Abstract
Fruit coatings serve a dual purpose in preserving the quality of fruits. Not only do they act as a barrier against water evaporation and fungal infiltration, but they also enhance the fruit’s visual appeal in the market. Yet, their influence on the fruit’s quality components, which play a crucial role in determining its nutritional value, taste, and overall flavor, has remained relatively unexplored. This study aimed to evaluate the effects of carnauba wax coating on the quality of Moro oranges during storage. The selected fruits were meticulously chosen for uniformity in size. The experiment involved applying carnauba wax, a commonly used type among local producers, at four different concentrations: 0%, 0.5%, 1%, and 1.5%. These treatments were applied during various storage periods, including immediately after fruits were harvested and after 40 and 80 days. Following the application of these treatments, the oranges were stored in a controlled environment (morgue) at a temperature of 4 ± 1 °C. Subsequently, several physicochemical parameters of both the fruit flesh and skin were examined. The results unveiled a decline in the overall ascorbic acid content of the fruits. In terms of phenol content, a general decreasing trend was observed after harvesting. At each sampling interval during storage, the phenol content in uncoated fruits consistently exceeded that of their waxed counterparts. Significant reduction in fruit weight was observed throughout the storage period. Both vitamin C and total acidity levels in the fruit exhibited decreases during the storage period. As time passed, fruit firmness gradually declined, while fruit decay increased during the 40- and 80-day storage periods for untreated Moro oranges. The anthocyanin content showed an increasing trend. The study also unveiled a decline in the antioxidant capacity of citrus fruits during storage. Strong significant positive correlations were observed between total phenol content and key parameters, such as antioxidant activity (0.941**), MDA (0.364*), vitamin C content, and total carbohydrate content (0.475**). Skin radiance showed a perfect correlation with chroma and hue (1.000**). Principal component analysis revealed that the first principal component accounted for 34.27% of the total variance, out of a total of five principal components that explained 77.14% of the variance. Through cluster analysis, the variables were categorized into three distinct groups; one associated with weight loss and another with ion leakage. Considering these findings, carnauba wax-based coating emerges as a promising solution for preserving Moro oranges. It effectively mitigates fruit weight loss and helps maintain fruit firmness during storage, making it a valuable tool for fruit preservation.
Keywords: Orange fruit, Coatings, Carnauba wax, Storage, Postharvest
Subject terms: Physiology, Plant sciences
Introduction
Mazandaran province holds the prestigious rank of being the top producer of citrus fruits in Iran, with the largest cultivated area dedicated to citrus orchards. Out of the total citrus production in the country, which exceeds four million tons1, more than two million tons are attributed to this region. However, only a portion of this citrus yield enters the market directly, with the majority being stored for approximately 3 to 4 months, especially in conventional (cold) storage facilities. Less than optimal conditions in these storage facilities lead to high post-harvest losses each year. To mitigate these losses and enhance both the visual and nutritional quality of citrus fruits during postharvest storage, application of various coatings and proper storage conditions have been suggested by different authors2–5. Marcilla et al.6 studied different concentrations of wax coating on oranges and found that fruits treated with wax coating at 70% concentration exhibited the least weight reduction during storage.
While citrus fruits are generally classified as non-climacteric, the composition of these fruits can undergo changes depending on storage conditions, including temperature and storage duration7. Miranda et al.8 observed a decline in the vitamin C content of oranges during extended storage periods. This reduction in vitamin C content often coincides with a decrease in antioxidant capacity and overall fruit quality9. Furthermore, investigations by Klimezak and Malecka10 revealed significant fluctuations in the levels of polyphenols within citrus fruits, a variation that is influenced by storage temperature and duration. The alteration in total polyphenol levels during storage is contingent on the specific storage conditions5. In the case of blood oranges, there is typically an increase in the content of anthocyanins as the storage period progresses11. Palou et al.12 reported anthocyanins, flavonoids, and vitamin C levels increased during the storage of blood oranges. Higher antioxidant activity in stored blood oranges can be attributed to the synthesis of various phenolic compounds, including anthocyanins.
The difference in anthocyanin levels during the storage of blood oranges is correlated with the orange variety13. LoScalzo et al.14 demonstrated a remarkable increase in anthocyanin levels, up to fivefold, in the Tarocco oranges. In contrast, Moro oranges exhibited a more modest twofold increase, while the Sanguinello variety showed no change during storage. Additionally, the research revealed that the titratable acidity (TA) experienced a decline at the conclusion of the storage period across all varieties, except for Valencia oranges, where an increase was observed.
The storage temperature also significantly influences the sensory characteristics and flavor of fruits15. Manzano et al.16 investigated the impact of different temperature regimes on the aroma and taste of Valencia oranges during storage, it was found that higher temperatures had a detrimental effect on the distinctive citrus fruit flavor, leading to an increase in undesirable tastes. Furthermore, Obenland et al.3 reported that the aroma and taste of fruits underwent deterioration and developed unpleasant characteristics after four weeks of storage at varying temperatures.
According to studies, fruit coatings serve a dual purpose. They not only act as a protective barrier, preventing water evaporation and fungal decay, but also enhance the visual appeal of the fruits. Furthermore, considering the potential beneficial effects of these coatings on the production and preservation of valuable nutritional compounds, their use can play a role in promoting the overall health and well-being of the community. The impact of these coatings on the quality-associated parameters of fruits, which are crucial in determining their nutritional value, taste, and flavor, has received limited attention in previous literature. Hence, there is a pressing need for further research to better understand and optimize the application of coatings to preserve nutritional compounds for different fruit varieties. Moreover, it’s essential to acknowledge that quality standards for fruits have evolved in recent years. This underscores the necessity of staying current with shifting quality criteria to meet the evolving expectations of consumers and comply with regulatory requirements in the fruit industry. These days the quality parameters of a fruits (e.g., no/low levels of accumulation of toxic or harmful chemicals) are pivotal factors in determining import and export licenses for fruits. Consequently, it is imperative to place a heightened focus on the production of organic fruits that not only possess visual appeal but also boast high nutritional value. Most Iranian citrus producers rely on conventional storage methods for preserving fruits like Moro oranges. While there has been limited research on changes in these compounds during postharvest storage, this study aimed to investigate the impact of carnauba wax coating-a commonly used type among local producers-on the postharvest quality of Moro blood oranges.
Materials and methods
Fruit samples and treatments
Healthy, uniform Moro blood oranges (grafted on Poncirus trifoliata rootstock) with no signs of physical damage were harvested and collected from the orchard of the National Citrus Research Institute Research Station.
The fruits were divided into 12 groups, each containing 60 samples (three replications, 20 fruits each). Treatments included four different concentrations: 0%, 0.5%, 1%, and 1.5%. The coated fruits were stored for three months under normal storage conditions, i.e., 1 ± 4 degrees Celsius and relative humidity of 75% to 85%). At intervals of 0, 40, and 80 days, 15 fruits from each treatment were randomly selected and fruit quality parameters were measured.
Physicochemical properties
To determine the reduction in fruit weight, three fruits from each replicate were individually labeled, weighed using a digital scale, and then placed in cold storage. At the end of the experimental period, the final weight of the fruits was determined again, and the weight loss17 was calculated using the following formula, expressed as a percentage:
To determine ion leakage, the electrolyte leakage index was utilized, following the method outlined by Sullivan and Ross18.
Fruit decay Incidence
The percentage of fruit decay incidence is determined using the following formula19:
In this equation, N signifies the total count of fruits placed in storage, and n is the number of rotten fruits. Each fruit is individually harvested and sealed in a freezer bag to prevent contact with other fruits. Subsequently, these bags are tightly sealed and stored in a cold storage facility at a temperature of 4 ± 1 degrees Celsius.
Fruit firmness
The fruit texture firmness was determined using a Penetrometer (Zwick, Germany)17.
Vitamin C (ascorbic acid)
The concentration of vitamin C was determined using the titration method with 2,6-dichlorophenolindophenol (DIP) as described by20 with little modification]. Initially, 15 ml metaphosphoric acid (3%) was added to 5 ml of orange juice. The mixture was titrated with DIP containing sodium bicarbonate until a pink color appeared. The amount of vitamin C was calculated and expressed in mg per 100 ml fruit juice.
Total phenol content
Total phenol content was determined utilizing the Folin-Ciocalteu method21. This entailed adding 470 μl of distilled water to 30 μl of fruit juice, followed by the addition of 2.5 ml of 10% Folin reagent. After a 3-min interval, 2 ml of 5.7% sodium carbonate were introduced. Following a 1.5-h incubation at room temperature, absorbance at a wavelength of 765 nm was recorded using a spectrophotometer. The results were expressed as mg of gallic acid equivalents per 100 ml fruit juice (mg GAE/100 g).
Total antioxidant activity
The total antioxidant activity was determined using the DPPH (2,2-diphenyl-picryl-hydrazyl) radical degradation method. Specifically, 10 μL of fruit juice was combined with 4 mL of distilled water in test tubes, followed by the addition of 1 mL of a 250 μM DPPH solution. Subsequently, the test tubes were left undisturbed in darkness for a 30-min incubation period. Afterward, the absorbance was measured at 517 nm using a spectrophotometer21. The antioxidant activity was then calculated as the percentage of inhibition relative to the control, employing the following formula:
where A blank is the absorbance of the control reaction, and A sample is the absorbance of the test compound in the sample.
Total sugars
The total sugar content was determined using the Anthrone method. In this procedure, 200 μl of concentrated alcoholic extract was combined with 3 ml of Anthrone reagent and placed in a boiling water bath for 20 min. After cooling, the absorbance at 620 nm was measured for each treatment. The total sugar content of each sample was quantified by comparing it to a standard curve22.
Titration analysis (TA)
Titration was carried out with 0.1 N NaOH using phenolphthalein as an indicator, and TA was expressed as grams of citric acid per 100 g of fruit juice according to the method discussed by23 with little modification].
Anthocyanins
Total anthocyanins were measured spectrophotometrically using pH differential method with two buffer systems24: potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M). Briefly, 0.4 ml of juice was mixed with 3.6 ml of corresponding buffers and read against water as blank at 510 and 700 nm. Absorbance (A) was calculated as
Then total anthocyanins content was calculated using the equation: Anthocyanin (mg · ml-1) = (A × MW × DF)/(ε × 1) Where A is the absorbance of the diluted sample and DF is the dilution factor. MW and in this formula correspond to the predominant anthocyanin in the sample. The pigment content was calculated as pelargonidin-3-glucoside, where MW = 433.38 and = 22.400.
Malondialdehyde content
Malondialdehyde (MDA) content was assessed following the thiobarbituric acid (TBA) reaction, as originally outlined by Campos et al.25, with minor adjustments. Initially, 200 ml fruit juice were homogenized in 2 mL of 0.1% trichloroacetic acid and subjected to centrifugation at 10,000 × g for 15 min. Next, 1 mL of the resulting supernatant was combined with 2.5 mL of 0.5% thiobarbituric acid in 20% trichloroacetic acid and subjected to incubation in hot water at 95 °C for 30 min. The reaction was promptly terminated by cooling the mixture on ice, followed by centrifugation at 10,000 × g for 30 min. The absorbance was measured at 532 and 600 nm. The concentration of MDA was determined by subtracting the nonspecific absorption at 600 nm from the absorption at 532 nm, using an absorbance coefficient of extinction (155 mM−1 cm−1).
Sensory assessment
Towards the conclusion of the storage period, a sensory evaluation was carried out with the participation of nine randomly selected assessors (untrained consumers of ages 18–45 years among students and employees). These assessors provided ratings on a scale from 1 to 10, considering various attributes including visual appearance (skin and flesh characteristics), aroma, taste, sweetness, tartness, bitterness, and overall acceptability of the fruit. A 10-point hedonic scale ranging from 10 for “like extremely” to 1 for “dislike extremely” was used to conduct the sensory test26.
Statistical analysis
The experiment was carried out in a factorial design within a completely randomized setup, evaluating two factors: coating concentration and the storage duration. Data were analyzed by SAS, and means were compared using Duncan’s multiple range tests at the 5% probability level. SPSS was used for correlation coefficients and cluster analysis.
Permission to collect fruit samples
The permission to collect Citrus sinensis cv. Moro fruits was acquired from Agricultural and Natural Resources Ministry of Iran.
Statement on experimental research and field studies on plants
The plants (Citrus sinensis cv. Moro) sampled comply with relevant institutional, national, and international guidelines and domestic legislation of Iran.
Results
Total phenol content in fruit flesh
The effect of carnauba coating treatment and storage time on the total phenol content of fruit juice was significant at the 5% and 1% probability levels, respectively, individually, but their interaction effect was non-significant (Table 1). In Table 1, descriptive statistics, including Mean ± SE (0.51 ± 0.025) and standard deviations (0.151), minimum (0.112) and maximum values (0.722), the range of variations (0.61), for various parameters, have been summarized.
Table 1.
Descriptive statistical analysis and analysis of variance for total phenol content of Moro oranges during storage
| Traits | Mean ± Standard error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Total phenol | 0.51 ± 0.025 | 0.151 | 0.61 | 0.112 | 0.722 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Total phenol | 0.041* | 9.485** | 0.096ns | 20.98 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
Notably, there were no significant differences observed among various levels of carnauba treatment, as depicted in Fig. 1. A comparison of mean values revealed that the highest and lowest total phenol content were observed at control (No wax) and 1% of storage, respectively (Fig. 1).
Figure 1.
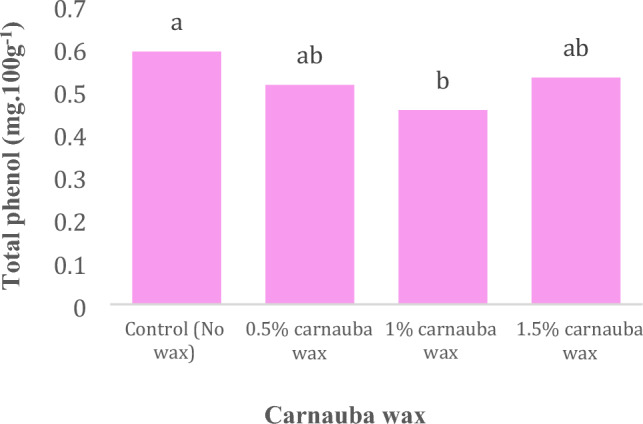
Effect of coating on total phenol content in Moro orange fruit juice. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Skin color, chroma, and hue
The effect of carnauba coating treatment on the skin color, chroma, hue and ion leakage of fruit juice was significant at the 1% probability levels, respectively, individually, but carnauba coating treatment and their interaction effect was non significant (Table 2). The highest percentages of phenotypic variation were exhibited by ion leakage (288), and characteristics related to skin radiance, chroma, and hue (28.29) (Table 2).
Table 2.
Descriptive statistical analysis and analysis of variance for ion leakage, skin radiance (L*), Chroma and Hue of Moro oranges during storage
| Traits | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Ion leakage | 132.26 ± 9.75 | 57.706 | 288 | 61 | 349 |
| Skin radiance (L*) | 38.2 ± 1.262 | 7.469 | 28.29 | 26.24 | 54.53 |
| Chroma | 43.4 ± 1.262 | 7.469 | 28.29 | 31.45 | 59.74 |
| Hue | 47.6 ± 1.262 | 7.469 | 28.29 | 35.57 | 63.86 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax*Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Ion leakage | 5496.4ns | 562,584.4** | 1837.5ns | 23.67 | |
| Skin radiance (L*) | 63.45ns | 48,561.5** | 13.38ns | 18.09 | |
| Chroma | 63.45ns | 62,700.3** | 0.626ns | 10.21 | |
| Hue | 63.45ns | 75,158.5** | 13.38ns | 16.65 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significan.
The results of the color analysis for both wax-coated and control (uncoated) oranges during the 90-day storage period are presented in Fig. 2A–C. According to Table 2, the effect of storage time on skin color, chroma, and hue was significant, whereas the carnauba treatment and its interaction with storage time did not yield significant effects. A comparison of mean values revealed that the highest and lowest skin color, chroma, and hue were observed at day 40 and day 80 of storage, respectively (Fig. 2A–C).
Figure 2.
Effect of Coating on hue (A), skin radiance (B), chroma (C), and ion leakage (D) in Moro orange fruit. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Ion leakage
Based on the results of the analysis of variance, neither the carnauba treatment nor its interaction with storage time had a significant impact on ion leakage. However, when considered individually, storage time exhibited a significant effect on ion leakage, with the highest and lowest levels observed on days 80 and 40 of storage, respectively (Fig. 2D).
The panel test, flavor, and overall acceptability
The effect of carnauba treatment, storage time, and their interaction on the panel test, flavor, and overall acceptability of oranges were significant (Table 3). The highest percentages of phenotypic variation were exhibited by panel test (2.42), and characteristics related to flavour and taste (2.3), little acceptance (2) (Table 3).
Table 3.
Descriptive statistical analysis and analysis of variance for panel test, flavour and taste and little acceptance of Moro oranges during storage.
| Traits | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Panel test | 1.39 ± 0.086 | 0.508 | 2.42 | 0.58 | 3 |
| Flavour and Taste | 1.29 ± 0.087 | 0.517 | 2.3 | 0.5 | 2.8 |
| Little acceptance | 1.43 ± 0.088 | 0.520 | 2 | 1 | 3 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Panel test | 0.481* | 63.13** | 1.5* | 26.51 | |
| Flavour and Taste | 0.492* | 55.93** | 0.51* | 27.67 | |
| Little acceptance | 0.478* | 66.7** | 1.26* | 27.61 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significan.
As illustrated in Table 4, the highest scores for the panel test and overall acceptability were associated with the carnauba treatment (0.5%) on day 40 of storage. Conversely, the lowest scores for these two parameters were observed in the control group as well as with a carnauba concentration of 1.0% at the beginning of storage and on day 40 of orange fruit storage. The highest and lowest taste and flavor scores, respectively, were documented at the carnauba concentration (0.5%) on day 40 of storage and the carnauba concentration (1.0%) on day 80 of storage (Table 4).
Table 4.
The interaction effect of carnauba wax coating and storage time on The panel test, flavor, and overall acceptability.
| Carnauba Wax Coating | Storage time (Days) | Little acceptance | Flavour and taste | Panel test |
|---|---|---|---|---|
| No wax | 0 (Before Postharvest) | 1c | 1cd | 1c |
| 0.5% carnauba wax | 0 (Before Postharvest) | 1.44abc | 1.16bcd | 1.62bc |
| 1% carnauba wax | 0 (Before Postharvest) | 2.08a | 1.91a | 2ab |
| 1.5% carnauba wax | 0 (Before Postharvest) | 1c | 1cd | 1c |
| No wax + 40 Day | 40 | 1.55abc | 1.46abc | 1.54bc |
| 0.5% carnauba wax | 40 | 2.15a | 2.1a | 2.3a |
| 1% carnauba wax | 40 | 1c | 1cd | 1c |
| 1.5% carnauba wax | 40 | 1.55abc | 1.05bcd | 1.24c |
| No wax | 80 | 1.78ab | 1.73ab | 1.48bc |
| 0.5% carnauba wax | 80 | 1.11bc | 1.11bcd | 1.22c |
| 1% carnauba wax | 80 | 1.26bc | 0.75d | 1.19c |
| 1.5% carnauba wax | 80 | 1.43abc | 1.65abc | 1.37bc |
Total sugars
The total sugar content in orange fruits was significantly influenced by the carnauba wax treatment, storage duration, and their interaction effects (Table 5). In Table 5, descriptive statistics, including Mean ± SE (14.9 ± 0.807) and standard variation (4.778), minimum (0.57) and maximum values (17.91), the range of variations (17.34), for various parameters, have been summarized.
Table 5.
Descriptive statistical analysis and analysis of variance for total sugar of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Total sugar | 14.9 ± 0.807 | 4.778 | 17.34 | 0.57 | 17.91 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Total sugar | 55.19** | 7448.7** | 195.1** | 10.32 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significan.
As depicted in Fig. 3, the highest total sugar content was observed in the following treatments: no carnauba wax treatment on both day 40 and day 80 of storage, carnauba wax treatment (1%) on day 40 of storage, and carnauba wax coating (1.5%) 80 days after storage. There were no statistically significant differences among these conditions.
Figure 3.
The interaction effect of carnauba wax coating and storage time on total sugars. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
TA
The statistical analysis of the data revealed significant effects of carnauba wax treatment and storage duration on fruit acidity levels (Table 6). In Table 6, descriptive statistics, including Mean ± SE (3 ± 0.249) and standard variation (1.47), minimum (1.29) and maximum values (8.3), the range of variations (7.01), for various parameters, have been summarized.
Table 6.
Descriptive statistical analysis and Analysis of variance for TA of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| TA | 3 ± 0.249 | 1.47 | 7.01 | 1.29 | 8.3 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| TA | 5.15** | 296.1** | 0.018* | 18.86 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significan.
As depicted in Fig. 4, the highest fruit acidity levels in oranges were observed in fruits treated with a 1.5% carnauba wax concentration, after 80 days of storage, followed by those treated with a 1% carnauba wax concentration after 40 days of storage. Other carnauba wax concentrations did not show significant differences. At the conclusion of the 80-day storage period, the lowest total acidity (TA) was recorded in fruits treated with carnauba wax (0.5%).
Figure 4.
The interaction effect of carnauba wax coating and storage time on total acids. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Vitamin C
As shown in Table 7, the impact of carnauba wax treatment on vitamin C content was non-significant. In Table 7, descriptive statistics, including Mean ± SE (0.84 ± 0.056) and standard variation (0.336), minimum (0.25) and maximum values (1.38), the range of variations (1.13), for various parameters, have been summarized.
Table 7.
Descriptive statistical analysis and analysis of variance for Vitamin C of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Vitamin C | 0.84 ± 0.056 | 0.336 | 1.13 | 0.25 | 1.38 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Vitamin C | 0.180ns | 24.3** | 0.006* | 26.92 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significan.
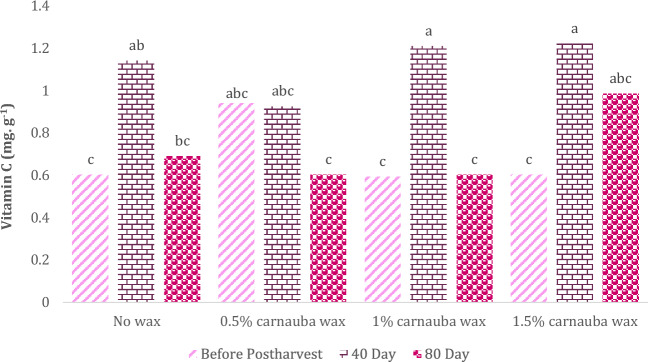
However, the storage duration and its interaction with carnauba treatment influenced this parameter significantly. As illustrated in Fig. 5, the highest vitamin C concentration was observed in fruits treated with 1% or 1.5% carnauba wax concentrations during both the 40 and 80 days of storage, with no significant difference between them. On the contrary, the lowest levels of this parameter were recorded in the control group and at the beginning of the storage period.
Figure 5.
The interaction effect of carnauba wax coating and storage time on vitamin C content in fruits. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Weight loss
The weight loss in orange fruits was significantly influenced by the carnauba wax treatment, storage duration, and their interaction effects (Table 8). In Table 8, descriptive statistics, including Mean ± SE (313.1 ± 7.535) and standard variation (44.579), minimum (221.27) and maximum values (406.9), the range of variations (185.63), for various parameters, have been summarized.
Table 8.
Descriptive statistical analysis and analysis of variance for weight loss of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Weight loss | 313.1 ± 7.535 | 44.579 | 185.63 | 221.27 | 406.9 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Weight loss | 3640.6** | 3,256,576.7** | 282.3** | 9.16 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
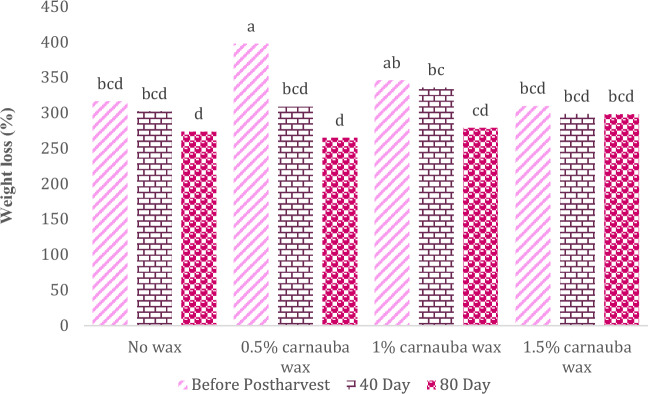
Figure 6 also shows that the highest and lowest rates of weight loss were observed in samples treated with carnauba wax at 0.5% concentration after 80 and 40 days of storage, respectively.
Figure 6.
The interaction effect of carnauba wax coating and storage time on fruit weight reduction. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Fruit peel firmness
The statistical analysis of the data revealed significant effects of carnauba wax treatment and storage duration on firmness (Table 9). In Table 9, descriptive statistics, including Mean ± SE (2.72 ± 0.097) and standard variation (0.574), minimum (1.91) and maximum values (3.81), the range of variations (1.9), for various parameters, have been summarized.
Table 9.
Descriptive statistical analysis and analysis of variance for firmness of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Firmness | 2.72 ± 0.097 | 0.574 | 1.9 | 1.91 | 3.81 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Firmness | 0.693** | 248.8** | 0.086* | 10.21 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
The carnauba treatment, storage time, and their interaction influenced this parameter significantly. The highest level of firmness was observed in control and fruit samples treated with carnauba wax concentrations (0.5% and 1%.) after 40 days of storage (Fig. 7).
Figure 7.
The interaction effect of carnauba wax coating and storage time on fruit peel firmness. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Fruit decay
Carnauba treatment, storage time, and their interaction significantly influence fruit decay (Table 10). In Table 10, descriptive statistics, including Mean ± SE (0.369 ± 0.101) and standard variation (0.199), minimum (0) and maximum values (1.75), the range of variations (1.75), for various parameters, have been summarized.
Table 10.
Descriptive statistical analysis and analysis of variance for decay of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Decay | 0.369 ± 0.101 | 0.199 | 1.75 | 0 | 1.75 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Decay | 0.101** | 11.67** | 0.232** | 19.32 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
As illustrated in Table 11 and Fig. 8, the highest fruit decay rate in oranges was observed after 40 and 80 days of storage without carnauba treatment.
Table 11.
The interaction effect of carnauba wax coating and storage time on fruit decay.
| Before postharvest | 40 | 80 day | |
|---|---|---|---|
| No wax | − | + | + |
| 0.5% carnauba | − | − | + |
| 1% carnauba wax | − | − | − |
| 1.5% carnauba wax | − | − | − |
Figure 8.
The interaction effect of carnauba wax coating and storage time on fruit decay. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Malondialdehyde
Carnauba treatment, storage duration, and their interaction significantly affected the MDA content (Table 12). In Table 12, descriptive statistics, including Mean ± SE (0.63 ± 0.062) and standard variation (0.369), minimum (0.060) and maximum values (1.157), the range of variations (1.097), for various parameters, have been summarized.
Table 12.
Descriptive statistical analysis and analysis of variance for MDA of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| MDA | 0.63 ± 0.062 | 0.369 | 1.097 | 0.060 | 1.157 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| MDA | 0.308** | 13.67** | 0.012** | 29.92 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
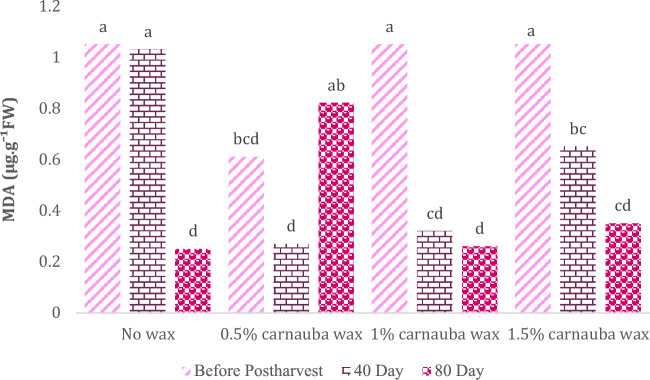
The highest MDA concentration was observed in the control group after fruit harvest. There were no differences in MDA content when carnauba was applied at concentrations of 0.5%, 1%, and 1.5% on days 80, 40, and at the beginning of cold storage, respectively. A steady decrease in MDA content was observed as the storage period progressed, indicating a beneficial effect of the storage period on mitigating this characteristic (Fig. 9).
Figure 9.
The interaction effect of carnauba wax coating and storage time on MDA level. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Anthocyanin
Carnauba treatment, storage duration, and their interaction significantly affected this parameter (Table 13). In Table 13, descriptive statistics, including Mean ± SE (1.44 ± 0.071) and standard variation (0.424), minimum (0.421) and maximum values (2.396), the range of variations (1.975), for various parameters, have been summarized.
Table 13.
Descriptive statistical analysis and analysis of variance for Anthocyanin of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Anthocyanin | 1.44 ± 0.071 | 0.424 | 1.975 | 0.421 | 2.396 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Anthocyanin | 0.280* | 70.23** | 0.018* | 23.83 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
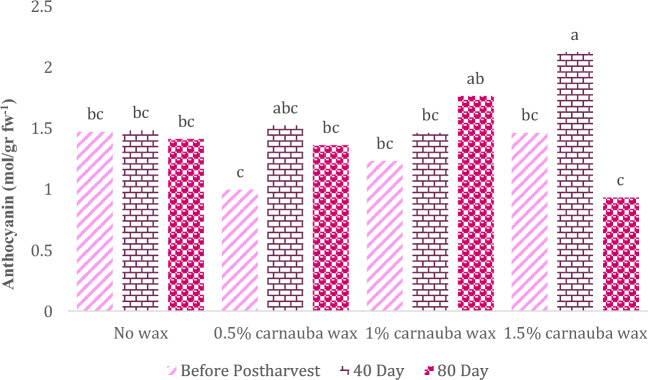
The highest and lowest levels of anthocyanin content in fruit were observed in the carnauba treatment (1.5%) on days 40 and 80 after cold storage, as illustrated in Fig. 10. As the storage period extended to 40 days, there was an increase in fruit anthocyanin levels.
Figure 10.
The interaction effect of carnauba wax coating and storage time on anthocyanin content in fruits. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
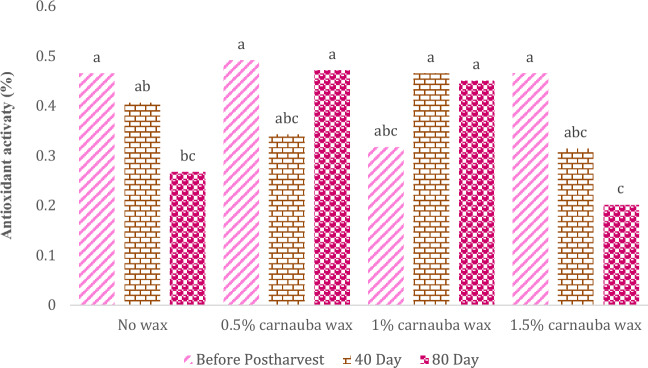
Antioxidant activity
This parameter was significantly influenced by Carnauba treatment, storage duration, and their interaction (Table 14). In Table 14, descriptive statistics, including Mean ± SE (0.38 ± 0.022) and standard variation (0.134), minimum (0.064) and maximum values (0.582), the range of variations (0.518), for various parameters, have been summarized.
Table 14.
Descriptive statistical analysis and analysis of variance for antioxidant activity of Moro oranges during storage.
| Trait | Mean ± Standard Error | Standard variation | Range | Minimum | Maximum |
|---|---|---|---|---|---|
| Antioxidant activity | 0.38 ± 0.022 | 0.134 | 0.518 | 0.064 | 0.582 |
| S.O.V | Carnauba wax | Storage time | Carnauba wax * Storage time | Cv | |
|---|---|---|---|---|---|
| df | 3 | 2 | 6 | – | |
| Antioxidant activity | 0.096* | 5.392** | 0.096* | 21.45 |
* and ** significant at 5% and 1% levels, respectively. ns: non-significant.
The peak antioxidant activity of fruits was observed in fruits treated with carnauba wax (0.5%) immediately after harvest. However, a general decline in antioxidant activity was observed when fruits were cold stored for longer periods, except for the carnauba treatment (1%), which showed an increase on days 40 and 80 (Fig. 11).
Figure 11.
The interaction effect of carnauba wax coating and storage time on antioxidant activity of fruits. Different letters above the bars indicate they are significantly different by Duncan’s multiple at P < 0.05.
Correlation coefficients for assessing relationships among characteristics
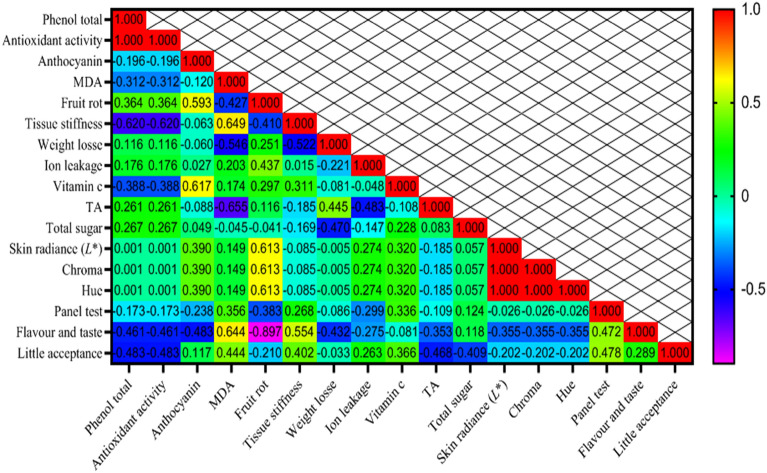
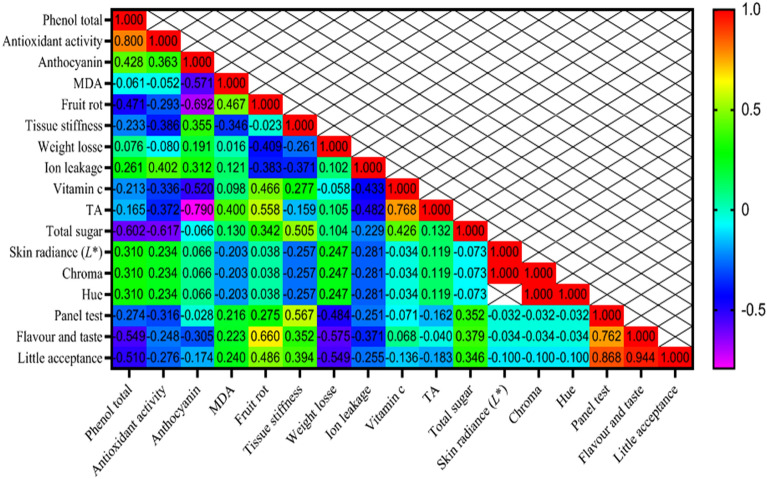
The Pearson correlation coefficients illustrating the relationships between the 40, 80 days and (combined) days traits studied are shown in Fig. 12. Here are the findings:
Total Phenolic compounds exhibited a robust positive correlation with Antioxidant activity (1.000**).
Anthocyanin exhibited a moderately positive correlation with Vitamin c (0.617*) and Fruit rot (0.593*).
MDA exhibited a moderately positive correlation with Flavour and taste (0.644*) and Fruit rot (0.649*).
Fruit rot exhibited a moderately positive correlation with Skin radiance (L*), Chroma and Hue (0.613*).
Tissue stiffness exhibited a moderately positive correlation with flavour and taste (0.554*).
Panel test exhibited a moderately positive correlation with little acceptance (0.478*) and flavour and taste (0.472*) (Fig. 12).
Total Phenolic compounds exhibited a robust positive correlation with Antioxidant activity (0.800**).
Fruit rot exhibited a moderately positive correlation with little acceptance (0.486*), flavour and taste (0.660*), TA (0.558*) and vitamin c (0.466*).
Tissue stiffness exhibited a moderately positive correlation with panel test (0.567*).
Vitamin c exhibited a moderately positive correlation with total sugar (0.426*).
Panel test exhibited a robust positive correlation with flavor and taste (0.762**).
Flavor and taste exhibited a robust positive correlation with little acceptance (0.944**) (Fig. 13).
Total Phenolic compounds exhibited a robust positive correlation with Antioxidant activity (0.941**) and a moderately positive correlation with MDA (0.364*).
Antioxidant activity displayed a moderate positive correlation with MDA (0.349*).
Anthocyanin content demonstrated a moderate positive correlation with Skin radiance (L*), Chroma, and Hue (0.395*).
MDA manifested a strong positive correlation with fruit peel firmness (0.668**).
Fruit decay rate showcased strong positive correlations with total acids (0.631**), Panel test (0.356*), Flavor and taste (0.513**), and Little acceptance (0.534**).
Firmness featured a moderate positive correlation with Skin radiance (L*), Chroma, and Hue (0.451*).
Vitamin C displayed a moderate positive correlation with Total Sugars (0.475**).
Skin radiance demonstrated a perfect correlation with Chroma and Hue (1.000**).
Chroma and Hue exhibited a perfect correlation with each other (1.000**).
Panel test revealed a strong positive correlation with Flavor and taste (0.729**) and Little acceptance (0.833**).
Flavor and taste showed a strong positive correlation with Little acceptance (0.791**) (Fig. 14).
Figure 12.
Heat map of relations among variables in correlation coefficient for Physicochemical indicators for 40 days.
Figure 13.
Heat map of relations among variables in correlation coefficient for Physicochemical indicators for 80 days.
Figure 14.

Heat map of relations among variables in correlation coefficient for Physicochemical indicators. # Y axis are respectively: 1—Total phenolic compounds, 2—Antioxidant, 3—Anthocyanin, 4—MDA, 5—Fruit decay rate, 6—Fruit peel firmness, 7—Weight reduction, 8—Ion leakage, 9—Vitamin C, 10—TA, 11—Total sugars, 12—Skin radiance (L*), 13—Chroma, 14—Hue, 15—Panel test, 16—Flavor and taste, 17—Little acceptance. # X axis are respectively: A—Total phenolic compounds, B—Antioxidant activity, C—Anthocyanin, D—MDA, E—Fruit decayrate, F—Fruit peel firmness, G—Weight reduction, H—Ion leakage, I—Vitamin C, J—TA, K—Total sugars, L—Skin radiance (L*), M—Chroma, N—Hue, O—Panel test, P—Flavor and taste, Q—Little acceptance.
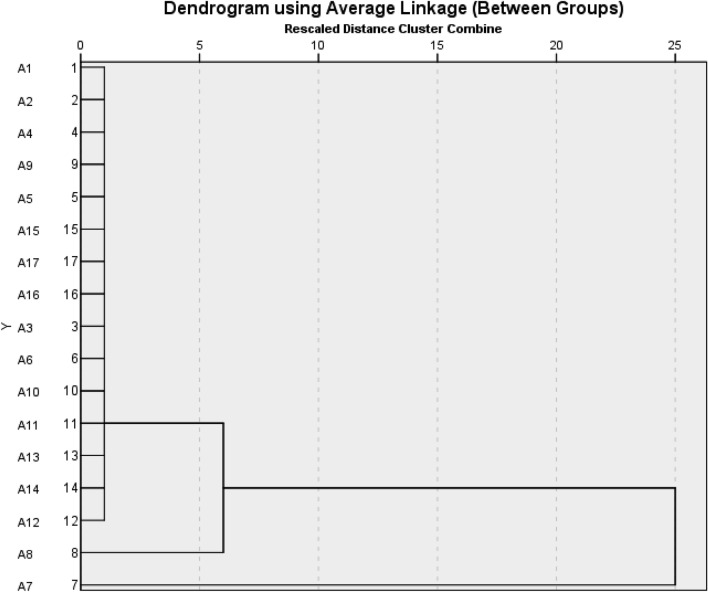
Cluster analysis
The cluster analysis dendrogram illustrates the segmentation of the 17 physicochemical parameters into three clusters (Fig. 15).
Figure 15.
Cluster analysis of physicochemical characteristics of Moro Orange fruit during storage. 1—Total phenolic compounds, 2—Antioxidant, 3—Anthocyanin, 4—MDA, 5—Fruit decay rate, 6—Fruit peel firmness, 7—Weight reduction, 8—Ion leakage, 9—Vitamin C, 10—TA, 11—Total sugars, 12—Skin radiance (L*), 13—Chroma, 14—Hue, 15—Panel test, 16—Flavor and taste, 17—Little acceptance.
The first cluster included one parameter: the friut weight reduction. The second cluster included Ion leakage. The remaining characteristics were positioned in the third cluster.
Discussion
Edible coatings are being considered as the packaging solution of the future, holding promise in minimizing postharvest losses attributed to moisture loss. These coatings consist of thin layers of food-grade biopolymers derived from proteins, polysaccharides, or lipids. They are directly applied to the product’s surface, seamlessly adhering to this surface as an integral part of the final product. These materials act as a protective shield against physical damage, microbial contamination, moisture loss, and nutritional oxidation26. Consequently, they play a pivotal role in preventing product spoilage, thereby elongating the product’s shelf life, preserving sensory quality, and ensuring safety27. What sets these coatings apart from traditional plastic packaging is their edibility, biodegradability, and the potential to partially or fully replace plastic alternatives28.
The utilization of carnauba wax as a coating on fruit surfaces serves a dual purpose—enhancing the visual appeal and safeguarding the fruit against decay. This can extend the fruit’s shelf life and mitigate post-harvest losses. Moro oranges, renowned for their export potential, demand meticulous attention during transportation and storage. The application of carnauba wax (1%) on the Moro oranges resulted in a reduced postharvest loss. Furthermore, a positive and significant correlation was discovered between the total phenol content and critical parameters such as antioxidant activity, vitamin C content, and total carbohydrate content. Various authors underscore the significance of carnauba wax application on oranges and persimmons as a post-harvest preservation method. This method, when integrated with other preservation strategies, emerges as a notable approach for maintaining fruit quality during the export process29,30.
A comparison of mean values revealed that the highest and lowest total phenol content were observed at control (No wax) and 1% of storage, respectively (Fig. 1). Align with our findings, Germano et al.31 has demonstrated that storage duration has a significant impact on the total phenol content in guava fruit. Nasirifar et al. 32 reported that the total phenol content decreases with increasing storage time, with a 10–20% reduction in total phenol content observed after 6 months of storage. Nazoori et al.5 attributed the decrease in phenolic compounds during storage to the senescence process.
One of the most notable changes in Moro oranges during storage is the alteration in skin color33. An increase in chroma value indicates a brighter and more transparent color or, in other words, higher color quality34. The chroma value of the samples was higher after 40 days of storage, indicating better preservation of skin color quality compared to the control (uncoated) group (Fig. 2A–C). However, skin color, chroma, and hue values decreased after day 40 (Fig. 2A–C), a trend similarly described by Braga et al.35 in fruit covered with chitosan-based edible coatings containing mint essential oil. Differences in skin color, chroma, and hue values likely result from carotenoid synthesis during ripening as reported by Jing et al.36. Nonetheless, the higher concentration of chitosan-based edible coatings (above 18%) led to delayed color changes37, possibly due to reduced gas exchange induced by the coating, resulting in altered enzymatic and chemical reactions involved in chlorophyll degradation and/or pigment synthesis38.
The application of carnauba wax coating effectively preserves the fruit peel’s lightness (L), chroma, and hue, ensuring the visual quality remains intact while preventing both weight loss and moisture loss up to a 1% concentration level. Over the 40-day storage period, there was a notable increase in acceptance, flavor, taste, and favorable results in the panel test. Biochemical analysis revealed that a 1% carnauba wax level led to a 6% increase in vitamin C content (compared to the no-wax treatment) and a 30% increase when using a 0.5% wax concentration.
In this study, apart from measuring and assessing some of the physicochemical properties using laboratory methods, the sensory and taste aspects of the fruits were tested. Some authors have reported a correlation between post-harvest treatments and the sensory quality of fruits39–41. The highest scores for the panel test and overall acceptability were associated with the carnauba treatment (0.5%) on day 40 of storage.Some authors have reported a correlation between post-harvest treatments and the sensory quality of fruits39–41.
Sensory tests typically aim to assess the organoleptic quality of a product in comparison to its reference sample. In the case of citrus fruits, the quantitative evaluation focuses on characteristics that define fruit quality. According to our findings, the optimal fruit quality should be characterized by both chemical and sensory parameters, which were influenced by the wax coating, despite some level of decline in quality occurring during the 40 and 80 days of storage. Typically, the accumulation of ethanol and ethyl acetate compounds is used as an indicator of off-flavors in fruit during storage42. This was more pronounced in fruits treated with wax during the 40-day storage period compared to other treatments, although it remained within acceptable limits. The taste of fruits is primarily determined by the sugar-to-acid ratio, volatile compounds, and their synergistic effects40. While the flavor and taste of fruits can be altered by thermal treatments, in this study, due to changes in acidity and soluble solids being independent of the treatment type, the sensory evaluation of fruits was influenced by the prolonged storage period under the impact of the carnauba wax treatment. These findings are consistent with those reported by Njombolwana et al.43.
Fruits coated with carnauba wax exhibited elevated levels of total sugar content throughout storage (Fig. 3). This increase in total sugar content may be attributed to reduced consumption of specific organic acids and their conversion to carbohydrates in these fruits during storage, likely due to delayed ripening progression44.
Titratable acidity (TA) stands out as one of the most crucial internal quality parameters in citrus fruits, naturally undergoing changes during the stages of ripening and storage45. The reduction in TA during fruit ripening is a consequence of the organic acids transforming into carbohydrates. However, fruit coatings prove effective in curbing the respiratory process, thus decelerating the conversion of organic acids. This is due to the fact that organic acids serve as a substrate for respiratory pathways46. As a result, these coatings demonstrate efficacy in preserving citrus TA over a 40-day storage period, in agreement with previous studies47,48. The highest fruit acidity levels in oranges were observed in fruits treated with a 1.5% carnauba wax concentration, after 80 days of storage, also lowest total acidity (TA) was recorded in fruits treated with carnauba wax (0.5%) (Fig. 7). Biolatto et al.49 found that TA levels remained relatively stable during the initial stages of storage but declined towards the end of the storage period in grapefruit. This reduction in TA during storage can be attributed to the utilization of organic acids in respiration pathways as an energy source for various metabolic processes50. Previous studies have also documented a decline in the quality parameters of harvested fruits during storage periods51–53 reported a decrease in TA in citrus fruits during extended storage.
In the case of Moro oranges, vitamin C concentration initially decreased, then increased after 40 days of storage, and subsequently decreased again at the end of the 80-day storage period. In this study, the increase in vitamin C content may be attributed to synthesis or moisture loss. Similarly, García-Betanzos et al.54 reported the influence of storage duration and temperature on vitamin C levels in fruits. The decline in vitamin C content during storage has been observed in various fruits, including strawberries 55, oranges56, and pears57, and is closely linked to the fruit variety.
The decline in fruit quality, manifested as surface wrinkling and diminished juiciness, is primarily attributed to fruit moisture loss, resulting in weight loss58. Fruit weight loss is subject to the influence of various storage conditions in cold storage facilities, including factors such as temperature, humidity, packaging methods, and pretreatment techniques59. Each of these factors can have a positive, negative, or neutral effect on the weight loss process. These results are in agreement with the observations of Strano et al.60, who reported that storage duration plays a crucial role in citrus fruit weight reduction, which is primarily due to moisture loss. In addition, Motamedi et al.61 reported significant differences in fruit weight reduction during storage among different citrus species. Their research found that these differences were related to the varying thickness of postharvest coatings or protective coverings on the fruit surface, such as the natural waxes on the cuticle, which act as a barrier to water loss. Previous studies have indicated that applying carnauba wax to fresh eggplants62 and Mandarin fruits63 contributes to mitigating weight loss in the treated or coated fruits.
As the storage duration increased, there was a decline in fruit peel firmness. This aligns with the findings of Meighani et al.64. Our findings suggest that carnauba wax treatment can play a role in preserving the internal tissue firmness of orange fruits. Fruit firmness is a critical factor for determining both fruit freshness and consumer acceptance. While the firmness of both coated and uncoated fruits experiences a decline during storage, particularly over the 80-day duration, the coated fruits exhibit higher firmness retention compared to the control samples or untreated fruits. This firmness preservation is likely attributable to the reduction in enzymatic degradation facilitated by the coating, which restricts oxygen availability. The reduction in fruit firmness is also associated with the loss of tissue moisture and turgor pressure65. The retention of firmness in coated fruits can be attributed to a decrease in microbial decay, a slower respiration rate, and a modified fruit shape. This has been observed in fruits such as wax-coated plums66, guavas67, bananas68, and tomatoes69.
The authors showed that untreated batches stored for 40 and 80 days exhibited decay symptoms. In contrast, oranges treated with the coating maintained their firmness and had a significantly lower weight loss of 27% compared to untreated samples. Carnauba wax, extracted from the leaves of the Copernicia prunifera palm and often dubbed the “queen of waxes”41, plays a crucial role in the post-harvest citrus fruit industry. The wax acts as a protective shield for oranges throughout the storage period. The application of carnauba wax on Valencia oranges and grapefruits has proven effective in reducing fruit decay70. The use of carnauba wax, in combination with beeswax, has been shown to prolong the shelf life of oranges during storage by mitigating respiration71. Employing carnauba wax during extended storage periods, especially at lower temperatures, limited respiration, reduced moisture loss, and maintained the fruit’s firmness32.
A number of studies have also reported significant reductions in decay when wax-based coatings were applied to various fruits. For example, the application of carnauba wax coating led to a 4% reduction in brown rot and a 9% reduction in rhizopus rot72. Moreover, the decay of Zizyphus mauritiana fruits was reduced with the application of carnauba wax coating73. The coating with carnauba wax, due to its strong antimicrobial properties, led to a significant reduction in the decay rate of the coated fruit.
When compared to untreated oranges, those with the 1% carnauba wax coating exhibited a reduced level of MDA during storage. Moreover, this coating contributed to an increase in total sugars, while the total acid levels remained unchanged at the 1% concentration. As a result, the application of carnauba wax coating emerges as a potentially effective strategy for prolonging the shelf life of oranges.
Carnauba treatment, storage duration, and their interaction significantly affected this parameter. As the storage period extended to 40 days, there was an increase in fruit anthocyanin levels. These findings resonate with the research of Paolo et al.74, who noted a similar uptick in anthocyanin levels in blood oranges during cold storage. The synthesis of anthocyanins during postharvest cold storage varies depending on the orange variety, with some displaying higher levels than others40. The synthesis of anthocyanins in blood oranges after harvest is intricately linked to the activity of enzymes like phenylalanine ammonia-lyase (PAL), which experiences an upsurge during storage75.
The reduced antioxidant capacity after 80 days of storage may be attributed to limited anthocyanin synthesis. Previous studies have consistently reported reductions in phenolic compounds76, antioxidant respose77, and vitamin C78 levels in oranges, mandarins, and grapefruits during storage. Similarly, prolonged storage of citrus fruits has been associated with decreased phenolic compounds and vitamin C levels. Tavarini et al.79 noted that the antioxidant activity of kiwifruit was highest at harvest but decreased during extended cold storage. Candir et al.80 reported a decrease in antioxidant capacity during pomegranate storage. The initial rise in antioxidant activity during the storage period in citrus fruits was also documented by Supapvanich et al.81. Policegoudra et al.82 observed that the antioxidant capacity of mangoes remained stable initially but declined with prolonged storage. Asanda et al.56 highlighted an increase in antioxidant activity during the cold storage of oranges in all varieties, primarily attributed to phenolic compounds and vitamin C, which presents an interesting contrast to the findings of our study.
Correlation coefficients are beneficial for measuring the interplay between two parameters and illuminating the extent and direction of their relationship. In the field of breeding, their application is of great importance83. The correlation coefficient showed a simultaneous significant increase in antioxidant activity 40 and 80 days after storage, which was accompanied by an increase in total phenol content. Similarly, both chroma and hue showed a significant increase associated with the increased skin radiance. These results highlight that most parameters were strongly positively correlated, meaning that improvements in one trait can positively and beneficially affect the others. Moreover, a significant and positive correlation between antioxidant activity and MDA levels in different citrus varieties has already been documented in the literature84–86. Cluster analysis serves as a statistical technique, for grouping the traits or the differentiation of various populations based on a variety of characteristics87–89. In our experiment, fruit weight reduction, formed individual clusters (Fig. 15).
Conclusions
In this study, edible carnauba wax was used to extend the shelf life of orange fruit. While wax coating increased the visual appeal of fruit, its impact on nutritional value during postharvest storage should not be overlooked. Carnauba wax is a practical choice due to its cost effectiveness and domestic production. Anthocyanin content in the fruit increased regardless of storage time. The naturally strong antibacterial properties of carnauba wax helped to maintain the weight, TA, and firmness of the coated or treated fruits over a storage period of 40 days. Under storage conditions, the carnauba wax coating proved to be highly effective in minimizing fruit decay and extended the shelf life of the fruits by up to 80 days at concentrations of 1% and 0.5%. The results of this study suggest that active wax-based coatings are a practical and sustainable alternative for preserving and extending the shelf life of citrus fruit during conventional cold storage.
Author contributions
A.S.S. supervised the research. M.B. and B.J. performed the experiment and collected the data. M.H. advised the research and revised the manuscript critically. All authors read and approved the final manuscript. All authors have agreed to submit the manuscript in its current form for consideration and possible publication in “Scientific reports”.
Data availability
All the data generated/ analyzed during the study are available with the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Salehi Sardoei, Email: alisalehisardoei@gau.ac.ir.
Mehrnaz Hatami, Email: m-hatami@araku.ac.ir.
References
- 1.Food and Agricultural Organization of the Iran. 2020. Crops—Extent, causes and prevention. 20 Jan. 2020. http://www.fao.org/faostat/en/#data/QC
- 2.Puttongsiri T, Haruenkit R. Changes in ascorbic acid, total polyphenol, phenolic acids and antioxidant activity in juice extracted from coated Kiew Wan tangerine during storage at 4, 12 and 20 °C. Agric. Nat. Resour. 2010;44:280–289. [Google Scholar]
- 3.Obenland D, Collin S, Mackey B, Sievert J, Arpaia ML. Storage temperature and time influences sensory quality of mandarins by altering soluble solids, acidity and aroma volatile composition. Postharvest Biol. Technol. 2011;59:187–193. doi: 10.1016/j.postharvbio.2010.09.011. [DOI] [Google Scholar]
- 4.Phothisuwan S, Koomhin P, Matan N, Matan N. Quality maintenance of salacca fruit with a carnauba wax coating containing orange oil and detection of sensory perception improvement with electroencephalography to appraise brain responses. LWT. 2021;147:111628. doi: 10.1016/j.lwt.2021.111628. [DOI] [Google Scholar]
- 5.Nazoori F, ZamaniBahramabadi E, Rafie A, Mirdehghan SH. Combined application of gamma-aminobutyric acid and carnauba wax as edible coating on pomegranates in cold storage. J. Agric. Sci. Technol. 2022;24(3):591–602. [Google Scholar]
- 6.Marcilla A, Martinez M, Carot JM, Palou L, Del Rio MA. Relationship between sensory and physicochemical quality parameters of cold-stored ‘Clemenules’ mandarins coated with two commercial waxes. Span. J. Agric. Res. 2009;7(1):181–189. doi: 10.5424/sjar/2009071-410. [DOI] [Google Scholar]
- 7.Arena E, Fallico B, Maccarine E. Evaluation of antioxidant capacity of blood orange juices as influenced by constituent’s concentration process and storage. Food Chem. 2001;74:423–427. doi: 10.1016/S0308-8146(01)00125-X. [DOI] [Google Scholar]
- 8.Miranda M, Sun X, Ference C, Plotto A, et al. Nano- and micro- carnauba wax emulsions versus shellac protective coatings on postharvest citrus quality. J. Am. Soc. Hortic. Sci. 2020 doi: 10.21273/JASHS04972-20. [DOI] [Google Scholar]
- 9.Shaabanian Z, Fattahi Moghadam J, Alavi SA. Investigation of quality preservation of Thomson navel orange (Citrus sinensis cv. Thomson Navel) using of coating treatments in common storage. Iran. Food Sci. Technol. Res. J. 2015;11:458–472. [Google Scholar]
- 10.Klimezak I, Malecka M. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2006;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- 11.Nayak SL, Sethi S, Shaarma RR, Prajapati U. Active edible coatings for fresh fruits and vegetables. In: Gutiérrez TJ, editor. Polymers for agri-food applications. Springer; 2019. pp. 417–432. [Google Scholar]
- 12.Palou L, Valencia-Chamorro SA, Pérez-Gago MB. Antifungal edible coatings for fresh citrus fruit: A review. Coatings. 2015;5:962–986. doi: 10.3390/coatings5040962. [DOI] [Google Scholar]
- 13.Kassim A, Workneh TS, Laing MD. A review of the postharvest characteristics and pre-packaging treatments of citrus fruit. Agric. Food. 2020;5:337–364. doi: 10.3934/agrfood.2020.3.337. [DOI] [Google Scholar]
- 14.LoScalzo R, Innoccari T, Summa C, Morelli R, Rapisarda P. effect of thermal treatment on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004;85:41–47. doi: 10.1016/j.foodchem.2003.05.005. [DOI] [Google Scholar]
- 15.Baltazari A, Mtui HD, Mwatawala MW, Chove LM, Msogoya T, Samwel J, Subramanian J. Effects of storage conditions, storage duration and post-harvest treatments on nutritional and sensory quality of orange (Citrus sinensis L. Osbeck) fruits. Int. J. Fruit Sci. 2019;20:732–749. doi: 10.1080/15538362.2019.1673278. [DOI] [Google Scholar]
- 16.Manzano JE, Diaz A. Effect of storage time, temperature and wax coating on the quality of fruits of ‘Valencia’ orange (Citrus sinensis L.) Int. Soc. Hortic. Sci. Leuven. 2011;44:24–29. [Google Scholar]
- 17.Basumatary IB, Mukherjee A, Katiyar V, Dutta J, Kumar S. Chitosan-based active coating for pineapple preservation: Evaluation of antimicrobial efficacy and shelf-life extension. LWT. 2022;168(1):113940. doi: 10.1016/j.lwt.2022.113940. [DOI] [Google Scholar]
- 18.Sullivan, C. Y. & Ross, W. M. Selecting for drought and heat resistance in grain sorghum. In Stress Physiology in Crop Plants 263–281 (1979).
- 19.Campbell CL, Madden LV. Introduction to Plant Disease Epidemiology. John Wiley & Sons; 1990. [Google Scholar]
- 20.Kashyap G, Gautam MD. Analysis of vitamin C in commercial and naturals substances by iodometric titration found in Nimar and Malwaregeion. J. Sci. Res. Pharm. 2012;1(2):77–78. [Google Scholar]
- 21.Moradi M, Tajik H, Razavi Rohani SM, Oromiehie AR, Malekinejad H, Aliakbarlu J, Hadian M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT Food Sci. Technol. 2012;46(2):477–484. doi: 10.1016/j.lwt.2011.11.020. [DOI] [Google Scholar]
- 22.McCready RM, Guggolz J, Silviera V, Owens HS. Determination of starch and amylose in vegetables. Anal. Chem. 1950;22:1156–1158. doi: 10.1021/ac60045a016. [DOI] [Google Scholar]
- 23.Nourozi F, Sayyari M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020;262(27):109041. doi: 10.1016/j.scienta.2019.109041. [DOI] [Google Scholar]
- 24.Wagner GJ. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids and anthocyanin in protoplasts. Plant Physiol. 1979;64:88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos P, Quartin V, Ramalho JC, Nunes MA. Electrolyte leakage and lipid degradation account for cold sensitivity inleaves of Coffea sp. Plants J. Plant Physiol. 2003;160:238–292. doi: 10.1078/0176-1617-00833. [DOI] [PubMed] [Google Scholar]
- 26.Susmita Devi L, Mukherjee A, Dutta D, Kumar S. Carnauba wax-based sustainable coatings for prolonging postharvest shelf-life of citrus fruits. Sustain. Food Technol. 2023;1:415–425. doi: 10.1039/d2b00049k. [DOI] [Google Scholar]
- 27.Müller MG, Lindner JA, Briesen H, et al. On the properties and application of beeswax, carnauba wax and palm fat mixtures for hot melt coating in fluidized beds. Adv. Powder Technol. 2018;29:781–788. doi: 10.1016/j.apt.2017.12.020. [DOI] [Google Scholar]
- 28.Celik N, Kiremitler NB, Ruzi M, Onses MS. Waxing the soot: Practical fabrication of all-organic superhydrophobic coatings from candle soot and carnauba wax. Prog. Org. Coat. 2021;153:106169. doi: 10.1016/j.porgcoat.2021.106169. [DOI] [Google Scholar]
- 29.Romani VP, Olsen B, Pinto Collares M, et al. Cold plasma and carnauba wax as strategies to produce improved bi-layer films for sustainable food packaging. Food Hydrocoll. 2020;108:106087. doi: 10.1016/j.foodhyd.2020.106087. [DOI] [Google Scholar]
- 30.Fei T, Leyva-Gutierrez FMA, Wan Z, Wang T. Development of a novel soy-wax containing emulsion with enhanced antifungal properties for the postharvest treatment of fresh citrus fruit. LWT Food Sci. Technol. 2021;141:110878. doi: 10.1016/j.lwt.2021.110878. [DOI] [Google Scholar]
- 31.Germano TA, Aguiar RP, Bastos MSR, Moreira RA, Ayala-Zavala JF, de Miranda MRA. Galactomannan-carnauba wax coating improves the antioxidant status and reduces chilling injury of ‘Paluma’ guava. Postharvest Biol. Technol. 2019;149:9–17. doi: 10.1016/j.postharvbio.2018.11.013. [DOI] [Google Scholar]
- 32.Nasirifar SZ, Maghsoudlou Y, Oliyaei N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci. Nutr. 2018;6(6):1508–1518. doi: 10.1002/fsn3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barragán-Iglesias J, Méndez-Lagunas LL, Rodríguez-Ramírez J. Ripeness indexes and physicochemical changes of papaya (Carica papaya L. cv. Maradol) during ripening on-tree. Sci. Hortic. 2018;236:272–278. doi: 10.1016/j.scienta.2017.12.012. [DOI] [Google Scholar]
- 34.Li-ying N, Ji-hong WU, Xiao-jun L, Fang C, Zheng-fu W, Guang-hua Z, Xiaosong H. Physicochemical characteristics of orange juice samples from seven cultivars. Agric. Sci. China. 2008;7(1):41–47. doi: 10.1016/S1671-2927(08)60020-6. [DOI] [Google Scholar]
- 35.Braga SP, Lundgren GA, Macedo SA, Tavares JF, dos Santos Vieira WA, Câmara MPS, de Souza EL. Application of coatings formed by chitosan and Mentha essential oils to control anthracnose caused by Colletotrichum gloesporioides and C. brevisporum in papaya (Carica papaya L.) fruit. Int. J. Biol. Macromol. 2019;139:631–639. doi: 10.1016/j.ijbiomac.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Jing G, Li T, Qu H, Yun Z, Jia Y, Zheng X, Jiang Y. Carotenoids and volatile profiles of yellow-and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biol. Technol. 2015;109:114–119. doi: 10.1016/j.postharvbio.2015.06.006. [DOI] [Google Scholar]
- 37.Miranda M, Sun X, Marín A, Dos Santos LC, Plotto A, Bai J, Baldwin E. Nano-and micro-sized carnauba wax emulsionsbased coatings incorporated with ginger essential oil and hydroxypropyl methylcellulose on papaya: Preservation of quality and delay of post-harvest fruit decay. Food Chem. X. 2022;13:100249. doi: 10.1016/j.fochx.2022.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Saini CS. Edible composite bi-layer coating based on whey protein isolate, xanthan gum and clove oil for prolonging shelf life of tomatoes. Meas. Food. 2021;2:100005. doi: 10.1016/j.meafoo.2021.100005. [DOI] [Google Scholar]
- 39.Tietel Z, Bar E, Lewinsohn E, Feldmesser E, Fallik E, Porat R. Effects of wax coatings and postharvest storage on sensory quality and aroma volatile composition of ‘Mor’ mandarins. J. Sci. Food Agric. 2010;90(6):995–1007. doi: 10.1002/jsfa.3909. [DOI] [PubMed] [Google Scholar]
- 40.Shahid MN, Abbasi NA. Effect of bee wax coatings on physiological changes in fruits of sweet orange CV “blood red”. Sarhad J. Agric. 2011;27:385–394. [Google Scholar]
- 41.Taghizadeh S, Sadeghi H, Hadadinejad M. Evaluation of Thamson–Navel orange young trees resistance to freezing, using carnauba wax and glycol potash. J. Hortic. Sci. 2020;34(1):33–44. doi: 10.22067/jhorts4.v34i1.78660. [DOI] [Google Scholar]
- 42.Tatari M, Bahrami HR, Mohammadpour I. The effect of different treatments on the storage life characteristics of ‘Siaverz’ local orange (Citrus sinensis L.) in storage conditions. J. Hortic. Postharvest Res. 2022;5(1):93–104. doi: 10.22077/jhpr.2022.4330.1210. [DOI] [Google Scholar]
- 43.Njombolwana NS, Erasmus A, van Zyl JG, du Plooy W, Cronje PJ, Fourie PH. Effects of citrus wax coating and brush type on imazalil residue loading, green mould control and fruit quality retention of sweet oranges. Postharvest Biol. Technol. 2013;86:362–371. doi: 10.1016/j.postharvbio.2013.07.017. [DOI] [Google Scholar]
- 44.Kelebek H, Selli S, Gubbuk H, Gunes E. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem. 2015;173:912–919. doi: 10.1016/j.foodchem.2014.10.116. [DOI] [PubMed] [Google Scholar]
- 45.Md Nor S, Ding P. Trends and advances in edible biopolymer coating for tropical fruit: a review. Food Res. Int. 2020;134:109208. doi: 10.1016/j.foodres.2020.109208. [DOI] [PubMed] [Google Scholar]
- 46.Lufu R, Ambaw A, Opara UL. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020;272:109519. doi: 10.1016/j.scienta.2020.109519. [DOI] [Google Scholar]
- 47.Álvarez-Barreto JF, Cevallos-Ureña A, Zurita J, Pérez J, León M, Ramírez-Cárdenas L. Edible coatings of aloe vera gel and carnauba wax microparticles to increase strawberry (Fragaria ananassa) shelf life. Int. J. Fruit Sci. 2023;23(1):181–199. doi: 10.1080/15538362.2023.2180129. [DOI] [Google Scholar]
- 48.Lin X, Zhang H, Guo X, Qin Y, Shen P, Peng Q. A novel sodium alginate–carnauba wax film containing calcium ascorbate: Structural properties and preservative effect on fresh-cut apples. Molecules. 2023;28:367. doi: 10.3390/molecules28010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biolatto A, Vazquez DE, Sancho AM, Carduza FJ, Pensel NA. Effect of commercial conditioning and cold quarantine storage treatments on fruit of ‘Rouge La Toma’ grapefruit (Citrus paradise Macf.) Postharvest Biol. Technol. 2005;35:167–176. doi: 10.1016/j.postharvbio.2004.08.002. [DOI] [Google Scholar]
- 50.Magashi AM, Bukar A. Antibacterial and antifungal effect of high pH and paraffin wax application on tomatoes, oranges and peppers. Afr. J. Biol. 2007;3:126–128. [Google Scholar]
- 51.Khedr EH. Application of different coating treatments to enhance storability and fruit quality of pomegranate (Punica granatum L., cv. Wonderful) during prolonged storage. Rev. Bras. Frutic. Jaboticabal. 2022;44(2):1–13. [Google Scholar]
- 52.Hassan ZH, Lesmayati S, Qomariah R, Hasbianto A. Effects of wax coating applications and storage temperatures on the quality of tangerine citrus (Citrus reticulata) var, Siam Banjar. Int. Food Res. J. 2014;21(2):641–648. [Google Scholar]
- 53.Khedr EH. Does the quality of valencia orange vary in response to different coatings during cold storage? Egypt. J. Hortic. 2017;44:219–233. doi: 10.21608/ejoh.2018.2220.1031. [DOI] [Google Scholar]
- 54.García-Betanzos CI, Hernández-Sánchez H, Bernal-Couoh TF, Quintanar-Guerrero D, de la Zambrano-Zaragoza ML. Physicochemical, total phenols and pectin methylesterase changes on quality maintenance on guava fruit (Psidium guajava L.) coated with candeuba wax solid lipid nanoparticles-xanthan gum. Food Res. Int. 2017;101:218–227. doi: 10.1016/j.foodres.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 55.Dhital R, Mora NB, Watson DG, Kohli P, Choudhary R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. Lebensm. Wiss. Technol. 2018;97:124–134. doi: 10.1016/j.lwt.2018.06.038. [DOI] [Google Scholar]
- 56.Mditshwa A, Magwaza LS, Tesfay SZ, Opara UL. Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci. Hortic. 2017;218:95–104. doi: 10.1016/j.scienta.2017.02.024. [DOI] [Google Scholar]
- 57.Adhikary T, Gill PPS, Jawandha SK, Kaur N. Postharvest quality response of pears with beeswax coatings during long term cold storage. J. Hortic. Sci. Biotechnol. 2022;97(6):785–798. doi: 10.1080/14620316.2022.2074321. [DOI] [Google Scholar]
- 58.Wang D, Huang J, Guo Z, Liu W. Durable mixed edible wax coating with stretching superhydrophobicity. J. Mater. Chem. A. 2021;9:1495–1499. doi: 10.1039/D0TA10638K. [DOI] [Google Scholar]
- 59.Singh S, Khemariya P, Rai A, Rai AC, Koley TK, Singh B. Carnauba wax-based edible coating enhances shelf-life and retain quality of eggplant (Solanum melongena) fruits. LWT. 2016;74:420–426. doi: 10.1016/j.lwt.2016.08.004. [DOI] [Google Scholar]
- 60.Strano MC, Altieri G, Allegra M, Di Renzo GC, Paterna G, Matera A, Genovese F. Postharvest technologies of fresh citrus fruit: Advances and recent developments for the loss reduction during handling and storage. Horticulturae. 2022;8(7):612. doi: 10.3390/horticulturae8070612. [DOI] [Google Scholar]
- 61.Motamedi E, Nasiri J, Malidarreh TR, Kalantari S, Naghavi MR, Safari M. Performance of carnauba wax-nanoclay emulsion coatings on postharvest quality of ‘Valencia’ orange fruit. Sci. Hortic. 2018;240:170–178. doi: 10.1016/j.scienta.2018.06.002. [DOI] [Google Scholar]
- 62.Eyng C, Nunes KC, Matumoto-Pintro PT, et al. Carnauba wax coating preserves the internal quality of commercial eggs during storage. Semin. Cienc. Agrar. 2021;42:1229–1244. doi: 10.5433/1679-0359.2021v42n3p1229. [DOI] [Google Scholar]
- 63.Won MY, Min SC. Coating Satsuma mandarin using grapefruit seed extract–incorporated carnauba wax for its preservation. Food Sci. Biotechnol. 2018;27:1649–1658. doi: 10.1007/s10068-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meighani H, Ghasemnezhad M, Bakhshi D. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2014;52(7):4507–4514. doi: 10.1007/s13197-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumoto A, Ono A, Murao S, Murakami M. Microparticles for sustained release of water-soluble drug based on a containment, dry coating technology. Drug Discov. Ther. 2018;12:347–354. doi: 10.5582/ddt.2018.01082. [DOI] [PubMed] [Google Scholar]
- 66.Tavassoli-Kafrani E, Gamage MV, Dumée LF, Kong L, Zhao S. Edible films and coatings for shelf life extension of mango: A review. Crit. Rev. Food Sci. Nutr. 2020;62(9):2432–2459. doi: 10.1080/10408398.2020.1853038. [DOI] [PubMed] [Google Scholar]
- 67.Murmu SB, Mishra HN. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018;245:820–828. doi: 10.1016/j.foodchem.2017.11.104. [DOI] [PubMed] [Google Scholar]
- 68.Odetayo T, Sithole L, Shez S, Nomngongo P, Tesfay S, Ngobese NZ. Effect of nanoparticle-enriched coatings on the shelf life of Cavendish bananas. Sci. Hortic. 2022;2022:111312. doi: 10.1016/j.scienta.2022.111312. [DOI] [Google Scholar]
- 69.López-Palestina CU, Aguirre-Mancilla CL, Raya-Pérez JC, Ramírez-Pimentel JG, Gutiérrez-Tlahque J, Hernández-Fuentes AD. The effect of an edible coating with tomato oily extract on the physicochemical and antioxidant properties of garambullo (Myrtillocactus geometrizans) fruits. Agronomy. 2018;8(11):248. doi: 10.3390/agronomy8110248. [DOI] [Google Scholar]
- 70.Sambav. An Integrated Approach to Peel Breakdown in Citrus. A Theses Presented to the Graduate School of University of Florida. Master of Science (2010).
- 71.Huyen, N. T. & Tanachai, P. Effects of mixed wax (beeswax and carnauba wax) on fruit quality and storage life of Vietnamese sweet orange'Canh'during low-temperature storage. In III Southeast Asia Symposium on Quality Management in Postharvest Systems 1179 77–86 (2015).
- 72.da Silva Andrade LB, da Silva Julião MS, Carneiro Vera Cruz R, et al. Antioxidant and antifungal activity of carnauba wax powder extracts. Ind. Crops Prod. 2018;125:220–227. doi: 10.1016/j.indcrop.2018.09.004. [DOI] [Google Scholar]
- 73.Adjouman YD, Nindjin C, Kouassi KN, Tetchi FA, N'Guessan GA, Sindic M. Effect of edible coating based on improved cassava starch on post-harvest quality of fresh tomatoes (Solanum lycopersicum L.) Int. J. Nutr. Sci. Food Technol. 2018;4(1):1–10. [Google Scholar]
- 74.Paolo R, Marisol LB. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes (Citrus sinensis (L.) Osbeck) Postharvest Biol. Technol. 2008;49:348–354. doi: 10.1016/j.postharvbio.2008.02.002. [DOI] [Google Scholar]
- 75.Klimczak I, Malecka M, Szlachta M, Gliszczynska-Swiglo A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- 76.Shi JX, Porat R, Goren R, Goldschmidt EE. Physiological responses of ‘Murcott’ mandarins and ‘Star Ruby’ grapefruit to anaerobic stress conditions and their relation to fruit taste, quality and emission of off-flavor volatiles. Postharvest Biol. Technol. 2005;38:99–105. doi: 10.1016/j.postharvbio.2005.06.008. [DOI] [Google Scholar]
- 77.Spreen, T. H., Barber, R. E., Brown, M. G., Hodges, A. W., Malugen, J. C., Mulkey, W. D., Muraro, R. P., Norberg, R. P., Rahmani, M., Roka, F. M. & Rouse, R. E. An economic assessment of the future prospects for the Florida citrus industry. In Presentation to the Special Industry Task Force, Florida Department of Citrus, Lakeland, FL (2006).
- 78.Shen Y, Yang H, Chen J, Liu D, Ye X. Effect of waxing and wrapping on phenolic content and antioxidant activity of citrus during storage. J. Food Process. Preserv. 2012;37(3):222–231. doi: 10.1111/j.1745-4549.2011.00639.x. [DOI] [Google Scholar]
- 79.Tavarini S, Remorini D, Massai R. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2007;107:282–288. doi: 10.1016/j.foodchem.2007.08.015. [DOI] [Google Scholar]
- 80.Candir E, Ozdemir AE, Aksoy MC. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018;235:235–243. doi: 10.1016/j.scienta.2018.03.017. [DOI] [Google Scholar]
- 81.Supapvanich, S., Megia, R. & Ding, P. Salak (Salacca zalacca (Gaertner) Voss). In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Mangosteen to White Sapote (ed. Yahia, E. M.) 334–352e (Elsevier, 2011). 10.1533/9780857092618.334
- 82.Policegoudra RS, Aradhya SM. Biochemical changes and antioxidant activity of mango ginger (Curcuma amada Roxb) rhizomes during postharvest storage at different temperatures. Postharvest Biol. Technol. 2007;46:189–194. doi: 10.1016/j.postharvbio.2007.04.012. [DOI] [Google Scholar]
- 83.Berry WD, Feldman S. Multiple Regression in Practice. Sage Publications; 1985. p. 96. [Google Scholar]
- 84.Abouzari A, Solouki M, Golein B, Fakheri B, Sabouri A. Frost stress tolerance in citrus genotypes under two subfreezing temperatures. Plant Prod. Technol. 2020;12(1):193–209. [Google Scholar]
- 85.Salehi Sardoei A, Sharifani M, Khoshhal Sarmast M, Ghasemnejhad M. Screening citrus cultivars for freezing tolerance by reliable methods. Int. J. Hortic. Sci. Technol. 2023;11(1):25–34. [Google Scholar]
- 86.Salehi Sardoei A, Sharifani M, Khoshhal Sarmast M, Ghasemnejhad M. Stepwise regression analysis of citrus genotype under cold stress. Gene Cell Tissue. 2022 doi: 10.5812/gct-126518. [DOI] [Google Scholar]
- 87.Hosseini SJ, Tahmasebi-sarvestani Z, Pirdashti H, Modarres Sanavi SAM, Mokhtassi-bidgoli A, Hazrati S. Study of diversity and estimation of leaf area in different mint ecotypes using artificial intelligence and regression models under salinity stress conditions. J. Crop Breed. 2019;11(32):59–73. doi: 10.29252/jcb.11.32.59. [DOI] [Google Scholar]
- 88.Simoes PHO, de Oliveira Neto CF, de Paula MT, et al. Ecophysiology and multivariate analysis for production of Tachigali vulgaris in Brazil: Influence of rainfall seasonality and fertilization. J. For. Res. 2023;34:1289–1305. doi: 10.1007/s11676-023-01611-8. [DOI] [Google Scholar]
- 89.Shahdadi F, Faryabi M, Khan H, Salehi Sardoei A, Fazeli-Nasab B, Goh BH, Goh KW, Tan CS. Evaluation of adding Mentha longifolia essential oil and pulegone in edible coatings based on alginate and chitosan on some pathogenic bacteria in lactic cheese. Molecules. 2023;28(11):4554. doi: 10.3390/molecules28114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated/ analyzed during the study are available with the corresponding author on reasonable request.