Abstract
Neuropeptide Y (NPY) signaling regulation of corticolimbic communication is known to modulate binge-like ethanol consumption in rodents. In this work we sought to assess the impact of intra-BLA NPY system modulation on binge-like ethanol intake and to assess the role of the NPY1R+ projection from the BLA to the mPFC in this behavior. We used “drinking-in-the-dark” (DID) procedures in C57BL6J mice to address these questions. First, the impact of intra-BLA administration of NPY on binge-like ethanol intake was assessed. Next, the impact of repeated cycles of DID intake on NPY1R expression in the BLA was assessed with use of immunohistochemistry (IHC). Finally, chemogenetic inhibition of BLA→mPFC NPY1R+ projections was assessed to determine if limbic communication with the mPFC was specifically involved in binge-like ethanol intake. Importantly, as both the BLA and NPY system are sexually dimorphic, both sexes were assessed in these studies. Intra-BLA NPY dose-dependently decreased binge-like ethanol intake in males only. Repeated DID reduced NPY1R expression in the BLA of both sexes. Silencing of BLA→mPFC NPY1R+ neurons significantly reduced binge-like ethanol intake in both sexes in a dose-dependent manner. We provide novel evidence that (1) intra-BLA NPY reduces binge-like ethanol intake in males; (2) binge-like ethanol intake reduces NPY1R levels in the BLA; and (3) chemogenetic inhibition of BLA→mPFC NPY1R+ neurons blunts binge-like drinking in male and female mice. These observations provide the first direct evidence that NPY signaling in the BLA, and specifically BLA communication with the mPFC, modulates binge-like ethanol consumption.
Subject terms: Reward, Motivation
Introduction
Nearly 25% of United States residents over the age of 12 report engaging in alcohol (ethanol) binge drinking behavior in the past month [1]. Binge drinking is defined as consumption of 5 servings of alcohol for males and 4 for females in approximately a two-hour period. The high prevalence of binge drinking is of national concern as it is associated with both acute and chronic negative health effects and, critically, is known to facilitate the transition to full alcohol dependence [2]. Additionally, 75% of the economic burden imposed on the United States by alcohol misuse is related to binge drinking [1]. Despite the impressive health and fiscal costs only four medications are currently approved by the U.S Food and Drug Administration (FDA) to assist individuals in decreasing alcohol intake levels. Identification of neural systems involved in regulating the motivation to binge consume ethanol is thus critical to both understanding how decrease this dangerous pattern of intake and in understanding how it facilitates the development of alcohol dependence.
The Neuropeptide Y (NPY) system is a potential therapeutic target for alcohol use disorders (AUDs) [3–5]. NPY is the most widely expressed neuropeptide in the brain [6]. NPY is densely expressed in regions related to reward and emotionality, including the: hippocampus, hypothalamus, amygdala, nucleus accumbens, and cortex [6–9]. Innate NPY levels are negatively correlated with ethanol intake, in that high ethanol consuming rodent genetic lines express lower brain levels of NPY, while low-consuming lines show relatively higher NPY expression [10–14]. Further, genetic knock-down of NPY increases ethanol intake relative to wildtype [15]. The critical role of NPY in regulating stress and anxiety related behaviors is a primary mechanism through which NPY is thought to impact ethanol intake [3, 16]. Indeed, previous work from our lab and others have shown NPY signaling significantly modulates binge ethanol intake through impacting activity in several brain regions associated with emotionality and reward [4, 5, 17–20].
The basolateral nucleus of the amygdala (BLA) serves as the primary input nucleus of the amygdala. The BLA acts as a processing hub for afferent sensory and cortical information to assign positive (rewarding) or negative (aversive) salience to incoming stimuli [21–28]. NPY plays an important role in modulating BLA activity, particularly in the context of anxiety, stress, and fear behaviors [29–31]. Intra-BLA NPY administration reduced cued, but not social, fear expression [32], fear-potentiated startle [33], anxiety-like behavior, while increasing resilience to stress [34–36]. Further, the BLA is one of the regions in which reduced NPY expression was detected in the ethanol preferring C57BL/6 J line relative to the ethanol non-preferring DBA/2 J line, suggesting a potential role of BLA NPY in alcohol-related behaviors [11]. While several additional NPY receptors (specifically 2 and 5) have been suggested to play a role in regulating BLA activity, the NPY receptor 1 (NPY1R) has been proposed to serve as the primary receptor modulating emotionality within BLA [35, 37, 38].
In this work, we therefore first sought to assess the impact of exogenous intra-BLA NPY administration on binge drinking behavior. We further assessed the impact of binge ethanol exposure on BLA NPY1R expression in total. While NPY1R is expressed on both BLA glutamatergic principal neurons and GABAergic interneurons, the vast majority is expressed on the glutamatergic population, with nearly all principal projections cells expressing NPY1R (NPY1R+) [39]. We therefore further sought to utilize designer receptors exclusively activated by designer drug (DREADD) technology to investigate the role BLA NPY1R+ glutamatergic projections may play in regulating binge ethanol intake. Previous work from our lab has demonstrated an important role of NPY1R+ mPFC projections to the BLA in reducing binge ethanol intake [19]. We therefore chose to assess the well-known reciprocal projection from the BLA back to the mPFC in regulating intake. This projection is known to play a critical role in anxiety, stress, and fear-related behaviors [23, 40–44] and is thus poised to significantly modulate binge drinking behavior. Finally, as both the NPY system and BLA are well known to be sexually dimorphic [for review see: [45, 46]], we performed all experiments in both male and female animals to assess potentially sex-specific effects.
Methods
Overall experimental design
There were four experiments that were performed: Experiment 1 involved giving male and female C57BL/6 J mice bilateral, site-directed infusion of vehicle or one of two doses of NPY (in a Latin design in which each mouse was exposed to all three doses in a counterbalanced order over 3 test days) into the BLA immediately before 2-hours of access to 20% ethanol on the test day. Similarly, in Experiment 2 mice were given bilateral infusion of vehicle or the high dose of NPY (in a Latin design) immediately before 2-hours of access to a 3% sucrose solution on test day. The effects of the high dose of NPY on open-field locomotor behavior, relative to vehicle, was also assessed over an hour test. Experiment 3 assessed the effects of 3, 4-day episodes of binge-like ethanol drinking in male and female C57BL/6 J mice on NPY1R protein expression in the BLA relative to mice that consumed only water. In Experiments 4 A and 4B, male and female NPY1R-Cre mice were used in tandem with chemogenetic tools to assess the effects of bilateral silencing of NPY1R + BLA to mPFC circuits on binge-like ethanol intake.
Animals
We used male and female C57BL/6 J mice (intra BLA: male N = 15, female N = 16; IHC: female N = 19, male N = 19) (Jackson Laboratories, Bar Harbor, ME) or NPY1R-Cre mice (male N = 16, female N = 20) (positive for Cre-recombinase expression under the NPY1R promoter, determined by standard polymerase chain reaction genotyping) on a C57BL/6 J background 8–10 weeks old at experiment start (bred in house heterozygous X C57BL/6 J (Jackson), (originally developed by [47]). Mice of the same sex were group-housed until stereotaxic surgeries began, at which point they were housed individually in an AAALAC accredited vivarium (22 °C with a reversed 12:12 h light:dark cycle, lights on at 20:00) until the end of the experiment. Animals had ad libitum access to Prolab® RMH 3000 (Purina LabDiet®; St. Louis, MO) and water, unless otherwise stated. Prior to experiments, animals acclimated to the experimental housing environment for ≥1 week. All procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee and followed the Guidelines for the Care and Use of Laboratory Animals.
“Drinking in the dark” procedures
Binge drinking was modeled using a 4-day DID paradigm as previously described [19]. Three hours into the dark cycle (11:00), water bottles were removed and replaced with sipper tubes containing 20% (v/v) ethanol or 3% (w/v) sucrose in tap water for 2 h. On the fourth day of each cycle (test day), animals were treated with drug or appropriate vehicle ~30 min prior to bottles on (except in the immunohistochemistry study, where no treatment was given). Drinking measures were collected after both 1- and 2-hours of drinking, and drinking data are displayed and analyzed at the 1- and 2-hour data points, as well as total consumption over the 2-hour tests. Each 4-day DID cycle was separated by a 3-day abstinence.
Immunohistochemistry (IHC)
Mice underwent 3 cycles of ethanol DID or 3 cycles with water alone (Fig. 1a). Three cycles were selected based on previous work in our lab [19, 48]. Immediately following the final DID session (at approximately 13:00 h) each mouse was administered 0.1 mL intraperitoneal (i.p.) injection ketamine/xylazine (6.67 mg/0.1 mL; 0.67 mg/0.1 mL; in 0.9% saline) and perfused transcardially using 0.1 M phosphate buffer saline (PBS; pH = 7.4) and 4% paraformaldehyde in PBS (pH = 7.4). After extraction, brains were post-fixed in 4% paraformaldehyde for 24–48 h then sectioned at 40 µm thickness (Leica VT1000S vibratome; Wetzlar, Germany). At this time random identification numbers were assigned to blind experimenter to treatment. Sections were blocked in 3% horse serum (0.005% Tween20 in phosphate buffered saline pH = 7.4) for 1 h then incubated in primary rabbit anti-NPY1R (1:500) (Immunostar cat#24506 polyclonal Hudson, Wisconsin) for 72 h at 5 °C. Sections were then incubated 2 h at room temperature in secondary solution (1:5000) (DI-3088 DyLight 488 horse anti-rabbit, Vector Laboratories, Burlingame, CA) then mounted onto glass slides and coverslipped in Shur Mount (General Data). One tissue set was run without primary antibody to confirm lack of non-specific fluorescence. Color images of BLA were captured through a digital camera (Roper Scientific), mounted on an optical microscope (Leica DM6000), and positive fluorescence was quantified using FIJI [49]. In short, the region of interest was selected, the image was converted to black and white, and the entropy threshold was used for fluorescence detection. As control mechanisms, images were also acquired for the mCherry wavelength (since IHC was GFP tagged with DyLight 488), and a set of slices with no primary antibody (secondary only) was run to make sure there was no autofluorescence in the tissue. Experimenter remained blinded to treatment group until following ImageJ quantification. Unilateral percent area of fluorescence for 2–4 sections per animal were averaged together and used for analysis (bregma −0.70 to −1.82). Representative images in figures were digitally adjusted (brightness/contrast/saturation) for best appearance in publication. All images were enhanced as a group (identical settings).
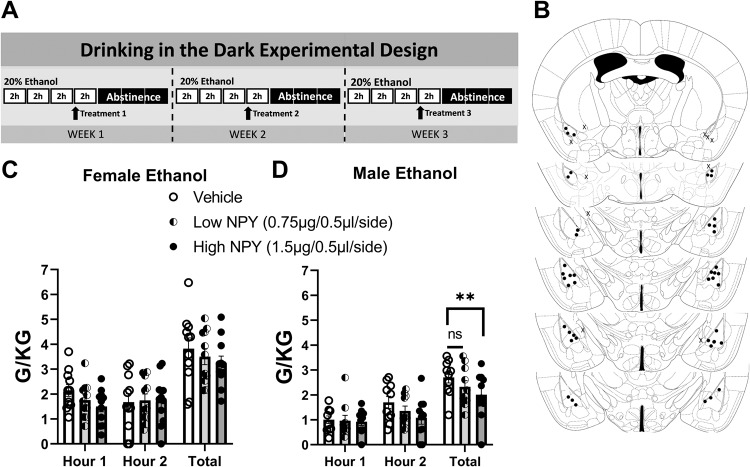
Fig. 1. Intra-BLA NPY dose dependently decreases binge-like ethanol intake in male mice.
A Experimental timeline. B Placement check of cannulae placement. C Neither NPY dose (low: 0.75 µg/0.5 µl/side; high: 1.5 µg/0.5 µl/side) impacted binge-like ethanol intake in female mice. D Higher (1.5 µg/0.5 µl/side) NPY dose decreased binge-like ethanol intake in males. **Dunnett’s multiple comparisons test p ≤ 0.01.
Surgeries
Immediately before surgery each mouse was administered 0.1 mL intraperitoneal (i.p.) injection of ketamine/xylazine (6.67 mg/0.1 mL; 0.67 mg/0.1 mL; in 0.9% saline). In cannulation studies, using an Angle II™ Stereotax (Leica Instruments, Buffalo Grove, IL), bilateral guide cannula (mPFC: 33GA double cannula cut 4 MM below pedestal; dummy cannula: 4 MM with 0.5 MM projection; injector: 4 MM with 2 MM projection) (BLA: 26 GA single cannula cut 5 MM below pedestal; dummy cannula: 5 MM with 0.5 MM projection; injector: 5 MM with 0.5 MM projection) (Plastics One; Roanoke, VA) were lowered into the mPFC (AP: 1.7, ML: ±0.4, DV: −2.6) or BLA (AP: -1.22, ML: ±3.01, DV: −4.75). Mice recovered for ≥1 week before experiment start.
In DREADD experiments the BLA was injected with ~0.4 µL/side (over a 5-minute period) of either a Cre-dependent control vector (AAV8-hSyn-DIO-mCherry) or the Cre-dependent Gi/o-coupled Designer Drug Exclusively Activated by Designer Drug (DREADD) vector (Gi-DREADD; AAV8-hSyn-DIO-hM4d-mCherry; Addgene, Watertown, MA)). The injection needle remained in place for 10–15 additional minutes before being withdrawn. Mice recovered for >3 weeks before experiment start.
Drug administration
On DID test days animals underwent micro-injection of the experiment-specific drug or appropriate vehicle ~30 min prior to test start. Drugs were as follows: NPY (Human, Rat; CAS Number:90880-35-6) (0.75 µg/0.5 µl/side or 1.5 µg/0.5 µl/side in saline; Cayman Chemicals, Ann Arbor, Michigan); Clozapine-N-Oxide (CNO; microinjection (900 pmol/0.3 µl/side or 2700 pmol/0.3 µl/side); Sigma-Aldrich, St. Louis, MO or gifted by the NIDA drug depositary; in 0.5% DMSO in Saline). A 2 × 2 Latin-square design was used in each experiment. Animals were randomly assigned to drug or vehicle treatment on test day 1, then received the alternative treatment on test day 2 of a second DID cycle [see individual figures]. In experiment 1 a third week was added, in which animals were randomly assigned to groups to receive one of three treatments on each of the three test days. This was done in a counter-balanced manner such that a third of the animals saw each of the three treatment conditions first. Infusions for microinjected drugs occurred as previously published at a rate of 0.5 μL/min using a Hamilton syringe (Reno, NV) attached to a Harvard Apparatus PHD 2000 infusion pump (Holliston, MS) [19]. After infusion, injectors remained in guide cannula for an additional 0.5–1 min for diffusion. As animals underwent a 3-day abstinence from ethanol or sucrose following each test day, potential carry-over effects 24 h following injection were not specifically evaluated. Following brain extraction and section mounting (see IHC methods) cannula and DREADD placements were verified through use of a digital camera (Roper Scientific), mounted on an optical microscope (Leica DM6000).
Statistical analysis
GraphPad Prism (GraphPad Software, Inc. La Jolla, Ca) was used to analyze and graph all data. As potential sex-specific effects were a main interest of the study, sexes were analyzed separately throughout this work. Two-way ANOVAs and Dunnett or Bonferroni post-hoc tests were used to determine the effect of binge cycle on NPY1R immunoreactivity and treatment versus time during individual DID drinking hours (see individual results). T-tests or One-way ANOVAs were used to evaluate the effect of treatment on total intake. All data are reported as the mean ± standard error of the mean and considered significant if p < 0.05. Animals were removed from analysis if: (1) they were found by a Grubbs test (Alpha = 0.05) to be a significant outlier; (2) due to cannula misplacement (uni or bilateral); or (3) due to inappropriate DREADD expression. Cannula placement was determined by locating the end of the guide cannula and adding injector projection length (0.5 MM). For the intra-BLA NPY manipulation 5 animals were excluded from analyses in the ethanol group, and 8 from analyses in the sucrose consumption group due to either unilateral or bilateral cannula misplacement. For the DREADD experiments 7 animals had to be excluded due to either cannula misplacement or unilateral/no DREADD expression.
Results
Experiment 1: intra-BLA NPY dose dependently decreases binge-like ethanol intake in male mice
Two-way (treatment × time) ANOVAs were used to analyze the counterbalanced treatment conditions (Vehicle, low NPY, high NPY) presented in a random order and One-way ANOVAs to analyze total intake. A preliminary analysis was conducted to determine whether order of drug presentation interacted with treatment effects on ethanol consumption, and no interaction effects were observed. Ethanol consumption for all animals across week 1, and week 1–3 test days is detailed in Supplementary Fig. 1. The treatment timeline is detailed in Fig. 1A. While BLA microinjections of 0.75 µg/0.5 µl/side (low NPY) did not alter total binge-like ethanol intake in female mice (N = 11) across the one- and two-hour time points [Time: F(1,10) = 0.093, p = 0.77; Treatment: F(2, 20) = 0.784, p = 0.47; interaction: F(2, 20) = 1.106, p = 0.35], nor when considering total ethanol intake [One-way ANOVA: F(2, 20) = 0.784, p = 0.47] (Fig. 1C), the highest NPY dose (1.5 µg/0.5 µl/side) did decrease intake in male mice compared to vehicle-treated males when considering total ethanol consumption (N = 10) [Time: F(1, 9) = 4.195, p = 0.07; Treatment: F(2, 18) = 3.884, p = 0.04; interaction: F(2, 18) = 0.775, p = 0.48; One-way ANOVA: F(2, 20) = 4.616, p = 0.02; Dunnett’s multiple comparisons test: Vehicle vs. Low NPY p = 0.12, Vehicle vs. High NPY p = 0.01] (Fig. 1D). Placement checks of cannulae placement are detailed in Fig. 1B.
Experiment 2: intra-BLA NPY does not alter sucrose intake or general locomotion
Given the lack of effect of the lower NPY dose, only the high dose of NPY was used to assess the impact of intra-BLA injection on consumption of 3% sucrose solution, a naturally rewarding and caloric dense liquid. The treatment timeline is detailed in Fig. 2A. In contrast to the impact on ethanol consumption, two-way ANOVA following NPY treatment did not detect alteration in sucrose consumption in either sex at the one hour and two our time point; a t-test comparing total sucrose intake in both sexes also detected no differences (N = 5/sex) [(Female: time: F(1, 8) = 1.581, p = 0.24; treatment: F(1, 8) = 0.02274, p = 0.88; interaction: F(1, 8) = 0.3118, p = 0.59; total t-test: t = 0.1298, df = 4, p = 0.9) (Male: time: F(1, 8) = 4.818, p = 0.06; treatment: F(1, 8) = 0.049, p = 0.83; interaction: F(1, 8) = 0.4956, p = 0.5; total t-test: t = 0.2493, df = 4, p = 0.82)] (Fig. 2B, C). The same male and female animals from the sucrose experiment were used for open field measures. Both sexes were analyzed together due to reduced N and the a priori hypothesis of no impact on locomotor activity suggested by the lack of impact on sucrose intake in either sex. Two-way ANOVA found a significant impact of time but not treatment following intra-BLA NP Y micro-injection (N = 6 (3 male; 3 female)) relative to vehicle (N = 4 (2 male, 2 female)) [time: F(11, 96) = 5.557, p = 0.0001; treatment: F(1, 96) = 0.8382, p = 0.36; interaction: F(11, 96) = 0.5422, p = 0.87] (Fig. 2D). Similarly, no impact of treatment on time spent in the center of the open field was detected by t-test in these animals [t = 0.4163, df = 8; p = 0.69, data not shown]. Placement checks of cannulae placement are presented in Fig. 2E.
Fig. 2. Intra-BLA NPY does not impact sucrose intake or general locomotion.
A Experimental timeline. B NPY (high dose: 1.5 µg/0.5 µl/side) did not impact sucrose intake in female or (C) male mice. D Treatment did not impact total distance traveled in the open field test (sexes combined). E Placement check of cannulae placement.
Experiment 3: three cycles of DID decreases BLA NPY1R IHC expression
NPY1R immunoreactivity in the BLA following water (female N = 9; male N = 10) or 3 cycles of binge ethanol (female N = 10; male N = 9) was assessed. A two-way ANOVA revealed a significant impact of treatment in which ethanol decreased NPY1R expression [F(1, 34) = 4.69, p = 0.04], but this effect occurred equally in males and females, suggesting no impact of sex [F(1, 34) = 0.325, p = 0.57; interaction: F(1, 34) = 0.102, p = 0.75] (Fig. 3A). Representative fluorescent immunoreactivity is detailed in Fig. 3B.
Fig. 3. Three cycles of ethanol DID decreases NPY1R expression in the BLA.

A 3 DID cycles decreases BLA NPY1R expression (sexes combined; circle = female, square = male). B Exemplar images of water and ethanol consuming animals. *Student’s t-test p ≤ 0.05. Brain areas delineated: BLA basolateral amygdala.
Experiment 4A: BLA→mPFC NPY1R+ Gi-DREADD inhibition decreases binge-like intake in a sex-dependent manner
All DREADD studies were performed in NPY1R-cre animals expressing Gi- or control-DREADDs in the BLA with cannulae directed at the mPFC. Treatment timeline details are presented in Fig. 4A. Two-way ANOVA detected no significant reduction in binge-like ethanol intake across the two-hour testing period in female animals following intra-mPFC microinjection of 3 nM/µl CNO (N = 7) [time: F(1, 6) = 14.31, p = 0.01; treatment: F(1, 6) = 1.16, p = 0.32; interaction: F(1, 6) = 2.66, p = 0.16]. There was also no significant change in ethanol consumption when considering total ethanol intake at the end of the two-hour session [t-test total intake: t = 1.079, df = 6, p = 0.32] (Fig. 4B). A significant reduction in ethanol consumption was detected in males after treatment with the 3 nM/µl CNO dose (N = 7) [time: F(1, 5) = 3.302, p = 0.13; treatment: F(1, 5) = 16.47, p = 0.01; interaction: F(1, 5) = 0.47, p = 0.52]. Similarly, t-test analysis comparing total ethanol intake indicated a significant decrease in consumption [t = 4.059, df = 5, p = 0.01] (Fig. 4C). In contrast, reduction in binge-like ethanol intake was observed only in females following an increased CNO dose (9 nM/µl) (N = 6) [time: F(1, 5) = 9.49, p = 0.03; treatment: F(1, 5) = 8.09, p = 0.04; interaction: F(1, 5) = 0.08, p = 0.78 (no hourly significance detected by Bonferroni’s multiple comparisons test)]; Again, t-test analysis considering total ethanol consumption indicated a significant decrease in intake of females treated with the high CNO dose vs vehicle [t-test: t = 2.844, df = 5, p = 0.04] (Fig. 4D). Such a decrease in ethanol consumption was not seen in males (N = 4) [time: F(1, 3) = 3.29, p = 0.17; treatment: F(1, 3) = 0.03, p = 0.88; interaction: F(1, 3) = 1.56, p = 0.3; t-test: t = 0.1650, df = 3, p = 0.88] (Fig. 4E). Exemplar images of control DREADD placement in the BLA and terminal presence in the mPFC of NPY1R-cre animal are presented in Fig. 4F. Supplementary Fig. 2 shows an example canulae placement photomicrograph in the mPFC, and Supplementary Fig. 3 shows an overlay schematic of the criteria the were used for acceptable bilateral virus expression in the BLA.
Fig. 4. Chemogenetic inhibition of BLA → mPFC NPY1R+ projections inhibits binge-like ethanol intake in a dose-dependent manner.
A Experimental timeline. B Low (3 nM/µl) CNO did not impact binge-like ethanol intake, while (C) this dose significantly decreased intake in males. D Higher (9 nM/µl) CNO decreased binge-like ethanol intake in females, but not (E) male animals. F Mapping of NPY1R+ projections from the BLA to the mPFC (both images from females). G High (9 nM/µl) CNO did not impact female binge-like ethanol intake in control DREADD animals. H Low (3 nM/µl) CNO did not decreased binge-like ethanol intake in in control DREADD male animals. I mPFC cannulae placement check. Brain areas delineated: BLA basolateral amygdala, mPFC medial prefrontal cortex, PL prelimbic cortex, IL infralimbic cortex. *p ≤ 0.05, **p ≤ 0.01, Two-way ANOVA.
Experiment 4B: BLA→mPFC NPY1R + CON-DREADD does not alter binge-like intake
Animals in this study received only the CNO microinjection dose found to impact binge-drinking behavior in the Gi-DREADD study (males = 3 nM/µL dose; females = 9 nM/µL dose). A two-way ANOVA detected no impact of treatment on binge-like drinking behavior in female (N = 7) [time: F(1, 6) = 1.53, p = 0.26; treatment: F(1, 6) = 0.59, p = 0.47; interaction: F(1, 6) = 2.98, p = 0.14; total t-test: t = 0.7516, df = 4, p = 0.51] (Fig. 4G) or male mice (N = 5) [time: F(1, 4) = 1.71, p = 0.26; treatment: F(1, 4) = 0.57, p = 0.49; interaction: F(1, 4) = 0.25, p = 0.64; total t-test: t = 0.7516, df = 4, p = 0.49] (Fig. 4H). The Placement check figure for mPFC cannulation and injector tip are presented in Fig. 4I.
Discussion
In this work we demonstrate an important role of the NPY system in binge ethanol consumption through modulation of the BLA. Both male and female mice showed reduced NPY1R expression in the BLA following three weeks of binge-like chronic ethanol consumption. In contrast, manipulations of BLA NPY signaling were notably impacted by sex. Administration of NPY into the BLA via cannulated microinjection reduced binge ethanol intake in male mice alone without impacting sucrose intake in either sex. DREADD manipulation of NPY1R + BLA terminals within the mPFC was likewise impacted by sex, in which a dose well-validated for use in male animals within our lab [19] reduced intake in male, but not female animals. Indeed, 3x this dose was required to reduce intake in females. These results add to the evidence of the importance of limbic NPY in regulating unhealthy alcohol consumption and underscores the importance of assessing the role of NPY in both male and female animals. A schematic of the circuit under investigation and the hypothetical mechanism is presented in Fig. 5. This research complements our previous finding indicating that the reciprocal NPY1R+ mPFC → BLA pathway, and NPY1R in the mPFC, modulate binge-like ethanol intake [19]. This work also adds to more historical work demonstrating that both NPY1R and NPY2R, within regions of the extended amygdala, such as the central amygdala and bed nucleus of the stria terminalis (BNST), modulate binge-like ethanol intake and elevated drinking stemming from dependence [5].
Fig. 5. Schematic representation of the neurocircuitry under investigation and hypothetical mechanisms of action.

Both chemogenetic (via Gi-DREADD) and pharmacological (via activation of Gi-coupled Y1 receptor (Y1R) silencing of mPFC-projecting BLA neurons blunts glutamate signaling in the pPFC, which theoretically promotes reductions of binge-like ethanol intake in mice.
Dysregulation of corticolimbic communication occurs in numerous psychopathologies, including anxiety and depressive disorders [50–53]. BLA→mPFC projections synapse on both glutamatergic and GABAergic cells to directly modulate fear, anxiety, and reward-directed behaviors [43, 44, 51, 52]. Nearly all BLA glutamatergic principal cells express NPY1R [39], suggesting a significant role NPY in modulating BLA activity and that a similar impact on binge drinking behavior would occur following non-specific inhibition of BLA→mPFC projections. However, given that it has been estimated that 99.9% of BLA principal neurons express NPY1R [39], it is unlikely that there is a significant contribution of a non-NPY1R circuit in this pathway. As BLA principal neurons predominantly project to a single downstream target, it is likely DREADD inhibition of mPFC projecting cells did not impact BLA communication with other target regions involved in binge drinking behavior, such as the nucleus accumbens or BNST [54, 55]. Of note, both the BLA and mPFC as a whole, rather than specific subregions or points along the rostral-caudal axis, were targeted in this work. Thus, while recent work has highlighted a fascinating specificity of BLA projections to the prelimbic versus infralimbic cortex along the rostral-caudal axis, as well as a differential contribution of these discrete circuits to aversive and reward behavior, at present we cannot speculate on the relative involvement of these populations to our observed results [39, 43]. Dissection of specific BLA→mPFC microcircuits in binge-like ethanol intake is an area of important future investigation.
Within the BLA NPY is expressed in ~40% of local GABAergic interneurons [56]. These interneurons are critical in processing incoming information and appropriately assigning valence to stimuli, with dysfunction in this process associated with anxiety-related pathology [30]. Interestingly, however, extrinsic afferent NPY projections also heavily innervate the BLA, arriving from regions including the: amygdalostriatal transition region, endopiriform nucleus, posterior piriform cortex, external capsule intercalated nuclei, and parahippocampal regions [57]. This extrinsic NPY is thought to play a critical role as lesioning of BLA NPY+ GABAergic cells did not reduce total BLA NPY+ fiber immunoreactivity density, suggesting a primarily extrinsic source of the peptide [56]. Notably, the anxiolytic actions of intra-BLA NPY were prevented following antagonism or knock-down of BLA NPY1R, demonstrating the necessity of BLA NPY1R activation in regulating anxiety-like behavior regardless of NPY source [38, 56]. Future work evaluating if intrinsic or extrinsic sources of NPY serve to modulate discrete projections involved in binge-like drinking behavior, such as the mPFC projection studied in this work, would be of great interest.
This present work adds to a growing literature demonstrating significant differences in both overall amygdala function, and specifically NPY system function, between male and female animals. The full extent of these differences is beyond the scope of the current work and have been recently outlined in several excellent reviews [see [45, 46]], however several key points will be summarized here. Multiple behavioral tests which are significantly impacted by alterations in BLA signaling show significant differences in between males and females, with females demonstrating: less anxiety-like behavior on the elevated plus maze at baseline and following chronic stress [58–63], less contextual fear conditioning, and higher levels of cued aversion [60, 61]. Principal glutamatergic cells within the female BLA have a higher rheobase and hyperpolarized membrane potential [64], paired with a higher glutamatergic drive onto these cells relative to males [58]. Further, the inhibitory tone within the female BLA is altered by estrous cycle, adding another layer of consideration in evaluating potential baseline differences [58]. In terms of alterations in BLA function, chronic stress induces near opposing effects in the male and female amygdala of rats, with females displaying dendritic hypotrophy and males showing hypertrophy [65]. Further, chronic stress induced alterations biasing the amygdala towards hyperexcitability are detectable 10 days following cessation of stress in males but not females [66]. Such stress resilience in females may be supported by increased estrogen expression [67–69]. Indeed, sex hormones may play a significant role in modulating both sexes as androgen and estrogen receptors are expressed within the BLA [66, 67]. Importantly, these sex-specific differences also occur in ethanol-induced alterations to BLA signaling. A more prolonged period of chronic ethanol exposure is required in females to induce increases in presynaptic glutamatergic release onto BLA principal neurons during withdrawal [70]. Further, only males develop postsynaptic increases in glutamatergic receptor expression on accumbens projecting BLA neurons [71]. This is in keeping with previous behavioral results suggesting female rodents need greater ethanol exposure to develop, and recover more quickly from, symptoms of withdrawal, such as increased anxiety or seizure susceptibility [72–76]. While behavioral studies must be taken with appropriate caution given they were primarily developed and validated for use in male rodents, such striking differences in BLA neuronal signaling at baseline and following chronic stress and alcohol exposure suggest important differences in male and female amygdala function worthy of further investigation.
The NPY system displays striking differences between sexes. NPY knockout increases anxiety-like behavior on the elevated plus maze and light-dark test in males alone, while increasing immobility time in the forced swim test in both sexes [73, 74]. NPY expression and cell number in the brain are overall lower in females [45]. However, the direct impact of expression level alone is difficult to interpret as NPY1Rs in females display higher binding-affinity for NPY than in males [77]. NPY1R receptor expression is further regulated by estrous cycle [78]. Interestingly, in BLA GABAergic cells NPY1R decreases cell excitability via two distinct pathways, (1) an increase of GABAA-mediated currents induced by decreased protein kinase A activity, and (2) by a decrease in NMDA-mediated currents from reduction in exchange protein activated by cAMP activity [79]. Thus, a potential bias in downstream signaling mechanisms between males and females may also contribute to the sex-dependent impact of NPY on behavior observed. A reduced sensitivity of females to exogenous NPY has recently been demonstrated by Nahvi and colleagues, in which females required an intra-nasal dose 8x that used in males to achieve a similar reduction in depressive-like behaviors following single prolonged stress exposure [80]. Given this and our previous findings that female mice are less sensitive to NPY1R-deletion induced increases of ethanol intake [81], we speculate that the sex differences noted in our present pharmacological manipulations reflects reduced sensitivity and downstream signaling of NPY1R in female mice. As such, it is possible that rather than intra-BLA NPY being ineffective in females, a higher dose than those used here is required to induce reductions in female ethanol intake. It will be important to characterize the impact of a broad dose-response sensitivity to NPY and related compounds in the BLA and directly compare these curves between male and female mice.
Intra-mPFC administration of CNO to activate Gi-DREADDS on NPY1R + BLA terminals dose-dependently decreased ethanol intake in male and female animals. Low dose CNO decreased intake in males alone while high dose CNO decreased intake only in females. While past literature supports the need for more robust DREADD inhibition in female rodents to achieve a similar behavioral end point observed in males [82], the lack of the higher CNO dose on male behavior is surprising. Innate differences between males and females in the excitatory:inhibitory balance outlined above exerted on NPY1R + BLA cells likely contributes to the significant differences in sexes observed in this work, however this is unlikely to fully account for the lack of impact of the higher CNO dose on male ethanol intake. One possibility is high and low CNO may induce distinct alterations in cellular signaling. CNO activation of Gq-DREADDs was recently shown to induce dose-dependent effects on plasticity and neuronal signaling within the hippocampus [83]. In this work, CNO application dose-dependently altered intracellular calcium dynamics in cultured cells. Further, bath application of low dose (1 µM) CNO facilitated an electrically evoked long-term potentiation in mouse hippocampal slices. In contrast, this potentiation was attenuated during bath application of a higher dose (20 µM) CNO, with electrical stimulation inducing a slight long-term depression instead. This higher dose of CNO further induced a significantly greater alteration in both AMPA and NMDA-mediated currents relative to the low dose. Thus, it is possible the low and high doses of CNO used in the present work may result in distinct effects on cellular signaling within the males, only the former of which induces reductions in ethanol intake. Finally, while we did not assess phases of the estrous cycle in the present work to avoid stress effects of dependent measure, it is also possible the different phases of the estrous cycle alter sensitivity to CNO in female mice, which may have impacted the present results. Together, these works highlight the need for further investigation into potential sex and agonist-dose differences in DREADD function.
There are a several of limitations to the present work that deserve addressing. First, we did not assess blood ethanol concentrations (BECs) in the present set of experiments, as the DID procedure in our hands consistently generates levels of consumption that are associated with pharmacologically relevant BECs. In the present set of studies, male mice drank ~2.75 g/kg/2 h and female mice drank about ~3.8 g/kg/2-h. As a point of reference, in recent published work from our laboratory mice that consumed 2 or great g/kg/2 h of ethanol achieved BECS of greater the 80 mg/dl on average [84, 85], thus we are confident that the mice in the present work achieved such levels of BEC or higher. Second, while we assessed NPY1R expression in the BLA we did not go into more detail regarding the anatomical location of these receptors in the BLA. However, as noted above previous research in rats provided evidence that nearly 100% of principal neurons of the BLA express NPY1R, and this same study showed that 81.4 to 88.7 of GABAergic interneurons expressed NPY1R, depending on the phenotype of co-expressed neurochemicals [39]. It will be important in future work to determine if the same expression pattern is evident in mice. Finally, we did not identify the innervation pattern of BLA NPY1R+ neurons in the mPFC, but as noted above pyramidal projection from the BLA to the mPFC synapse on both glutamatergic and GABAergic cells types [43, 44, 51, 52].
In conclusion, in this work we demonstrate (1) a role for BLA NPY and specifically NPY1R+ projections to the mPFC in modulating binge-like ethanol intake and (2) a notable difference in sensitivity of this projection to experimental manipulation between male and female animals. These findings, combined with our previous work, suggest an important role of NPY in regulating corticolimbic communication during binge-like ethanol intake and add to the growing body of literature supporting the NPY system as an important target for pharmacotherapeutic development. Of direct translational relevance, there is an emerging literature implicating the utility of intranasally administered NPY to treat disorders that are often co-morbid with AUD, including posttraumatic stress disordered (PTSD)-like phenotypes [86, 87]. Further, these results further highlight the critical importance of investigating potential differences in drug action between males and females, as treatments viable in both sexes may be overlooked as only impacting behavior in males when doses previously validated in male animals alone are utilized.
Supplementary information
Acknowledgements
Special thanks to Rhiannon Thomas, MS, for technical assistance and feedback.
Author contributions
SLR: conception and design of the work; acquisition, analysis, and interpretation of data; and drafting and revising of manuscript. SCB: design of work; acquisition of data; manuscript review. EMY: acquisition of data. TET: conception and design of the work; revising of manuscript; final approval of the version to be published.
Funding
This work was supported by NIH grants AA022048, AA013573, AA025809, F32AA025811, and K99AA028268.
Competing interests
The authors declare no competing financial interests. Dr. Thiele owns shares of Glauser Life Sciences, a company focusing on the development of therapeutics for mental health disorders. The work that is presented in this paper is not directly related to the scientific aims of Glauser Life Sciences.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01742-w.
References
- 1.National Institute on Alcohol Abuse and Alcoholism. No Title. Alcohol Facts Stat. 2022.
- 2.Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav. 2012;106:325–31. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilpin NW. Neuropeptide Y (NPY) in the extended amygdala is recruited during the transition to alcohol dependence. Neuropeptides. 2012;46:253–9. doi: 10.1016/j.npep.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson SL, Thiele TE. The role of neuropeptide Y (NPY) in alcohol and drug abuse disorders. Int Rev Neurobiol. 2017;136:177–97. doi: 10.1016/bs.irn.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y–a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–60. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 7.Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, et al. Neuropeptide Y distribution in human brain. Nat. 1983;306:584–6. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 8.Allen YS, Bloom SR, Polak JM. The neuropeptide Y-immunoreactive neuronal system: discovery, anatomy and involvement in neurodegenerative disease. Hum Neurobiol. 1986;5:227–34. [PubMed] [Google Scholar]
- 9.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, et al. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–9. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 10.Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytiä P, Heilig M. Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2001;25:1564–9. [PubMed] [Google Scholar]
- 11.Hayes DM, Knapp DJ, Breese GR, Thiele TE. Comparison of basal neuropeptide Y and corticotropin releasing factor levels between the high ethanol drinking C57BL/6J and low ethanol drinking DBA/2J inbred mouse strains. Alcohol Clin Exp Res. 2005;29:721–9. doi: 10.1097/01.ALC.0000164375.16838.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–9. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 13.Hwang BH, Suzuki R, Lumeng L, Li TK, McBride WJ. Innate differences in neuropeptide Y (NPY) mRNA expression in discrete brain regions between alcohol-preferring (P) and -nonpreferring (NP) rats: a significantly low level of NPY mRNA in dentate gyrus of the hippocampus and absence of NPY mRNA in the medial habenular nucleus of P rats. Neuropeptides. 2004;38:359–68. doi: 10.1016/j.npep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li TK. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–30. doi: 10.1111/j.1530-0277.1999.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharm Biochem Behav. 2000;67:683–91. doi: 10.1016/S0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–63. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Badia-Elder NE, Gilpin NW, Stewart RB. Neuropeptide Y modulation of ethanol intake: effects of ethanol drinking history and genetic background. Peptides. 2007;28:339–44. doi: 10.1016/j.peptides.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–69. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson SLL, Marrero IMM, Perez-Heydrich CAA, Sepulveda-Orengo MTT, Reissner KJJ, Thiele TEE. Medial prefrontal cortex neuropeptide Y modulates binge-like ethanol consumption in C57BL/6J mice. Neuropsychopharmacology. 2019;44:1132–40. doi: 10.1038/s41386-018-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, et al. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology. 2012;37:1409–21. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron. 2017;93:1464–1479.e5. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J Neurosci. 2009;29:2486–95. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA. 2010;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff SBE, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–8. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 25.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–92. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–8. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyeler A, Chang CJ, Silvestre M, Lévêque C, Namburi P, Wildes CP, et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 2018;22:905–18. doi: 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH. Inhibitory networks of the amygdala for emotional memory. Front Neural Circuits. 2013;0:129. doi: 10.3389/fncir.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med. 2018;50:1–16. doi: 10.1038/s12276-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012;107:699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornhuber J, Zoicas I. Brain region-dependent effects of neuropeptide Y on conditioned social fear and anxiety-like behavior in male mice. Int J Mol Sci. 2021;22:3695. doi: 10.3390/ijms22073695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic–adrenal–pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitermann RJ, Rostkowski AB, Urban JH. Neuropeptide Y input to the rat basolateral amygdala complex and modulation by conditioned fear HHS public access. J Comp Neurol. 2016;524:2418–39. doi: 10.1002/cne.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, et al. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacol (Berl) 2008;195:547–57. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- 37.Morales-Medina JC, Dumont Y, Benoit CE, Bastianetto S, Flores G, Fournier A, et al. Role of neuropeptide Y Y1 and Y2 receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology. 2012;62:200–8. doi: 10.1016/j.neuropharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharm. 1999;368:143–7. doi: 10.1016/S0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 39.Rostkowski AB, Teppen TL, Peterson DA, Urban JH. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. J Comp Neurol. 2009;517:166–76. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGinty VB, Grace AA. Activity-dependent depression of medial prefrontal cortex inputs to accumbens neurons by the basolateral amygdala. Neuroscience. 2009;162:1429–36. doi: 10.1016/j.neuroscience.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little JP, Carter AG. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2013;33:15333–42. doi: 10.1523/JNEUROSCI.2385-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dilgen J, Tejeda HA, O’Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol. 2013;110:221–9. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheriyan J, Kaushik MK, Ferreira AN, Sheets PL. Specific targeting of the basolateral amygdala to projectionally defined pyramidal neurons in prelimbic and infralimbic cortex. ENeuro. 2016;3:489–94. doi: 10.1523/ENEURO.0002-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2015. 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed]
- 45.Nahvi RJ, Sabban EL. Sex differences in the neuropeptide Y system and implications for stress related disorders. Biomolecules. 2020;10:1–21. doi: 10.3390/biom10091248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price ME, McCool BA. Structural, functional, and behavioral significance of sex and gonadal hormones in the basolateral amygdala: a review of preclinical literature. Alcohol. 2022;98:25–41. doi: 10.1016/j.alcohol.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–41. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, et al. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–95. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killgore WDS, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL, et al. Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety. 2014;31:150–9. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandra Kredlow M, Fenster RJ, Laurent ES, Ressler KJ, Phelps EA. Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology. 2022;47:247–59. doi: 10.1038/s41386-021-01155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun T, Song Z, Tian Y, Tian W, Zhu C, Ji G, et al. Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc Natl Acad Sci USA. 2019;116:3799–804. doi: 10.1073/pnas.1814292116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chefer VI, Wang R, Shippenberg TS. Basolateral amygdala-driven augmentation of medial prefrontal cortex GABAergic neurotransmission in response to environmental stimuli associated with cocaine administration. Neuropsychopharmacology. 2011;36:2018–29. doi: 10.1038/npp.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang L, Chen Y, Jin S, Lin L, Duan S, Si K, et al. Organizational principles of amygdalar input-output neuronal circuits. Mol Psychiatry. 2021;26:7118–29. doi: 10.1038/s41380-021-01262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manoorcheri K, Carter AG. Rostral and caudal basolateral amygdala engage distinct circuits in the prelimbic and infralimbic prefrontal cortex. Elife. 2022;11:e82688. [DOI] [PMC free article] [PubMed]
- 56.Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–94. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience. 2015. 10.1016/j.neuroscience.2015.03.004. [DOI] [PMC free article] [PubMed]
- 58.Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, et al. Sex- and estrus-dependent differences in rat basolateral amygdala. J Neurosci. 2017;37:10567–86. doi: 10.1523/JNEUROSCI.0758-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Mitra R, Vyas A, Chatterjee G, Chattarji S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci Lett. 2005;383:278–83. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 61.Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24:3245–54. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 62.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 63.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guily P, Lassalle O, Chavis P, Manzoni OJ. Sex-specific divergent maturational trajectories in the postnatal rat basolateral amygdala. IScience. 2022;25:103815. doi: 10.1016/j.isci.2022.103815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blume SR, Padival M, Urban JH, Rosenkranz JA. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci Rep. 2019;9:12292. doi: 10.1038/s41598-019-48683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta K, Chattarji S. Sex differences in the delayed impact of acute stress on the amygdala. Neurobiol Stress. 2021;14:100292. doi: 10.1016/j.ynstr.2020.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 68.Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–87. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- 69.Kiss J, Csaba Z, Csáki Á, Halász B. Demonstration of estrogen receptor α protein in glutamatergic (vesicular glutamate transporter 2 immunoreactive) neurons of the female rat hypothalamus and amygdala using double-label immunocytochemistry. Exp Brain Res. 2013;226:595–602. doi: 10.1007/s00221-013-3474-8. [DOI] [PubMed] [Google Scholar]
- 70.Morales M, McGinnis MMM, Robinson SLL, Chappell AMM, McCool BAA. Chronic intermittent ethanol exposure modulation of glutamatergic neurotransmission in rat lateral/basolateral amygdala is duration-, input-, and sex-dependent. Neuroscience. 2018;371:277–87. doi: 10.1016/j.neuroscience.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Price ME, McCool BA. Chronic alcohol dysregulates glutamatergic function in the basolateral amygdala in a projection-and sex-specific manner. Front Cell Neurosci. 2022;16:857550. doi: 10.3389/fncel.2022.857550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharm Exp Ther. 2007;320:427–36. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- 73.Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–96. doi: 10.1111/j.1530-0277.2001.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 74.Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharm Biochem Behav. 2004;78:459–64. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharm. 2011;163:1302–14. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–80. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- 77.Michel MC, Leweljohann K, Farke W, Bischoff A, Feth F, Rascher W. Regulation of NPY/NPY Y1 receptor/G protein system in rat brain cortex. Am J Physiol. 1995;268:R192–200. doi: 10.1152/ajpregu.1995.268.1.R192. [DOI] [PubMed] [Google Scholar]
- 78.Martini M, Sica M, Gotti S, Eva C, Panzica GC. Effects of estrous cycle and sex on the expression of neuropeptide y Y1 receptor in discrete hypothalamic and limbic nuclei of transgenic mice. Peptides. 2011;32:1330–4. doi: 10.1016/j.peptides.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar ANPY. y 1 receptors differentially modulate gaba a and nmda receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology. 2013;38:1352–64. doi: 10.1038/npp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nahvi RJ, Tanelian A, Nwokafor C, Hollander CM, Peacock L, Sabban EL. Intranasal neuropeptide Y as a potential therapeutic for depressive behavior in the rodent single prolonged stress model in females. Front Behav Neurosci. 2021;15:705579. doi: 10.3389/fnbeh.2021.705579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levine OB, Skelly MJ, Miller JD, Rivera-Irizarry JK, Rowson SA, DiBerto JF, et al. The paraventricular thalamus provides a polysynaptic brake on limbic CRF neurons to sex-dependently blunt binge alcohol drinking and avoidance behavior in mice. Nat Commun. 2021;12:5080. doi: 10.1038/s41467-021-25368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pati S, Salvi SS, Kallianpur M, Vaidya B, Banerjee A, Maiti S, et al. Chemogenetic activation of excitatory neurons alters hippocampal neurotransmission in a dose-dependent manner. 2019. 10.1523/ENEURO.0124-19.2019. [DOI] [PMC free article] [PubMed]
- 84.Dornellas APS, Burnham NW, Luhn KL, Petruzzi MV, Thiele TE, Navarro M. Activation of locus coeruleus to rostromedial tegmental nucleus (RMTg) noradrenergic pathway blunts binge-like ethanol drinking and induces aversive responses in mice. Neuropharmacology. 2021;199:108797. doi: 10.1016/j.neuropharm.2021.108797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burnham NW, Chaimowitz CN, Vis CC, Segantine Dornellas AP, Navarro M, Thiele TE. Lateral hypothalamus-projecting noradrenergic locus coeruleus pathway modulates binge-like ethanol drinking in male and female TH-ires-cre mice. Neuropharmacology. 2021;196:108702. doi: 10.1016/j.neuropharm.2021.108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 87.Sabban EL, Serova L, Nahvi RJ, Liu X. Potential benefits of intranasal neuropeptide Y include sustained extinction of fear memory. J Neuroendocrinol. 2023;e13279. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.