19 years, 2.8%; 20s, 8.6%; 30s, 19.7%; and 40s, 25.9%; P < 0.001). In the 12–19 years group, most patients exhibited no evidence of pneumonia, and the pneumonia status did not significantly differ according to vaccination status (P = 0.577). In the 20s and 40s age groups, the prevalence of pneumonia was higher among non-vaccinated patients than among those who received complete primary vaccine series (20s, 28.6% vs. 5.1%; P = 0.001 and 40s, 61.5% vs. 13.0%; P < 0.001).
Table 2. Prevalence of pneumonia according to the age and vaccination status of COVID-19 patients.
| Age groups | All patients | Non-vaccinated | Incomplete primary vaccine series | Complete primary vaccine series | P value |
|---|---|---|---|---|---|
| 12–19 yr (n = 72) | 2 (2.8) | 1/18 (5.6) | 0/7 (0.0) | 1/47 (2.1) | 0.577 |
| 20–29 yr (n = 175) | 15 (8.6) | 8/28 (28.6) | 0/9 (0.0) | 7/138 (5.1) | 0.001 |
| 30–39 yr (n = 127) | 25 (19.7) | 12/36 (33.3) | 0/4 (0.0) | 13/87 (14.9) | 0.056 |
| 40–49 yr (n = 205) | 53 (25.9) | 32/52 (61.5) | 3/15 (20.0) | 18/138 (13.0) | < 0.001 |
Data are presented as the number of patients (%).
COVID-19 = coronavirus disease 2019.
The chest X-ray severity score among patients with pneumonia is summarized in Table 3. The chest X-ray severity score increased with age (P < 0.001). The chest X-ray severity score was significantly higher in non-vaccinated patients than in complete primary vaccinated patients in the 20s to 40s age groups (all P < 0.05). However, the chest X-ray severity score did not differ according to vaccination status in the 12–19 years group (P = 0.678).
Table 3. Pneumonia score on chest radiograph according to the age and vaccination status of COVID-19 patients.
| Age groups | Non-vaccinated | Incomplete primary vaccine series | Complete primary vaccine series | P value |
|---|---|---|---|---|
| 12–19 yr (n = 72) | 0.2 ± 0.9 | 0 ± 0 | 0.1 ± 0.6 | 0.678 |
| 20–29 yr (n = 175) | 2.4 ± 5.2 | 0 ± 0 | 0.1 ± 0.6 | < 0.001 |
| 30–39 yr (n = 127) | 2.6 ± 4.3 | 0.4 ± 1.3 | 0.3 ± 1.3 | < 0.001 |
| 40–49 yr (n = 205) | 3.6 ± 1.9 | 0.9 ± 1.9 | 0.4 ± 1.5 | < 0.001 |
Data are presented as mean ± standard deviation.
COVID-19 = coronavirus disease 2019.
Transfer to tertiary hospital due to COVID-19 aggravation
Among the 579 patients, 13 (2.2%) experienced progression to severe disease and were transferred to a tertiary hospital (when high-flow oxygen or mechanical ventilation was necessary or multiorgan dysfunction occurred). The rate of such events did not differ according to age or vaccination status of patients with COVID-19 (all P > 0.05) (Table 4). There were no fatalities in our hospital.
Table 4. Progression to severe disease (transfer to tertiary hospital) according to the age and vaccination status of COVID-19 patients.
| Age groups | Total | Non-vaccinated | Incomplete primary vaccine series | Complete primary vaccine series | P value |
|---|---|---|---|---|---|
| 12–19 yr (n = 72) | 1 (1.4) | 1/18 (5.6) | 0/7 (0.0) | 0/47 (0.0) | 0.137 |
| 20–29 yr (n = 175) | 1 (0.6) | 1/28 (3.6) | 0/9 (0.0) | 0/138 (0.0) | 0.158 |
| 30–39 yr (n = 127) | 6 (4.7) | 3/36 (8.3) | 0/4 (0.0) | 3/87 (3.4) | 0.216 |
| 40–49 yr (n = 205) | 5 (2.4) | 3/52 (5.8) | 0/15 (0.0) | 2/138 (1.4) | 0.081 |
Data are presented as the number of patients (%).
COVID-19 = coronavirus disease 2019.
Risk factors of pneumonia occurrence
We investigated whether the occurrence of pneumonia differed between patients with risk factors and those without. Among patients with one or more risk factors, the prevalence of pneumonia was significantly higher at 29.3% compared to 12.9% in patients without risk factors (P < 0.001). The rate of transfer to a tertiary hospital due to progression to severe disease was also higher in patients with risk factors at 4.9% compared to those without risk factors at 1.5% (P = 0.038) (Supplementary Table 2). Multivariate analysis with various factors such as risk factors, age, sex, and vaccination status was performed, and male sex, increasing age (from 12–19 years to 40s), and vaccination status were revealed as significant factors (Table 5).
Table 5. Univariate and multivariate logistic regression analysis of factors associated with pneumonia.
| Variables | Univariate analysis OR (95% CI) | P value | Multivariate analysis OR (95% CI) | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | Reference | Reference | |||
| Male | 2.200 (1.397–3.463) | 0.007 | 2.719 (1.612–4.588) | < 0.001 | |
| Age, yr | |||||
| 12–19 | Reference | Reference | Reference | Reference | |
| 20–29 | 3.281 (0.731–14.734) | 0.121 | 5.278 (1.098–25.365) | 0.038 | |
| 30–39 | 8.578 (1.968–37.387) | 0.004 | 11.150 (2.388–52.0412) | 0.002 | |
| 40–49 | 12.204 (2.892–51.506) | < 0.001 | 18.051 (3.977–81.924) | < 0.001 | |
| ≥ 1 risk factor | 2.784 (1.732–4.477) | < 0.001 | 1.868 (0.842–4.145) | < 0.001 | |
| Vaccinated status | |||||
| Complete primary vaccine series | Reference | Reference | Reference | Reference | |
| Incomplete primary vaccine series | 0.892 (0.261–3.045) | 0.855 | 1.138 (0.310–4.177) | 0.846 | |
| Not vaccinated | 6.224 (3.858–10.042) | < 0.001 | 7.460 (4.378–12.714) | < 0.001 | |
OR = odds ratio, CI = confidence interval.
DISCUSSION
In this study, patients aged 20 to 49 years with the omicron variant of COVID-19 who received complete primary vaccine series had a lower prevalence of pneumonia and a lower chest X-ray severity score than those who were not vaccinated against COVID-19. The prevalence of pneumonia was very low (2%) among patients aged 12–19 years and did not differ according to vaccination status. Progression to severe disease did not significantly differ according to vaccination status in any age group (12 to 49 years).
The most important turning point in COVID-19 treatment has been the development of vaccines and therapies. COVID-19 vaccines have dramatically reduced the mortality rate of COVID-19.17 Patients with COVID-19 exhibit different courses, from mild symptoms to a poor prognosis, the latter leading to intensive care unit (ICU) admission and a mortality rate of approximately 40%.18 COVID-19 has various manifestations, such as cardiac, thromboembolic, and neurological complications; however, viral pneumonia and acute respiratory distress syndrome are major complications, resulting in a severe and critical disease course.19 In COVID-19 treatment, preventing progression to pneumonia may be an important factor in the patient’s prognosis. In our study, vaccination against COVID-19 was related to a significantly lower prevalence of COVID-19 pneumonia in patients aged 20–49 years. Determining the vaccine’s effectiveness for patients aged 12–19 years was difficult, as only two cases of COVID-19 pneumonia occurred in this age group in our study.
Similarly, in a recent report from China, among patients infected with the omicron variant, the risk of COVID-19 pneumonia did not significantly differ between the vaccinated (Chinese vaccine) and unvaccinated groups for patients aged 3–17 years (OR, 1.44; 95% confidence interval [CI], 0.32–6.37; P = 0.63) and 18–59 years (OR, 0.97; 95% CI, 0.54–1.74; P = 0.91).20 However, in patients ≥ 60 years, the risk of pneumonia was significantly lower in the vaccinated group than in the unvaccinated group (OR, 0.28; 95% CI, 0.12–0.68; P = 0.01). In addition, among patients infected with the delta variant, vaccinated patients in all three age groups (3–17, 18–59, ≥ 60 years) had a significantly lower risk of pneumonia than non-vaccinated patients.20 In our study, vaccination was related to a reduction in the prevalence of pneumonia in patients aged 20–49 years (most received the ChAdOx1 nCoV-19, BNT162b2, or mRNA-1273 vaccine) in the omicron era.
In a recent study, the vaccine’s effectiveness against infection and symptomatic COVID-19 was lower in patients with the omicron variant than in patients with the alpha and delta variants.21 A systematic review revealed that the overall vaccine-induced protection against COVID-19 (omicron variant) was as low as 33%, lower than that against the alpha and delta variants.7 In the omicron variant era, younger age groups exhibited a relatively less severe disease course, and the efficacy of COVID-19 vaccines was reduced against the omicron variant; therefore, the benefit of the COVID-19 vaccine is unclear for younger persons. However, during the first three weeks of the onset of the omicron wave, two doses of the BNT162b2 vaccine administration reduced the hospitalization rate for COVID-19 by 70%.7 These results agree with ours; we discovered that the COVID-19 vaccine also reduces the incidence of pneumonia among patients aged 20–49 years. The occurrence of pneumonia is an important factor in the prognosis of patients with COVID-19. In a previous study, patients with COVID-19 who developed pneumonia exhibited more severe clinical features during hospitalization, a higher rate of admission to an ICU, a higher rate of application of mechanical ventilation, and a higher mortality rate than patients without pneumonia.22
In a study from Brazil, the vaccine protection rate against severe COVID-19 remained > 80% from 28 days after the second dose of BNT162b2 administration in adolescents.23 In another study, the BNT162b2 vaccine efficaciously prevented COVID-19 six months after vaccination in patients ≥ 12 years and patients of various age groups.24 In our study, the average time from vaccine administration to COVID-19 diagnosis for individuals in their 20s, 30s, and 40s was 93-100 days; we confirmed that patients who received the vaccine had a lower risk of pneumonia. Moreover, 3.1–9.7% of patients were administered by incomplete primary vaccine series in this study, and all groups except the 12–19-year age group had a decreasing risk of pneumonia occurrence in the order non-vaccination, incomplete primary vaccine series, and complete primary vaccine series.
Our research has shown that the prevalence rate of COVID-19 pneumonia increases as individuals age from 12–19 years to their 40s, in males, and who do not receive the vaccine. This is a similar trend to the risk factors for severe COVID-19 occurrence reported in previous cases.15,16
Guardians may be reluctant to let their children and adolescents undergo COVID-19 vaccination because of serious events, such as myocarditis, that may occur after mRNA-based COVID-19 vaccine in young men.25 However, the Global Advisory Committee on Vaccine Safety concluded that in all age groups, the benefits of mRNA COVID-19 vaccines in reducing hospitalizations and deaths due to COVID-19 outweigh the risks.26 The direct health benefit of COVID-19 vaccination in younger people is lower than that in older adults and high-risk groups. However, the benefits of vaccinating young people go beyond the direct health benefits to those people; it prevents transmission to society, especially the high-risk groups.27 Therefore, the World Health Organization and CDC recommend COVID-19 vaccination for people ≥ 6 months old.27,28 In addition, a study conducted in the United States observed that the risk of myocarditis or pericarditis due to COVID-19 infection in individuals ≥ 12 years is 6.4–31.2 times higher than the risk of myocarditis or pericarditis resulting from the second dose of the COVID-19 vaccine.29
We did not observe significant differences in mortality and progression to severe disease based on vaccine status across all age groups < 50 years. According to the KDCA, as of August 2023, the mortality rate due to COVID-19 in the 40s age group was as low as 0.01%, decreasing even further with younger age groups.30 Our results are consistent with the findings in the KDCA report.
Our study had several limitations. First, it was a single-center, retrospective study. The number of patients aged 12–19 years with pneumonia was lower than that for other age groups, with only two cases reported. Therefore, this study did not determine whether the COVID-19 vaccine reduces the occurrence of pneumonia in patients aged 12–19 years. In addition, this study was conducted on patients with relatively mild disease, and the percentage of patients requiring ICU admission was low (1.4–4.7%). Therefore, we could not determine the vaccine’s effectiveness in reducing the risk of ICU admission among patients with the omicron variant. Moreover, only one radiologist interpreted chest radiographs, although this radiologist had > 12 years of diagnostic experience, had previously interpreted chest radiography for patients with COVID-19, and had conducted various COVID-19-related studies.
In conclusion, among patients aged 20–49, vaccinated patients exhibited a significantly lower prevalence of pneumonia and chest X-ray severity score than non-vaccinated patients.
Footnotes
Funding: This research was supported by a grant of the Information and Communications Promotion Fund (ICT promotion fund) through the National IT Industry Promotion Agency (NIPA), funded by the Ministry of Science and ICT (MSIT), Republic of Korea, 2023.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park SH, Kim HJ, Park JS, Kwon YS.
- Data acquisition: Kwon YS, Kim JY, Lee JY, Kim MA, Kim TH.
- Formal analysis: Kwon YS, Kim JY.
- Investigation: Kwon YS, Kim HJ, Kim MA, Kim TH.
- Methodology: Park SH, Park JS.
- Writing - original draft: Kwon YS, Kim JY, Lee JY.
- Writing - review & editing: Kwon YS, Kim JY.
SUPPLEMENTARY MATERIALS
Clinical data of COVID-19 patients according to the vaccination status
Difference in the occurrence of pneumonia and progression to severe disease according to risk factors
References
- 1.Daria S, Bhuiyan MA, Islam MR. Detection of highly muted coronavirus variant omicron (B.1.1.529) is triggering the alarm for South Asian countries: associated risk factors and preventive actions. J Med Virol. 2022;94(4):1267–1268. doi: 10.1002/jmv.27503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021;36(50):e346. doi: 10.3346/jkms.2021.36.e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigal A. Milder disease with omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022;22(2):69–71. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385(26):2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilboa M, Mandelboim M, Indenbaum V, Lustig Y, Cohen C, Rahav G, et al. Early immunogenicity and safety of the third dose of BNT162b2 messenger RNA coronavirus disease 2019 vaccine among adults older than 60 years: real-world experience. J Infect Dis. 2022;225(5):785–792. doi: 10.1093/infdis/jiab584. [DOI] [PubMed] [Google Scholar]
- 7.Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review. J Med Virol. 2022;94(7):2969–2976. doi: 10.1002/jmv.27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Stay up to date with COVID-19 vaccines. [Accessed June 30, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html .
- 9.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Estimated effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5(9):e2232760. doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nham E, Song JY, Noh JY, Cheong HJ, Kim WJ. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci. 2022;37(47):e351. doi: 10.3346/jkms.2022.37.e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JD, Leonard S, Hoggatt KJ, Boscardin WJ, Lum EN, Moss-Vazquez TA, et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA. 2022;328(14):1427–1437. doi: 10.1001/jama.2022.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghesi A, Zigliani A, Masciullo R, Golemi S, Maculotti P, Farina D, et al. Radiographic severity index in COVID-19 pneumonia: relationship to age and sex in 783 Italian patients. Radiol Med (Torino) 2020;125(5):461–464. doi: 10.1007/s11547-020-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. People with certain medical conditions. [Accessed September 20, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html .
- 16.Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16(3):e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu N, Joyal-Desmarais K, Ribeiro PA, Vieira AM, Stojanovic J, Sanuade C, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. 2023;11(5):439–452. doi: 10.1016/S2213-2600(23)00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 19.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213(2):54–56.e1. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Liu Q, Wu D, Tang L, Wang X, Yan T, et al. Association of COVID-19 vaccination and clinical severity of patients infected with delta or omicron variants - China, May 21, 2021-February 28, 2022. China CDC Wkly. 2022;4(14):293–297. doi: 10.46234/ccdcw2022.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melendi SE, Pérez MM, Salas CE, Haedo MF, Xavier FB, Saltos Navarrete JD, et al. COVID-19 with and without pneumonia: clinical outcomes in the internal medicine ward. Medicina (B Aires) 2020;80(Suppl 6):56–64. [PubMed] [Google Scholar]
- 23.Florentino PT, Millington T, Cerqueira-Silva T, Robertson C, de Araújo Oliveira V, Júnior JB, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22(11):1577–1586. doi: 10.1016/S1473-3099(22)00451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated statement regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. [Updated 2021]. [Accessed June 30, 2023]. https://www.who.int/news/item/27-10-2021-gacvs-statement-myocarditis-pericarditis-covid-19-mrna-vaccines-updated .
- 27.World Health Organization. Interim statement on COVID-19 vaccination for children. [Updated 2022]. [Accessed December 11, 2022]. https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children .
- 28.Centers for Disease Control and Prevention. COVID-19 vaccine safety in children and teens. [Updated 2023]. [Accessed June 30, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-safety-children-teens.html .
- 29.Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ministry of Health and Welfare. COVID-19 occurrence status. [Accessed August 29, 2023]. https://ncov.kdca.go.kr/bdBoardList_Real.do .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical data of COVID-19 patients according to the vaccination status
Difference in the occurrence of pneumonia and progression to severe disease according to risk factors