Abstract
To study tenofovir transfer into milk, two lactating macaques were given a subcutaneous dose of tenofovir (30 mg/kg of body weight). Peak concentrations and area under the curve values of tenofovir in milk were ∼3 and ∼20% of those detected in serum, respectively.
For lactating mothers taking medications, the drug concentration in breast milk is usually lower than that in plasma; it is generally of negligible concern for the nursing infant, but some exceptions exist (reviewed in reference 2). A growing number of human immunodeficiency virus (HIV)-infected people in developing countries are gaining access to treatment with anti-HIV drugs, including lactating mothers for whom avoidance of breast-feeding is not always an option. Thus, questions are raised regarding possible biological implications of anti-HIV drugs that may be transferred to the nursing infant (4, 5). It is difficult to predict drug transfer into milk based on physicochemical properties (6). Accordingly, animal models can be useful to gather preliminary information on the transfer of compounds into breast milk prior to obtaining such data from human studies (1).
We performed a pilot study to determine the transfer of tenofovir {9-[2-(phosphonomethoxy)propyl]adenine; PMPA} in breast milk of rhesus macaques. Two healthy lactating adult rhesus macaques (Macaca mulatta), which were multiparous and 5 to 11 years of age, were used. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards, and we strictly adhered to the Guide for the Care and Use of Laboratory Animals (9). When necessary, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, New Jersey) at a concentration of 10 mg/kg of body weight injected intramuscularly. Both female macaques had been lactating for 10 to 11 weeks, and their infants had been weaned the day prior to the pharmacokinetic study. A single dose of tenofovir (30 mg/kg) was administered subcutaneously. Pre- and postdose blood samples (without anticoagulant) were collected over a 24-h time period (at 0, 0.5, 1, 2, 4, 6, 8, and 24 h, with an additional time point at 10 h for animal 24964) and were spun immediately for the collection of serum; at the same times, all milk that could be expressed manually from both nipples was collected (up to 7.5 ml per time point). Our study has the caveat that this milk collection schedule (especially the absence of sample collections between 10 and 24 h of dosing) does not completely mimic the more regular drinking activity of a nursing infant macaque. Serum and milk samples were stored at −70°C and subsequently analyzed by MDS Pharma Services (Montreal, Canada) using high-performance liquid chromatography methods with mass spectrometry detection (liquid chromatography-mass spectrometry-mass spectrometry), previously validated for monkey plasma and rat milk (unpublished data). For monkey plasma, the limit of quantitation was 10 ng/ml, standard curve linearity r2 was 0.999, and within- and between-run quantitative comparison [QC] accuracy and precision were <2% bias and 95%, respectively; for monkey milk, the limit of quantitation was 10 ng/ml, r2 was 0.9932, and within-run QC accuracy was <3% bias. Tenofovir concentrations were measured in whole milk (i.e., without prior separation of the different milk fractions). The values of the pharmacokinetic parameters were derived by noncompartmental analysis with WinNonlin software (version 3.1; Pharsight Corporation, Mountain View, California).
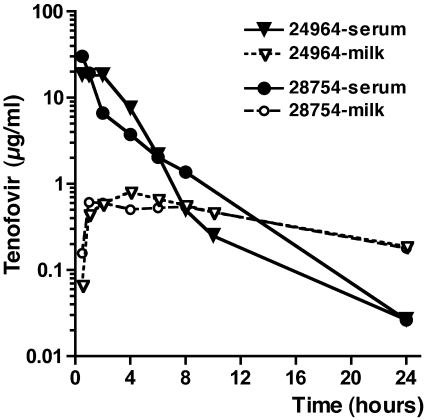
Tenofovir concentrations in serum and milk are shown in Fig. 1, while pharmacokinetic parameters are presented in Table 1. Tenofovir was detected in the milk of both animals, but the peak concentrations (∼0.6 to 0.8 μg/ml, corresponding to ∼2 to 3 μM) were ∼2 to 4% of those detected in serum, with milk area under the curve (AUC) values being ∼20% of the serum AUC values.
FIG. 1.
Concentrations of tenofovir in serum and milk following a single subcutaneous dose of 30 mg of tenofovir/kg of body weight.
TABLE 1.
Tenofovir pharmacokinetics in lactating rhesus macaques
| Animal no.a | Sample | Cmax (μg/ml) | Tmax (h) | C24h (μg/ml) | AUC0-∞ (μg · h/ml) | t1/2 (h) | CL/F (ml/h/kg) | Vz/F (ml/kg) |
|---|---|---|---|---|---|---|---|---|
| 24964 | Serum | 18.3 | 0.5 | 0.026 | 68.9 | 3.97 | 435 | 2,489 |
| Milk | 0.808 | 4 | 0.188 | 12.8 | 10.3 | |||
| 28754 | Serum | 30.2 | 0.5 | 0.026 | 56.2 | 2.85 | 534 | 2,191 |
| Milk | 0.610 | 1 | 0.179 | 12.1 | 10.9 |
Animals 24964 and 28754 were 11 and 5 years of age, respectively. A single dose of tenofovir (30 mg/kg) was administered subcutaneously. Abbreviations: Cmax, maximum concentration; Tmax, the time to Cmax; C24h, concentration at 24 h; AUC0-∞, area under the concentration-versus-time curve extrapolated to infinity; t1/2, half-life of terminal elimination phase; CL/F, apparent clearance; Vz/F, apparent volume of distribution based on terminal phase. CL/F and Vz/F were not calculated for milk. Each individual concentration profile was reviewed to assure accuracy of the pharmacokinetic analysis.
Other anti-HIV drugs (nevirapine, zidovudine, and lamivudine) are also found in breast milk (7, 8). Treatment of HIV-infected lactating mothers with anti-HIV drugs is expected to benefit the infant indirectly (by improving the mother's health and reducing maternal systemic virus levels, thus potentially lowering the infectivity of the breast milk). However, could such levels of anti-HIV drugs in breast milk have any direct biological effects, either harmful or beneficial for the infant? It was recently reported that concentrations of lamivudine and nevirapine in breast milk were high enough to give detectable serum levels in their nursing infants, which may provide prophylactic effects but may also have toxic effects (R. Shapiro et al., 42nd Ann. Meet. Infect. Dis. Soc. Am., Boston, Late Breaker abstr. LB-1, 2004). Concerns have also been raised that infants who become infected may be exposed for relatively long periods to subtherapeutic levels of drug, which may lead to resistance and limit the future treatment options for the infant (4, 5).
For breast-feeding mothers taking the orally bioavailable prodrug tenofovir disoproxyl fumarate, breast milk is expected to contain almost exclusively the parental compound tenofovir, which due to its charged anionic nature exhibits low oral bioavailability in animals (5% in cynomolgus macaques) and is expected to also show low oral bioavailability after ingestion by the nursing infant (3, 10). A previous study in macaques suggests that the concentration of tenofovir in milk is unlikely to give topical prophylaxis against oral HIV infection (13). Instead, infant macaque studies suggest that direct administration of tenofovir disoproxyl fumarate to the nursing infant at regimens that give systemic drug levels is needed to prevent infection through breast-feeding (12; K. Van Rompay, J. Lawson, R. Colón, N. Bischofberger, and M. Marthas, XV Int. AIDS Conf., Bangkok, Late Breaker abstr. LbOrB10, 2004). Considering the volume of ingested breast milk, the tenofovir concentrations we observed in breast milk of lactating macaques are unlikely to be toxic for the infant, especially because our previous studies demonstrated a favorable safety profile of prolonged daily treatment of infant macaques with a dose of tenofovir (10 mg/kg subcutaneously) that is much higher than the daily amount of tenofovir likely to be ingested and absorbed from breast milk (11). Because of its low oral bioavailability, small amounts of tenofovir in milk are also very unlikely to select for resistance in an already infected infant, thus preserving future treatment options.
In conclusion, this pilot pharmacokinetic study in lactating rhesus macaques demonstrates that tenofovir, similar to most other drugs, is found in milk but at lower levels than in maternal blood. The available data suggest that such low tenofovir levels in milk will most likely have no biological effects whatsoever for the nursing infant.
Acknowledgments
We thank L. Hirst and the staff of the Veterinary & Colony Services of the California National Primate Research Center for expert technical assistance and M. Marthas for critical review of the manuscript.
This research was supported by Gilead Sciences and E. Glaser Pediatric AIDS Foundation grant PG-51014 to K.K.A.V.R.
REFERENCES
- 1.Alcorn, J., and P. J. McNamara. 2002. Acyclovir, ganciclovir, and zidovudine transfer into rat milk. Antimicrob. Agents Chemother. 46:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. 2001. The transfer of drugs and other chemicals into human milk. Pediatrics 108:776-789. [DOI] [PubMed] [Google Scholar]
- 3.Cundy, K. C., C. Sueoka, G. R. Lynch, L. Griffin, W. A. Lee, and J.-P. Shaw. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob. Agents Chemother. 42:687-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaillard, P., M.-G. Fowler, F. Dabis, H. Coovadia, C. van der Horst, K. Van Rompay, A. Ruff, T. Taha, T. Thomas, I. de Vicenzi, and M.-L. Newell for the Ghent IAS Working Group on HIV in Women and Children. 2004. Use of antiretroviral drugs to prevent HIV-1 transmission through breastfeeding: from animal studies to randomized clinical trials. J. Acquir. Immune Defic. Syndr. 35:178-187. [DOI] [PubMed] [Google Scholar]
- 5.John-Stewart, G., D. Mbori-Ngacha, R. Ekpini, E. N. Janoff, J. Nkengasong, J. S. Read, P. Van de Perre, and M.-L. Newell. 2004. Breastfeeding and transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 35:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen, L. A., S. Ito, and G. Koren. 2003. Prediction of milk/plasma concentration ratio of drugs. Ann. Pharmacother. 37:1299-1306. [DOI] [PubMed] [Google Scholar]
- 7.Moodley, J., D. Moodley, K. Pillay, H. Coovadia, J. Saba, R. van Leeuwen, C. Goodwin, P. R. Harrigan, K. H. Moore, C. Stone, R. Plumb, and M. A. Johnson. 1998. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J. Infect. Dis. 178:1327-1333. [DOI] [PubMed] [Google Scholar]
- 8.Musoke, P., L. A. Guay, D. Bagenda, M. Mirochnick, C. Nakabiito, T. Fleming, T. Elliott, S. Horton, K. Dransfield, J. W. Pav, A. Murarka, M. Allen, M. G. Fowler, L. Mofenson, D. Hom, F. Mmiro, and J. B. Jackson. 1999. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS 13:479-486. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 10.Shaw, J. P., C. M. Sueoka, R. Oliyai, W. A. Lee, M. N. Arimilli, C. Kim, and K. C. Cundy. 1997. Metabolism and pharmacokinetics of a novel oral prodrug of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 14:1824-1829. [DOI] [PubMed] [Google Scholar]
- 11.Van Rompay, K. K. A., L. L. Brignolo, D. J. Meyer, C. Jerome, R. Tarara, A. Spinner, M. Hamilton, L. L. Hirst, D. R. Bennett, D. R. Canfield, T. G. Dearman, W. Von Morgenland, P. C. Allen, C. Valverde, A. B. Castillo, R. B. Martin, V. F. Valerie, F. Samii, R. Bendele, J. Desjardins, M. L. Marthas, N. C. Pedersen, and N. Bischofberger. 2004. Biological effects of short-term and prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 48:1469-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 13.Van Rompay, K. K. A., K. A. Schmidt, J. R. Lawson, R. Singh, N. Bischofberger, and M. L. Marthas. 2002. Topical administration of low-dose tenofovir disoproxyl fumarate to protect infant macaques against multiple oral exposures of low doses of simian immunodeficiency virus. J. Infect. Dis. 186:1508-1513. [DOI] [PubMed] [Google Scholar]