Summary
Olfactory-mediated behaviors in fish are often examined in artificial microcosms that enable well-controlled treatments but fail to replicate environmental and social contexts. However, observing these behaviors in nature poses challenges. Here, we describe a protocol for recording sea lamprey (Petromyzon marinus) behaviors in a natural system. We describe steps for administering and verifying accurate odorant concentrations, surveying sea lamprey abundance, and tracking sea lamprey movements. We also detail procedures to analyze treatment effects on pheromone-mediated spawning in a high-density population.
For complete details on the use and execution of this protocol, please refer to Scott et al.1
Subject areas: Behavior, Chemistry, Environmental sciences
Graphical abstract

Highlights
-
•
Protocol to survey sea lamprey abundance and monitor behaviors within a natural system
-
•
Procedure to track pheromone-mediated movements and spawning of tagged female sea lamprey
-
•
Approach to quantify and analyze treatment effects on pheromone-mediated spawning
-
•
Can be modified to test olfactory-mediated behaviors of fishes to other stimuli
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Olfactory-mediated behaviors in fish are often examined in artificial microcosms that enable well-controlled treatments but fail to replicate environmental and social contexts. However, observing these behaviors in nature poses challenges. Here, we describe a protocol for recording sea lamprey (Petromyzon marinus) behaviors in a natural system. We describe steps for administering and verifying accurate odorant concentrations, surveying sea lamprey abundance, and tracking sea lamprey movements. We also detail procedures to analyze treatment effects on pheromone-mediated spawning in a high-density population.
Before you begin
Protocol overview
Sea lamprey (Petromyzon marinus) are a jawless fish species that invaded the Laurentian Great Lakes and contributed to the collapse of native fish communities in the 1900s.2 Sea lamprey rely on conspecific odors to locate suitable spawning habitat and mates3 during their terminal reproductive phase. Considerable efforts have been made to characterize sea lamprey pheromone components and associated spawning behaviors.3,4 During the spring spawning period, spermiated males swim upstream, construct nests from rocks, and release a multi-component sex pheromone to attract mates. After spawning with multiple mates, sea lamprey perish in the early summer.5 Recent advances in the characterization of the sea lamprey chemical ecology have established the framework to develop control strategies that exploit the reliance on the sex pheromone to reduce reproduction.4,6 We developed a protocol that provides methods on how to survey free-ranging (non-released) sea lamprey abundance and monitor spawning behaviors of tagged females in a high-density natural population within a stream. The protocol has been used to quantify the disruption of pheromone-mediated attraction of ovulated females to nesting males during application of pheromone behavioral antagonist, which is a possible strategy to inhibit spawning of sea lamprey. This protocol can be readily adapted to investigate olfactory-mediated behavioral responses of fishes to other stimuli in natural systems.
Institutional permissions
This experiment requires approval from the Institutional Animal Use and Care Committee (IACUC). Fish care and experiments for this study were performed in accordance with the IACUC at Michigan State University, East Lansing, MI (Animal use forms: 03/12-063-00, 12/14-223-00, 03/11-053-00, 05/09-088-00, and 02/17-031-00). All experiments conform to the Committee’s regulatory standards. Before releasing tagged fishes or chemosensory cues into lakes or streams, contact your local natural resource and regulatory agencies to determine what permissions are required. In our case, the overall work plan was coordinated by the Great Lakes Fishery Commission (GLFC) and conducted in coordination with U.S. Fish and Wildlife Service (USFWS) and the U.S. Environmental Protection Agency (USEPA). All research activities should be carried out by trained personnel. Before you begin, acquire permissions from the relevant institutions and agencies.
Acquire, maintain, and mature female sea lamprey
Timing: 12–14 days before the start of trials and continued daily throughout the experiment to maintain consistent supply of ovulated females
This section describes the acquisition of pre-spawning adult sea lamprey and husbandry procedures used to house the sea lamprey at the laboratory and river. This section also describes the maturation of pre-ovulated to ovulated female sea lamprey that will be used in the experiment.
-
1.
Acquire pre-spawning phase adult female (pre-ovulated female) sea lamprey.
Note: We acquired pre-ovulated female sea lamprey from the USFWS– Marquette Biological Station (MBS; Marquette, Michigan) that captured sea lamprey in barrier-integrated traps on the Ocqueoc River (Presque Isle County, MI) and Cheboygan River (Cheboygan County, MI), two tributaries of Lake Huron, with scientific collection permits from the State of Michigan, USA. Sea lamprey were transported in oxygenated tanks to the U.S. Geological Survey, Great Lakes Science Center, Hammond Bay Biological Station (HBBS), Presque Isle County, MI. Both the MBS and HBBS are contracted agents of the GLFC.
-
2.
Maintain pre-spawning adult sea lamprey at HBBS separated by sex in different 1000 L flow-through tanks (flow rate: 1 L/min) supplied with aerated (dissolved oxygen > 8 mg/L), ambient water (7°C–20°C) pumped from Lake Huron (maximum stocking density: 0.4 sea lamprey/L).
Note: Pre-ovulated females have a softer, more distended abdomen than males. Pre-spermiated males have a ridge of tissue on the dorsal side that becomes more prominent as they mature. Pre-spawning adult sea lamprey have ceased feeding so it is not necessary to provide food.
Note: The tanks are in the laboratory with windows to maintain the light-dark period with natural light.
-
3.Mature the pre-ovulated female sea lamprey in rearing cages in a river.
-
a.Transfer 50 pre-ovulated female sea lamprey from the 1000 L flow-through tanks into rearing cages (1 m × 0.7 m × 0.7 m) constructed of polyurethane mesh and polyvinyl chloride (PVC) frame (0.5 m3) in a river (maximum stocking density: 20 sea lamprey/cage).Note: We held our rearing cages in the lower Ocqueoc River (Presque Isle County, MI) where water temperatures varied from 15°C to 24°C.
- b.
-
c.Remove ovulated females from the in-stream cages.
-
i.Transport them back to the laboratory in a 200 L aerated tank.
-
ii.Hold them in 200 L flow-through tanks supplied with aerated, ambient Lake Huron water until experimentation.
-
iii.Return females deemed pre-ovulated to the in-stream cages.
-
i.
-
d.Daily, replenish the rearing cages with pre-ovulated females to ensure there are 50 females maturing in the rearing cages. This will provide a continual supply of ovulated females for the duration of the experiment.
-
a.
Tag ovulated female sea lamprey
Timing: <90 s per sea lamprey tagged
This step details the attachment of an externally mounted light-emitting diode to the dorsal region and a streamer tag to the dorsal fin a female sea lamprey.
-
4.Tag 5–8 ovulated females per trial depending on sea lamprey availability (Figure 1).
-
a.Insert a light-emitting diode (LED) light (color options: red, yellow, white, green, or blue) into silicon tubing (inner diameter: 4.75 mm).Note: The LED should fit securely in the silicon tubing so that it does not fall out.
-
b.Externally mount the silicon tubing with the LED light to the mid-dorsal region of each female, orientated longitudinally.
-
i.Suture both ends to the dermis with non-absorbable monofilament suture material while another person holds the lamprey that attaches to a flat surface (i.e., lab bench top, plastic folding table, etc.) with its suction cup-like mouth.
-
ii.Cut the excess filament with scissors.Note: We used a modified surgeon’s suture knot that consisted of three triple-wrap throws. The suture material was wrapped around the hemostat three times, and the direction of the wraps was alternated between throws to secure the knot.
-
i.
-
c.Attach a streamer tag (color options: red, yellow, white, green, blue, or pink) to the dorsal fin to enable visual identification of each individual female.
 CRITICAL: Minimize the amount of time the female is out of water. Practice suture knot techniques on a silicon pad to increase efficiency before proceeding to tagging females for experiments (Figure 1).
CRITICAL: Minimize the amount of time the female is out of water. Practice suture knot techniques on a silicon pad to increase efficiency before proceeding to tagging females for experiments (Figure 1). -
d.After LED light and tag attachment, return the tagged ovulated female to the 200 L flow-through tanks supplied with aerated, ambient Lake Huron water.
-
i.Observe for at least 5 h to ensure the light and tag remain attached and the tagging had no adverse impacts (i.e., writhing in an effort to remove the tag).
-
i.
-
a.
Figure 1.
Tagged ovulated female sea lamprey (Petromyzon marinus)
Each ovulated female has a unique color combination of a light-emitting diode (LED) light (red, yellow, white, green, or blue) and a streamer tag (red, yellow, white, green, blue, or pink) to enable visual identification. The identifiers consist of an externally mounted silicon tubing with a LED light (red shown here) affixed to the mid-dorsal region by suturing both ends to the dermis and a streamer tag (yellow shown here) attached to the dorsal fin. Photo credit Andrea Miehls, Great Lakes Fishery Commission. Tagged ovulated sea lamprey, related to before you begin step 4.
Estimate total stream discharge
Timing: 2 h for initial setup to determine the amount of test article needed to activate total stream discharge; 30 min on subsequent days after the initial setup is completed
This section describes the steps to estimate the daily total stream discharge and determine the amount of test article needed to activate the stream to achieve the target concentration.
-
5.Measure stream discharge with a portable flow meter and staff gauge height to establish a total discharge and staff gauge height relationship (Figure 2).
-
a.For initial setup, select the site for stream discharge estimate.Note: Site selection and setup occurs on the first day and then the same site is used for the duration of the experiment. Select a site free of obstacles (i.e., large rocks, down trees, etc.). An ideal site to measure discharge is one where the stream channel is rectangular in shape with relatively consistent depth and velocity across the channel. Avoid areas with deep holes (slow water), back eddies, or bends in the stream.Note: We conducted our experiments at a field site on Carp Lake Outlet, a tributary of Lake Michigan, in Emmet County, MI. The site we selected for stream discharge estimate was located 135 m downstream of the sea lamprey barrier (45° 44′43.0″ N 84° 49′45.0″ W).
-
b.Anchor a discharge string.
-
i.Anchor a string on the stream bank.
-
ii.Walk across the stream at a right angle to the current.
-
iii.Anchor the string to the opposite bank such that the string is taut above the water surface and perpendicular to the flow direction.
-
i.
-
c.Measure and section the string that stretches the stream width.
-
i.Divide the stream width into 10 evenly spaced sections.
-
ii.Add flagging tape to denote these sections on the string (e.g., if the stream width is 10 m, each section width is 1 m. Flagging tape is added at 0.5, 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, 7.5, 8.5, and 9.5 m from the bank.)
-
i.
-
d.Measure the water depth and velocity at 60% of the water depth at each of the 10 sections denoted by the flagging tape marks using a portable flow meter while holding the wading rod vertically with the meter positioned parallel to the direction of flow.Note: To check the calibration of the flow meter, place the flow meter in a 19-L bucket of water and ensure the velocity reading is 0.00 m/s. If it does not read 0.00 m/s, calibrate the flow meter following the manufacturer's instructions.Note: Stand 0.5 m to the side and 0.5 m downstream of the wading rod to avoid influencing the velocity measurement.
-
e.Calculate each section discharge by multiplying the section width, depth, and velocity.
-
f.Calculate the total stream discharge by summing the discharges of the 10 sections measured.
-
g.If there is an established stream-specific relationship between total discharge and staff gauge height, subsequently estimate total stream discharge by recording staff gauge height instead of directly measuring with a portable flow meter.
-
h.Use the total stream discharge, length of trial, molecular weight of the test article, and the percent of inert components of the test article, specific to the lot number, to calculate the amount of test articles needed to achieve the target in stream concentration.Note: In 2019, we applied either vehicle (water/methanol, 1:1, v:v) or a mixture of 6.21 × 10–12 M petromyzonol sulfate (PZS) and 6.21 × 10–12 M petromyzonol tetrasulfate (3sPZS) to the river. In 2020, we applied either vehicle (water/methanol, 1:1, v:v) or a mixture of 6.21 × 10–11 M PZS and 6.21 × 10–11 M 3sPZS to the river.Note: For example, if a trial is 3 h long (10800 s) and the stream discharge is 1 cubic meter per second (1000 L per second), the test article needs to activate 10800000 L of water over the duration of a trial. To achieve a target in stream concentration of 6.21 × 10–12 M PZS (molecular weight: 491.68 g/mol), you need 33.0 mg of the active ingredient PZS plus an additional 1.91 mg of PZS (accounting for 1.5% water and 4.3% other inert components according to the lot number we used) for a grand total of 34.91 mg of PZS ammonium salt. Measure 3.491 mL of the 10 mg/mL stock solution of PZS (prepared below in Materials and equipment).
-
a.
Figure 2.
Illustration of stream cross-section setup and measurements to estimate total stream discharge
The river width is measured and divided into 10 evenly spaced sections denoted with an “X.” The dashed line rectangles indicate the section area, which is calculated as the section width multiplied by the section depth. The section discharge is calculated as the section area multiplied by the section velocity measured at 60% of the water depth with a portable flow meter. The total stream discharge is calculated by summing the discharge of the 10 sections.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Petromyzonol sulfate (PZS) | Bridge Organics | CAS: 1271318-61-6 |
| Petromyzonol tetrasulfate (3sPZS) | Bridge Organics | N/A |
| 5-Deuterated 3-keto petromyzonol sulfate (2H5 3kPZS) internal standard | Bridge Organics | N/A |
| Methanol | Sigma-Aldrich | Cat#34860 |
| Deposited data | ||
| All data | This paper | Mendeley Data repository: http://doi.org/10.17632/k6h7vz6dvy.1 |
| All related code | This paper | Zenodo Data repository: https://doi.org/10.5281/zenodo.7412008 |
| Experimental models: Organisms/strains | ||
| Pre-ovulated female sea lamprey | U.S. Fish and Wildlife Service | N/A |
| Software and algorithms | ||
| R (version 4.1.0) | R Core Team | https://www.r-project.org/ |
| R package effects (version 4.2.2) | Fox and Weisberg8 | https://cran.r-project.org/web/packages/effects/index.html |
| R package brglm (version 0.7.2) | Kosmidis and Firth9 | https://cran.r-project.org/web/packages/brglm/index.html |
| R package lme4 (version 1.1.26) | Bates et al.10 | https://cran.r-project.org/web/packages/lme4/index.html |
| R package bbmle (version 1.0.20) | Bolker11 | https://cran.r-project.org/web/packages/bbmle/index.html |
| Other | ||
| Peristaltic pump | Cole-Parmer | Masterflex L/S; Cat#EW-07554-90 |
| Non-absorbable monofilament suture material | Ethilon | Cat#662G |
| LED light | Thill Nite Brite | N/A |
| Colored streamer tags | Hallprint | PST2S |
| Portable flow meter | Marsh-McBirney | Cat#Flo-Mate 2000 |
| Syringe filter, 25 mm diameter, 2 μm pore size | Whatman | Cat#WHA67832520 |
| Cartridge for solid-phase extraction, 6cc, 500 mg sorbent, 60 μm | Waters Corp. | Oasis MCX; Cat#186000776 |
| Freeze dryer | Labconco | Cat#7310021 |
| Triple quadrupole mass spectrometer | Waters Corp. | Xevo TQ-S |
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Materials and equipment
Prepare test articles and internal standard stock solutions
Petromyzonol tetrasulfate (3sPZS) stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 3sPZS | 10 mg/mL | 1 g |
| Methanol | 50% | 50 mL |
| Deionized H2O | N/A | 50 mL |
| Total | N/A | 100 mL |
Storage conditions: −20°C, maximum storage time: 1 year.
CRITICAL: Methanol is flammable. Store the prepared solution in an explosion proof freezer that can safely store flammable materials. Keep away from heat, sparks, and open flames. Methanol is toxic if swallowed. Methanol can cause eye damage, skin irritation, and breathing difficulties if inhaled. To prevent exposure, wear protective gloves, protective clothing, and eye protection and use in a well-ventilated area.
Petromyzonol sulfate (PZS) stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| PZS | 10 mg/mL | 1 g |
| Methanol | 50% | 50 mL |
| Deionized H2O | N/A | 50 mL |
| Total | N/A | 100 mL |
Storage conditions: −20°C, maximum storage time: 1 year.
CRITICAL: See the hazards and precautions when handling methanol and the prepared solution with methanol/deionized water (1:1, v:v) stated above.
50% methanol vehicle stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Methanol | 50% | 50 mL |
| Deionized H2O | N/A | 50 mL |
| Total | N/A | 100 mL |
Storage conditions: −20°C, maximum storage time: 1 year
CRITICAL: See the hazards and precautions when handling methanol and the prepared solution with methanol/deionized water (1:1, v:v) stated above.
5-deuterated 3-keto petromyzonol sulfate (2H5 3kPZS) internal standard stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 2H5 3kPZS | 5 ng/mL | 5 μg |
| Methanol | 50% | 500 mL |
| Deionized H2O | N/A | 500 mL |
| Total | N/A | 1 L |
Storage conditions: Pipet 1 mL aliquots into vials; −20°C, maximum storage time: 1 year.
CRITICAL: See the hazards and precautions when handling methanol and the prepared solution with methanol/deionized water (1:1, v:v) stated above.
Step-by-step method details
Prepare field site for trials
Timing: 2 h
This step describes the transportation of supplies to the field site and the preparation of the peristaltic pump used to apply the test article or vehicle to the river.
-
1.Transport supplies to field site.
-
a.Transport two 1 mL aliquots of 2H5 3kPZS internal standard in a cooler on ice for river water sample collection.
-
b.Transport the necessary volume of test article needed to activate the stream to the target concentration given the daily total stream discharge or vehicle in a cooler on ice (calculated in estimate total stream discharge, before you begin step 5h).
-
c.Transport tagged female sea lamprey to field site in 250 L aerated tank and transfer females to release cage in the river upon arrival to ensure acclimation to the stream conditions.
-
i.Push the LED to make contact with the associated battery assembly to illuminate the LED lights as the females are added to the release cage.
-
i.
-
a.
Note: Acclimate females in release cage for at least 2 h (2000 h–2200 h) prior to release.
-
2.Prepare peristaltic pump operation.
-
a.Install a charged 12 V deep cycle battery and connect battery inverter cables from the battery to the peristaltic pump to power the pump.Alternatives: A deep cycle battery is recommended because it will provide more sustained power over a longer period; however, a 12 V deep cycle battery can be replaced with a 12 V traditional battery.
-
b.Turn on, prime, and calibrate the peristaltic pump at 21:00 h.
-
i.Dip and fill the 10 L graduated beaker into an area of the river with flowing water.Note: Minimize collecting water with sediment and debris to prevent it from clogging the peristaltic pump tubing.
-
ii.Place the intake tubing line into the 10 L beaker, turn on the pump, and prime the pump.Note: If the tubing is on correctly, the pump head will squeeze the tubing and force the liquid through as it rotates. Allow the pump to run until the air bubbles are purged from the tubing.
-
iii.Set the desired pump rate by adjusting the drive speed dial. The target pump rate is 1000 ± 25 mL/min.
-
iv.Calibrate the pump by observing how much water is pumping into a 1 L graduated cylinder for 1 min.Note: If the pump rate needs to be adjusted, change the drive speed dial and check the pump calibration again. Repeat until two consecutive calibrations fall within the target pump rate (1000 ± 25 mL/min).
-
v.Turn the calibrated pump off.
-
i.
-
c.Affix the pump outlet tubing to the perforated diffuser tubing in the river designed to mix the test article with the discharge quickly and thoroughly.Note: We positioned the diffuser tubing centered bank to bank 15 m upstream of the barrier because the turbulence created on the drop facilitates mixing the test article.
-
d.Rinse the treatment bins with at least 5 L river water.
-
i.Swirl the water such that it rinses the entire inside of the bin for at least 30 s and dump into the river downstream.
-
ii.Repeat 10 times to ensure the bin is well rinsed.
-
i.
-
e.Measure and add 90 L river water into each treatment bin (2 bins total) using a 10 L graduated beaker.
-
i.Dip and fill the 10 L graduated beaker into an area of the river with flowing water. Repeat 9 times per bin for a total of 90 L per bin.
-
i.
-
f.Add vial of measured test article or vehicle to each bin.
-
i.Put on latex gloves.
-
ii.Unscrew the capped vial and pour the test article or vehicle into the treatment bin.
-
iii.Rinse the vial three times with water in the bin and drop the vial and cap in the bin.
-
iv.Mix the treatment bin thoroughly.
-
v.Add the inlet tubing to the bin ensuring it is at the lowest point in the bin.
-
i.
-
a.
Conduct pre-trial survey of spawning grounds to count free-ranging (non-released) sea lamprey and locate sea lamprey spawning nests
Timing: 30 min
This step describes how to locate and identify free-ranging (non-released) sea lamprey and sea lamprey spawning nests while safely wading in the stream during the pre-trial survey of the spawning grounds.
-
3.Beginning at the release cage at 21:00 h, walk upstream towards the barrier while zig-zagging bank to bank to ensure the entire field site is surveyed by at least two researchers.Note: Wear a dim red headlamp (< 5 lumens) to aid visibility in low-light conditions while minimizing disturbance to sea lamprey.Note: Start the trials at dusk because sea lamprey spawning activity increases substantially at nightfall.5Note: Use caution when wading streams and wear a personal floatation device. Ensure researchers are well trained and aware of the river conditions. Avoid high, fast, or turbulent flow. If the substrate of the river is not visible, it is not feasible to survey for sea lamprey and nests.
-
a.Identify and record the location, number, and sex of free-ranging (non-released) sea lamprey and the location of sea lamprey nests in the survey area on a scaled map of the field site.
-
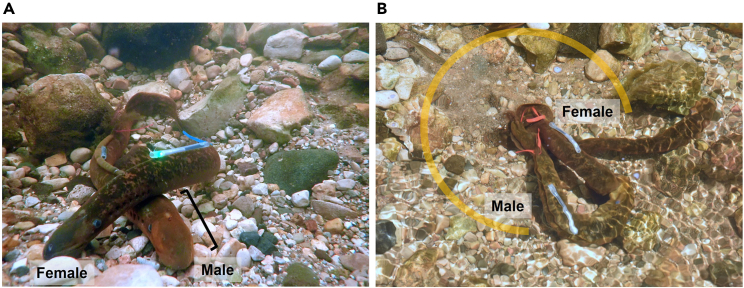
i.Spermiated males have a distinct ridge of tissue along the dorsal ridge in front of the dorsal fin and often have an enlarged head and gill region compared to the rest of the body (Figure 3A). Ovulated females have a soft, distended abdomen and more pronounced myomeres that appear as thin white vertical lines along the mid-section (Figure 3A).
-
ii.Nests are classified as a depression in the stream bed with a buildup of rocks on the back end of the circle (45 cm in width, 20 cm deep below substrate; Figure 3B).Note: Nests are constructed from gravel substrate (0.9–5.1 cm in diameter) in water with unidirectional flow (10–170 cm deep with velocities of 50–150 cm/sec).12,13 A few large stones typically remain at the upstream rim of the nest as oral disk attachment points during spawning. Nests are commonly observed at the upstream end of riffles, just downstream of pools.Note: Take caution to minimize possible disturbance of the sea lamprey or nests while conducting the survey.
-
i.
-
a.
Figure 3.
Spermiated male and ovulated female sea lamprey (Petromyzon marinus)
(A) The spermiated male (red streamer tag on dorsal fin) has a characteristic distinct ridge of tissue along the dorsal ridge in front of the dorsal fin and an enlarged head and gill region compared to the rest of the body (denoted with a black bracket). The ovulated female (green LED light in silicon tubing on mid-dorsal region and blue streamer tag on dorsal fin) has a soft, distended abdomen. Compared to males, females tend to have pronounced myomeres that appear as thin white vertical lines along the mid-section.
(B) A tagged spermiated male spawning with a tagged ovulated female on a nest. The male sea lamprey shown here is attached to female’s head with his tail wrapped around her tail. The male and female are vigorously vibrating their intertwined tails and simultaneously releasing gametes into the water. The nest (defined by yellow semi-circle) is a depression in the stream bed with a buildup of rocks on the back end. A free-ranging (non-released and untagged) ovulated female is also pictured in the upper right. Note: Tagged males and females were released into a stream during the daytime to photograph an example of spawning behavior. Males were not tagged in the described protocol. Photo credit Andrea Miehls, Great Lakes Fishery Commission. Spawning sea lamprey on nest, related to Steps 3 and 7.
Collect environmental metadata related to trial
Timing: 15 min
This step describes the procedures to collect and process the river water samples to estimate the concentration of lamprey pheromones in the samples. It also describes where to collect the additional abiotic stream data including water temperature, water staff gauge height, and water clarity.
-
4.Collect duplicate 100 mL water samples in the middle of the water column 5 m upstream of female release cage at 21:45 h to estimate the concentration of lamprey pheromones.Note: Wear latex gloves to reduce contamination during the water sample collection.
-
a.Label two 125 mL plastic bottles with the river name, date, time, and replicate number.
-
b.Rinse a 100 mL graduated cylinder and two labeled 125 mL plastic bottles ten times by filling each with river water and dumping it out at the designated collection site.
-
c.Rinse the closed internal standard vials by submerging them in the river water for 30 s at the designated collection site.
-
d.Measure 100 mL of river water from the middle of the water column and add it to the labeled bottle.
-
e.Spike each water sample with a 1 mL aliquot of 2H5 3kPZS internal standard by opening the vial and dropping the vial directly into the bottle.Note: Take care not to spill any of the 2H5 3kPZS internal standard as this would result in an inaccurate estimate of the 3kPZS concentration in the river water. Spiking the water sample with a known amount of 2H5 3kPZS allows you to accurately quantify the analyte of interest (e.g., 3kPZS). By taking the ratio of the analyte 3kPZS signal to the 2H5 3kPZS signal and plotting it against the 3kPZS concentration in calibration solutions, you can quantify the unknown concentration of 3kPZS in the river water sample in Step 4i.
-
f.Close the bottle containing the spiked river water sample and mix by inverting the bottle ten times.
-
g.Store on ice in a cooler until samples can be frozen at −20°C.
-
h.Freeze the spiked river water samples at −20°C as soon as possible until they are processed at a later date.Note: Filtering, extracting, and quantifying of the river water samples is often done after all behavioral trials have concluded allowing all samples to be processed at the same time. For accurate results, process samples within 6 months.14
-
i.Process river water samples to estimate the concentration of lamprey pheromones.
-
i.Thaw the frozen river water samples and filter with a syringe filter.
-
ii.Extract the water sample using an activated solid phase extraction cartridge by passing the water sample through the extraction system (filter and cartridge) at a flow rate of 10 mL/min.
-
iii.Wash the cartridge by passing 8 mL of deionized water through the extraction system.
-
iv.Dry the cartridge by pushing air through cartridge with a syringe for 1 min.
-
v.Elute the sample from the cartridge into a 15 mL tube with 8 mL of methanol at a flow rate of 4 mL/min.
-
vi.Freeze-dry the eluent, dissolve in 1 mL of methanol, and centrifuge (13,800 × g for 15 min at 4°C) in the laboratory.15
-
vii.Collect the supernatant, freeze-dry, and reconstitute in 100 μL methanol/ water (1:1, v/v).
-
viii.Quantify the background lamprey pheromone concentration in river water (3kPZS, PZS, 3,12-diketo-4,6-petromyzonene-24-sulfate (DkPES)) with liquid chromatography tandem mass spectrometry.15
-
i.
-
a.
-
5.Collect additional abiotic stream data that may influence sea lamprey behavior.
-
a.Measure and record the water temperature at the release cage.
-
b.Measure the water staff gauge height at the barrier.
-
c.Observe and record the water clarity (Good: very clear; Average: can see river bottom but not easily; Poor: cannot see river bottom) in the spawning grounds.
-
a.
Track movement and observe behavior of tagged females
Timing: 2.5 h
This step describes the introduction of the test article or vehicle to the river with the peristaltic pump followed by the release of the tagged females. This step outlines the procedures for tracking the movement and observing the behavior of the tagged females while checking on the pump operation to ensure continual application of the treatment at the target concentration.
-
6.After females acclimate in the release cage for 90 min, introduce the test article or vehicle treatment by turning on the calibrated pump at 21:30 h.
-
a.Apply the treatment for a 30-min period before releasing the tagged females to ensure the test article has reached a steady state with the stream flow and has arrived at the release cage.
-
a.
-
7.Release, track the movements, and observe the behaviors of the LED-tagged ovulated females with dim red headlamps for 2.5 h after release while continuing to apply the test articles (22:00 h- 00:30 h).
-
a.When a female exits the release cage, record the identity of the female (LED and streamer tag color combination), the time the female exits the cage, and the swim track movement on a scaled map of the field site with georeferenced landmarks (e.g., release cage, culvert, and barrier) to show the relative location of the fish’s position in the river based on visual observations.
-
i.Draw a solid line for upstream movement, dashed line for downstream movement, and arrowheads to denote directionality.
-
ii.Draw an “X” to denote if the female stops swimming.
-
iii.Record time stamps on swim tracks, especially when the female stops or passes landmarks on the map (i.e., large boulders, river access points, down trees, culverts, etc.).
-
i.
-
b.If the female enters a nest, record presence or absence of a male (e.g., male-occupied nest) and the behaviors of interest including enter nest, exit nest, and spawn (Table 1; Figure 3B) with corresponding time stamp.
-
i.Sea lamprey spawning follows a typical succession of behaviors that start when a female attaches to larger rock often at the upstream rim of the nest.
-
ii.A spermiated male glides along the female’s dorsal region, attaches to the female’s head, and wraps around the female.
-
iii.The male and female vigorously vibrate their intertwined tails and simultaneously release gametes into the water.
-
iv.Refer to Johnson et al. to see images of the typical succession of sea lamprey spawning behaviors.5
-
i.
-
c.Additional behaviors related to nest building or maintenance5 including rock movement, tail fan, etc. can be documented if those are of interest.
-
i.A rock movement is when a lamprey uses its oral disc to move a rock in the nest.
-
ii.A tail fan is when a lamprey rapidly moves its tail laterally to stir up and remove the sediment from its nest that is carried away with the water current.
-
i.
-
a.
-
8.Check peristaltic pump operation at the middle and end of trial.
-
a.Check the calibration of the peristaltic pump mid-trial and re-calibrate if needed.
-
b.Move the pump tubing line to the second bin of test article solution mid-trial at 23:00 h.
-
c.Check the peristaltic pump operation at the end of the trial at 00:30 h.
-
i.Measure and record the volume of any remaining behavioral antagonist solution with a 1 L graduated cylinder to determine the actual amount pumped into the river.
-
ii.Rinse the treatment bins with at least 5 L river water.
-
iii.Swirl the water such that it rinses the entire inside of the bin for at least 30 s and dump into the river downstream.
-
iv.Repeat 10 times to ensure the bin is well rinsed.
-
i.
-
a.
-
9.
At the end of the trial at 00:30 h, return to the release cage and record the LED and streamer tag color combination for alive females remaining in the release cage.
Table 1.
Ethogram used for behavioral observations
| Behavior | Description |
|---|---|
| Enter nest | Lamprey enters within the perimeter of the nest, which is classified as a depression in the stream bed with a buildup of rocks on the back end of the circle (45 cm in width, 20 cm deep below substrate). |
| Exit nest | Lamprey exits the perimeter of the nest, which is classified as a depression in the stream bed with a buildup of rocks on the back end of the circle (45 cm in width, 20 cm deep below substrate). |
| Spawn | A female lamprey attaches to larger rock often at the upstream rim of the nest and a spermiated male glides along the female’s dorsal region, attaches to the female’s head, and wraps around the female. The male and female vigorously vibrate their intertwined tails and simultaneously release gametes into the water. |
| Rock movement | Lamprey uses its oral disc to move a rock in the nest. |
| Tail fan | Lamprey rapidly moves its tail laterally to stir up and remove the sediment from its nest that is carried away with the water current. |
Conduct post-trial survey of spawning grounds to count free-ranging (non-released) sea lamprey and locate sea lamprey spawning nests
Timing: 30 min
This step describes how to locate and identify free-ranging (non-released) sea lamprey and sea lamprey spawning nests while safely wading in the stream during the post-trial survey of the spawning grounds. It also describes how to level all observed sea lamprey spawning nests and recover tagged females.
-
10.Conduct post-trial survey of spawning grounds following the same procedure as the pre-trial survey of spawning grounds.
-
a.Level all nests with a metal garden rake to ensure only the nests constructed within 24 h will be counted in the pre-trial survey the next day.
-
b.Recover all tagged females and return these fish to the laboratory to ensure in subsequent trials you only track the females for the trial in which they are released.
-
a.
Expected outcomes
The expected outcomes of observations include a pre-trial and post-trial survey of the spawning grounds for each trial (Figure 4A), and a swim track denoting the fine-scale movements of each tagged female from the release cage annotated with the behaviors observed and corresponding time stamps based on visual observations (Figure 4B). The raw observational data from the spawning ground nest surveys and behavioral observations for each trial is tabulated and compiled (Table 2; Table 3). The compiled survey and behavior data along with the water chemistry data are the input file for statistical models used to analyze the data. Please see below for how survey and behavior data are analyzed to determine if free-ranging sea lamprey abundance and pheromone-mediated attraction and spawning of tagged female in a high-density population is influenced by pheromone behavioral antagonist application (see quantification and statistical analysis section).
Figure 4.
Representative expected outcomes from a pre-trial survey of spawning grounds and swim track movements and behaviors of tagged female sea lamprey (Petromyzon marinus)
(A) The pre-trial survey of spawning grounds denotes the location of sea lamprey nests and the number of free-ranging (non-released) male (M) and female (F) sea lamprey observed on a scaled map of the field site.
(B) The swim track (blue) denotes the movements of a tagged female from the release cage on a scaled map of the field site with georeferenced landmarks (e.g., release cage, culvert, and barrier) to show the relative location of the fish’s position in the river. The swim track is annotated with the behaviors observed and corresponding time stamps based on visual observations.
Table 2.
Example of tabulated raw data from pre- and post-trial spawning ground nest surveys from two trials
| Julian. Date | Water. TempC | GaugeHt | Treatment | Raw data |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-trial survey |
Post-trial survey |
||||||||||
| # Nest | # Male | # Female | # Total | # Nest | # Male | # Female | # Total | ||||
| 19184 | 23.3 | 1.39 | Ant_6.21E-12M | 15 | 26 | 14 | 40 | 14 | 24 | 9 | 33 |
| 19186 | 24.7 | 1.38 | Vehicle | 9 | 16 | 6 | 22 | 9 | 24 | 9 | 33 |
For each trial, the Julian date, water temperature in degrees Celsius (Water.TempC), stream discharge with staff gauge height as a surrogate (GaugeHt), and treatment (Antagonist (Ant_6.21E-12) or Vehicle) are recorded. The number of sea lamprey (Petromyzon marinus) nests observed, number of free-ranging (untagged) male sea lamprey, number of free-ranging female sea lamprey, total number of free-ranging sea lamprey are tabulated from the pre- and post-trial spawning ground surveys for each trial.
Table 3.
Example of tabulated raw data compiled from behavioral observations from two trials
| Julian. Date | Water. TempC | GaugeHt | Treatment | Fish.ID | Raw data |
||||
|---|---|---|---|---|---|---|---|---|---|
| Remain. Cage | Down | Up | Male.occupied. Nest | Spawn | |||||
| 19184 | 23.3 | 1.39 | Ant_6.21E-12M | 5/Yellow/Yellow | 0 | 1 | 0 | 0 | 0 |
| 19184 | 23.3 | 1.39 | Ant_6.21E-12M | 7/Red/Red | 1 | 0 | 0 | 0 | 0 |
| 19184 | 23.3 | 1.39 | Ant_6.21E-12M | 8/Blue/Blue | 0 | 0 | 1 | 0 | 0 |
| 19186 | 24.7 | 1.38 | Vehicle | 2/Green/Yellow | 0 | 0 | 1 | 1 | 1 |
| 19186 | 24.7 | 1.38 | Vehicle | 3/White/White | 0 | 0 | 1 | 1 | 1 |
| 19186 | 24.7 | 1.38 | Vehicle | 4/Red/Blue | 0 | 0 | 1 | 1 | 1 |
For each trial, the Julian date, water temperature in degrees Celsius (Water.TempC), stream discharge with staff gauge height as a surrogate (GaugeHt), and treatment (Antagonist (Ant_6.21E-12) or Vehicle) are recorded. For each sea lamprey (Petromyzon marinus) (Fish.ID: fish number/LED light color/streamer tag color) within a trial, the outcome of each behavioral parameter (Remain.Cage: Remained in the release cage for duration of trial, Down: Swam downstream of the release cage and remained downstream for duration of trial, Up: Swam upstream of the release cage and remained upstream for duration of trial, Male.occupied.nest: Found at least one male-occupied nest, and Spawn: Spawned at least once) is denoted as a binary response (1: yes, 0: no).
Females’ ability to locate and spawn with free-ranging males is expected to decrease when behavioral antagonists are applied relative to vehicle. Our experimental findings indicate behavioral antagonist treatment reduced the proportion of females that swam upstream, found a male-occupied nest, and spawned (Figures 5A–5E). The relative number of free-ranging sea lamprey increased over the duration of a trial when vehicle was applied, which was expected given their nocturnal tendencies.16 Relative to the number of sea lamprey present at the beginning of a trial, we observed fewer free-ranging sea lamprey in the spawning grounds after behavioral antagonist application than after vehicle application, suggesting sea lamprey vacated the spawning grounds (Figure 5F).
Figure 5.
A mixture of behavioral antagonist reduced the proportion of released females that spawned and reduced the number of free-ranging sea lamprey (Petromyzon marinus) occupying the spawning grounds
The results display the percent of the tagged female sea lamprey that (A) spawned, (B) found a male-occupied nest, (C) moved upstream of the release cage, (D) moved downstream of the release cage, and (E) remained in the release cage at the end of the trial when exposed to vehicle (50% methanol) or behavioral antagonist (petromyzonol tetrasulfate (3sPZS) and petromyzonol sulfate (PZS), each at 6.2 × 10-12 M) over 3 h at Carp Lake Outlet based on the top ranked model selected by corrected Akaike information criterion (AICc). Each movement response parameter was evaluated using a generalized linear mixed-effect model with a binomial distribution. (F) Behavioral antagonist application decreased free-ranging sea lamprey abundance over 3-h trials. The plot shows the mean difference between the total number of free-ranging (untagged) sea lamprey observed before and after trials based on the top ranked linear regression model selected by AICc. A positive value indicates the number of sea lamprey increases during a trial. Error bars represent standard error. Figure modified from Scott et al.1
Quantification and statistical analysis
This section describes the procedures for quantification and statistical analysis of the behavioral responses of released ovulated females and the change in free-ranging sea lamprey abundance. We used R 4.1.0 for all statistical analyses. All data and R scripts used in this study are listed in the key resources table. We excluded data from trials if no free-ranging males were observed during the pre-trial survey to ensure there was a possibility for the released females to find a male-occupied nest and mate (2019: 0 trials; 2020: 2 trials). We recommend excluding trials from further analysis if more than 10% of the prepared solution from the peristaltic pump operation remains at the end of the trial because this indicates the target concentration of the test article was not achieved (2019: 0 trials, 2020: 0 trials).
-
1.
Analysis 1: Testing if behavioral antagonist application influenced behavior of released ovulated females.
The analysis determines the mean percentage of released female sea lamprey that spawned, found an occupied nest, moved upstream, moved downstream, and remained in the release
cage during the 3-h trials as a function of environmental covariates (water temperature, stream discharge with staff gauge height used as a surrogate, background lamprey pheromone concentration in river water), treatment applied (vehicle or antagonist), and Julian date based on the top ranked generalized linear mixed-effect model selected by corrected Akaike information criterion (AICc).-
a.Open the R program (version 4.1.0), set the working directory, and load the necessary packages (lme4 package,10 version 1.1.26; bbmle package,11 version 1.0.20; effects package,8 version 4.2.2; brglm package,9 version 0.7.2).setwd() #Enter file path.# Load the necessary packages.library(lme4) #used to fit lmer & glmer modelslibrary(bbmle) #used to rank competing models and calculate AICclibrary(effects) #used to determine the effect size and standard error of top modellibrary(ggplot2) #used to plot data of top model to visualize resultslibrary(brglm) #bias-reduction binomial generalized linear model used to account for zero-inflated data
-
b.Read the CSV data file into R.unplugged_19<-read.csv("Females_unplugged_2019_20221201.csv")
-
c.Set categorical covariates, random effects covariates, and binomial response variables as factors and confirm the CSV data file was read in correctly.#Set variables as factors.unplugged_19$Treatment<-factor(unplugged_19$Treatment) #categorical covariateunplugged_19$JulianDate<-factor(unplugged_19$JulianDate) #random effectunplugged_19$Cage<-factor(unplugged_19$Cage) #binomial responseunplugged_19$Upstream<-factor(unplugged_19$Upstream) #binomial responseunplugged_19$Downstream<-factor(unplugged_19$Downstream) #binomial responseunplugged_19$Nest<-factor(unplugged_19$Nest) #binomial responseunplugged_19$Mating<-factor(unplugged_19$Mating) #binomial responsesummary(unplugged_19) #Confirm CSV was read in correctly.
-
d.Evaluate the behavioral response of each female using a generalized linear mixed-effect model with a binomial distribution in the lme4 package (version 1.1.26).10Note: Shown here are a subset of models (models 1–5 of 14) for the analysis of the proportion of released females that spawned during the 3-h trial. See the complete list of models in the ‘All related code’ file in the Key resource table.
-
i.Fit models with covariates related to environmental conditions including water temperature, stream discharge, background lamprey pheromone concentration in river water (3kPZS, DkPES, and PZS), treatment application (vehicle, 3sPZS and PZS each at 6.2 × 10−12 M, or 3sPZS and PZS each at 6.2 × 10−11 M), and Julian date as a random effect.Note: If the data is zero-inflated (e.g., the proportion of females that remained in the release cage in 2019), evaluate the behavioral response with a bias-reduction binomial generalized linear model (brglm package; version 0.7.2).9#Generalized linear mixed-effect models with a binomial distribution fit environmental covariates and Julian date as a random effectglm.mate.m1 <- glmer (Mating∼ Treatment + (1 | JulianDate),family= binomial, data= unplugged_19, na.action=na.omit)glm.mate.m2 <- glmer (Mating∼ Treatment + tkPZS_ng_L + (1 | JulianDate),family= binomial, data= unplugged_19, na.action=na.omit)glm.mate.m3 <- glmer (Mating∼ Treatment + Temp + tkPZS_ng_L+ (1 | JulianDate), family= binomial, data= unplugged_19, na.action=na.omit)glm.mate.m4 <- glmer (Mating∼ Treatment + Temp + (1 | JulianDate),family= binomial, data= unplugged_19, na.action=na.omit)glm.mate.m5 <- glmer (Mating∼ Treatment + Temp + tkPZS_ng_L + GaugeHt+ (1 | JulianDate), family= binomial, data= unplugged_19, na.action=na.omit)
-
i.
-
e.Construct a list of the generalized linear mixed-effect models and rank the competing models using AICc in the bbmle package (version 1.0.20).11#Construct a list of the models and rank with AICcSelect_model_mate <- list("m1" = glm.mate.m1, "m2" = glm.mate.m2,"m3" = glm.mate.m3, "m4" = glm.mate.m4, "m5" = glm.mate.m5)AICctab(Select_model_mate, base = TRUE)
-
f.Interpret the results of the top model to determine statistical significance between treatment groups and the mean and standard error of each treatment group.#View results of top model to determine statistical significance.summary(glm.mate.m1) #glm.mate.m1 was the top ranked model based on AICc#Determine mean and standard error of each treatment group of top model.effects_Trt <- effect(term = c("Treatment"), mod = glm.mate.m1) #Using the top modelsummary(effects_Trt)x_Trt <- as.data.frame(effects_Trt)x_Trt
-
g.Plot the top model to visualize results.#View results of top model to determine statistical significance.ggplot(data = x_Trt, aes(x= Treatment, y = fit ))+geom_point(data = x_Trt, aes(x = Treatment, y = fit), size = 3.5)+geom_errorbar(data = x_Trt, aes(x = Treatment, ymin = fit-se,ymax = fit+se),alpha = 0.7, width = 0.2 )+labs(x = "Treatment", y = "Spawn (%)")+ylim(min = 0, max = 1)+theme_classic()+theme(text=element_text(size= 15))
-
h.Repeat Analysis 1 steps 1d- 1g for the additional behavioral responses recorded (remained in the release cage, swam downstream of the release cage, swam upstream of the release cage, and found a male-occupied nest during the 3-h trial).
-
a.
-
2.
Analysis 2: Testing if behavioral antagonist application influenced free-ranging sea lamprey abundance.
The analysis determines the mean difference between the total number of free-ranging (untagged) sea lamprey observed before and after 3-h trials as a function of environmental covariates (water temperature, stream discharge with staff gauge height used as a surrogate, background lamprey pheromone concentration in river water) and treatment applied (vehicle or antagonist) based on the top ranked linear regression model selected by AICc. A positive value for change in sea lamprey abundance indicates the number of sea lamprey increases during a trial.-
a.Open the R program (version 4.1.0), set the working directory, and load the necessary packages (lme4 package,10 version 1.1.26; bbmle package,11 version 1.0.20; effects package,8 version 4.2.2; brglm package,9 version 0.7.2).setwd() #Enter file path.# Load the necessary packages.library(bbmle) #used to rank competing models and calculate AICclibrary(effects) #used to determine the effect size and standard error of top modellibrary(ggplot2) #used to plot data of top model to visualize results
-
b.Read the CSV data file into R.atlarge_19<-read.csv("At_Large_2019.csv")
-
c.Set categorical covariate (treatment) as a factor and confirm the CSV data file was read in correctly.#Set variables as factors.atlarge_19$Treatment<-factor(atlarge_19$Treatment) #Categorical covariatesummary(atlarge_19) #Confirm CSV was read in correctly.
-
d.Evaluate the change in free-ranging sea lamprey abundance over the duration of a trial as a linear regression model for each year in the lme4 package (version 1.1.26).10Note: Shown here are a subset of models (models 1–5 of 14) for the analysis of the proportion of released females that spawned during the 3-h trial. See the complete list of models in the ‘All related code’ file in the Key resource table.
-
i.Fit models with covariates related to environmental conditions including water temperature, stream discharge, background lamprey pheromone concentration in river water (3kPZS, DkPES, and PZS), and treatment application (vehicle, 3sPZS and PZS each at 6.2 × 10−12 M, or 3sPZS and PZS each at 6.2 × 10−11 M).#Linear regression models fit environmental covariates and treatmentlm.diffatlargetotal.m1 <- lm (DIFFnumbermalesandfemales ∼ Treatment,data= atlarge_19, na.action=na.omit)lm.diffatlargetotal.m2 <- lm (DIFFnumbermalesandfemales ∼ Treatment+ tkPZS_ng_L, data= atlarge_19, na.action=na.omit)lm.diffatlargetotal.m3 <- lm (DIFFnumbermalesandfemales ∼ Treatment + Temp+ tkPZS_ng_L, data= atlarge_19, na.action=na.omit)lm.diffatlargetotal.m4 <- lm (DIFFnumbermalesandfemales ∼ Treatment+ Temp, data= atlarge_19, na.action=na.omit)lm.diffatlargetotal.m5 <- lm (DIFFnumbermalesandfemales ∼ Treatment + Temp+ tkPZS_ng_L + GaugeHt, data= atlarge_19, na.action=na.omit)
-
i.
-
e.Construct a list of the linear regression models and rank the competing models using AICc in the bbmle package (version 1.0.20).11#Construct a list of the models and rank with AICcSelect_model_diffatlarge <- list("m1" = lm.diffatlargetotal.m1,"m2" = lm.diffatlargetotal.m2, "m3" = lm.diffatlargetotal.m3,"m4" = lm.diffatlargetotal.m4, "m5" = lm.diffatlargetotal.m5) AICctab(Select_model_diffatlarge, base = TRUE)
-
f.Interpret the results of the top model to determine statistical significance between treatment groups and the mean and standard error of each treatment group.#View results of top model to determine statistical significance.summary(lm.diffatlargetotal.m1) # lm.diffatlargetotal.m1was the top rankedmodel based on AICc#Determine mean and standard error of each treatment group of top model.effects_Trt <- effect(term = c("Treatment"), mod = lm.diffatlargetotal.m1) #Using the top modelsummary(effects_Trt)x_Trt <- as.data.frame(effects_Trt)x_Trt
-
g.Plot the top model to visualize results.#View results of top model to determine statistical significance.ggplot(data = x_Trt, aes(x= Treatment, y = fit ))+geom_point(data = x_Trt, aes(x = Treatment, y = fit), size = 3.5)+geom_errorbar(data = x_Trt, aes(x = Treatment, ymin = fit-se,ymax = fit+se), alpha = 0.7, width = 0.2 )+labs(x = "Treatment", y = "Difference in # At large male + female")+ylim(min = -1, max = 10)+theme_classic()+theme(text=element_text(size= 15))
-
a.
Limitations
The protocol relies extensively on manually locating nests and tracking sea lamprey. Manually tracking sea lamprey movements necessitates many well-trained observers to minimize disturbing or losing sight of the focal subjects that are tracked with dim red headlamps at night when sea lamprey spawning activity increases substantially.5 Given the labor-intensive nature of data collection, the observation time for each trial was limited to 3 h during the peak spawning period. We cannot conclude whether the behaviors observed during 3 h trials would continue over the course of a sustained pheromone behavioral antagonist application. Interpretation of the results should be limited to the 3 h duration.
In addition, environmental conditions can affect sea lamprey nest surveys and behavioral observations. If the substrate of the river is not visible, then it is not feasible to survey for nests and spawning sea lamprey. Precipitation events that result in high, fast, or turbulent flow often dramatically reduce visibility and hinder data collection. Sea lamprey spawning behaviors coincide with increases in spring water temperatures to those that are suitable for embryonic development. Spawning activity has been observed in water temperature ranging from 10°C- 26°C and is limited outside this range.5
Troubleshooting
Problem 1
The pump is not operating correctly because debris has accumulated in the tubing (related to Step 2).
Potential solution
-
•
Check to make sure the pump head tubing is encircling the pump head. Release and remove the head tubing from the pump head. Dislodge any accumulated debris in the tubing. Collect a new beaker of river water free of debris and resume priming and calibrating the pump.
Problem 2
The pump is not operating correctly with air bubbles persisting in the tubing after priming (related to Step 2).
Potential solution
-
•
Release and remove the head tubing from the pump head. Inspect for cracks in the tubing. If detected, replace with a new piece of head tubing and resume priming and calibrating the pump. The head tubing should be replaced approximately every two weeks as the pump roller heads wear the material of the tubing.
-
•
Release and remove the head tubing from the pump head. Ensure all connections at barbed couplers are airtight.
Problem 3
The inlet tubing isn’t staying in the lowest point of the behavioral antagonist bin (related to Step 2).
Potential solution
-
•
Use a cable tie to affix washers to the end to weigh down the tubing.
Problem 4
The peristaltic pump power switch is not illuminated despite being in the on position (related to Step 2).
Potential solution
-
•
Switch the pump off. Visually inspect for loose connections between the battery cables and the battery and the battery cables and the pump. Ensure the battery, cables, and battery terminals are clean and free of corrosion. Tighten all connections.
-
•
If you suspect the battery is the problem, check the charge of the battery and replace it with a charged 12 V deep cycle battery.
Problem 5
The streamer tag falls out of the dorsal fin in transit to the field site (related to Step 1).
Potential solution
-
•
Keep a few spare streamer tags (at least one of each color) in the vehicle that transports supplies to the field site. Replace the fallen-out tag with a spare tag before the females are added to the release cage. When you arrive back at the laboratory, replenish any tags that are used to ensure you have spares when needed.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Weiming Li (liweim@msu.edu).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Anne Scott (scottan7@msu.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data have been deposited to Mendeley Data: http://doi.org/10.17632/k6h7vz6dvy.1.
The code generated during this study is available at Zenodo: https://doi.org/10.5281/zenodo.7412008.
Acknowledgments
The U.S. Fish and Wildlife Service – Marquette Biological Station provided sea lamprey. U.S. Geological Survey, Great Lakes Science Center, Hammond Bay Biological Station provided access to their research facilities. Trisha Searcy, Braden Idalski, Melissa Pomranke, Dan Durbin, Shane Kammerman, and Ethan Ready assisted with behavioral experiments. Lijun Chen, Sonam Tamrakar, Belinda Huerta, and Ke Li measured concentrations of pheromone components in the water samples at Michigan State University’s Mass Spectrometry and Metabolomics Core. Andrea Miehls (Great Lakes Fishery Commission) provided photos for Figures 1 and 3. This study was funded by grants from the Great Lakes Fishery Commission (LIW_54075). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Author contributions
Conceptualization, A.M.S., N.S.J., M.J.S., and W.L.; methodology, A.M.S. and N.S.J.; writing – original draft, A.M.S.; writing – review and editing, A.M.S., N.S.J., M.J.S., and W.L.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Anne M. Scott, Email: scottan7@msu.edu.
Weiming Li, Email: liweim@msu.edu.
References
- 1.Scott A.M., Johnson N.S., Siefkes M.J., Li W. Synergistic behavioral antagonists of a sex pheromone reduce reproduction of invasive sea lamprey. iScience. 2023;26 doi: 10.1016/j.isci.2023.107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsden J.E., Siefkes M.J. In: Docker M.F., editor. Vol. 2. Springer Netherlands; 2019. Control of Invasive Sea Lamprey in the Great Lakes, Lake Champlain, and Finger Lakes of New York; pp. 411–479. (Lampreys: Biology, Conservation and Control). [DOI] [Google Scholar]
- 3.Buchinger T.J., Siefkes M.J., Zielinski B.S., Brant C.O., Li W. Chemical cues and pheromones in the sea lamprey (Petromyzon marinus) Front. Zool. 2015;12:32. doi: 10.1186/s12983-015-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fissette S.D., Buchinger T.J., Wagner C.M., Johnson N.S., Scott A.M., Li W. Progress towards integrating an understanding of chemical ecology into sea lamprey control. J. Great Lakes Res. 2021;47:S660–S672. doi: 10.1016/j.jglr.2021.02.008. [DOI] [Google Scholar]
- 5.Johnson N.S., Buchinger T.J., Li W. In: Lampreys: Biology, Conservation and Control. Docker M.F., editor. Springer; 2015. Reproductive ecology of lampreys; pp. 265–303. [Google Scholar]
- 6.Siefkes M.J. Use of physiological knowledge to control the invasive sea lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conserv. Physiol. 2017;5 doi: 10.1093/conphys/cox031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikitina N., Bronner-Fraser M., Sauka-Spengler T. Culturing lamprey embryos. Cold Spring Harb. Protoc. 2009;2009 doi: 10.1101/pdb.prot5122. pdb.prot5122. [DOI] [PubMed] [Google Scholar]
- 8.Fox J., Weisberg S. Sage Publications; 2018. An R Companion to Applied Regression. [Google Scholar]
- 9.Kosmidis I., Firth D. Jeffreys-prior penalty, finiteness and shrinkage in binomial-response generalized linear models. Biometrika. 2021;108:71–82. doi: 10.1093/biomet/asaa052. [DOI] [Google Scholar]
- 10.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 11.Bolker B., Team R.C. R Foundation for Statistical Computing; 2017. Bbmle: Tools for General Maximum Likelihood Estimation. R Package Version 1.0. 20. [Google Scholar]
- 12.Applegate V.C. US Fish and Wildlife Service; 1950. Natural history of the sea lamprey, Petromyzon marinus, in Michigan. [Google Scholar]
- 13.Manion P.J., Hanson L.H. Spawning behavior and fecundity of lampreys from the upper three Great Lakes. Can. J. Fish. Aquat. Sci. 1980;37:1635–1640. doi: 10.1139/f80-211. [DOI] [Google Scholar]
- 14.Xi X., Johnson N.S., Brant C.O., Yun S.-S., Chambers K.L., Jones A.D., Li W. Quantification of a male sea lamprey pheromone in tributaries of Laurentian Great Lakes by liquid chromatography–tandem mass spectrometry. Environ. Sci. Technol. 2011;45:6437–6443. doi: 10.1021/es200416f. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Johnson N., Bernardy J., Hubert T., Li W. Monitoring sea lamprey pheromones and their degradation using rapid stream-side extraction coupled with UPLC-MS/MS. J. Sep. Sci. 2013;36:1612–1620. doi: 10.1002/jssc.201300110. [DOI] [PubMed] [Google Scholar]
- 16.Walaszczyk E.J., Goheen B.B., Steibel J.P., Li W. Differential effects of sex pheromone compounds on adult female sea lamprey (Petromyzon marinus) locomotor patterns. J. Biol. Rhythms. 2016;31:289–298. doi: 10.1177/074873041662924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data have been deposited to Mendeley Data: http://doi.org/10.17632/k6h7vz6dvy.1.
The code generated during this study is available at Zenodo: https://doi.org/10.5281/zenodo.7412008.