Abstract
The innate genetic variability characteristic of chronic hepatitis C virus (HCV) infection makes drug resistance a concern in the clinical development of HCV inhibitors. To address this, a transient replication assay was developed to evaluate the replication fitness and the drug sensitivity of NS5B sequences isolated from the sera of patients with chronic HCV infection. This novel assay directly compares replication between NS5B isolates, thus bypassing the potential sequence and metabolic differences which may arise with independent replicon cell lines. Patient-derived NS5B sequences were similar to those of the established HCV genotypes, but isolates from each patient shared genetic variability specific to that patient, with additional genetic variability observed across the individual isolates. Every sample provided functional NS5B isolates which supported subgenomic replication, frequently to levels comparable to that of laboratory-optimized replicons. All isolates were equivalently sensitive to an active-site nucleoside inhibitor, but the sensitivities to a panel of nonnucleoside inhibitors which targeted three distinct sites on NS5B varied among the isolates. In con1, the original laboratory-optimized replicon, the NS5B S282T substitution confers resistance to the nucleoside inhibitor but impairs replication. This substitution was engineered into both genotype 1a and genotype 1b isolates. Replication was severely debilitated, demonstrating that no compensatory residues were encoded within these genetically diverse sequences to increase the replication fitness of the mutated replicons. This work describes a transient replicon-based assay that can support the clinical development of compounds which target NS5B and demonstrates its utility by examining several patient-derived NS5B isolates for replication fitness and differential sensitivity to NS5B inhibitors.
Persistent infection with hepatitis C virus (HCV) is a primary cause of several debilitating liver diseases, including chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (11, 15, 27). Approximately 170 million individuals are afflicted worldwide, and more than half are likely to develop severe liver disorders (50). The current preferred treatment is pegylated alpha interferon administered with ribavirin (33, 34, 41). Treatment, however, is poorly tolerated and of limited efficacy, with less than 50% of those individuals infected with the most prevalent genotype, HCV genotype 1b (HCV 1b), likely to respond. Recently, several new inhibitors of the virus-encoded RNA-dependent RNA polymerase have been identified, and clinical trials of anti-HCV inhibitors have already begun (7-10, 14, 21-23, 32, 35, 44, 48, 49).
HCV chemotherapy must address the broad genetic diversity encountered in clinical settings (13). HCV genetic variation is characterized both by numerous distinct genotypes and by a high degree of genetic diversity among the viruses circulating in infected individuals (16). The latter arises in part from the error-prone mechanism of the gene product of the HCV-encoded NS5B gene, the RNA-dependent RNA polymerase. In the infected population this enzyme misincorporates nucleotides at an estimated rate of 10−4 and thus provides an inherent mechanism to generate diversity among circulating variants within a patient (39). Particular variants within the pretreatment virus population may show reduced sensitivity to a specific class of antiviral compound, can be selected by the drug regimen, and should cause the reemergence of the viral load, resulting in antiviral treatment failure. In clinical trials of antivirals with activity against HCV, it is therefore important to characterize the genetic diversity of the viruses within an HCV-infected individual prior to initiation of drug therapy and to monitor variants which arise during treatment. Clinical trials will be aided by simple cell-based assays that can be used to quantify the efficacies of drug candidates against a diverse panel of HCV variants which may arise during the course of therapy.
The advent of the HCV replicon enabled measurement of HCV subgenomic RNA replication in a cell-based format. HCV subgenomic RNA replication was first achieved with a specific genotype 1b sequence, con1, which conferred neomycin resistance through expression of a bicistronic neomycin resistance gene within the replicon (1, 31). Subsequent study of HCV replication was modified through the characterization of “adaptive” mutations within replicon-encoded HCV sequences and isolation of “enhanced” cell lines (2, 17, 19, 24, 28-30, 36, 40). Both developments increased the efficiency with which replication was established with laboratory-optimized HCV replicons. Replacement of the replicon-encoded neomycin resistance gene with nonselective reporter genes, such as those for luciferase and β-lactamase, enabled cell-based replication to better model persistent replication due to the absence of selective pressure to maintain the replicon copy while also increasing the sensitivity of the assay (36, 47). Recently, cell-based replication of genotype 1a subgenomic replicons has been achieved, and additional compensatory changes which increase genotype 1a subgenomic replication have been described (3, 17, 18, 51). Other developments include the use of of replicon-harboring Huh7 cells to quantify interferon sensitivity, isolation of mutant con1 replicons competent for replication in HeLa cells, and development of a novel genotype 2a subgenomic replicon (20, 26, 47, 53).
In this work a transient cell-based assay was developed to evaluate clinical NS5B isolates for their replication fitness, their sensitivities to NS5B polymerase inhibitors, and the presence of compensatory residues that confer a replication advantage to drug-resistant mutants. We sequenced multiple NS5B isolates from several patients and noted genetic variation specific to the isolates of individual patients. We provide examples of patient-derived NS5B isolates that supported subgenomic replication, and the replication of the replicons of several of these isolates was comparable to that of the parental laboratory-optimized replicons. A mechanism-based nucleoside analogue targeted to the polymerase active site (7) was broadly active against a genetically diverse panel of isolates. Three nonnucleoside inhibitors of the polymerase which interact with distinct sites outside the catalytic center (8-10, 14, 32, 44-46, 49) showed variable inhibition. In addition, a mutation within the polymerase gene which conferred resistance to the nucleoside analogue in the con1 replicon but which impaired replication (35) also debilitated replication when it was introduced directly into clinical NS5B isolates, demonstrating that no compensatory residues were encoded within the genetically diverse backgrounds of these isolates to increase the replication fitness of mutants encoding this resistance mutation. This work characterizes the HCV subgenomic replication driven by NS5B sequences derived from multiple patients and uses a novel adaptation of the replicon system to quantify replication fitness and sensitivity to antiviral compounds and to assess the isolates for the presence of compensatory residues which facilitate replication fitness.
MATERIALS AND METHODS
Rescue and characterization of NS5B genes from the sera of patients and chimpanzees.
Serum samples of patients chronically infected with HCV were the generous gift of M. Lucey and K. R. Reddy, Gastroenterology Division, University of Pennsylvania. These clinical samples were collected prior to 2001. Total RNA was isolated by using the QIAGEN RNeasy Mini kit, according to the manufacturer's instructions (QIAGEN, Inc. Valencia, CA). The RNA was used as a template for the reverse transcriptase reaction (Superscript II RT; Invitrogen Life Technologies, Carlsbad, CA), which was primed with a 34-nucleotide dA primer. Following reverse transcription, the reaction mixtures were heat inactivated at 65°C for 15 min and then digested with RNase H and RNAse T1 (Roche Applied Science, Indianapolis, IN) at 37°C for 20 min to remove RNA prior to PCR. Nested PCR was performed with an Expand High Fidelity PCR system (Roche Applied Science) and the following primers: primer PCR1, forward (5′-GAGGACGTCGTSTGCTGCTGCTCRATGTC) and reverse (34mer poly-dA), and PCR2, forward (5′-CGATATGATCACRCCATGCGCYGCGGA) and reverse (5′-AGCTCCCCGTTCACCGGTTGG). PCR products were cloned into pGEM-T (Promega, Madison, WI), and individual NS5B isolates were sequenced. A genotype was assigned on the basis of the closest sequence homology upon pairwise alignments with the NS5B sequences for genotype 1a H77 (GenBank accession no. AF009606), genotype 1b con1 (GenBank accession no. AJ238799), and prototype sequences for genotypes 2a through 6 (genotype 2a HCV HC-J6, GenBank accession no. D00944; genotype 2b HCV HC-J8, GenBank accession no. D10988; genotype 3a HCV NZL1, GenBank accession no. D17763; genotype 4a HCV ED43, GenBank accession no. Y11604; genotype 5a HCV EVH1480, GenBank accession no. Y13184; genotype 6a HCV EUHK2, GenBank accession no. Y12083). For genotypes 2, 3, 4, and 6, NS5B was rescued from infected chimpanzee sera (provided by Jens Bukh, National Institutes of Health). The methodology was the same as that described above, except that genotype-specific primers were designed on the basis of the published sequence of the known input virus. Primer sequences are available upon request.
Construction of chimeric replicons harboring NS5B isolates from chronically infected patients.
The β-lactamase bicistronic subgenomic replicons of con1, BK, and H77 have been described previously (17, 36). Briefly, all replicons contain an NS5A S232I “adaptive” mutation. BK encodes the additional NS3 “adaptive” mutations S196T and R470M, and H77 encodes NS3 “adaptive” mutations S196T and P470L (17). Chimeric replicons with patient-derived NS5B genes were cloned by using a previously described strategy (17) which placed unique BclI and ClaI restriction sites flanking the NS5B gene. The replicon sequences were flanked at the 5′ end with a T7 transcriptional start site and at the 3′ end with an XbaI site. Plasmids were linearized by XbaI digestion, and RNA for transfections was generated with MEGAscript (Ambion, Austin, TX).
Description of NS5B isolates and chimeric replicons.
For all isolates, at least six polymerase genes were cloned, sequenced, and compared to the reference sequences listed above. NS5B isolates tested in the replicon assay are designated with a patient sample number (ps), followed by an isolate number when more than one isolate from the same serum sample was analyzed. The genotypes of the isolates, determined as described above, are listed in Table 1. All isolates were cloned in the genotype 1b con1 background. Selected isolates were also cloned and tested within the genotype 1a H77 background. NS5B isolates derived from infected chimpanzee sera are designated with the prefix cs, followed by the genotype and clone number.
TABLE 1.
Clinical NS5B isolates support subgenomic replication
The result for the designated genotype is in boldface.
Replication fitness was determined by direct comparison of A460 values to those for con1 replicons, which were normalized to a relative fitness of 1, as described in Materials and Methods. All experiments were performed in triplicate, and the values are means ± standard deviations.
Establishment of persistently replicating cell lines and analysis of replication.
Transfections were performed by using the enhanced-replication Huh7-derived cell line MR2 and a DMRIE-C (Life Technologies, Rockville, MD) transfection protocol, as described previously (36). On the morning following transfection, the cells were expanded and cultured for an additional 3 days. On day 4, the cells were collected, counted, and seeded into a 96-well Costar black-walled, clear-bottom plate in the presence of 1 μM clavulanic acid at a seeding density of 7,500 cells/well. Compounds were added immediately in an equal volume, thus adjusting the final clavulanic acid concentration to 0.5 μM and the final dimethyl sulfoxide concentration to 1.0%. Cells harboring the nonreplicating GAA replicon (36) were analyzed at that time to verify that the background β-lactamase activity from the residual input RNA was undetectable. GAA is nonfunctional due to Asp-to-Ala substitutions within the active site of NS5B. Cells were incubated with an inhibitor for 2 days and analyzed as described below.
Analysis of β-lactamase activity.
The medium was removed by aspiration, and the cells were stained for 2 h with CCF4-AM (Invitrogen Corp., Carlsbad, CA) in Dulbecco modified Eagle medium supplemented with 25 mM HEPES, pH 8.0. Fluorescence due to β-lactamase activity was quantified through excitation at 405 nm, followed by measurement of the emission at 460 nm with a CytoFluor 4000 fluorescence plate reader. Emission at 530 nm was measured to control for cell viability and plating efficiency, with a tolerance of ±10% accepted. The background emission value, defined as the emission at 460 nm in the presence of 10 μM 2′C-methyladenosine (a concentration greater than the 95% effective concentration [EC95], which resulted in no blue cells), was subtracted from all values. The values of the emission at 460 nm for the con1 replicon were assigned a fitness value of 1, and the values for all patient-derived replicons were assessed in relation to the value for con1. EC50 measurements were calculated by fitting the emission data as a percentage of that for the dimethyl sulfoxide control to a sigmoidal four-parameter fit function (minimum, maximum, slope, inflection point) by using Kaleidagraph software (Synergy Software, Reading, PA), as described previously (35). Titrations were performed in triplicate, and the values were averaged. All titrations were repeated at least once in entirety with new transfections to further verify the reproducibilities. The EC50s for con1 are presented with their standard deviations, and the values for patient-derived isolates are compared in relation to the con1 values.
NS5B inhibitors.
The nucleoside inhibitor 2′C-methyladenosine (7) was synthesized by ISIS Pharmaceuticals (Carlsbad, CA). The nonnucleoside benzimidazole inhibitor NNI 1 (45) was obtained from Frank Narjes (Istituto di Ricerche di Biologia Molecolare P. Angeletti [IRBM]), the nonnucleoside benzothiadiazine inhibitor NNI 2 (14) was purchased from Interbioscreen (Moscow, Russia), and the nonnucleoside thiophene inhibitor NNI 3 was obtained from Frank Narjes (IRBM).
Nucleotide sequence accession numbers.
The novel NS5B sequences described in this work (see also the supplemental material) have been deposited in GenBank and assigned accession numbers AY973846 to AY973866.
RESULTS
NS5B sequences isolated from individual patients are genetically diverse and encode patient-specific variation.
To examine the genetic variability of NS5B within individual patients, multiple isolates from serum samples of several patients chronically infected with HCV were sequenced. At least six isolates per patient were individually sequenced, and the sequence variability among these isolates is presented in Fig. 1.
FIG. 1.
Sequence diversity among NS5B isolates isolated from patients chronically infected with HCV. The NS5B isolates were sequenced and their genotypes were evaluated as described in Materials and Methods. An amino acid sequence comparison was made with NS5B from H77 (genotype 1a) and con1 (genotype 1b). Those residues which differed between H77 and the genotype 1a isolates or con1 and the genotype 1b isolates are shown. NS5B of BK is included in the genotype 1b alignment for comparison. (Top) Residues which differ among genotype 1a NS5B isolates of patients ps2, ps3, and H77. ps2-2 encodes a frame shift, indicated by hyphens, and only those residues included up to the frame shift are included in the alignment. (Bottom) Residues which differ among genotype 1b NS5B isolates of patients ps1, ps4, ps5, and con1. Boxed residues are those positions for which analysis of the genotype 1b sequences in GenBank suggests separation into two groups.
The ps2 sequences (genotype 1a) were the most heterogeneous of all patient isolate samples examined, and six sequences are presented to illustrate the degree of variation (Fig. 1, top). Three types of sequence variations were noted. In one type all isolates from an individual patient encoded a pattern which appeared to be patient specific within that genotype. For example, all ps2 sequences encode S46 and L47, which do not usually appear among other genotype 1a sequences listed in GenBank and which more commonly appear among genotype 1b sequences. Similarly, the R81 and M82 pair in all genotype 1a ps3 sequences typically does not occur in other genotype 1 sequences but is found within some genotype 3 sequences (unpublished observations). In a second type of sequence variation, all sequences of isolates from a patient had one of two amino acids distributed equally at a specific position. For example, residue 242 of the ps1 isolates was distributed equally between C242 and S242. Finally, there was variation within individual isolates which may have been caused by the NS5B polymerase or, alternatively, by a reverse transcription-PCR-induced change. An example of this is the R451 in ps2-3, which was C451 in the other sequences of this patient. Interestingly, C451R in con1 has been implicated in benzothiadiazine resistance (46).
Analysis of genotype 1b sequences present in GenBank suggests that >90% of these sequences are split into two major groups of A218, C316, and Q464 (ACQ), which appear in con1, and S218, N316, and E464, which appear in BK (Fig. 1, bottom). All genotype 1b sequences that we isolated were of the con1 ACQ type.
Patient-derived NS5B sequences support subgenomic replication.
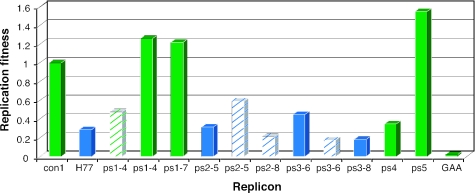
To quantify replication, the subgenomic replicon with the β-lactamase reporter was used in a cell-based assay to transiently measure replication. Isolates from the first three patient sets were tested for replication activity in both genotypically homologous and heterologous replicons (Table 1). The ps1 and ps2 isolates functioned in either replicon. In contrast, the ps3 isolates functioned only in the homologous 1a replicon. Interestingly, the activity of ps2-5 was greater in the genotypically heterologous 1b (con1) replicon than in the genotypically homologous 1a (H77) replicon. Studies with the ps4 and ps5 isolates were conducted only with the homologous 1b replicon; the activity of ps5 was more robust than that of the con1 replicon, while ps4 was approximately one-third as active as con1. Functional sequences were isolated from all serum samples tested, and the replication fitness of the sequences is summarized in Fig. 2.
FIG. 2.
Replication fitness of chimeric genotype 1a and 1b replicons encoding patient NS5B isolates. Replication was measured as described in Materials and Methods, and replication levels were normalized to that of con1. The genotype of NS5B is indicated by color: blue for genotype 1a and green for genotype 1b. Solid bars represent a genotypically homologous replicon between the replicon backbone (NS3-5A) and NS5B. Hatched bars indicate a genotypically heterologous replicon between NS5B and the replicon backbone, with the color of the hatched bars representing the genotype of the polymerase.
Patient-derived isolates are sensitive to both nucleoside and nonnucleoside inhibitors of NS5B.
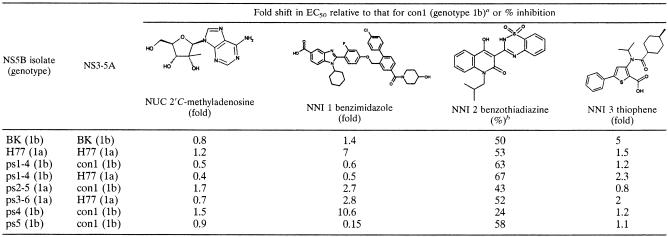
Cell culture assessment of sensitivity to inhibition by compounds has generally relied upon EC50s derived from laboratory-optimized con1 replicons (7-9, 35, 42, 43, 45, 46). To extend these observations to patient-derived polymerases and to evaluate the differential responses among the isolates, sequences from all patients were tested for their sensitivities to a panel of four compounds. Four distinct NS5B inhibitor binding sites have been suggested by binding or resistance studies, and a compound known to interact with each of these four sites was selected. Only the most robust isolate of each patient was used in these sensitivity studies, and the results are summarized in Table 2.
TABLE 2.
Sensitivities of patient NS5B isolates to a panel of NS5B inhibitors
The EC50s of NUC, NNI 1, NNI 2, and NNI 3 for con1 are 1.04 ± 0.19, 0.47 ± 0.07, 11.6 ± 3.9, and 0.82 ± 0.28 μM, respectively.
NNI 2 was tested at 40 μM.
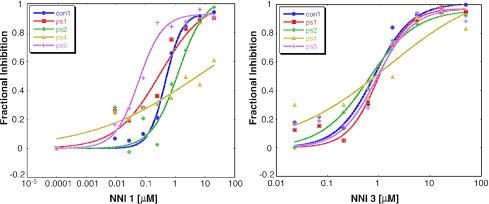
We previously showed that the nucleoside analogue 2′C-methyladenosine is an active-site inhibitor of con1 replication (35). In the present studies all replicons were sensitive to this compound. The con1 replicon had an EC50 of 1 μM, and the EC50s of all patient isolates were essentially equivalent, as demonstrated for genotype 1a isolates in the left panel of Fig. 3 and genotype 1b isolates in the right panel of Fig. 3.
FIG. 3.
Titrations of nucleoside inhibitor 2′C-methyladenosine (2′C-Me) against subgenomic replicons encoding patient NS5B isolates. The enhanced Huh7 cell line MR2 was transfected with RNA encoding subgenomic replicons and expanded as described in Materials and Methods. On day 4 the cells were collected and plated in the presence of compound and cultured for 2 days. Inhibition was normalized for each replicon by determination of its fluorescence emission at 460 nm in the absence of drug and subtraction from this value the emission in the presence of 10 μM 2′C-methyladenosine (10 times the con1 EC50). (Left panel) Titrations against H77 and genotype 1a NS5B isolates ps2 (ps2-5) and ps3 (ps3-6); (right panel) titrations against con1 and genotype 1b isolates ps1 (ps1-4), ps4 (ps4-6), and ps5 (ps5-11).
The sensitivities of the isolates to antiviral compounds were further evaluated by using three nonnucleoside NS5B inhibitors which are thought to target distinct binding sites on NS5B. The benzimidazole compound NNI 1 inhibited replication (Fig. 4, left panel), but variable sensitivity among the isolates was observed. This was more pronounced among the genotype 1b isolates, which ranged from almost 7-fold more sensitive (ps5) to more than 10-fold less sensitive (ps4) than con1. The EC50s of the genotype 1a isolates were similar to each other and were slightly more sensitive than the genotype 1a H77 replicon. However, all were intermediate in sensitivity relative to the extremities shown by some of the genotype 1b sequences.
FIG. 4.
Titrations of the benzimidazole NNI 1 (left panel) and thiophene NNI 3 (right panel) inhibitors against chimeric subgenomic replicons encoding patient NS5B isolates. MR2 cells were transfected with RNA-encoding chimeric replicons, expanded, and titrated as described in Materials and Methods. Shown are con1 and chimeric replicons of patient isolates in con1: ps1 (ps1-4), ps2 (ps2-5), ps4 (ps4-6), and ps5 (ps5-11).
A nonnucleoside benzothiadiazine compound, NNI 2, inhibited the replication of con1 (EC50, 11 μM) but was poorly active against all the other replicons at 40 μM, the highest concentration tested (Table 2). Solubility problems prevented testing of this compound at higher concentrations, and therefore, reliable EC50s could not be calculated. The nonnucleoside thiophene compound NNI 3 inhibited replication of both genotype 1a and genotype 1b isolates with submicromolar potency (Fig. 4, right panel). Only BK (genotype 1b) responded differently, and it was fivefold less sensitive than con1.
The robust replication of genotype 1b isolate ps1-4 in either the genotype 1a (H77) or genotype 1b (con1) replicon enabled us to address whether differences in the host replication complex affect sensitivities to these NS5B inhibitors (Table 2). The sensitivities of ps1-4 to the nucleoside inhibitor 2′C-methyladenosine and two of the nonnucleoside inhibitors were nearly identical in either the genotype 1a or the genotype 1b replicon background, while the sensitivities to the nonnucleoside thiophene inhibitor showed only minor variances (twofold). Thus, the sensitivity of NS5B to these compounds is little influenced by the replicon background.
Assessment of clinical NS5B isolates engineered to encode a known resistance mutation.
Antiviral therapy for HCV infection would benefit from a method that could be used to assess the replication fitness and compound sensitivities of variants which encode known resistance mutations. We addressed this by introducing a known resistance mutation directly into patient-derived NS5B sequences to evaluate its effects in the genetic background of that isolate.
We previously showed that the con1 replicon cultured in the presence of 2′C-methyladenosine developed resistance by selection for replicons that encoded NS5B S282T and that these replicons were debilitated for replication (35). All our patient isolates encoded S282 and were sensitive to 2′C-methyladenosine. To characterize the effects of this known resistance mutation on sensitivity and replication fitness, we introduced S282T into selected genotype 1a and genotype 1b patient sequences. We also introduced S282T into the BK sequence to include an example of the SNE NS5B linkage group described above.
Relative to the respective parental replicons, BK S282T, ps2-5 S282T, and ps1-4 S282T were all more debilitated than con1 S282T (Fig. 5). Such low levels of replication made quantitative analysis difficult, and therefore, the relative levels of resistance to 2′C-methyladenosine could not be addressed. The severe debilitation demonstrates that none of the polymerases tested carried innate variable amino acids which would restore fitness should this resistance mutation arise.
FIG. 5.
Patient isolates encoding 2′C-methyladenosine resistance mutation S282T are debilitated for replication. S282T was introduced by mutagenesis into NS5B of BK, con1, and patient isolates ps1-4 and ps2-5. RNAs of the parental and S282T mutant replicons were transfected into MR2 cells, expanded, and assayed as described in Materials and Methods. Replication of S282T mutants is shown as a fraction of that of their own parental S282 replicon normalized to that of con1, which had a fitness level of 1.
Non-genotype 1 NS5B isolates support weak replication in a genotype 1b background.
To determine whether the robust con1 and BK replicons also provide a backbone for functional chimeric replicons of non-genotype 1 polymerases, NS5B of genotypes 2a, 2b, 3a, 4a, and 6a was rescued from infected chimpanzee sera and introduced into the con1 and the BK replicons.
Eight genotype 2b sequences were examined. All showed single-residue variations from each other, with the sequence of one isolate matching the sequence of the prototype genotype 2b isolate (unpublished observations). The latter sequence was selected for replicon studies. Sequence differences between the rescued and the prototype sequences are shown for the other genotypes (Fig. 6). Although numerous differences from the prototype sequences were detected, we noted two specific sequence differences among our isolates that were implicated in compound resistance (boxed in Fig. 6). The prototype for genotype 4a encodes T282, while the prototype for genotype 6a encodes L495. Resistance studies have implicated these residues in conferring resistance to the nucleoside inhibitor (35) and the benzimidazole NNI 1 inhibitor (45), respectively. The sequences we rescued from infected chimpanzee sera that encode S282 and P495.
FIG. 6.
Variability among NS5B isolates from infected chimpanzee serum. The complete sequences of multiple NS5B isolates from infected chimpanzees were determined. Positions which differ from those in the prototype sequences are shown. cs2a con is the consensus sequence generated among our rescued genotype 2a sequences. The boxed residues in the genotype 4a and genotype 6a alignments are the positions within con1 that conferred resistance to the nucleoside inhibitor 2′C-methyladenosine and the nonnucleoside benzimidazole inhibitor NNI 1, respectively.
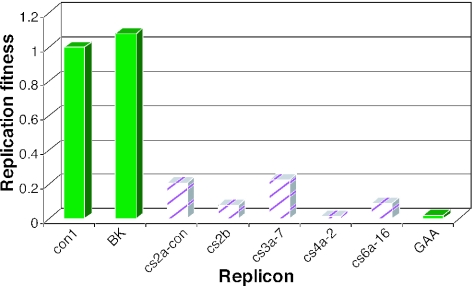
Representative polymerases for genotypes 2a, 2b, 3, 4, and 6 were cloned into the genotype 1b con1 background. We also compared a genotype 2b consensus NS5B sequence cloned into both the BK and the con1 NS3-5A backgrounds and found that the BK genotype 2b replicon showed greater fitness than the equivalent con1 chimera (data not shown). When the genotype 2 and 3 chimeras were assayed for replication fitness, low levels of replication were observed (Fig. 7). Although replication was not sufficient to allow quantitative compound EC50s to be determined, introduction of the genotype 2 or genotype 3 polymerase into a Neor replicon may enable selection of mutants with enhanced replication to a level suitable for replication and drug sensitivity studies.
FIG. 7.
Replication fitness of non-genotype 1 chimeric replicons. NS5B was rescued from HCV-infected chimpanzee sera of genotypes 2a, 2b, 3a, 4a, and 6a and cloned into the con1 replicon; cs2a and cs2b, however, were cloned into the BK replicon. RNA was transfected into MR2 cells and evaluated for replication, as described in Materials and Methods. Replication was normalized to that of con1, setting it to a relative fitness level of 1. For genotype 2a NS5B, the consensus sequence was generated and tested, while for genotype 2b, the rescued sequence matched the prototype sequence. Only one sample per genotype is shown, but all sequences shown in Fig. 6 were tested.
DISCUSSION
Simple cell-based assays which measure the sensitivities and replication fitnesses of patient-derived NS5B sequences would aid with the clinical evaluation of antiviral compounds for the treatment of HCV infection. The present work describes the adaptation of β-lactamase replicons (36) for the assessment of patient-derived NS5B sequences in a transient assay. Clinical isolates of both genotypes 1a and 1b cloned into genotype 1a (H77) or genotype 1b (con1) replicons generated chimeras which replicated to levels comparable to those of the parental replicons. The replication assay described in this work enabled analysis of isolates for fitness and compound sensitivity in a short-term assay, prior to the development of any additional genetic diversity within replicon sequences which may occur upon long-term maintenance of replicon-harboring cell lines. Importantly, this method allows one to assess the effect of specific amino acid substitutions on replication or drug efficacy, which enables the direct comparison of two replicons which differ by a single residue. The use of a single cell line also eliminates differences in metabolism or compound uptake among clonal cell lines, either of which may complicate interpretation of the results.
The NS5B isolates of our individual clinical samples showed considerable sequence variability, with most of the variable residues predicted to reside along the outer surfaces of the thumb, fingers, and palm domains of NS5B (4, 5), as shown for the genotype 1b isolates (Fig. 8). Several of these variable positions occur at or near surface regions implicated in the NNI binding pockets. Given the error rate of NS5B polymerase (39), the high viral turnover rate (37), and viral loads which can exceed 109 genomes per patient, variants that have altered sensitivities to compounds that bind at these sites will likely arise. Reduced replication fitness may limit the spread of such resistant viruses, and they may even be unnoticed unless drug treatment reduces the titer of the more prevalent, drug-sensitive viral population. If these resistant variants are debilitated, it may be that compensatory substitutions are required to restore fitness. It is useful, therefore, to have a means for the identification of sequences with reduced sensitivities to compounds of clinical interest.
FIG. 8.
Variable amino acid positions among the patient NS5B isolates. The model of NS5B is derived from the crystal structure of the genotype 1b (BK) Δ55 enzyme (4, 5). The thumb, fingers, and palm regions are colored blue, red, and yellow, respectively. The catalytic Asp318 within the active site is shown as a brown ball and stick and is located toward the center of the figure. The NNI nonnucleoside binding sites are indicated with boldface numbers. The locations of the variable amino acids encoded within the genotype 1b patient NS5B isolates are shown in light green. Because the model is based upon the Δ55 crystal structure, variable residues within the last 55 amino acids are not shown.
One utility of the assay described in this work is that it enables patient-derived NS5B sequences to be directly evaluated for their sensitivities to HCV inhibitors. This was demonstrated with a panel of four compounds, each of which binds to one of the four known compound binding sites on the viral polymerase (Fig. 8). All isolates were sensitive to the active-site inhibitor 2′C-methyladenosine, with only modest differences in EC50s. There was greater variability in the sensitivities to nonnucleoside inhibitors which target other binding sites on the polymerase. Given the structural constraints necessary to maintain the catalytic function of the active-site polymerase reaction, it is not surprising that greater variability in sensitivity to inhibition by compounds which bind outside the active site was observed. At least with these isolates, the variability in the response to a compound that binds at the NNI 1 site was most pronounced. Both genotype 1a and genotype 1b isolates showed reduced sensitivities to the NNI site 1 inhibitor relative to that of con1, and the spread in sensitivity between the most sensitive and the least sensitive genotype 1b sequences exceeded 70-fold. Our patient-derived NS5B sequences were all sensitive to the NNI site 3 inhibitor, with only modest differences in response. Interestingly, among the replicons tested the sensitivity of only BK differed, and it was fivefold less sensitive than con1. The responses to the benzothiadiazine NNI 2 inhibitor were poor, and reliable EC50s could not be determined due to poor compound solubility. Although these patient-derived polymerases show differential EC50s in the replicon system, due to the importance of pharmacokinetics, it remains to be determined how predictive of clinical efficacy the cell culture-derived values are.
A given resistance-conferring substitution in one strain may result in altered replication levels when it is present in different genetic backgrounds, as is likely encountered among isolates of individual patients. The transient replication assay enabled us to address this by engineering a known resistance mutation into a patient isolate and directly testing the isolate for fitness within the NS5B genetic background of the virus circulating in that patient. When ps1-4 S282T and ps2-5 S282T were engineered into ps1-4 and ps2-5, they both exhibited severely impaired replication compared to those of the respective parental replicons, demonstrating that no innate adaptability is encoded within either of these polymerases to overcome the deleterious effect of an S282T substitution on replication. In this model study, we engineered S282T into one of the more robust replicons for both a genotype 1a polymerase and a genotype 1b polymerase. Although we tested only one variant each, we recognize that a resistance-determining amino acid substitution may develop in any one of the patient's variants.
NS5B sequences ps1 and ps2 supported replication in both the genotype 1a and the genotype 1b genetic backgrounds. Thus, there is no intrinsic block to a functional intertypic replicon, even though intertypic recombinant viruses are rare (25). However, non-genotype 1 NS5B sequences did not support replication to quantifiable levels in functional genotype 1b replicons. We do not attribute this to a severe defect in the enzymatic function of the polymerases, because cloning, expression, and purification of NS5B from these sequences yielded active enzymes (Steve Carroll, personal communication). Recently, a description of NS5B cis-acting regulatory elements has been presented (52). The results of the analysis of our patient sequences across the 5BSL3.2 cis-acting regulatory element are consistent with those of the previously the published analysis (52), and a disruption of this element likely does not account for any low fitness observed. Because of the presence of low numbers of replicating cells for the genotype 2 and 3 chimeras, selection of clonal cell lines by use of a Neor marker in place of β-lactamase may reveal novel adaptive substitutions suitable for these genotypes, as recently demonstrated for genotype 1a (51).
We also note that only a minor number of sequence differences distinguish isolates ps2-5 and ps3-6, yet there was a pronounced biological consequence. Whereas both isolates supported replication nearly equivalently in a homologous genotype 1a backbone, ps2-5 supported replication better in a heterologous genotype 1b backbone. The positions which vary between these two isolates reside principally on the outer surfaces of the polymerase (5). It is reasonable to speculate that a small number of the positions which differ between ps2-5 and ps3-6 affect surface interactions critical for the establishment or maintenance of a replication complex.
Clinical development of human immunodeficiency virus reverse transcriptase and protease inhibitors required a means to identify and analyze resistant mutants which arose during treatment (6, 12, 38). The high rate of random nucleotide misincorporation which is characteristic of HCV RNA-dependent RNA polymerase raises the concern that it will be necessary to monitor resistance during the clinical development of HCV inhibitors. The present work demonstrates a cell-based methodology useful for the characterization of the intrinsic genetic diversity of NS5B within chronically infected patients, the monitoring of novel variants during treatment, and the evaluation of the effects of specific mutations within patient isolates on the response to treatment.
Supplementary Material
Acknowledgments
We thank Andrea Carfi and Stefania DiMarco (IRBM, Rome, Italy) for valuable insights into the binding of NS5B inhibitors, Frank Narjes and Michael Rowley (IRBM) for nonnucleoside inhibitors, ISIS Pharmaceuticals (Carlsbad, CA) for the nucleoside analogue, M. Lucey and K. R. Reddy (Division of Gastroenterology, University of Pennsylvania) for serum samples of chronically infected HCV patients, J. Bukh (National Institutes of Health) for the chimpanzee HCV-passaged serum samples, and Meiqing Lu and Steven Carroll (Merck Research Laboratories, West Point, PA) for helpful comments on the manuscript. We thank Susan Barr for help with the preparation of the manuscript.
The authors have no competing interests.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 82:1972-1974. [DOI] [PubMed] [Google Scholar]
- 2.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blight, K. J., J. A. McKeating, J. Marcotrigiano, and C. M. Rice. 2003. Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. J. Virol. 77:3181-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bressanelli, S., L. Tomei, F. A. Rey, and R. De Francesco. 2002. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J. Virol. 76:3482-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bressanelli, S., L. Tomei, A. Roussel, I. Incitti, R. L. Vitale, M. Mathieu, R. De Francesco, and F. A. Rey. 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl. Acad. Sci. USA 96:13034-13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrnes, V. W., V. V. Sardana, W. A. Schleif, J. H. Condra, J. A. Waterbury, J. A. Wolfgang, W. J. Long, C. L. Schneider, A. J. Schlabach, B. S. Wolanski, D. J. Graham, L. Gotlib, A. Rhodes, D. L. Titus, E. Roth, O. M. Blahy, J. C. Quintero, S. Stasewski, and E. A. Emini. 1993. Comprehensive mutant enzyme and viral variant assessment of HIV-1 reverse transcriptase resistance to nonnucleoside inhibitors. Antimicrob. Agents Chemother. 37:1576-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolansi, Z. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. [DOI] [PubMed] [Google Scholar]
- 8.Chan, L., O. Pereira, T. J. Reddy, S. K. Das, C. Poisson, M. Courchesne, M. Proulx, A. Siddiqui, C. G. Yannopoulos, N. Nguyen-Ba, C. Roy, D. Nasturica, C. Moinet, R. Bethell, M. Hamel, L. L'Heureux, M. David, O. Nicolas, P. Courtemanche-Asselin, S. Brunette, D. Bilimoria, and J. Bedard. 2004. Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 1. Sulfonamides. Bioorg. Med. Chem. Lett. 14:793-796. [DOI] [PubMed] [Google Scholar]
- 9.Chan, L., O. Pereira, T. J. Reddy, S. K. Das, C. Poisson, M. Courchesne, M. Proulx, A. Siddiqui, C. G. Yannopoulos, N. Nguyen-Ba, C. Roy, D. Nasturica, C. Moinet, R. Bethell, M. Hamel, L. L'Heureux, M. David, O. Nicolas, P. Courtemanche-Asselin, S. Brunett, D. Bilimoria, and J. Bedard. 2004. Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2. Tertiary amides. Bioorg. Med. Chem. Lett. 14:797-800. [DOI] [PubMed] [Google Scholar]
- 10.Chan, L., T. J. Reddy, M. Proulx, S. K. Das, O. Pereira, W. Wang, A. Siddiqui, C. G. Yannopoulos, C. Poisson, N. Turcotte, A. Drouin, M. H. Alaoui-Ismaili, R. Bethell, M. Hamel, L. L'Heureux, D. Bilimoria, and N. Nguyen-Ba. 2003. Identification of N,N-disubstituted phenylalanines as a novel class of inhibitors of hepatitis C NS5B polymerase. J. Med. Chem. 46:1283-1285. [DOI] [PubMed] [Google Scholar]
- 11.Colombo, M. 1998. The role of hepatitis C virus in hepatocellular carcinoma. Recent Results Cancer Res. 154:337-344. [DOI] [PubMed] [Google Scholar]
- 12.Condra, J. H., D. J. Holder, W. A. Schleif, O. M. Blahy, R. M. Danovich, L. J. Gabryelski, D. J. Graham, D. Laird, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, T. Yang, J. A. Chodakewits, P. J. Deutsch, R. Y. Leavitt, F. E. Massari, J. W. Mellors, K. E. Squires, R. T. Steigbigel, H. Teppler, and E. A. Emini 1996. Genetic correlates of in vivo resistance to indinavir, a human immunodeficiency virus type I protease inhibitor. J. Virol. 70:8270-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, G. L. 1999. Hepatitis C virus genotypes and quasispecies. Am. J. Med. 107:21S-26S. [DOI] [PubMed] [Google Scholar]
- 14.Dhanak, D., K. J. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonenmacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H. Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 15.Di Biscegli, A. M. 1997. Hepatitis C and hepatocellular carcinoma. Hepatology 26:34S-38S. [DOI] [PubMed] [Google Scholar]
- 16.Farci, P. 2001. Hepatitis C virus: the importance of viral heterogeneity. Clin. Liver Dis. 5:895-916. [DOI] [PubMed] [Google Scholar]
- 17.Grobler, J. A., E. J. Markel, J. F. Fay, D. J. Graham, A. Simcoe, E. M. Muray, S. Ludmerer, G. Migliaccio, and O. A. Flores. 2003. Key determinants of HCV cell culture replication map to the NS3 helicase. J. Biol. Chem. 278:16741-16746. [DOI] [PubMed] [Google Scholar]
- 18.Gu, B., A. T. Gates, S.-E. Behrens, and R. T. Sarisky. 2003. Replication studies using genotype 1a subgenomic hepatitis C virus replicons. J. Virol. 77:5352-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo, J. T., Z. Zhu, and C. Seeger. 2003. Cytopathic and noncytopathic interferon responses in cells expressing hepatitis C virus subgenomic replicons. J. Virol. 77:10769-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto, H., K. Mizutani, and A. Yoshida. Jan. 2003. Fused cyclic compounds and medicinal use thereof patent. WO patent 0300254.
- 22.Hashimoto, H., K. Mizutani, and A. Yoshida. July. 2001. Preparation of heterocyclic compounds as remedies for hepatitis C patent. WO patent 0147883.
- 23.Hashimoto, H., K. Mizutani, and A. Yoshida. March. 2003. Preparation of substituted 1-cyclohexyl-2-phenylbenzimidazole-5-carboxylic acids as remedies for hepatitis C patent. U.S. patent 2,003,050,320.
- 24.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinina, O., H. Norder, S. Mukomolov, and L. O. Magnius. 2002. A natural intergenotypic recombinant of hepatitis C virus identified in St. Petersburg. J. Virol. 76:4034-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato, T., T. Date, M. Miyamoto, A. Furusaka, K. Tokushige, M. Mizokami, and T. Wakita. 2003. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology 125:1808-1817. [DOI] [PubMed] [Google Scholar]
- 27.Kiyosawa, K., E. Tanaka, and T. Sodeyama. 1998. Hepatitis C virus and hepatocellular carcinoma. Curr. Studies Hematol. Blood Transfusion 62:161-180. [DOI] [PubMed] [Google Scholar]
- 28.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohmann, V., S. Hoffmann, U. Herian, F. Penin, and R. Bartenschlager. 2003. Viral and cellular determinant of hepatis C virus RNA replication in cell culture. J. Virol. 77:3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohmann, V., A. Korner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmann, V., F. Korner, J.-O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 32.Love, R. A., H. E. Parge, X. Yu, M. J. Hickey, W. Diehl, J. Gao, H. Wriggers, A. Ekker, L. Wang, J. A. Thomson, P. S. Dragovich, and S. A. Fuhrman. 2003. Crystallographic identification of a noncompetitive inhibitor binding site on the hepatitis C virus NS5B RNA polymerase enzyme. J. Virol. 77:7575-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 34.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 35.Migliaccio, G., J. E. Tomassini, S. S. Carroll, L. Tomei, S. Altamura, B. Bhat, L. Bartholomew, M. R. Bosserman, A. Ceccacci, L. F. Colwell, R. Cortese, R. De Francesco, A. B. Eldrup, K. L. Getty, X. S. Hou, R. L. LaFemina, S. W. Ludmerer, M. MacCoss, D. R. McMasters, M. W. Stahlhut, D. B. Olsen, D. J. Hazuda, and O. A. Flores. 2003. Characterization of resistance to non-obligate chain terminating ribonucleoside analogs which inhibit HCV replication in vitro. J. Biol. Chem. 278:49164-49170. [DOI] [PubMed] [Google Scholar]
- 36.Murray, E. M., J. A. Grobler, E. J. Markel, M. F. Pagnoni, G. Paonessa, A. J. Simon, and O. A. Flores. 2003. Persistent replication of hepatitis C virus replicons expressing the β-lactamase reporter in subpopulations of highly permissive Huh7 cells. J. Virol. 77:2928-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 38.Nunberg, J. H., W. A. Schleif, E. J. Boots, J. A. O'Brien, J. C. Quintero, J. M. Hoffman, E. A. Emini, and M. E. Goldman. 1991. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J. Virol. 65:4887-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata, N., H. J. Alter, R. H. Miller, and R. H. Purcell 1991. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc. Natl. Acad. Sci. USA 88:3392-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 75:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 42.Stuyver, L. J., T. R. McBrayer, T. Whitaker, P. M. Tharnish, M. Ramesh, S. Lostia, L. Cartee, J. Shi, A. Hobbs, R. F. Schinazi, K. A. Watanabe, and M. J. Otto. 2004. Inhibition of the subgenomic hepatitis C virus replicon in Huh-7 cells by 2′-deoxy-2′-fluorocytidine. Antimicrob. Agents Chemother. 48:651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuyver, L. J., T. Whitaker, T. R. McBrayer, B. I. Hernandez-Santiago, S. Lostia, P. M. Tharnish, M. Ramesh, C. K. Chu, R. Jordan, J. Shi, S. Rachakonda, K. A. Watanabe, M. J. Otto, and R. F. Schinazi 2003. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob. Agents Chemother. 47:244-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Summa, V., A. Petrocchi, P. Pace, V. G. Matassa, R. De Francesco, S. Altamura, L. Tomei, U. Koch, and P. Neuner. 2004. Discovery of alpha, gamma-diketo acids as potent selective and reversible inhibitors of hepatitis C virus NS5b RNA-dependent RNA polymerase. J. Med. Chem. 47:14-17. [DOI] [PubMed] [Google Scholar]
- 45.Tomei, L., S. Altamura, L. Bartholomew, A. Biroccio, A. Ceccacci, L. Pacini, F. Narjes, N. Gennari, M. Bisbocci, I. Incitti, L. Orsatti, S. Harper, I. Stansfield, M. Rowley, R. De Francesco, and G. Migliaccio. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francesco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vrolijk, J. M., A. Kaul, B. E. Hansen, V. Lohmann, B. L. Haagmans, S. W. Schalm, and R. Bartenschlager. 2003. A replicon-based bioassay for the measurement of interferons in patients with chronic hepatitis C. J. Virol. Methods 110:201-209. [DOI] [PubMed] [Google Scholar]
- 48.Walker, M. P., N. Yao, and Z. Hong. 2003. Promising candidates for the treatment of chronic hepatitis C. Expert Opin. Investig. Drugs 12:1269-1280. [DOI] [PubMed] [Google Scholar]
- 49.Wang, M., K. K. Ng, M. M. Cherney, L. Chan, C. G. Yannopoulos, J. Bedard, N. Morin, N. Nguyen-Ba, M. H. Alaoui-Ismaili, R. C. Bethell, and M. N. G. James. 2003. Non-nucleoside analogue inhibitors bind to an allosteric site on HCV NS5B polymerase. Crystal structures and mechanism of inhibition. J. Biol. Chem. 278:9489-9495. [DOI] [PubMed] [Google Scholar]
- 50.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 51.Yi, M., and S. M. Lemon. 2004. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J. Virol. 78:7904-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You, S., D. D. Stump, A. D. Branch, and C. M. Rice. 2004. A cis-acting replication element in the sequence encoding the NS5B RNA-dependent RNA polymerase is required for hepatitis C virus RNA replication. J. Virol. 78:1352-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, Q., J.-T. Guo, and C. Seeger. 2003. Replication of hepatitis C virus subgenomes in nonhepatic epithelial and mouse hepatoma cells. J. Virol. 77:9204-9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.