Abstract
Cladophialophora bantiana is associated with central nervous system infection and a poor outcome. C. bantiana tends to be resistant to amphotericin B. Accordingly, we evaluated amphotericin B and three triazoles—posaconazole, itraconazole, and fluconazole—for treatment of C. bantiana infection in mice. In immunosuppressed ICR mice infected intravenously, posaconazole, itraconazole, and amphotericin B prolonged survival. This improvement in survival corresponded with a reduction in brain fungal concentrations for mice which were given itraconazole and posaconazole, but not amphotericin B. In nonimmunosuppressed BALB/c mice infected intracerebrally, posaconazole showed dose-dependent responses in survival and reduction of brain tissue counts. These responses were observed for short, delayed, and prolonged therapy. Although posaconazole prolonged the survival of mice with reductions in brain fungal counts, it did not sterilize brain tissue with continuous therapy for 8 weeks. We concluded that posaconazole shows promise for the treatment of C. bantiana brain infections.
Cladophialophora bantiana (synonyms: Xylohypha bantiana and Cladosporium trichoides) is a member of the dematiaceous (phaeohyphomycete) group of mycelial fungal pathogens. These fungi, which are characterized by black melanin pigment, may belong to many genera (3). They may be inoculated subcutaneously, causing soft tissue infection, or inhaled, causing pneumonia or sinusitis (3, 8). Immunocompetent patients tend to have chronic localized subcutaneous infections. In a few immunocompetent patients and in more immunosuppressed patients, dematiaceous fungi may extend widely locally or disseminate hematogenously (10). Some phaeohyphomycetes have a predilection to infect the brain, where they may form fungal nodules or abscesses (4, 6, 7, 11, 14). C. bantiana is the most common fungus reported to cause primary brain abscesses with associated high mortality (5, 7, 10). Ramichloridium mackenziei is virtually exclusively a central nervous system pathogen, with mortality verging on 100% (1, 9). Limited animal data suggest no benefit of amphotericin B or fluconazole to experimental C. bantiana infection (4). Because phaeohyphomycetes may be resistant to amphotericin B, and because amphotericin B penetrates poorly into the central nervous system (CNS), we considered the possibility that broad-spectrum triazole antifungals may be more effective against C. bantiana. Clinical experience with phaeohyphomycosis supports the use of itraconazole against this class of pathogen (3, 13, 15). We evaluated C. bantiana infection in two mouse models, ICR mice immunosuppressed with corticosteroids and nonimmunosuppressed BALB/c mice, and treatment with posaconazole, itraconazole, fluconazole, or amphotericin B.
MATERIALS AND METHODS
Pathogen.
C. bantiana strain 96-1955 (The Fungus Testing Laboratory, San Antonio, Tex.) was used for the in vitro testing and experimental infection of two strains of mice, ICR and BALB/c. C. bantiana was grown on potato dextrose agar medium plates (BBL; Baltimore Biologics, Cockeysville, Md.) for 10 days at 37°C. Surface growth was washed with sterile 0.85% normal saline and filtered through layers of sterile glass wool. Homogeneous suspensions of the appropriate conidial concentration were prepared by use of hemacytometer counts. The conidial suspension was administered in concentrations of 0.2 ml for intravenous inoculation and 0.06 ml for intracranial inoculations. The actual inoculation dose was determined by quantitative cultures.
In vitro susceptibility. In vitro testing was done according to the M-38P document of the National Committee for Laboratory Standards (NCCLS) as reported previously (1).
Animals.
Age-matched 4- to 6-week-old female inbred BALB/c mice (Veterinary Medical Unit Breeding Colony, Audie Murphy Veterans Administration Hospital, San Antonio, Tex.) were used in the therapeutic studies after intracranial infection. Ten mice were included in each group for each survival and tissue burden study. The mice were housed up to five per cage with free access to water and food. Eighty 4- to 6-week-old ICR female mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used in a therapeutic study after intravenous (i.v.) inoculation of C. bantiana.
Brain fungal burden.
Brains were harvested from the mice that succumbed during the course of treatment. Mice that survived until the end of the treatment protocol, or until a designated time in some experiments, were euthanized under methoxyflurane anesthesia. The brains were harvested, weighed, and homogenized in 2 ml of sterile normal saline containing piperacillin and amikacin. The homogenate was diluted by serial 10-fold dilutions in saline. The dilutions (0.1 ml each) and the undiluted homogenates were plated on potato dextrose agar in duplicate and incubated at 37°C. Numbers of CFU were determined, and numbers of CFU per gram of organ were calculated.
Drugs.
Posaconazole is an investigational triazole antifungal that was provided by Schering-Plough Research Institute, Kenilworth, N.J. The drug was reconstituted from a powder that was suspended in 0.3% Noble agar and was given in a 0.2-ml volume orally by gavage. Amphotericin B was purchased commercially (Bristol-Myers-Squibb, Princeton, N.J.) and injected in 0.2 ml of water intraperitoneally. Itraconazole in cyclodextrin solution (Janssen Pharmaceutica, Titusville, N.J.) and fluconazole (Pfizer Inc.) were diluted in sterile water to the appropriate concentrations and administered in a 0.2-ml volume by gavage.
Experimental therapy of C. bantiana in ICR mice (intravenous challenge).
Eight groups (10 per group) of ICR mice were immunosuppressed with cortisone acetate at a concentration of 100 mg/kg of body weight subcutaneously at 1 day before and 1 day after infection. On the day of infection, the mice were inoculated with 1.2 × l06 CFU of C. bantiana in a 0.2-ml volume i.v. through the lateral tail vein. Mice were selected randomly for treatment with posaconazole at a dose of 30 mg/kg/day given orally, amphotericin B at a dose of 3 mg/kg/day given intraperitoneally, itraconazole at a dose of 30 mg/kg three times daily, or 0.3% Noble agar given orally (control). Half of the mice (40 mice) were monitored for survival for up to 30 days postchallenge, and the other half were terminated at day 8 postchallenge for the determination of brain fungal concentrations. Brain fungal burdens were performed as described above.
Dose-finding study of experimental therapy of C. bantiana in BALB/c nu+ mice after intracranial challenge.
Because our primary goal was a study of CNS infection, we wished to establish CNS infection reliably in all animals. Therefore, we switched from i.v. infection (in which CNS dissemination is common but varied) to a model with a fixed, direct CNS inoculum. We also wished to use, if possible, a less-manipulated, nonimmunosuppressed animal. A successful model of intracranial infection with C. bantiana was established with nonimmunosuppressed BALB/c mice. This model was consistent and reproducible and therefore was used in all subsequent studies. The infection dose was 5.6 × 104 CFU/mouse injected directly into the brain. Intracranial inoculation was done under methoxyflurane anesthesia. The scalp of the mouse was swabbed with 70% alcohol. Using a 1-ml syringe and a 27-gauge needle, 0.06 ml of conidial suspension was injected through the skull at a midpoint between the ears. In a dose-finding study, five groups of 21 BALB/c mice each were selected randomly to receive posaconazole at a dose of 5, 15, 25, or 50 mg/kg/day orally or 0.3% Noble agar (control) orally for 4 weeks. Treatment was started at day 1 postchallenge. Seven mice from each treatment or control group were terminated for determination of fungal brain concentrations as they died or at days 1, 14, and 28 postinfection for surviving animals.
Delayed experimental therapy of C. bantiana in BALB/c nu+ mice.
Six groups of 10 BALB/c nu+ mice each were selected randomly to receive posaconazole at a dose of 50, 25, 15, or 5 mg/kg/day orally, fluconazole at a dose of 15 mg/kg twice daily, or 0.3% Noble agar orally. The mice were injected with 5.6 × 103 CFU of C. bantiana in a 0.06-ml volume directly into the brain. Treatments were started at day 6 postinfection and continued for 28 days. Mice were monitored for survival for up to 35 days postchallenge. Brain fungal burden was done as animals died or at 5 weeks for survivors.
Prolonged therapy with posaconazole in BALB/c mice.
To determine whether prolonged therapy with posaconazole can eradicate experimental intracranial infection with a low infective dose of C. bantiana, six groups of BALB/c mice (10 mice per group) were injected with 9.1 × 102 CFU of C. bantiana in a 0.06-ml volume directly into the brain. One group was terminated at day 1 postchallenge to determine actual brain infection burden. The other five groups were monitored for up to 8 weeks. Therapy was started at day 1 postchallenge and continued for 8 weeks. Brain fungal burden was determined as animals died or at 8 weeks for survivors. Two groups (20 mice each) received posaconazole at a dose of 50 mg/kg/day orally, another two groups received posaconazole at a dose of 25 mg/kg/day orally, and a single control group received 0.3% Noble agar orally.
Statistical analysis.
The Wilcoxon matched-pairs test was used to determine the difference between survival groups, and the Mann-Whitney U test was used to determine significance between treatment and control groups for brain tissue burden. A P of ≤0.05 was required for statistically significant differences between groups.
RESULTS
In vitro data.
The first noticed growth in the drug-free tube was at 48 h. MICs at 48 and 72 h were as follows: for amphotericin B, 0.5 and 1.0 μg/ml; for itraconazole, <0.03 and <0.03 μg/ml; for fluconazole, 16 and 16 μg/ml; and for 5-flucytosine, <0.125 and <0.125 μg/ml. MICs of posaconazole were <0.03 and <0.03 μg/ml at 48 and 72 h, respectively.
Experimental therapy of C. bantiana infection in ICR mice.
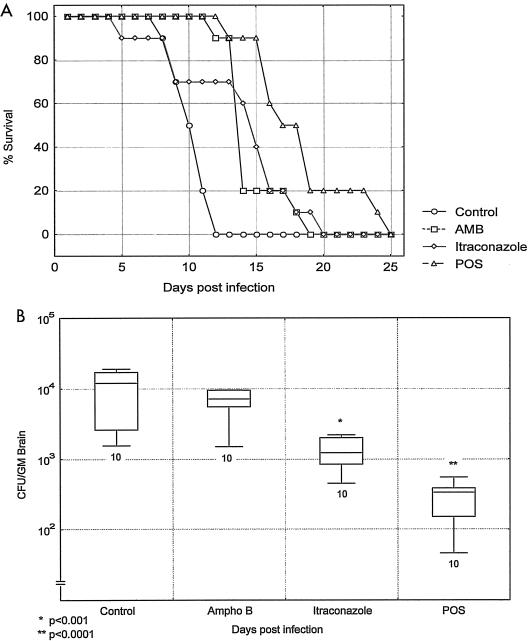
In immunosuppressed ICR mice that were infected i.v. with C. bantiana, survival was significantly prolonged with itraconazole (P = 0.01), posaconazole (P = 0.0003), and amphotericin B (P = 0.003). The best response was to posaconazole (Fig. 1A). The fungal tissue burdens of mice that were sacrificed 1 day after the end of therapy showed significant reductions with itraconazole (P < 0.001) and posaconazole (P < 0.0001) but not with amphotericin B (P = 0.911) (Fig. 1B).
FIG. 1.
Survival (A) and brain fungal burdens (B) of immunosuppressed ICR mice infected with C. bantiana i.v. and treated with amphotericin B (AMB and Ampho B) at 3 mg/kg/day, itraconazole at 30 mg/kg three times daily, or posaconazole at (POS) 30 mg/kg/day for 7 days. The box and whiskers graph for this figure and those in other figures show medians and the ranges of values, with the number of animals indicated below the whisker.
Experimental therapy of C. bantiana infection in BALB/c mice.
In the remaining studies, C. bantiana was given through direct intracranial injection.
Dose-finding study for posaconazole.
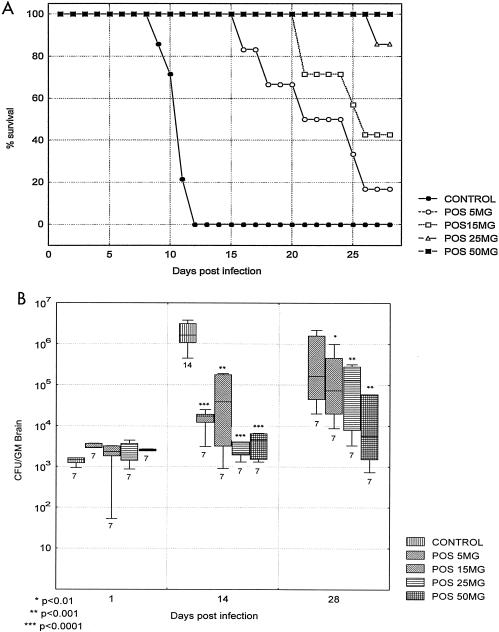
This study was done to elucidate the effect of graded doses of posaconazole in short-term therapy of C. bantiana CNS infection. We wished to explore a broad range of drug doses, from a minimally effective dose to a maximal tolerable dose, which we would use for later studies of prolonged therapy to determine whether the brain could be sterilized of C. bantiana. In this study, the control mice succumbed by day 11, but the treated mice survived much longer. All of the treated groups survived significantly longer than the controls (P was <0.001 for each group). There was a dose response all the way up to a dose of 50 mg of posaconazole per kg, which resulted in 100% survival at day 28 (Fig. 2A). This improvement in survival correlated well with a progressive, significant reduction in brain fungal counts for animals sacrificed at days 14 and 28 (Fig. 2B). A few mice on lower doses of posaconazole died before day 28 and had high brain fungal counts.
FIG. 2.
Survival (A) and brain fungal burden (B) of BALB/c mice infected with C. bantiana intracranially and treated with posaconazole (POS) at a dose of 5, 15, 25, or 50 mg/kg/day for 28 days starting at day 1 postinfection. Mice were sacrificed for determination of tissue burden at days 1, 14, and 28 postchallenge. All control mice succumbed by day 14.
Delayed experimental therapy.
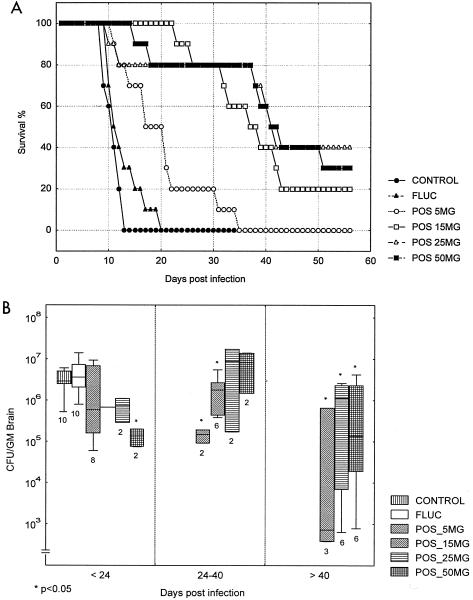
The major difference between this study and the above studies was the initiation of treatment at day 6 following infection. Survival was monitored for prolonged treatment through 4 weeks. Fluconazole did not prolong survival or reduce brain fungal concentration. All doses of posaconazole significantly prolonged survival, again with a dose-dependent response up to 50 mg/kg. In this study, no group of mice had 100% survival with any dose of drug, despite prolonged therapy (Fig. 3A). When brain tissue burden was measured, the control groups had counts up to 107 CFU/g, and the treatment groups had a very broad range of counts ranging from 107 to 102 CFU/g. In this study, posaconazole at a concentration of 5 mg/kg did not significantly reduce counts, but higher doses did. However, it is apparent that not all mice benefited. Despite higher doses of drug, some mice succumbed, and these animals had counts of C. bantiana cells in the same range as those of the control animals (Fig. 3B).
FIG. 3.
Survival (A) and brain fungal burdens (B) of BALB/c mice infected with C. bantiana intracranially and treated with posaconazole (POS) at a dose of 5, 15, 25, or 50 mg/kg/day or fluconazole (FLUC) at a dose of 50 mg/kg/day for 28 days starting at day 6 postinfection. Fungal brain concentrations were determined as mice succumbed.
Prolonged therapy with posaconazole.
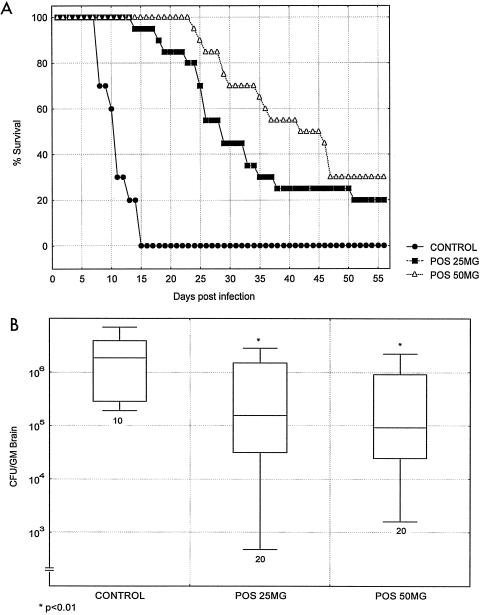
The last study was conducted with a lower dose of C. bantiana delivered intracranially. The purpose was to allow even longer survival and to determine whether prolonged treatment could sterilize brain tissues. The treatment commenced 1 day after infection and continued through 2 months. The control animals succumbed by day 14, while those who received doses of posaconazole at concentrations of both 25 and 50 mg/kg showed markedly extended survival (Fig. 4A). However, even while treatment continued, half of the mice given doses of 50 mg/kg succumbed. Brain tissue counts for the dead and sacrificed animals again had two populations, and the mice that succumbed had higher counts than those of mice that survived until the end of the treatment period (Fig. 4B). Posaconazole did not sterilize brain tissue.
FIG. 4.
Survival (A) and brain fungal burdens (B) of BALB/c mice infected with C. bantiana intracranially and treated with posaconazole (POS) at doses of 25 and 50 mg/kg/day for 8 weeks starting at day 1 postinfection. Fungal brain concentrations were determined as mice succumbed.
DISCUSSION
C. bantiana is clearly a therapeutic challenge. In our mouse model, we found fluconazole to be of no value, which is consistent with in vitro testing of fluconazole for most phaeohyphomycetes and with an earlier study of C. bantiana cerebral infection with a mouse model (4, 5). Amphotericin B prolonged survival but did not significantly lower brain counts. This finding mirrors to some degree the clinical situation, wherein amphotericin B may slow the course but is ultimately ineffective in many patients with phaeohyphomycosis. Dixon and Polak found high doses of amphotericin B to have a modest effect on the survival of mice infected with C. bantiana (4). In our study, both itraconazole and posaconazole were more beneficial, showing both prolonged survival and reduced tissue burdens of treated mice.
Because of diarrhea caused by the cyclodextrin vehicle, itraconazole could not be administered at doses higher than 30 mg/kg three times daily. Therefore, we could not use higher doses of itraconazole. Posaconazole was the most effective drug in our studies. As shown in Fig. 2, with survival and 28-day tissue burden results, we found a dose-response curve that favored posaconazole in doses from 5 to 50 mg/kg/day. With relatively brief observations of 1 month, survival was prolonged through most of the treatment period. The dose-dependent responses with regard to survival and tissue burden in animals which succumbed or were sacrificed before day 24 are shown in Fig. 3. However, when treatment was stopped at day 28, there was a relatively rapid acceleration of mortality in all surviving groups, such that more than half of the mice had succumbed by day 58. The tissue burdens of some mice also began to rise, resulting in higher counts in days 24 to 40 and after day 40. The dose-response effect was also lost.
When the inoculum was lowered and the treatment was extended to 2 months, posaconazole was again quite beneficial, but only during the length of treatment and then only incompletely. Mice receiving 50 mg of posaconazole/kg/day survived to day 40 at a greater rate than mice which received 25 mg/kg/day. However, as the observation continued, the survival rates narrowed and decreased so that, by day 56, the survival rates of both groups were similar. None of the brains of the treated animals were sterilized. Indeed, in some treated animals, the tissue burden was not reduced, and most of these animals succumbed. In those which survived through 56 days, the tissue burden was reduced. However, none of the brains were free of C. bantiana, and we suspect that these animals also would eventually have succumbed. The range of counts was very broad and may have reflected a late regrowth of C. bantiana in the brain.
Our studies had the most encouraging results for posaconazole, and we believe that this drug may be a useful agent for this infection. However, it is clear that posaconazole acts in a fungistatic, not a fungicidal, capacity; when the drug is stopped, despite high doses and prolonged treatment, animals succumb and high brain organism counts are recorded. Indeed, some animals have progressive disease and succumb during posaconazole therapy. These findings suggest that the drug may have a maximal threshold effect, despite increases in dosage and duration of treatment. There are several possible explanations for this. First, posaconazole is highly protein bound and may transfer poorly or slowly into the brain (Schering-Plough Research Institute, internal document on SN 97458, 2001). Second, C. bantiana can form brain abscesses, into which penetration of posaconazole (or any other antifungal) may be variable. Third, the kinetics of absorption in humans are varied, and bioavailability is less than 100% (2). This is reminiscent of itraconazole, which has activity against cryptococcosis despite the absence of measurable drug in the cerebrospinal fluid (CSF). However, the activity is less than that of fluconazole, in that itraconazole given at 200 mg/day as capsules resulted in more relapses than fluconazole, which is very well absorbed after oral administration and penetrates the CNS very well (12). There has been an assumption that this result may be related to poor CSF penetration by itraconazole. However, with the well-known irregular absorption of oral itraconazole from capsules, it is just as likely that poor oral absorption was as or more important than poor brain penetration. Indeed, amphotericin B is effective against C. neoformans meningitis even though it is also rarely detectable in the CSF. Fourth, it is possible that resistance may have emerged during prolonged therapy. We did not recheck the in vitro susceptibilities of isolates that were cultured from mice which succumbed late in the study.
Nevertheless, the clearly beneficial response in CNS infection suggests that this may be the best agent tested so far. Our results with C. bantiana are somewhat similar to results with another neurotropic fungus, Ramichloridium mackenziei (1). However, the inability to sterilize the brain, despite very prolonged treatment with high doses of posaconazole, suggests that we have not yet reached optimal therapy. It may be that even higher doses of posaconazole, perhaps administered in an i.v. formulation under development, combinations of antifungals, or combined antifungal and surgical resection may be more beneficial. Our results also suggest that more-prolonged suppressive therapy may be required.
Acknowledgments
This work was supported by the Schering-Plough Research Institute, Kenilworth, N.J.
REFERENCES
- 1.Al-Abdely, H. M., L. Najvar, R. Bocanegra, A. Fothergill, D. Loebenberg, M. G. Rinaldi, and J. R. Graybill. 2000. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziei”). Antimicrob. Agents Chemother. 44:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Hoog, G. S., F. Queiroz-Tellez, G. Haase, G. Fernandez-Zeppenfeldt, D. Attili Angelis, A. H. Gerrits van den Ende, T. Matos, H. Peltroche-Llacsahuanga, A. A. Pizzirani-Kleiner, J. Rainer, N. Richard-Yegres, V. Vicente, and F. Yegres. 2000. Black fungi: clinical and pathogenic approaches. Med. Mycol. 38(Suppl. 1):243-250. [PubMed] [Google Scholar]
- 4.Dixon, D. M., and A. Polak. 1987. In vitro and in vivo drug studies with three agents of central nervous system phaeohyphomycosis. Chemotherapy 33:129-140. [DOI] [PubMed] [Google Scholar]
- 5.Fothergill, A. W., D. A. Sutton, and M. G. Rinaldi. 1996. An in vitro head-to-head comparison of Schering 56592, amphotericin B, fluconazole, and itraconazole against a spectrum of filamentous fungi. Program Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F89.
- 6.Mukherji, S. K., and M. Castillo. 1995. Cerebral phaeohyphomycosis caused by Xylohypha bantiana: MR findings. Am. J. Roentgenol. 164:1304-1305. [DOI] [PubMed] [Google Scholar]
- 7.Nieto-Rodriguez, J. A., and S. Kusne. 1996. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava. Clin. Infect. Dis. 23:211. [DOI] [PubMed] [Google Scholar]
- 8.Patterson, J. W., N. G. Warren, and L. W. Kelly. 1999. Cutaneous phaeohyphomycosis due to Cladophialophora bantiana. J. Am. Acad. Dermatol. 40:364-366. [DOI] [PubMed] [Google Scholar]
- 9.Podnos, Y. D., P. Anastasio, L. De la Maza, and R. B. Kim. 1999. Cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (Ramichloridium mackenziei): case report. Neurosurgery 45:372-375. [DOI] [PubMed] [Google Scholar]
- 10.Revankar, S. G., J. E. Patterson, D. A. Sutton, R. Pullen, and M. G. Rinaldi. 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 34:467-476. [DOI] [PubMed] [Google Scholar]
- 11.Revankar, S. G., D. A. Sutton, and M. G. Rinaldi. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206-216. [DOI] [PubMed] [Google Scholar]
- 12.Saag, M. S., G. A. Cloud, J. R. Graybill, J. D. Sobel, C. U. Tuazon, P. C. Johnson, W. J. Fessel, B. L. Moskovitz, B. Wiesinger, D. Cosmatos, L. Riser, C. Thomas, R. Hafner, W. E. Dismukes, et al. 1999. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 28:291-296. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey, P. K., J. R. Graybill, M. G. Rinaldi, D. A. Stevens, R. M. Tucker, J. D. Peterie, P. D. Hoeprich, D. L. Greer, L. Frenkel, G. W. Counts, J. Goodrich, S. Zellner, R. W. Bradsher, C. M. van der Horst, K. Israel, G. A. Pankey, and C. P. Barranco. 1990. Itraconazole treatment of phaeohyphomycosis. J. Am. Acad. Dermatol. 23:577-586. [DOI] [PubMed] [Google Scholar]
- 14.Vukmir, R. B., S. Kusne, P. Linden, W. Pasculle, A. W. Fothergill, J. Sheaffer, J. Nieto, R. Segal, H. Merhav, A. J. Martinez, and M. G. Rinaldi. 1994. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava in a liver transplant recipient. Clin. Infect. Dis. 19:714-719. [DOI] [PubMed] [Google Scholar]
- 15.Whittle, D. I., and S. Kominos. 1995. Use of itraconazole for treating subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei. Clin. Infect. Dis. 21:1068. [DOI] [PubMed] [Google Scholar]