Abstract
The activity of nerolidol, a sesquiterpene used as a food-flavoring agent and currently under testing as a skin penetration enhancer for the transdermal delivery of therapeutic drugs, was evaluated against Leishmania species. Nerolidol inhibited the growth of Leishmania amazonensis, L. braziliensis, and L. chagasi promastigotes and L. amazonensis amastigotes with in vitro 50% inhibitory concentrations of 85, 74, 75, and 67 μM, respectively. The treatment of L. amazonensis-infected macrophages with 100 μM nerolidol resulted in 95% reduction in infection rates. Inhibition of isoprenoid biosynthesis, as shown by reduced incorporation of [2-14C]mevalonic acid (MVA) or [1-14C]acetic acid precursors into dolichol, ergosterol, and ubiquinone, was observed in nerolidol-treated promastigotes. This drug effect can be attributed to the blockage of an early step in the mevalonate pathway, since incorporation of the precursor [1(n)-3H]farnesyl pyrophosphate in polyisoprenoids is not inhibited by nerolidol. L. amazonensis-infected BALB/c mice were treated with intraperitoneal doses of 100 mg/kg/day for 12 days or topically with 5 or 10% ointments for 4 weeks. Significant reduction of lesion sizes in nerolidol treated mice was observed for both treatment routes. However, long-term follow up indicated that the disease was not cured in this highly susceptible animal model. Nonetheless, the in vitro activity of nerolidol against these parasites may prove a useful tool for the development of new drugs for the treatment of leishmaniasis. In addition, biosynthesis of dolichols with 11 and 12 isoprene units was identified in Leishmania, as described for other trypanosomatids and Apicomplexa.
Leishmaniasis is a vector-borne infection afflicting 14 million people in the world. The clinical manifestations include a spectrum of disease from predominantly cutaneous to visceral syndromes, depending on the parasite species and the host's immune responses. Visceral leishmaniasis is fatal if left untreated, and the cutaneous forms are often disfiguring and mutilating (19). Uncommon clinical presentations and a great deal of resistance to the classical therapeutic approaches are observed when leishmaniasis presents as an opportunistic infection in association with immunodepression, especially AIDS (3).
Pentavalent antimonials, first used clinically at the beginning of last century, are still the first choice drugs for the treatment of leishmaniasis. Antimonials are toxic and poorly tolerated, require daily injections for up to 28 days and are becoming ineffective due to the proliferation of resistant parasites (reviewed in references 8 and 26).
Efforts to improve the therapeutic arsenal against leishmaniasis have led to the application of amphotericin B in liposomes and, more recently, to the study of miltefosine activity in visceral patients in India (36). Nonetheless, the need for alternative drugs remains.
Early studies on sterol biosynthesis in Leishmania revealed that these parasites cannot synthesize cholesterol de novo but are able to produce ergosterol through the mevalonate pathway (15, 16). Antifungal drugs targeting the final steps of ergosterol biosynthesis were consequently tried as antileishmanial agents (2, 38). Although azoles were shown to be effective against L. major infections, the results were not uniform in the treatment of mucocutaneous leishmaniasis (9, 27).
In most eukaryotic cells, the isoprenoid biosynthetic pathway generates other essential metabolic products in addition to cholesterol and ergosterol, such as the dolichols, which are present in all membranes in variable amounts (4) and, in a modified phosphorylated form, are required for the asparagine-linked glycosylation of proteins (39). The pathway also generates the isoprene side chains attached to the benzoquinone ring of ubiquinone (13), prenyl groups transferred to prenylated proteins (32), and prenylated transfer RNAs. Therefore, the blockage of the initial steps in this pathway could potentially have serious effects for the parasite.
In addition to ergosterol, other products of the mevalonate pathway have been identified in Leishmania: coenzyme Q9 (CoQ9) was detected as the predominant species of ubiquinone in promastigotes and amastigotes of L. amazonensis, whereas CoQ7 and CoQ8 were also identified in promastigotes (12); prenylated proteins were observed in Leishmania mexicana after incorporation of labeled mevalonate (43), and phosphorylated dolichol has been detected as a sugar donor for glycosylation of proteins in L. mexicana (30), although the structure of dolichol in these organisms was still to be characterized.
In plants, the isoprenoid biosynthetic pathway also generates compounds known as terpenes, components of many essential oils possessing antibacterial, antifungal, and antiparasitic properties (7).
Nerolidol is a sesquiterpene present in essential oils of several plants, approved by the U.S. Food and Drug Administration as a food flavoring agent. Nerolidol exhibits antineoplastic activity (41), and it has also been tested as a skin penetration enhancer for the transdermal delivery of therapeutic drugs (5, 42). Lopes et al. (23) reported the activity of nerolidol against the malaria parasite. In the present study we describe the leishmanicidal activity of nerolidol and its inhibitory effect on the biosynthesis of isoprenoids. We also show that L. amazonensis promastigotes synthesize dolichols of 11 and 12 isoprene units.
MATERIALS AND METHODS
Parasites.
Leishmania promastigotes were grown in liquid culture as previously described (20). The strains used were L. amazonensis MHOM/BR/1973/M2269, L. chagasi MHOM/BR/1974/M2682, and L. braziliensis MHOM/BR/1975/M2903. Amastigotes were obtained from experimentally infected BALB/c mice as described previously (37).
Drugs.
Nerolidol (a mixture of cis- and trans-nerolidol) (Fig. 1) was purchased from Sigma-Aldrich (St. Louis, Mo.). A 19.6 mM stock solution prepared in methanol was used for in vitro experiments. Intraperitoneal injections were done by diluting the drug in 10% ethanol in 0.01 M phosphate buffer (pH 7.4); ointments containing 5 or 10% (wt/wt) nerolidol were prepared in a 70% vaseline-30% lanolin base.
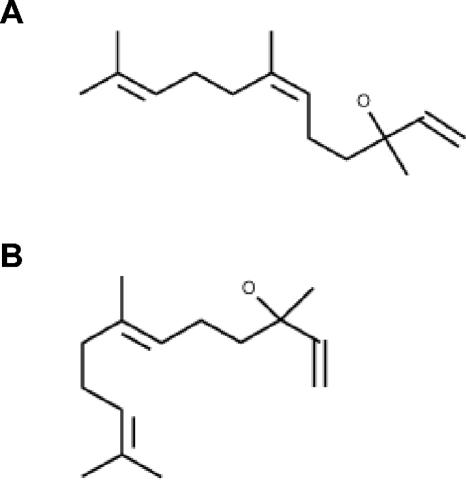
FIG. 1.
Chemical structure of nerolidol. (A) cis-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol and (B) trans-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol.
Assay of in vitro antiproliferative activity.
Inhibition of cell growth was tested in vitro by cultivating promastigotes (1 × 106) or amastigotes (5 × 106) in the presence of increasing concentrations of nerolidol in 24-well culture dishes (Corning Life Sciences, Corning, N.Y.) for 2, 24, and 48 h. Cell viability was assessed by measuring the cleavage of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) with metabolically active cells as described by Barcinski et al. (1). Briefly, cells were incubated in 3-(N-morpholino) propanesulfonic acid (MOPS)-buffered saline (30 mM MOPS [pH 7.2], 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 5.5 mM d-glucose) containing 5 mg of MTT ml−1 for 2 h (at 25°C for promastigotes and 33°C for amastigotes and macrophages). MTT cleavage was measured by using a multiwell scanning spectrophotometer (Labsystems; Multiskan EX) with a reference wavelength of 690 nm and a test wavelength of 595 nm. Assays were performed in triplicate, and results are expressed as the mean percent reduction of parasite numbers compared to untreated control wells calculated for at least three independent experiments. The 50% inhibitory concentration (IC50) was determined from sigmoidal regression of the concentration-response curves.
The inhibition of amastigote intracellular growth was assayed by analyses of the number of infected cells in macrophage monolayers. J774.A1 macrophages were plated in round glass coverslips inside the wells of a 24-well culture dish at a concentration of 5 × 105 cells per coverslip in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 50 mg of gentamicin ml−1. After 2 h of incubation at 37°C in an atmosphere of 5% CO2, L. amazonensis stationary-phase promastigotes were added to the wells (2.5 × 106 per well), and the cultures were incubated at 33°C in a 5% CO2 atmosphere. After 3 h, free promastigotes were removed by extensive washing with RPMI medium without fetal calf serum, and infected cultures were treated with the different drug concentrations for 48 h. The monolayers were washed, fixed, and stained with the Instant Prov kit (Newprov, Pinhais, Brazil), and the percentage of infected macrophages was assessed by light microscopy observation by counting 100 cells in triplicate coverslips.
Cytotoxicity was evaluated by cultivating 5 × 105 J774.A1 macrophages or human foreskin fibroblasts in 24-well plates for 24 h in the presence of increasing concentrations of nerolidol. Cell viability was assessed by the MTT assay as described above, and results are expressed as the percent reduction in cell viability compared to untreated control cultures. The 50% cytotoxic concentration was determined as described above for the IC50 values.
Metabolic labeling.
Promastigotes (2.5 × 108) were incubated in normal medium or in medium with different concentrations of nerolidol for 2 h and labeled for 18 h (in the presence of the drug) with 2 μCi of [2-14C]mevalonic acid (MVA; 72 mCi/mmol; Amersham International, Buckinghamshire, United Kingdom) ml−1, 1.87 μCi of [1-14C]acetic acid (60 mCi/mmol; Amersham) ml−1, or with 1 μCi of [1(n)-3H]farnesyl pyrophosphate (FPP; 17 Ci/mmol; Amersham) ml−1 at 25°C. The same protocol was used for labeling parasites with 1.5 μCi of l-[35S]methionine (>1,000 Ci/mmol; Amersham) ml−1 in 10 mM methionine-deficient RPMI medium. To verify whether control and treated cultures contained the same quantity of cells, parasites were counted in aliquots of labeled cultures and their viabilities were determined by MTT assay. Parasite pellets were stored at −70°C until extraction.
Lipid extraction.
Freeze-dried pellets were extracted with hexane (three extractions of 1 ml). The pooled extracts were dried under a nitrogen stream and resuspended in 3 ml of hexane. Aliquots of each extract were monitored for radioactivity in a Beckman LS5000 TD β-Counter (6).
HPTLC.
Hexane extracts obtained from the same number of treated or untreated parasites were analyzed by high-performance thin-layer chromatography (HPTLC). Silica Gel 60 plates (Merck) were developed as described previously (28) with hexane-diethyl ether-acetic acid (80:20:1 [vol/vol/vol]) at room temperature. Authentic standards of CoQ10, farnesol, geraniol, dolichol (11 to 12 isoprene units), and ergosterol were run on the same plate. Standards were visualized with iodine vapor. Plates were exposed to phosphor screen (Molecular Dynamics) for 10 days and scanned by using a Storm 840 apparatus (Molecular Dynamics). Product levels were quantified by using the ImageQuant program (Molecular Dynamics), and inhibition was calculated by comparing the areas under each curve. Plates were also exposed to Kodak X-Omat films.
Reversed-phase HPLC (RP-HPLC).
Samples were analyzed on an Ultrasphere ODS C18 Beckman column (250 by 4.6 mm; particle size, 5 μm) with a Gilson high performance liquid chromatography (HPLC) apparatus equipped with UV/VIS 151 and 152 detectors. For dolichol and ergosterol analysis, samples were resuspended in methanol, and the eluent was monitored at 210 nm. A gradient elution system was used, with methanol-water (9:1 [vol/vol]) as solvent A and hexane-propan-2-ol-methanol (1:1:2 [vol/vol/vol]) as solvent B. A linear gradient from 5 to 100% B over a period of 25 min was run; 100% B was then pumped through for additional 5 min. The flow rate was 1.5 ml min−1. Standards of prenols of 6, 7, 10, 11, and 12 isoprene units, a mixture of dolichol of 9, 11, and 12 units (kindly provided by Tadeusz Chojnacki, Institute of Biochemistry and Biophysics of the Polish Academy of Sciences, Warsaw, Poland), and ergosterol were coinjected (6). Fractions of 0.5 min were collected, and aliquots were subjected to liquid scintillation counting.
In order to obtain a better purification of samples to be analyzed by mass spectrometry (see below), samples purified by RP-HPLC corresponding to the retention times of the dolichol standard of 11 isoprene units and polyisoprenoid standard of 60 carbons were resuspended in 200 μl of 100% methanol and subjected to a new analytical RP-HPLC separation performed in the same Gilson apparatus on a Phenomenex Luna C18 column (250 by 4.6 mm; particle size, 5 μm). Methanol-propan-2-ol-water (12:8:1 [vol/vol/vol]; solvent A) and hexane-propan-2-ol (1:1 [vol/vol]) (solvent B) were used as solvents. A linear gradient was run from 0 to 70% of solvent B over a period of 40 min; 70% of solvent B was then pumped through for an additional 5 min with a flow rate of 1.0 ml min−1 (the method was adapted from that described in reference 35). The UV detector was set at 210 nm.
For the analysis of isoprene side chains attached to benzoquinone rings, parasite extracts were resuspended in methanol and coinjected with a mixture of known amounts of authentic standards of Q6 and Q10. Methanol-hexane (75:25 [vol/vol]) was used as a solvent system at a flow of 1 ml min−1. Standards were detected at 275 nm (10). Each 30-s fraction was collected, and aliquots were monitored for radioactivity. In order to compare the effect of nerolidol on the biosynthesis of dolichol and derivates or on the isoprene side chains of ubiquinones, the same quantities of treated or nontreated parasites were injected. Percent inhibition was determined as follows: [(cpm control − cpm treated)/cpm control] × 100.
ESI-MS and MS/MS analysis of dolichols of 11 e 12 isoprene units.
Samples purified from the RP-HPLC in the Phenomenex Luna C18 column with retention times corresponding to authentic standards of dolichol with 11 isoprene units and polyisoprenoid with 12 units (2 μM each) were resuspended in 30 μl of chloroform/methanol (1:1 [vol/vol]), containing 2 mM lithium iodide. The samples (10 μl) were injected into the mass spectrometer (ESI-ion trap-MS, LCQ-Duo, ThermoFinnigan, San Jose, Calif.) ion source through a 10-μl loop, with an OMNIFIT N2 pressure system (Omni Fit, Ltd., Cambridge, United Kingdom), set at 10 lb/in2 and a flow rate of 10 μl min−1, running with the same mixture as used for the dilution of the samples. All spectra were acquired in positive-ion mode with spray voltage, capillary voltage and capillary temperature set at 4.52 kV, 17 V, and 250°C, respectively. Scans were collected at 700 to 800 mass-to-charge (m/z) range for the sample corresponding to the dolichol of 11 isoprene units and at 800 to 900 m/z range for the dolichol of 12 isoprene units. For electrospray ionization-tandem mass spectrometry (ESI-MS/MS) analysis, a relative collision energy of 40% (2 eV) was applied in both analyses, and the sheath (N2) and collision (He) gas pressures were 1.5 mTorr and 4 lb/in2, respectively. These parameters were optimized for the highest intensity of the [M + Li]+-ion by using the same authentic dolichol standards as described above.
In vivo assays.
Female BALB/c mice were infected subcutaneously at the left hind footpad or at the base of the tail with 106 stationary-phase promastigotes or 106 freshly harvested L. amazonensis amastigotes. After 4 to 8 weeks, swelling at the inoculation site developed, and the treatment was initiated.
Lesion size was recorded once a week by measuring the thickness of the lesion with a caliper. All animal experiments were approved by the Ethical Committee. The data on the lesion progression were analyzed for statistical significance by using the two-tailed Student t test for unpaired samples. A result was considered significant at P < 0.05.
Limiting dilution.
Parasites from tissue were quantified as described previously (22).
RESULTS
Effect of nerolidol on promastigotes and amastigotes of Leishmania in vitro.
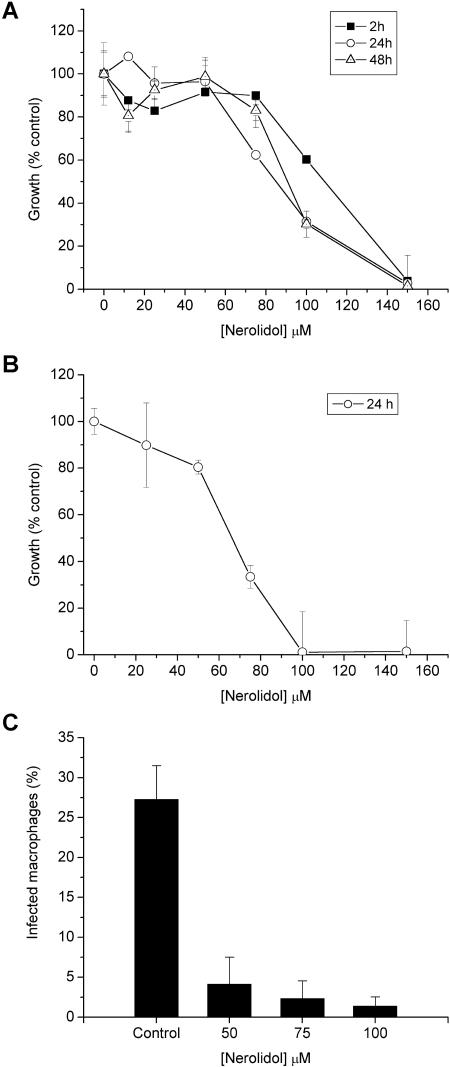
The antileishmanial activity of nerolidol was evaluated in cultures of L. amazonensis promastigotes. A dose-dependent effect was already evident after 2 h and increased in intensity when cultures were incubated for 24 h, especially at lower drug concentrations. Longer assays (48 h) resulted in only minor increments of activity (Fig. 2A), probably due to the high volatility of the drug. Promastigotes incubated with 0.76% (vol/vol) methanol (the concentration necessary to deliver the highest drug concentration used in the test) showed growth rates equivalent to 93.3% ± 5.5% of the control cultures, indicating that the drug diluent was not toxic to the parasites in the conditions applied in the test. The calculated IC50 of nerolidol for L. amazonensis promastigotes was 85.22 ± 5.45 μM. Nerolidol was also active against amastigotes purified from lesions and grown in vitro for 24 h with an IC50 of 67.73 ± 3.79 μM (Fig. 2B).
FIG. 2.
Effect of nerolidol on the survival of L. amazonensis. The cell viability of promastigotes (A) and amastigotes (B) was evaluated after the indicated periods of incubation with different concentrations of nerolidol. The growth of treated cultures is shown as the percentage of control parasites incubated in medium alone. (C) Effect of nerolidol on intracellular amastigotes. Macrophages were infected with L. amazonensis promastigotes for 3 h. After infection, nerolidol was added at final concentrations of 50, 75, or 100 μM for 48 h. Cells were fixed and stained, and the percentage of infected macrophages was determined by counting at least 100 cells/coverslip in triplicate coverslips. Cultures were tested in triplicates, and the results shown are the average of at least three independent experiments.
The activity of nerolidol was also tested against two other Leishmania species. The IC50s determined for L. braziliensis and L. chagasi promastigotes were 74.15 ± 10.51 and 75.10 ± 22.90 μM, respectively, indicating a broad in vitro susceptibility in the Leishmania genus.
Toxicity to mammalian cells.
50% cytotoxic concentrations of 125.69 ± 14.40 μM and 134.94 ± 32.19 μM were established for J774.A1 macrophages and human foreskin fibroblast cell cultures treated with nerolidol.
Effect of nerolidol against intracellular amastigotes.
Drug concentrations of 50, 75, and 100 μM were used to treat macrophage cultures infected with L. amazonensis. The treatment reduced the intracellular parasitism by 85, 91, and 95%, respectively (Fig. 2C).
Biosynthesis of isoprenoids in L. amazonensis promastigotes.
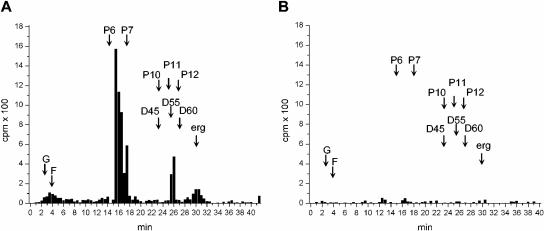
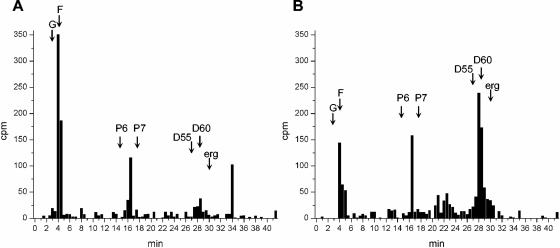
In order to verify whether the leishmanicidal effect of nerolidol was associated with an inhibition of the biosynthesis of isoprenoids, we first had to characterize the dolichol synthesized by control untreated parasites. The neutral lipid fraction obtained from [14C]MVA-labeled promastigotes was analyzed by RP-HPLC. Incorporated radioactivity was detected in molecules with retention times identical to a dolichol standard containing 11 isoprene units (26 min) (Fig. 3A), indicating that this was probably the dolichol species synthesized by L. amazonensis promastigotes. Synthesis of labeled molecules cochromatographing with the ergosterol standard was also observed, as well as a peak with a retention time similar to the hexaprenol standard (16 min), probably corresponding to an intermediary metabolite of the mevalonate pathway.
FIG. 3.
HPLC analysis of [14C]MVA-labeled L. amazonensis promastigotes. Labeled parasite extracts were analyzed by RP-HPLC by using methanol-water-hexane-propan-2-ol-methanol as a solvent system. The retention times of coinjected standards are indicated by arrows as follows: G, geraniol; F, farnesol; Pn, prenols of “n” isoprene units; D45, D55, and D60, dolichols with 45, 55, and 60 C; erg, ergosterol. Fractions of 0.5 ml (0.5 min) were collected and monitored for radioactivity. (A) Control; (B) promastigotes treated with 30 μM nerolidol. Identical numbers of cells were used to prepare the extracts analyzed in panels A and B.
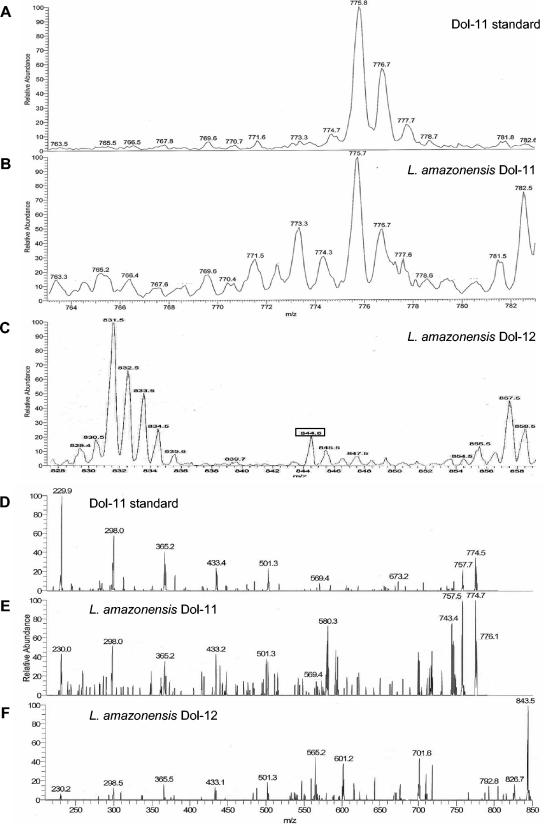
The identity of the molecules cochromatographing with the dolichol standards was verified by ESI-MS and ESI-MS/MS analysis (Fig. 4). Dolichol containing 11 isoprene units (monoisotopic mass = 768.7 Da) was detected in samples purified from L. amazonensis promastigotes as a major singly charged lithium-adduct ([M + Li]+) ion species at m/z 775.7 (Fig. 4B). This same ion species was detected when the authentic dolichol standard of 11 isoprene units was analyzed (Fig. 4A). The molecular identity was confirmed by comparing the MS/MS spectrum of the ion of m/z 775.7 [M + Li]+ (Fig. 4E) of L. amazonensis promastigotes with that of the standard (Fig. 4D), revealing the same dissociation profile with major fragment ions at m/z 757.7 [M + Li -H2O]+, 569.4 [M + Li -206]+, 501.3 [M + Li -274]+, 433.4 [M + Li -342]+, 365.2 [M + Li -410]+, 298.0 [M + Li -478]+, and 229.9 [M + Li -546]+. These ions (except for the ion at m/z 757.7) represent the initial molecule with the successive loss of isoprene (68 Da) units (17).
FIG. 4.
ESI-MS analysis of dolichol. ESI-MS (A to C) and MS/MS (D to F) spectra of an authentic dolichol standard composed of 11 isoprene units (A and D), L. amazonensis sample with the same retention time of the dolichol standard of 11 isoprene units (B and E), and L. amazonensis sample with the same retention time of a polyisoprenoid standard of 60 carbons (C and F). (A and B, m/z 775.8 [M + Li]+; C, m/z 844.6 [M + Li]+).
In addition, a dolichol composed of 12 isoprene units (monoisotopic mass = 836.8 Da) was also detected in samples purified from L. amazonensis promastigotes as a singly charged lithium-adduct [M + Li]+ ion species at m/z 844.6 (Fig. 4C, box). Due to the unavailability of a purified authentic dolichol standard of 12 isoprene units, we tried to confirm the molecular identity by comparing the MS/MS spectrum of the ion of m/z 844.6 [M + Li]+ (Fig. 4F) with that of the dolichol standard of 11 isoprene units (m/z 757.7) (Fig. 4D). The first revealed the same dissociation profile as the latter, giving rise to the major fragment ions at m/z 501.3 [M + Li -274]+, 433.1 [M + Li -342]+, 365.5 [M + Li -410]+, 298.5 [M + Li -478]+, and 230.2 [M + Li -546]+ and a dehydrated ion species at m/z 826.7 (Fig. 4F). The identity of the molecule cochromatographing with the ergosterol standard was also confirmed by ESI-MS and MS/MS analysis (data not shown).
Lipids extracted from [14C]MVA-labeled promastigotes were also analyzed by HPLC for the isoprene side chains attached to the benzoquinone rings. However, labeling of ubiquinones by this precursor was very poor, and labeled fractions were not detected (data not shown).
Inhibition of the biosynthesis of dolichol, ergosterol, and ubiquinones by nerolidol in L. amazonensis.
The drug concentration chosen to evaluate the effect of nerolidol on the incorporation of isoprenyl precursors into isoprenoids was 30 μM because under these conditions only 10% of parasites died after 48 h of treatment and overall protein synthesis was not affected, as shown by metabolic labeling with [35S]methionine (data not shown). L. amazonensis promastigotes treated with 30 μM nerolidol and labeled with [14C]MVA had the synthesis of dolichol and ergosterol inhibited by 90 and 75%, respectively (Fig. 3B). The synthesis of geraniol, farnesol, and the putative hexaprenol intermediate were also inhibited by nerolidol in these conditions (Fig. 3B).
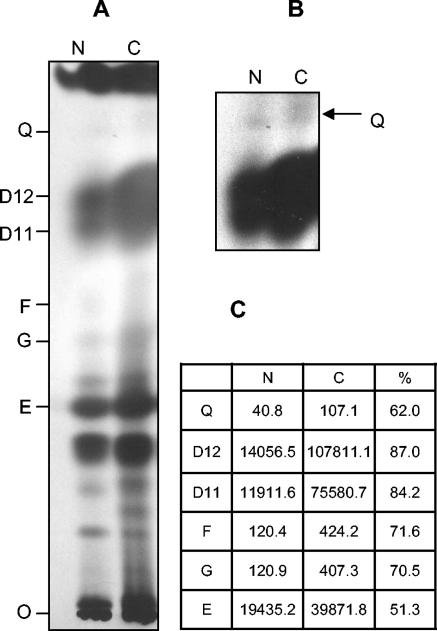
The inhibition of isoprenoid biosynthesis by nerolidol was also investigated by labeling L. amazonensis promastigotes with [1-14C]acetic acid. Synthesized products were analyzed by HPTLC (Fig. 5). Products with Rf values equivalent to dolichols containing 11 and 12 isoprene units and ergosterol standards were strongly labeled. The treatment with 30 μM nerolidol resulted in decreased synthesis of dolichol and ergosterol (Fig. 5A and C). Bands with Rf values corresponding to geraniol, farnesol, and ubiquinones were also detected, as well as bands at Rf values smaller than ergosterol standards. These labeled products were also inhibited by nerolidol. Ubiquinones were detected as a faint band in untreated promastigotes and decreased in intensity in treated parasites (Fig. 5B and C), indicating that these molecules are either not abundant or very weakly labeled by this precursor but have their synthesis inhibited by nerolidol.
FIG. 5.
HPTLC analysis of the synthesis of isoprenoids in the presence of nerolidol. (A) Hexane extracts from L. amazonensis promastigotes incubated in the absence (C) or in the presence of 30 μM nerolidol (N) and metabolically labeled with [1-14C]acetic acid were separated by HPTLC. The position of the authentic standards ran in the same plate is shown (E, ergosterol; G, geraniol; F, farnesol; D11 and D12, dolichol of 11 and 12 isoprene units; Q, mixture of standards of CoQ7-10). Identical numbers of cells were used to prepare the extracts loaded in each lane. Exposure time, 4 weeks. (B) The upper region of the plate after 8 weeks of exposure. The arrow indicates bands with Rf values equivalent to the ubiquinone standards. (C) Counts measured for radioactive spots, scanned after 10 days of exposure in a phosphor screen, for the nerolidol-treated sample (N) and control parasites (C) and the calculated percentage of inhibition (%).
These data indicated that nerolidol is probably an inhibitor at an early step in the pathway, since decreased synthesis of geraniol and farnesol were observed. To confirm this hypothesis, another precursor of isoprenoid biosynthesis, [1(n)-3H]FPP, was used. HPLC analysis of nerolidol-treated parasites labeled with [1(n)-3H]FPP revealed that the drug did not inhibit the incorporation of [1(n)-3H]FPP into dolichol and ergosterol (Fig. 6). In fact, from two- to fivefold increases in labeled dolichol and ergosterol were observed in nerolidol-treated parasites compared to control cultures in two independent experiments. These findings indicate that nerolidol is an inhibitor of FPP biosynthesis.
FIG. 6.
HPLC analysis of [3H]FPP-labeled L. amazonensis promastigotes. Labeled parasite extracts were analyzed by RP-HPLC by using methanol-water-hexane-propan-2-ol-methanol as a solvent system. The retention times of coinjected standards are indicated by arrows: G, geraniol; F, farnesol; Pn, prenols of “n” isoprene units; D55 and D60, dolichols with 55 and 60 C; erg, ergosterol. Fractions of 0.5 ml (0.5 min) were collected and monitored for radioactivity. (A) Control; (B) promastigotes treated with 30 μM nerolidol. Identical numbers of cells were used to prepare the extracts analyzed in panels A and B.
In vivo assays.
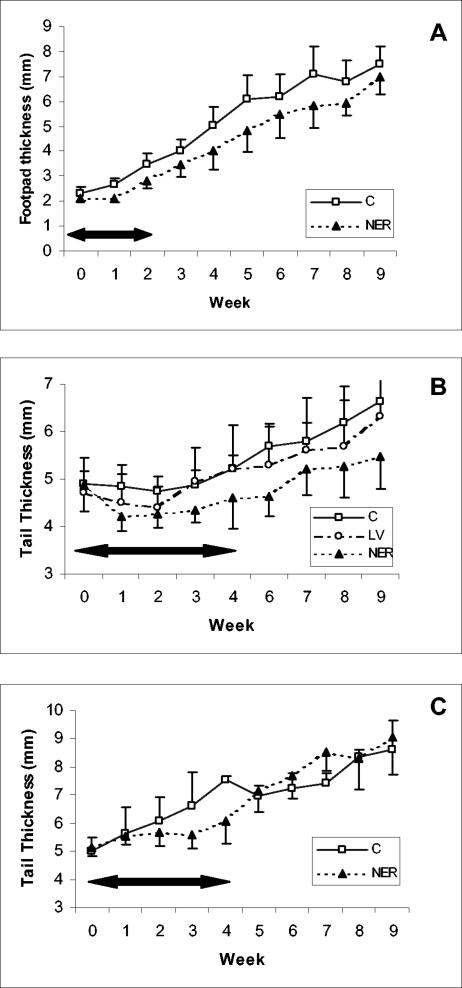
BALB/c mice infected with L. amazonensis at the hind footpad were treated with 100 mg of nerolidol kg−1 day−1 intraperitoneally. The treatment was initiated 30 days after the experimental infection, by which time all mice had measurable swelling in the footpad, and the mean size of lesions in all groups was comparable. Each animal received 10 daily injections over a period of 12 days. Control infected mice received the diluent alone. The treatment with nerolidol did not induce detectable toxic effects in control uninfected mice. A significant reduction (P < 0.05) of lesion size in nerolidol-treated mice was observed up to week 9 (Fig. 7A). Treated mice, however, had a continuous increase in the size of their lesions, even if at a slower rate.
FIG. 7.
Treatment of infected mice with nerolidol. Mice were infected with L. amazonensis at the left hind footpad (A) or at the basis of the tail (B and C) and treated with nerolidol for the periods indicated by horizontal arrows. (A) Mice treated intraperitoneally with 100 mg of nerolidol/kg/day (NER, ▴) or 10% ethanol in phosphate-buffered saline (C, □) for 12 days. The results shown are the mean and standard deviations for groups of five animals. Standard deviations are shown as half bars for clarity (above the symbols for control treated mice and below the symbols for nerolidol-treated mice). (B) Mice treated with ointment containing 5% (wt/wt) nerolidol in a 70% vaseline, 30% lanolin basis (LV) for 4 weeks. Control infected mice treated with the ointment basis without drug are indicated as open circles (LV). The mean for groups of five animals is shown. (C) Mice treated with ointment containing 10% (wt/wt) nerolidol in 70% vaseline, and 30% lanolin basis for 4 weeks. The mean for groups of five animals is shown. Symbols are as defined for panel A.
In a second series of experiments, nerolidol was used topically. Treatment was initiated 2 months after inoculation of 106 promastigotes at the base of each tail, and lesions were apparent in all mice. An ointment containing 5% nerolidol was applied daily over the swelling or the ulcer in L. amazonensis-infected mice for 4 weeks. Control groups included untreated mice, animals receiving the ointment without nerolidol, and uninfected mice receiving nerolidol containing cream. The topical application of nerolidol resulted in no side effects on uninfected mice (data not shown). The use of ointment without nerolidol induced a small and temporary decrease in the lesion size (Fig. 7B), and disease in these mice progressed as in the control group (statistical analysis for these groups showed no significant differences). The treatment with 5% nerolidol resulted in a significant reduction in the average lesion size up to week 9 (P < 0.001, Fig. 7B). These mice were monitored for up to 4 months after the end of the treatment and by then lesions were of equivalent size in both control and treated groups (data not shown).
For the third experiment, mice were infected with 106 amastigotes at the basis of the tail and lesions developed quickly. Topical treatment with 10% nerolidol was initiated 6 weeks after infection. A reduction in the size of lesions was observed while the ointment was being applied (Fig. 7C). Upon completion of the therapeutic scheme, two mice in each group were euthanized, the lesions were recovered, and parasites were quantified by limiting dilution. Lesions from treated animals contained 108 and 1012 parasites, whereas 1014 parasites were recovered from both untreated mice. Parasites recovered from treated animals were transformed in vitro to promastigotes and tested for their sensitivity to nerolidol. The IC50 against recovered parasites was 60.1 μM, indicating that the recrudescence of lesions was not due to resistant parasites. Remaining treated mice were monitored for up to week 9 but, after the interruption of treatment, lesions progressed as in the control group (Fig. 7C).
DISCUSSION
In this study we show that nerolidol is active against promastigotes, in vitro-cultured amastigotes, and intracellular amastigotes of L. amazonensis. The sensitivity of different Leishmania species to this drug is homogeneous, as inferred from the results obtained with L. braziliensis and L. chagasi, agents related to cutaneous and visceral leishmaniasis. The drug was very effective in reducing the intracellular infection on cultured macrophages, in spite of the relatively high cytotoxicity observed in vitro.
The sesquiterpene nerolidol was shown to inhibit carcinogenesis on azoxymethane-induced neoplasia in rats (41) by an uncharacterized mechanism. This drug also hinders Plasmodium falciparum growth in vitro (23) by inhibiting the synthesis of ubiquinone and dolichol (10, 33).
Dolichols composed of 11 to 13 isoprene units had been previously characterized in Trypanosoma brucei, Trypanosoma cruzi, and Crithidia fasciculata (24, 29, 31), but the structure of these molecules had not been described for Leishmania species. So, to investigate the mode of action of nerolidol against Leishmania, we first established that the L. amazonensis promastigotes synthesize polyisoprenoids containing 11 and 12 isoprene units. The identity of the RP-HPLC-purified polyisoprenoids was confirmed by mass spectrometry with the detection of ion peaks with masses corresponding to dolichol-11 (Fig. 4B and E) and dolichol-12 (Fig. 4C and F).
Previously published data described the presence of CoQ9 as the main species of ubiquinone in L. amazonensis promastigotes (12). Interestingly, the incorporation of [14C]MVA and [3H]acetic acid into the isoprene chain of ubiquinone in L. amazonensis promastigotes was very poor. Low levels of ubiquinone in trypomastigotes of T. brucei had been previously reported by Löw et al. (24).
Different prenyl transferases are involved in the biosynthesis of the various isoprenoid molecules: polyprenyl-trans-transferases are responsible for the synthesis of the isoprenoid chain of ubiquinone and polyprenyl-cis-transferases synthesize dolichol, whereas farnesyl-P2-farnesyl-transferase is implicated in the synthesis of squalene (18, 40). All of these enzymes utilize FPP as a substrate. So FPP is the last common substrate for the synthesis of all of the end products.
By labeling Leishmania promastigotes with two different early precursors of the isoprenoid biosynthetic pathway and evaluating the results by HPLC and HPTLC, we were able to observe unequivocal inhibition of both dolichol and ergosterol biosynthesis in the presence of nerolidol. Interestingly, the synthesis of geraniol and farnesol was also inhibited in these conditions, suggesting that, in Leishmania, the target for nerolidol inhibition might be an enzyme responsible for the synthesis of FPP precursors. This was confirmed by the lack of dolichol and ergosterol biosynthesis inhibition when parasites were treated with nerolidol and labeled with [1(n)-3H]FPP.
In contrast, nerolidol's inhibitory activity on the synthesis of dolichol and ubiquinone in P. falciparum was evident when [1(n)-3H]FPP was used as a precursor, leading to the suggestion that nerolidol could act in this system by inhibiting the elongation of isoprene chains (33). Our observations in Leishmania suggest that more than one mechanism could be responsible for the antiparasitic activity of nerolidol.
The conversion of mevalonate into geranyl pyrophosphate depends on the enzymes mevalonate kinase, diphosphomevalonate decarboxylase, isopentenyl isomerase, and dimethylallyl-trans-transferase. Further studies will be necessary to establish the precise action of nerolidol in this pathway and to verify whether effects in other cell targets might be relevant for the drug activity.
A previously reported antileishmanial activity of a linalool-enriched plant extract was correlated with an increased production of nitric oxide (NO) by infected macrophages (34). We did not detect differences in the accumulation of nitrate on supernatants of infected macrophages treated or not treated with nerolidol (data not shown). The different molecular structures of linalool (a cyclic monoterpenic alcohol) and nerolidol might explain this discrepancy. It is also possible that contaminants present in the plant extract utilized in the above-mentioned experiments were responsible for the increased production of NO in that system.
Toxic effects were not observed in vivo after intraperitoneal or topical administration of nerolidol. The treatment of infected mice with 100 mg of nerolidol kg−1 day−1 intraperitoneally for 12 days resulted in a significant decrease in lesion size, but the response was not long lasting.
Since nerolidol has been found to increase the transcutaneous permeation of several drugs (11, 42), it seemed reasonable to test its activity as a topical agent for the treatment of cutaneous leishmaniasis. A significant decrease in the size of lesions in treated animals was observed, particularly while the drug was being administered. However, a permanent cure was not achieved and treated mice eventually developed lesions as extensive as their controls. Since parasites recovered from treated mice were as sensitive to nerolidol as control parasites, an irregular absorption and tissue distribution of the drug could justify the results obtained.
BALB/c mice infected with L. amazonensis develop extensive, ulcerating lesions that eventually give rise to distant metastasis and death. Therefore, transient results observed in this experimental system should not be disregarded since this represents an extreme model of susceptibility. In addition, the topical treatment in these mice was difficult to control since animals quickly rubbed or licked off the cream. Different vehicles for oral or parenteral administration, as well as different bases for ointments could be tried to achieve a better bioavailability.
The inhibition of enzymes in the sterol biosynthesis pathway has been explored by using azoles in the therapy of leishmaniasis. However, these are inhibitors of the final steps in the synthesis of ergosterol. At least some species of Leishmania seem to be able to overcome their effect, possibly by using the host's steroids. On the other hand, besides the synthesis of ergosterol instead of cholesterol, there are other peculiar steps in the isoprenoid pathway in Leishmania: leucine is the main precursor of mevalonate, as opposed to acetyl coenzyme A in mammals (14); dolichols containing 11 to 12 isoprene units are synthesized instead of 19 to 20 observed in human dolichol. These differences could be explored in the identification of new targets for chemotherapy.
The description of the activity of nerolidol opens a new window for investigation. The association of nerolidol and other drugs targeting the same pathway at different points may prove a valuable tool for the treatment of leishmaniasis.
Acknowledgments
We thank Tadeusz Chojnacki from the Institute of Biochemistry and Biophysics of the Polish Academy of Sciences for kindly providing the isoprenoid standards and for valuable advice. We also thank Igor C. de Almeida for critical review of the manuscript. We are grateful to Herbert Rodrigues Goulart and Maria Belén Cassera for assistance with HPLC experiments, Antonio Carlos Franco da Silveira for help with the STORM 840, and Jenicer K. U. Yokoyama-Yasunaka for technical assistance.
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisa (CNPq). D.C.A. and F.L.A. are the recipients of FAPESP's fellowships.
REFERENCES
- 1.Barcinski, M. A., D. Schechtman, L. G. Quintao, D. de A. Costa, L. R. Soares, M. E. Moreira, and R. Charlab. 1992. Granulocyte-macrophage colony-stimulating factor increases the infectivity of Leishmania amazonensis by protecting promastigotes from heat-induced death. Infect. Immun. 60:3523-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach, D. H., L. J. Goad, and G. G. Holz, Jr. 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31:149-162. [DOI] [PubMed] [Google Scholar]
- 3.Berhe, N., D. Wolday, A. Hailu, Y. Abraham, A. Ali, T. Gebre-Michael, P. Desjeux, A. Sonnerborg, H. Akuffo, and S. Britton. 1999. HIV viral load and response to antileishmanial chemotherapy in coinfected patients. AIDS 13:1921-1925. [DOI] [PubMed] [Google Scholar]
- 4.Chojnacki, T., and G. Dallner. 1988. The biological role of dolichol. Biochem. J. 251:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornwell, P. A., and B. W. Barry. 1994. Sesquiterpene components of volatile oils as skin penetration enhancers for the hydrophilic permeant 5-fluorouracil. J. Pharm. Pharmacol. 46:261-269. [DOI] [PubMed] [Google Scholar]
- 6.Couto, A. S., E. A. Kimura, V. J. Peres, M. L. Uhrig, and A. M. Katzin. 1999. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem. J. 341:629-637. [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends. Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 9.Dedet, J. P., P. Jamet, P. Esterre, P. M. Ghipponi, C. Genin, and G. Lalande. 1986. Failure to cure Leishmania braziliensis guyanensis cutaneous leishmaniasis with oral ketoconazole. Trans. R. Soc. Trop. Med. Hyg. 80:176. [DOI] [PubMed] [Google Scholar]
- 10.de Macedo, C. S., M. L. Uhrig, E. A. Kimura, and A. M. Katzin. 2002. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol. Lett. 207:13-20. [DOI] [PubMed] [Google Scholar]
- 11.El-Kattan, A. F., C. S. Asbill, N. Kim, and B. B. Michniak. 2001. The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int. J. Pharm. 215:229-240. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, J. E., K. D. Setchell, and E. S. Kaneshiro. 1994. Detection of ubiquinone in parasitic and free-living protozoa, including species devoid of mitochondria. Mol. Biochem. Parasitol. 65:213-224. [DOI] [PubMed] [Google Scholar]
- 13.Faust, J. R., J. L. Goldstein, and M. S. Brown. 1979. Synthesis of ubiquinone and cholesterol in human fibroblasts: regulation of a branched pathway. Arch. Biochem. Biophys. 192:86-99. [DOI] [PubMed] [Google Scholar]
- 14.Ginger, M. L., M. L. Chance, I. H. Sadler, and L. J. Goad. 2001. The biosynthetic incorporation of the intact leucine skeleton into sterol by the trypanosomatid Leishmania mexicana. J. Biol. Chem. 276:11674-11682. [DOI] [PubMed] [Google Scholar]
- 15.Goad, L. J. 1985. Biosynthesis and interconversion of sterol in plants and marine invertebrates, p. 311-332. In J. H. Law and H. C. Rilling (ed.), Steroids and isoprenoids, vol. 3. Academic Press, Inc., New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 16.Goad, L. J., G. G., Jr. Holz, and D. H. Beach. 1984. Sterols of Leishmania species: implications for biosynthesis. Mol. Biochem. Parasitol. 10:161-170. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths, W. J., M. Hjertman, A. Lundsjo, J. Wejde, J. Sjövall, and O. Larsson. 1996. Analysis of dolichols and polyprenols and their derivatives by electron impact, fast atom bombardment and electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 10:663-675. [Google Scholar]
- 18.Grünler, J., J. Ericsson, and G. Dallner. 1994. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone, and prenylated proteins. Biochim. Biophys. Acta 1212:259-277. [DOI] [PubMed] [Google Scholar]
- 19.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 20.Kapler, G. M., C. M. Coburn, and S. M. Beverley. 1990. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 10:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lima, H. C., J. A. Bleyenberga, and R. G. Titus. 1997. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol. Today 13:80-82. [DOI] [PubMed] [Google Scholar]
- 23.Lopes, N. P., M. J. Kato, E. H. Andrade, J. G. Maia, M. Yoshida, A. R. Planchart, and A. M. Katzin. 1999. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J. Ethnopharmacol. 67:313-319. [DOI] [PubMed] [Google Scholar]
- 24.Löw, P., G. Dallner, S. Mayor, S. Cohen, B. T. Chait, and A. K. Menon. 1991. The mevalonate pathway in the bloodstream forms of Trypanosoma brucei. J. Biol. Chem. 266:19250-19257. [PubMed] [Google Scholar]
- 25.Montalvetti, A., J. Peña-Dias, R. Hurtado, L. M. Ruiz-Pérez, and D. González-Pacanowska. 2000. Characterization and regulation of Leishmania major 3-hydroxy-3-methylglutaryl-CoA reductase. Biochem. J. 349:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, H. W. 2000. Treatment of visceral leishmaniasis (kala-azar): a decade of progress and future approaches. Int. J. Infect. Dis. 4:158-177. [DOI] [PubMed] [Google Scholar]
- 27.Navin, T. R., B. A. Arana, F. E. Arana, J. D. Berman, and J. F. Chajon. 1992. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J. Infect. Dis. 165:528-534. [DOI] [PubMed] [Google Scholar]
- 28.Palmer, D. N., D. R. Husbands, P. J. Winter, J. W. Blunt, and R. D. Jolly. 1986. Ceroid lipofuscinosis in sheep. I. Bis(monoacylglycero)phosphate, dolichol, ubiquinone, phospholipids, fatty acids, and fluorescence in liver lipopigment lipids. J. Biol. Chem. 261:1766-1772. [PubMed] [Google Scholar]
- 29.Parodi, A. J., and L. A. Quesada-Allue. 1982. Protein glycosylation in Trypanosoma cruzi. J. Biol. Chem. 257:7637-7640. [PubMed] [Google Scholar]
- 30.Parodi, A. J., J. Martin-Barrientos, and J. C. Engel. 1984. Glycoprotein assembly in Leishmania mexicana. Biochem. Biophys. Res. Commun. 118:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Quesada-Allue, L. A., and A. J. Parodi. 1983. Novel mannose carrier in the trypanosomatid Crithidia fasciculata behaving as a short α-saturated polyprenyl phosphate. Biochem. J. 212:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rilling, H. C., E. Breunger, W. W. Epstein, and P. F. Crain. 1990. Prenylated proteins: the structure of the isoprenoid group. Science 247:318-320. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues Goulart, H., E. A. Kimura, V. J. Peres, A. S. Couto, F. A. Aquino Duarte, and A. M. Katzin. 2004. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 48:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa, M. S. S., R. R. Mendonca-Filho, H. R. Bizzo, I. de Almeida Rodrigues, R. M. Soares, T. Souto-Padron, C. S. Alviano, and A. H. Lopes. 2003. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 47:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skorupinska-Tudek, K., T. Bienkowski, O. Olszowska, M. Furmanowa, T. Chojnacki, W. Danikiewicz, and E. Swiezewska. 2003. Divergent pattern of polyisoprenoid alcohols in the tissues of Coluria geoides: a new electrospray ionization MS approach. Lipids 38:981-990. [DOI] [PubMed] [Google Scholar]
- 36.Sundar, S., L. B. Gupta, M. K. Makharia, M. K. Singh, A. Voss, F. Rosenkaimer, J. Engel, and H. W. Murray. 1999. Oral treatment of visceral leishmaniasis with miltefosine. Ann. Trop. Med. Parasitol. 93:589-597. [DOI] [PubMed] [Google Scholar]
- 37.Uliana, S. R. B., N. Goyal, E. Freymüller, and D. F. Smith. 1999. Leishmania: overexpression and comparative structural analysis of the stage-regulated meta 1 gene. Exp. Parasitol. 92:183-191. [DOI] [PubMed] [Google Scholar]
- 38.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114:S91-S99. [PubMed] [Google Scholar]
- 39.Waechter, C. J., and W. J. Lennarz. 1976. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu. Rev. Biochem. 45:95-112. [DOI] [PubMed] [Google Scholar]
- 40.Wang, K. C., and S. Ohnuma. 2000. Isoprenyl diphosphate synthases. Biochim. Biophys. Acta 1529:33-48. [DOI] [PubMed] [Google Scholar]
- 41.Wattenberg, L. W. 1991. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis 12:151-152. [DOI] [PubMed] [Google Scholar]
- 42.Yamane, M. A., A. C. Williams, and B. W. Barry. 1995. Terpene penetration enhancers in propylene glycol/water co-solvent systems: effectiveness and mechanism of action. J. Pharm. Pharmacol. 47:978-989. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama, K., P. Trobridge, F. S. Bruckner, J. Scholten, K. D. Stuart, W. C. V. Voorhis, and M. H. Gelb. 1998. The effects of protein farnesyltransferase inhibitors on trypanosomatids: inhibition of protein farnesylation and cell growth. Mol. Biochem. Parasitol. 94:87-97. [DOI] [PubMed] [Google Scholar]