Summary
Organ-on-a-chip technology incorporating stem cell techniques represents a promising strategy to improve modeling of human organs. Here, we present a protocol for generating a standardized 3D placenta-on-a-chip model using trophoblast derived from human induced pluripotent stem cells (hiPSCs). We describe steps for seeding hiPSCs into multi-chip OrganoPlate devices and on-chip differentiation into trophoblasts against an extracellular matrix under perfused conditions. We then detail procedures for conducting a functional barrier integrity assay, immunostaining, and collecting protein or RNA for molecular analysis.
For complete details on the use and execution of this protocol, please refer to Lermant et al. (2023).1
Subject areas: Biotechnology and bioengineering, Organoids, Systems biology
Graphical abstract

Highlights
-
•
Placenta-on-a-chip model is generated by differentiating hiPSCs in a microfluidic plate
-
•
The obtained 3D placental barrier is cultured against an ECM under perfused conditions
-
•
Multi-chip plate format allows for high-throughput functional and molecular studies
-
•
Model of the early placenta can be applied to toxicology or disease-related studies
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Organ-on-a-chip technology incorporating stem cell techniques represents a promising strategy to improve modeling of human organs. Here, we present a protocol for generating a standardized 3D placenta-on-a-chip model using trophoblast derived from human induced pluripotent stem cells (hiPSCs). We describe steps for seeding hiPSCs into multi-chip OrganoPlate devices and on-chip differentiation into trophoblasts against an extracellular matrix under perfused conditions. We then detail procedures for conducting a functional barrier integrity assay, immunostaining, and collecting protein or RNA for molecular analysis.
Before you begin
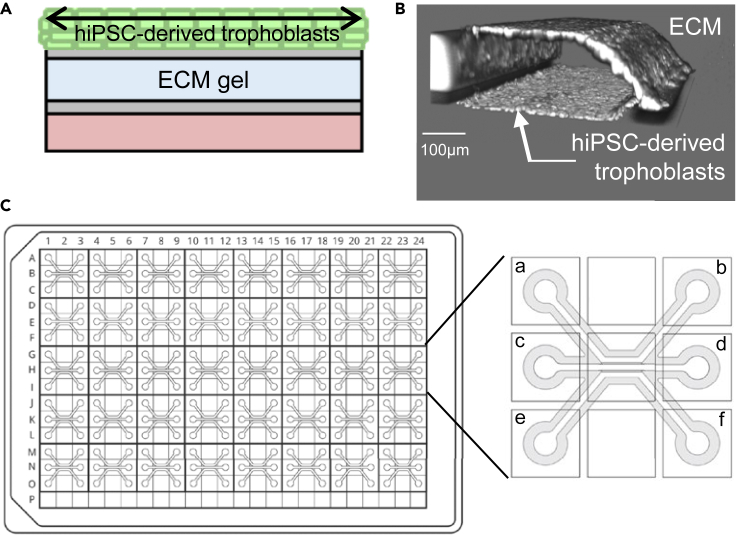
By using microfluidic plates (OrganoPlate) the differentiated trophoblasts develop into a 3D tubule, forming a structural barrier in direct contact with an ECM gel in the absence of a physical barrier. Cultured under perfused conditions, the trophoblasts take cues from physical interactions with the environment e.g., extracellular matrix and flow from the media (Figures 1A and 1B).
Figure 1.
Overview of the hiPSC-derived placenta-on-a-chip platform
(A) Schematic view of an individual chip containing hiPSC-derived trophoblasts cultured under perfusion in the top channel against an ECM gel loaded in the middle channel.
(B) 3D reconstructions of confocal images of the hiPSC-derived placental barrier after 4 days of differentiation showing the formation of a closed tubule in direct contact with the ECM. Trophoblasts were stained with nuclei marker Hoechst.
(C) Diagram showing the entire 3-lane OrganoPlate layout (left) and an individual chip (right) with detailed location of perfusion channel inlet (a) and outlet (b), gel channel inlet (c) and outlet (d), and bottom channel inlet (e) and outlet (f).
Various in situ assays can be carried out in this model, including live cell imaging, immunostaining, functional barrier permeability and transport assays. Alternatively, trophoblasts can be lysed and collected for downstream molecular analyses. As an alternative to the barrier integrity assay described here, trans-epithelial electrical resistance measurements can also be carried out in this model.
The presented protocol was optimized with ChiPS4, a hiPSC cell line derived from primary human dermal fibroblasts, and was shown efficient to produce a syncytium phenotype.1 By adapting this protocol, one can generate placenta-on-a-chip models from various hiPSC cell lines, i.e., using hiPSC from patients to build a diseased placental model. The third channel can be used to integrate co-cultures e.g., endothelial cells, as we previously demonstrated, or perfuse with other factors.2
Establishing and maintaining the hiPSC cell culture
Timing: weeks (30 min daily)
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
-
1.
Dilute the concentrated Geltrex solution into DMEM culture medium to achieve a final concentration of 10 μg/cm2.
-
2.
Add 8 mL of diluted gel into a 75 cm2 culture flask and let it incubate for at 37°C and 5% CO2 for 1 h.
-
3.
Remove and discard culture medium from the cells.
-
4.
Briefly rinse the cell layer with PBS.
-
5.
Add 2–3 mL of TrypLE select and incubate for 5 min at 37°C.
-
6.
Resuspend cells in 10 mL of complete ChiPS4 culture medium (TeSR1 containing 30 ng/mL FGF-2 and 10 ng/mL Noggin).
-
7.
Counts the cells and centrifuge at 200 g for 5 min.
-
8.
Resuspend cells in 10 mL complete ChiPS4 culture medium further supplemented with 10 μM Y-27632 immediately before use to achieve a final cell density of 3–5×104 cells/cm3.
-
9.
Aspirate the Geltrex coating solution from the new culture flask and immediately plate cells.
-
10.
Change culture medium daily and passage when they reach approximately 80% confluence.
Note: Cells will typically reach 80% confluence in 6–8 days depending upon the split ratio.
Preparation of solutions and cell culture media
Timing: 30 min
-
11.Prepare the 37 g/L NaHCO3 solution for Collagen-I gel preparation. The solution can be prepared ahead of time and kept at 4°C for 1–2 months.
-
a.Dissolve 3.7 g of NaHCO3 powder in 100 mL of deionized H2O.
-
b.Adjust pH to 9.5 using a 2 M NaOH solution.
-
c.Sterile filter the solution with a 0.22 μm membrane filter.
-
a.
-
12.
Prewarm the ChiPS4 seeding medium, trophoblast pre-differentiation medium and final trophoblast differentiation medium at 37°C before use.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Geltrex LDEV-free, hESC-qualified, reduced growth factor basement membrane matrix | Thermo Fisher Scientific | Cat#A1413301 |
| Recombinant human FGF-2 | PeproTech | Cat# 100-18B |
| Recombinant human Noggin | PeproTech | Cat# 120-10C |
| Recombinant human BMP-4 (E. coli derived) | PeproTech | Cat#120-05ET |
| PD 173074 | Sigma-Aldrich | Cat#P2499 |
| A 83-01 | Tocris Bioscience | Cat#2939 |
| NaHCO3 | Sigma-Aldrich | Cat#S5761 |
| Cultrex 3D Culture Matrix Rat Collagen I | Bio-Techne | Cat#3447-020-01 |
| Fluorescein isothiocyanate-dextran, 10 kDa | Sigma-Aldrich | Cat#FD10S |
| Tetramethylrhodamine isothiocyanate-dextran, 155 kDa | Sigma-Aldrich | Cat#T1287 |
| TrypLE Select (1X) | Thermo Fisher Scientific | Cat#12563011 |
| ROCK inhibitor (Y-27632) | Sigma-Aldrich | Cat#SCM075 |
| Hank’s balanced salt solution (HBSS) | Sigma-Aldrich | Cat#H6648 |
| HEPES, 1 M buffer solution | Thermo Fisher Scientific | Cat#15630049 |
| FBS | Gibco/ATCC | Cat#A13450 |
| Critical commercial assays | ||
| TeSR1 medium | In-house. Ludwig et al.3; also available from STEMCELL Technologies | N/A |
| Buffer RLT | QIAGEN | Cat#79216 |
| Cell lysis buffer (10X) | Cell Signaling Technology | Cat#9803 |
| Halt protease and phosphatase inhibitor cocktail (100X) | Thermo Fisher Scientific | Cat#78440 |
| Experimental models: Cell lines | ||
| ChiPS4 hiPSC cell line | Cellartis | CVCL_RM97 |
| Software and algorithms | ||
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Fiji (ImageJ) | ImageJ | https://imagej.nih.gov/ij/download.html |
| Other | ||
| OrganoPlate 3-lane 40 | MIMETAS | Cat#4003-400B |
| OrganoFlow | MIMETAS | Cat#MI-OFPR-L |
| Incucyte S3 live-cell analysis instrument | Sartorius | N/A |
| ErgoOne E single-channel pipette electronic, 0.5–10 μL | Starlab | Cat#G9001-0010 |
| ErgoOne E 8-channel electronic pipette, 15–300 μL | Starlab | Cat#G9008-0300 |
| Sorvall ST 8 small benchtop centrifuge | Thermo Fisher Scientific | Cat#75007200 |
| Centrifuge 5425 R | Eppendorf | Cat#5425 R |
| Galaxy Mini centrifuge | VWR | C1413-230 EU |
Materials and equipment
Recipes
-
•
Complete medium for undifferentiated ChiPS4 culture: TeSR1 medium4 with 30 ng/mL FGF-2 and 10 ng/mL Noggin.
Store at 4°C for up to 2 weeks.
-
•
ChiPS4 seeding medium: TeSR1 medium with 30 ng/mL FGF-2 and 10 μM Y-27632.
Prepare immediately before use.
-
•
Trophoblast pre-differentiation medium: TeSR1 with 20 ng/mL FGF-2.
Prepare immediately before use.
-
•
10 μg/mL BMP4 stock solution: BMP-4 reconstituted in 0.1% BSA.
Aliquot and store at ‒20°C. Thaw immediately before use and do not re-freeze.
-
•
1 mM A83-01 stock solution: A83-01 reconstituted in DMSO.
Aliquot and store at ‒20°C for up to one month. Thaw immediately before use and do not re-freeze.
-
•
0.1 mM PD173074 stock solution: PD173074 reconstituted in DMSO.
Aliquot and store at 4°C. Bring to 18°C–23°C immediately before use.
Final trophoblast differentiation medium (BAP)
| Reagent | Final concentration | Amount |
|---|---|---|
| BMP4 (10 μg/mL) | 10 ng/mL | 10 μL |
| A83-01 (1 mM) | 1 μM | 10 μL |
| PD173074 (0.1 mM) | 0.1 μM | 10 μL |
| TeSR1 medium (without FGF-2) | N/A | q.s. 10 mL |
| Total | N/A | 10 mL |
Prepare immediately before use.
-
•
37 g/L NaHCO3 solution: sterile deionized water with 37 g/L of NaHCO3, pH 9.5.
Store at 4°C for few months.
ECM gel
| Reagent | Final concentration | Amount |
|---|---|---|
| HEPES (1 M) | 0.1 M | 10 μL |
| NaHCO3 (37 g/L) | 3.7 g/L | 10 μL |
| Collagen-I (5 mg/mL) | 4 mg/mL | 80 μL |
| Total | N/A | 100 μL |
Use immediately (within 10 min).
-
•
0.2 mg/mL Geltrex coating solution: ice-cold DMEM culture medium with 0.2 mg/mL Geltrex.
Thaw the Geltrex solution at 4°C for 12–18 h. Use the diluted Geltrex solution immediately or aliquot and store at ‒20°C for up to 18 months.
-
•
RNA extraction buffer: RLT buffer (QIAGEN) added with 1% β-mercaptoethanol.
Prepare immediately before use.
-
•
Protein extraction buffer: Cell lysis buffer added with 1X halt protease & phosphatase inhibitor cocktail.
Prepare immediately before use.
-
•
FITC-dextran 10 kDa stock solution: 25 mg/mL FITC-Dextran in HBSS.
Store at 4°C for up to 6 months, protected from light.
-
•
TRITC-dextran 155 kDa stock solution: 25 mg/mL TRITC-Dextran in HBSS.
Store at 4°C for up to 6 months, protected from light.
FITC-dextran 10 kDa + TRITC-dextran 155 kDa working solution
| Reagent | Final concentration | Amount |
|---|---|---|
| FITC-Dextran 10 kDa (25 mg/mL) | 0.5 mg/mL | 60 μL |
| TRITC-Dextran 155 kDa (25 mg/mL) | 0.5 mg/mL | 60 μL |
| TeSR1 medium | N/A | q.s. 3 mL |
| Total | N/A | 3 mL |
Prepare immediately before use.
-
•
Fixative solution: 3.7% paraformaldehyde in PBS.
Store at 4°C for up to two weeks, or aliquot and store at ‒20°C for up to 6 months.
-
•
Permeabilization buffer: 0.3% Triton X-100 in PBS.
Store at 4°C for 3–4 weeks.
-
•
Washing solution: 2% FBS in PBS.
Store at 4°C for 3–4 weeks.
Blocking buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 2% | 300 μL |
| BSA | 2% | 300 mg |
| Tween 20 | 0.1% | 15 μL |
| PBS | N/A | q.s. 15 mL |
| Total | N/A | 15 mL |
Prepare immediately before use.
CRITICAL: Potential hazard
Paraformaldehyde is moderately toxic by skin contact. It has recently been designated as a probable human carcinogen. Skin contact with paraformaldehyde may cause itching and rash that may lead to skin allergy upon repeated exposure. It has also been reported to cause reproductive and mutagenic problems in humans exposed long term. All weighing and handling of paraformaldehyde should be done with adequate ventilation using chemicals fume hoods, vented balance enclosures or other local exhaust ventilation. Pre-weighed packets or purchase of prepared formalin solutions should be substituted if possible to minimize potential exposures.
β-Mercaptoethanol causes skin irritation, serious eye damage and may cause damage to organs through prolonged or repeated exposure. Use an extractor hood and wear protective gloves, eye and face protection when handling it. Avoid release to the environment.
Alternatives: Fixing Methods
-
•
Fixative solution: other fixation methods can be used with the OrganoPlate depending on the application and the targeted antigen to be stained. Other fixation methods shown to be compatible with the OrganoPlate include 0.4% formaldehyde in HBSS, ‒20°C 100% acetone, ‒20°C 100% methanol and ‒20°C 95% methanol, 5% acetic acid. Adjust incubation time according to the table below:
| Fixative | Incubation time |
|---|---|
| 0.4% formaldehyde in HBSS | 10–15 min at 18°C–23°C |
| ‒20°C 100% acetone | 5 min at 18°C–23°C |
| ‒20°C 100% methanol | 10–15 min at 18°C–23°C |
| ‒20°C 95% methanol, 5% acetic acid | 5–10 min at 18°C–23°C |
-
•
RNA extraction buffer: TRIzol reagent (Invitrogen, Cat#12034977) has also been successfully used to extract RNA in the OrganoPlate following similar protocol as described with the RLT extraction buffer.
-
•
Fluorescent compounds: FITC-dextran 10 kDa and TRITC-dextran 155 kDa are used in this protocol to assess the barrier permeability to compounds of different sizes. Other fluorescent dyes can be used for assessing barrier integrity of the model. Commonly used fluorescent dyes include FITC-dextran 150 kDa (Sigma-Aldrich, Cat#46946) or FITC-dextran 20 kDa (Sigma-Aldrich, Cat#FD20S). Sodium fluorescein (10 μg/mL; Sigma-Aldrich, Cat#F6377)", which is < 400 Da (physiologically, placental barrier is impermeable to 10 kDa but permeable to molecules <400 Da; that’s a good dye to test the model.
-
•
Animal free extracellular matrix: more companies are developing animal free matrix as an alternative to extracellular matrix components. Where possible we have used animal free matrix, yet in the future new developments can be incorporated into the model upon testing.
-
•
Electronic pipettes: any repeating pipette allowing for dispensation of 1–3 μL volumes can be used for gel loading and cell seeding in the OrganoPlate. Higher total volumes will allow user to load more chips in one go before having to reload the pipette. Pipettes allowing whole-microliter volumes only will make it more difficult to correct incomplete gel filling or overflow. Any 8-tip multichannel repeating pipette allowing for dispensation of 15–50 μL volumes can be used for changing medium, adding lysis buffers or other solutions in the OrganoPlate.
-
•
Microscope: a microscope with an automatic stage is needed to conduct high-throughput assays on multiplex images of the whole OrganoPlate (i.e., barrier integrity assay). In our study we used the Incucyte S3 (Sartorius), although any other microscope that allows rapid automatic multiplex acquisition of the whole OrganoPlate while maintaining the cells in the incubator conditions can be used instead.
TeSR1 culture medium: basal TeSR1 culture medium used for ChiPS4 routine culture and differentiation was made based on a recipe published in the literature.4 Pre-made TeSR1 media is commercially available can be used instead.
-
•
Centrifuges: a centrifuge is needed for routine culture of hiPSC and the preparation of the hiPSC suspension to be seeded in the OrganoPlate. The reference mentioned here is given as an example. Any benchtop centrifuge providing a 200 g centrifugal force and fitting 15 mL tubes can be used instead. A refrigerated centrifuge is needed for processing protein lysates for downstream analyses. The reference mentioned here is given as an example, but any refrigerated benchtop centrifuge providing a 14,000 g force and fitting 1.5 mL Eppendorf tubes can be used instead.
Step-by-step method details
The first four steps of this protocol describe the step-by-step procedures to seed and differentiate hiPSC cells into trophoblasts directly within a 3-lane OrganoPlate microfluidic device.
The last three steps outline standard analysis methods that can be implemented to assess functional and molecular features of the placenta-on-a-chip. These include functional barrier integrity assay in the chip, immunostaining and extracting RNA or proteins for downstream molecular analyses. These steps have been successfully used previously for characterizing the model.1
Extracellular matrix (ECM) gel loading in the OrganoPlate
Timing: 45 min
In this step, a collagen-I based gel is prepared and loaded into the middle channel of all chips, and left to polymerize for 15 min. This ECM fills the middle channel and will be in direct contact with the cultured cells.
-
1.
Add 50 μL HBSS to all wells of the middle column of the chips (columns 2, 5, 8, 11, 14, 17, 20 and 23).
Note: if not using all chips on the whole plate, place a sealing film (i.e. SealPlate film, Sigma-Aldrich cat#Z369667) on the rest of the wells for later use. Care needs to be taken in keeping chips sterile.
-
2.Prepare the required amount of 4 mg/mL Collagen-I (2 μL gel per chip + 40% extra). Prepare at least 100 μL of total gel volume to ensure proper mixing of all components.
-
a.Place an Eppendorf tube on ice.
-
b.Prepare the ECM gel by mixing 1 M HEPES, 37 g/L NaHCO3 and 5 mg/mL collagen-I in a 1:1:8 ratio.
-
c.Mix well by pipetting up and down at least 20 times while keeping it on ice.
-
d.If bubbles formed, quickly spin the tube down (∼5 s) max speed of 2000 g (Galaxy Mini Centrifuge).
-
e.Use the gel within 10 min after preparation.
-
a.
Note: Ensure all components are kept cool during the whole procedure. Hint. Bring your ice bucket containing the collagen tube close to centrifuge to minimize the time spent out of the ice.
-
3.Dispense the gel into the gel channel inlets (columns 1, 4, 7, 10, 13, 16, 19, 22; rows B, E, H, K, N) using a multi-dispenser or electronic pipette.
-
a.Gently place your pipette tip on top of the hole in the bottom of the well and dispense the gel. Troubleshooting 1 and 2.
-
a.
CRITICAL: Contact between the droplet and the hole is essential for gel loading. Correct positioning of the gel on top of hole allows capillary forces to pull the gel into the microfluidic gel channel.
Note: Optimal gel volume to get a correct gel filling might need to be adjusted depending on several factors, such as the gel viscosity and temperature in the room. In case of incomplete gel filling, increase the loading volume (i.e. to 2.3 μL). In case the gel overflows from the gel channel into the adjacent medium channel, reduce the loading volume (i.e. to 1.7 μL).
Note: load a maximum of 20 chips at once before emptying and reloading the pipette tip with cold gel. This will avoid gelation of the gel while it is in the pipette tip.
-
4.
Place the OrganoPlate in a humidified incubator at 37%, 5% CO2 for 15 min to allow gel polymerization.
-
5.
Add 30 μL of HBSS to the gel channel inlets (columns 1, 4, 7, 10, 13, 16, 19, 22; rows B, E, H, K, N) to prevent the gel from drying out.
-
6.
Place the OrganoPlate back in the incubator and proceed to cell seeding on the same day.
OrganoPlate coating
Timing: 1.5 h
Before seeding hiPSC into the perfusion channel, coating it with a Geltrex solution is necessary to maintain cell attachment, tubule formation and biological performance of the model.
-
7.Prepare the required amount of 0.2 mg/mL Geltrex coating solution (40 μL gel per chip + 40% extra).
-
a.Mix Geltrex stock solution with ice-cold DMEM culture medium at a 1:75 ratio in pre-chilled tubes.
-
a.
-
8.
Add 40 μL of Geltrex 0.2 mg/mL coating solution to the perfusion channel inlets (columns 1, 4, 7, 10, 13, 16, 19, 22; rows A, D, G, J, M).
Note: Place the tips in the corner of the well and allow them to touch the bottom of the plate. Avoid placing the tips immediately on top of holes as this may favor air bubble formation.
-
9.
Place the OrganoPlate in a humidified incubator at 37°C, 5% CO2 for at least 1 h and proceed to cell seeding in the OrganoPlate.
Cell seeding in the OrganoPlate
Timing: 5 h
After polymerization of the gel and coating of the perfusion channel, a hiPSC suspension is seeded in the perfusion channel in direct contact with the ECM filling the middle channel. After cell attachment to the ECM, medium perfusion is started to initiate cell culture and 3D tubule formation.
-
10.Prepare your ChiPS4 cell suspension for seeding.Note: Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
-
a.Harvest cells according to their dissociation protocol.
-
i.Remove and discard culture medium from the cells.
-
ii.Briefly rinse the cell layer with PBS.
-
iii.Add 2–3 mL of TrypLE select and incubate for 5 min at 37°C.
-
iv.Resuspend cells in 10 mL of TeSR1 medium supplemented with 30 ng/mL FGF-2 and 10 μM Y-27632 (seeding medium).
-
i.
-
b.Count cells and centrifuge at 200 g for 5 min.
-
c.Calculate the required number of cells for seeding in the OrganoPlate according to the number of chips used. We recommend seeding 20,000 cells per chip when using ChiPS4.Note: The optimal cell density for seeding against ECM in the OrganoPlate is cell type dependent and might need to be adjusted when using alternative cell lines according to their size, proliferation potential, doubling time and adhesive properties (generally between 10,000 and 40,000 per chip).
-
d.Resuspend cells in seeding medium to obtain a 10,000 cells/μL cell suspension.
-
a.
-
11.
Remove HBSS from gel channel inlets (columns 1, 4, 7, 10, 13, 16, 19, 22; rows B, E, H, K, N).
-
12.
Aspirate the coating solution from inlet and outlet wells of the perfusion channels (columns 1, 3, 4, 6, 7, 9, 10, 12, 13, 15, 16, 18, 19, 21, 22, 24; rows A, D, G, J, M).
-
13.
Wash by adding 50 μL PBS to inlets and outlets of the perfusion channels and aspirate dispensed PBS thoroughly.
-
14.
Add 50 μL of seeding medium in perfusion channel outlets (columns 3, 6, 9, 12, 15, 18, 21, 24; rows A, D, G, J, M).
Note: Place the tips in the corner of the well and allow them to touch the bottom of the plate. Avoid placing the tips immediately on top of holes as this may favor air bubble formation.
-
15.
Add 2 μL of cell suspension in the perfusion channel inlets (columns 1, 4, 7, 10, 13, 16, 19, 22; rows A, D, G, J, M) using the same pipetting procedure as previously used for gel loading.
Note: regularly resuspend the cell suspension throughout seeding to ensure homogeneous cell density.
Note: Check under the microscope whether the cell suspension has entered the channel. This step can be repeated if initial seeding is not satisfactory by adding another 2 μL droplet.
-
16.
Place the OrganoPlate on its side using the supplied plate stand in a humidified incubator at 37°C, 5% CO2 for at least 4 h to allow cells to settle onto the ECM gel and attach.
-
17.
When cells have attached, add 50 μL of seeding medium in the inlets of seeded perfusion channels (columns 1, 4, 7, 10, 13, 16, 19, 22; rows A, D, G, J, M).
Note: cell attachment to the ECM can be checked using a phase-contrast microscope.
Optional: If desired (i.e. creating a gradient) 50 μL of medium can also be added to the bottom channel inlets and outlets at this stage.
-
18.
Place the OrganoPlate on an OrganoFlow interval rocker platform set at 7-degree inclination and 8-min cycle time in a humidified incubator at 37°C, 5% CO2 and proceed to trophoblast differentiation protocol the day after.
Trophoblast differentiation in the OrganoPlate
Timing: 5–8 days (30 min daily)
A sequence of two culture media is used to drive trophoblast differentiation from hiPSC as described in previous methods.5 The first one, referred to as “pre-differentiation medium” and reduced in FGF-2, is applied to the hiPSC for the first 24 h. The day after, referred to as differentiation day 0, the final trophoblast differentiation medium without FGF-2 and added with BMP4, A83-01 and PD173074 (BAP) is applied to the cells and replenished daily to drive intact placental barrier formation.
-
19.24 h after seeding, change culture medium to trophoblast pre-differentiation culture medium (TeSR1 with 20 ng/mL FGF-2).Note: Pipette tips should have a maximum volume of 200 μL as larger tips cannot be properly placed in the well corner.
-
a.Aspirate medium from perfusion channel inlets and outlets Add 50 μL of PBS to perfusion channel inlets and outlets and aspirate.
-
b.Add 50 μL of pre-differentiation media in perfusion channel inlets.
-
c.Add 50 μL of pre-differentiation media in perfusion channel outlets.
-
d.Place the OrganoPlate back on the OrganoFlow interval rocker platform set at 7-degree inclination and 8-min cycle time in a humidified incubator and proceed with culture.
-
a.
-
20.After a further 24 h, change culture medium to BAP trophoblast differentiation medium (TeSR1 without FGF-2, added with 10 ng/mL BMP4, 1 μM A83-01 and 0.1 μM PD173074) using an 8-tip multichannel pipette and P1000 tips.
-
a.Aspirate medium from perfusion channel inlets and outlets.
-
b.Add 50 μL of freshly made differentiation media in perfusion inlets.
-
c.Add 50 μL of freshly made differentiation media in perfusion outlets.
-
d.Place the OrganoPlate back on the OrganoFlow interval rocker platform set at 7-degree inclination and 8-min cycle time in a humidified incubator and proceed with culture.
-
a.
-
21.
Refresh medium daily for 7 days by aspirating old medium and adding 50 μL of fresh BAP differentiation medium in both perfusion channel inlets and outlets.
Note: Place the tips in the corner of the well and allow them to touch the bottom of the plate. Avoid placing the tips immediately on top of holes as this may disturb cultured cells or favor air bubble formation.
Note: Continuous culture of ChiPS4 into BAP medium has proved efficient to generate a functional 3D placental barrier after 4 days. The placental barrier structure has been successfully maintained for a total of 7 days after adding the BAP differentiation medium, although longer culture might be implemented upon testing.
Barrier integrity assay
Timing: 1.5–2 h
This step describes a standard assay used to assess the permeability of the obtained placental barrier by monitoring the diffusion of fluorescent compounds from the perfusion channel to the gel channel over time as described in detail before.6 This assay is non-destructive and can be performed at any point throughout the differentiation protocol.
-
22.
Set the Incucyte microscope settings to allow image acquisition to start immediately after the dyes are added to the chips.
Note: A microscope with an automatic stage is needed to conduct the barrier integrity assay on multiplex images of the whole OrganoPlate for a higher throughput. In our study we used the Incucyte (Sartorius) which provides rapid automatic multiplex acquisition of the whole OrganoPlate while maintaining the cells in the incubator conditions.
-
23.Prepare the fluorescent working solution containing TRITC-dextran and FITC-dextran dyes.
-
a.Calculate the required amount of fluorescent working solution (70 μL working solution per chip + 40% extra).
-
b.Dilute 25 mg/mL FITC-dextran 10 kDa and TRITC-dextran 155 kDa stock solutions in TeSR1 culture medium at a 1:50 ratio to achieve a final concentration of 0.5 mg/mL.
-
a.
Note: Skip step 24 and proceed immediately to step 25 if all channels, including the opposite channel to the tubule, are perfused with media.
-
24.Perform a “wetting” step to ensure proper flow profiles and successful readouts.
-
a.Add 50 μL TeSR1 medium to gel and bottom channel inlets.
-
b.Add 50 μL TeSR1 medium to gel and bottom channel outlets.
-
c.Place the plate under an angle by placing one side on an object, and perfuse for 5 min.
-
d.Aspirate medium from all inlets and outlets.
-
a.
-
25.Start the assay by pipetting TeSR1 medium and fluorescent working solutions in the following order:
-
a.Pipette 20 μL of TeSR1 medium in the gel inlet and outlet.
-
b.Pipette 20 μL of TeSR1 medium in the bottom channel inlet and outlet.
-
c.Pipette 40 μL of fluorescent working solution in the perfusion channel inlet.
-
d.Pipette 30 μL of fluorescent working solution in the perfusion channel outlet.
-
a.
-
26.Proceed to image acquisition immediately on a fluorescent microscope.
-
a.To monitor barrier integrity over time, image each chip of the OrganoPlate every 2 min for the total duration of the assays (i.e., 30 min). Troubleshooting 6.
-
a.
Note: Make sure not to over-expose the fluorescent dyes when setting the exposure times. Saturated signals will hamper correct quantification of the assay in a later stage.
-
27.
After acquisition, aspirate all solutions from the wells and add 50 μL of fresh trophoblast differentiation medium to perfusion channel inlets and outlets. Place the OrganoPlate back on the OrganoFlow interval rocker platform set at 7-degree inclination and 8-min cycle time in a humidified incubator to continue culture.
Note: After data acquisition is completed, cells can be safely put back in culture, fixed or lysed for protein or RNA collection.
Extracting RNA or proteins for downstream analyses
Timing: 20 min
This section describes the procedure to extract RNA or proteins from cells cultured in the OrganoPlate in order to conduct downstream molecular analyses. The cells are lysed directly within the OrganoPlate and the lysate collected for further processing.
-
28.Prepare the required amount of lysis buffer (50 μL per chip + 40% extra).
-
a.To prepare RNA extraction buffer, mix β-mercaptoethanol into buffer RLT at a 1:100 ratio.
-
b.To prepare protein extraction buffer, add 1 μL of halt protease & phosphatase inhibitor cocktail to 100 μL of cell lysis buffer.
-
a.
Note: RNA and protein extraction buffers should be prepared immediately before use.
Alternative: TRIzol reagent can be used instead of buffer RLT for RNA extraction.
-
29.
Aspirate culture medium from all perfusion channel inlet and outlet wells of the chips.
-
30.
Add the lysis buffer to the perfusion channels by dispensing 35 μL to the inlet wells and 15 μL to the outlet wells.
-
31.
Incubate at 18°C–23°C for 30–60 sec.
-
32.
Collect cell lysates from all inlet and outlet wells and transfer to regular Eppendorf tubes when extracting proteins, or RNase-free Eppendorf tubes when extracting RNA. Troubleshooting.
Note: samples from replicate chips can be pooled together for increased RNA yields. For RNA extraction, we recommend pooling 5 replicate chips.
-
33.Process your RNA lysates for downstream analyses.
-
a.Add RNA extraction buffer to the tubes containing RNA lysates to reach a final volume of 350 μL per tube.
 Pause point: Sample lysates can be stored at ‒80°C for several months before extraction.
Pause point: Sample lysates can be stored at ‒80°C for several months before extraction. -
b.Proceed with RNA extraction procedure according to the manufacturer’s protocol.
 Pause point: Isolated RNA samples can be stored at ‒80°C for several months before being assayed.
Pause point: Isolated RNA samples can be stored at ‒80°C for several months before being assayed. -
c.Proceed with subsequent analyses according to the manufacturer’s protocols.
-
a.
-
34.Process your protein lysates for downstream analyses.
-
a.Sonicate protein lysates for 10 sec.
-
b.Centrifuge at 14,000 g for 10 min in a chilled centrifuge set at 4°C.
-
c.Collect supernatant and transfer to clean Eppendorf tubes.
 Pause point: Isolated protein samples can be stored at ‒20°C to ‒80°C for several months before being assayed.
Pause point: Isolated protein samples can be stored at ‒20°C to ‒80°C for several months before being assayed. -
d.Proceed with subsequent analyses according to the manufacturer’s protocols.
-
a.
Immunostaining analysis
Timing: 1.5 days
This section describes step-by-step procedure to perform fixation, blocking and antibody labeling directly within the OrganoPlate for fluorescence detection of specific cellular biomolecules in the placental barrier model. Perform all steps at 18°C–23°C unless specified otherwise.
-
35.Fix cells in the OrganoPlate.
-
a.Aspirate culture medium from all perfusion channel inlets and outlets.
-
b.Add 3.7% paraformaldehyde in PBS to the chips’ inlets and outlets: 100 μL to the perfusion channel inlet, 50 μL to all other inlets and outlets.
-
c.Incubate for 10–15 min at 18°C–23°C under static conditions.
-
d.Aspirate fixative solution from all inlets and outlets and wash the chips twice (5 min each) with PBS using same volumes as for the fixative solution.
-
e.Remove the HBSS from the observation windows and replace with PBS.
-
a.
Pause point: Add 50 μL of PBS to all wells, seal the plate with Parafilm and wrap in aluminum foil. The fixed plate can be stored at 18°C–23°C for up to 2 weeks. Do not freeze the plate after fixation, as this will cause the glass bottom and the microfluidics to delaminate from the plate.
-
36.
Prepare permeabilization buffer, blocking solution and washing solution as indicated in materials section.
-
37.Permeabilize the cells.
-
a.Wash chips 2 × 5 min with washing solution by adding 100 μL to perfusion channel inlet and 50 μL to all other inlets and outlets.
-
b.Aspirate the washing solution and permeabilize cells for 10 min with permeabilization buffer using the volume scheme described in 3.a.
-
a.
-
38.Block the cells.
-
a.Aspirate the permeabilization buffer and wash chips 2 × 5 min with washing solution using the volume scheme described in 3.a.
-
b.Aspirate washing buffer and block cells for 45 min with blocking buffer using the volume scheme described in 3.a.
-
a.
-
39.Incubate with primary antibodies.
-
a.Prepare primary antibody solutions in blocking buffer at the desired concentration (80 μL primary antibody solution per chip).
-
b.Aspirate blocking buffer and add primary antibody solutions using the volume scheme described in 3.a.
-
c.Incubate the primary antibodies for 12–18 h at 4°C under static conditions.
-
a.
-
40.Incubate with secondary antibodies.
-
a.Prepare secondary antibody solutions in blocking buffer at the desired concentration (80 μL secondary antibody solution per chip).
-
b.Aspirate the primary antibody solutions and wash chips 3x (3 min each) with the washing solution using the volume scheme described in 3.a.
-
c.Aspirate the washing solution and add secondary antibody solutions using the volume scheme described in 3.a.
-
d.Incubate with secondary antibodies at 18°C–23°C for 30 min in the dark on the OrganoFlow rocker platform.
-
a.
-
41.
Aspirate the secondary antibody solutions and wash twice (3 min each) with the washing solution using the volume scheme described in 3.a.
-
42.
Wash chips once (5 min) with PBS using the volume scheme described in 3.a., and add 50 μL PBS to all wells before starting image acquisition.
Expected outcomes
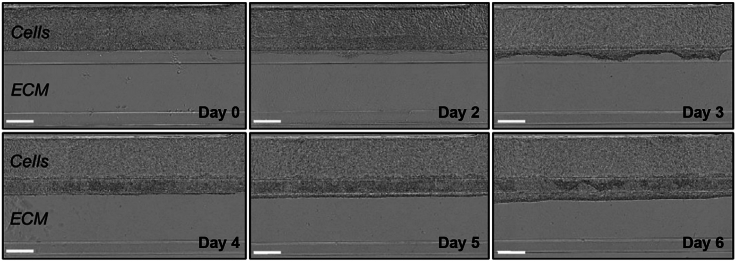
Successful application of this protocol allows users to generate a hiPSC-derived, perfused placental barrier in a multi-chip OrganoPlate device. After seeding, cells should attach to the Collagen-I ECM and proliferate, progressively self-arranging into a 3D, hollow tubular structure after 4 days of differentiation (i.e., 4 days after addition of the final differentiation medium) (Figures 2 and 3A). From day 4–7, cells were shown to form a uniform front at the apical side in contact with the ECM and progressively invade into the Collagen-I ECM (Figure 2).
Figure 2.
Representative phase-contrast images of hiPSCs differentiating into trophoblasts in the OrganoPlate device
Pictures were taken on the day when differentiation medium was first added to the cells (Day 0) and daily throughout 6 days of differentiation. Scale bar = 200 μm.
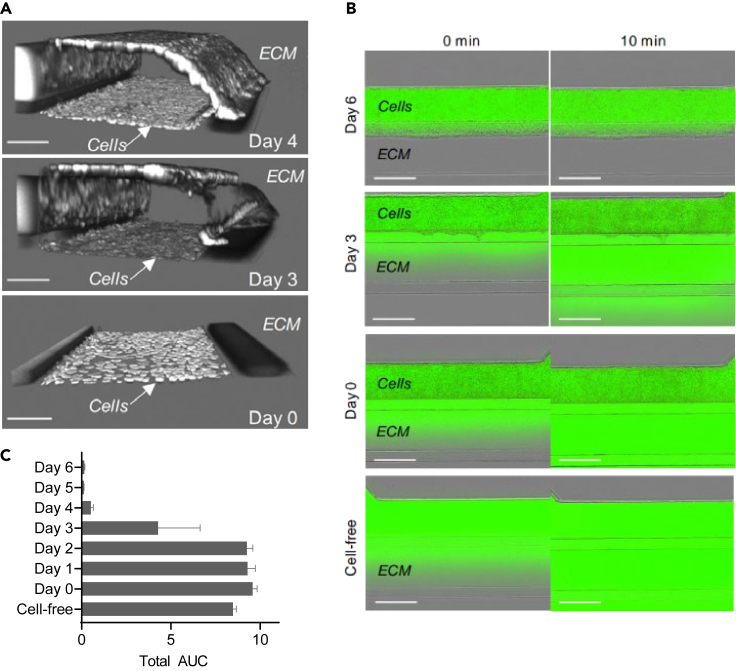
Figure 3.
Formation of a leak-tight barrier after 4 days of differentiation in the OrganoPlate
(A) 3D reconstructions of confocal images of ChiPS4 stained with nuclei marker Hoechst at day 0, 3 and 4 of trophoblast differentiation. Scale bar = 100 μm.
(B) Representative images showing fluorescence signal 10 min after adding 10 kDa FITC-Dextran compound in the perfusion channel of day 0, 3 and 6 cultures compared to a cell-free channel. Scale bar = 400 μm.
(C) Total area under the curve (AUC) quantified from ratio values of 10 kDa FITC-Dextran fluorescence signal in the ECM channel to signal in the cell compartment calculated daily from independent experiments (n = 3). Values are shown as mean ± SEM. (Figure adapted from Lermant et al., iScience 20231).
Successful formation of a structural barrier can be confirmed by performing a barrier integrity assay as described above. The placental barrier model is expected to show consistent and complete leak-tight properties to both 155 kDa and 10 kDa compounds starting from day 4 and up to day 6 of differentiation. Structural integrity of the model after that time point has not been assessed. Up to day 3, fluorescent compounds are expected to leak from the perfusion channel (Figure 3). Check Lermant et al. for detailed results and quantifications of 155 kDa and 10 kDa compound leakage over 6 days of differentiation.1
Successful trophoblast differentiation can be verified by gene and protein expression analyses of positive and negative trophoblast markers. Depending on the timing of extraction, RNA yields should be between 50 and 200 ng/μL and protein yields between 2 and 10 μg/μL when following the procedures described above. After 4 days of differentiation, the obtained cell population should show strong and concomitant expression of all transcription factors making up the trophectoderm core transcriptional circuitry. In contrast, pluripotency, mesoderm, endoderm and ectoderm lineage markers are expected to be strongly downregulated as shown by the RNA-seq results obtained in Lermant et al. 2023. The expression timeline of several trophoblast and pluripotency markers has been assessed by qPCR and immunostaining over 7 days of differentiation. Trophoblast markers KRT-7, GATA3, PGF and HLA-G were shown to be gradually expressed in time while pluripotency markers NANOG and POU5F1 were drastically down-regulated until reaching nearly null expression at day 6 (Figure 4).
Figure 4.
Expression pattern of common trophoblast markers in the hiPSC-derived placenta model
(A) Expression levels of KRT-7, GATA3, PGF, HLA-G, NANOG and POU5F1 after 2, 4 and 6 days of differentiation compared to day 0 levels assayed by qRT-PCR and normalized to GAPDH. Relative mRNA expression is shown as mean ± SEM (n = 3). Blue lines = positive trophoblast markers; orange lines = negative trophoblast markers.
(B) Representative images of ChiPS4 fixed at day 0, day 3 or day 7 of differentiation and stained with nuclei marker Hoechst (blue) and KRT-7 (red). Scale bar, 100 μm. (Figure adapted from Lermant et al., iScience 20231).
Transcriptomics analysis of the whole cell population highlighted the presence of both cytotrophoblasts (CTB) and syncytiotrophoblasts (STB) markers after 4 days of differentiation in the model obtained. Expressed CTB markers include genes related to stemness, and STB markers include genes related to hormone synthesis, cell fusion, L-galectin production and placental transporters. Single-cell RNA-seq analysis has not been conducted to confirm these findings. Our recent publication provides a comprehensive list of measured CTB and STB markers and their relative increase compared to day 01. Syncytins and hCG expression show an exponential expression pattern with a peak expression reached at day 6 (Figure 5A). Moreover, immunostaining analysis revealed signs of a spatio-temporal regulation characterized by hCG protein expression being mostly restricted to a defined apical layer in proximity to the ECM (Figure 5B). Similarly, fusion events can be detectable by immunostaining with local loss of plasma membrane protein E-cadherin matching hCG production areas (Figure 5B).
Figure 5.
Expression pattern of common syncytium markers in the hiPSC-derived placenta model
(A) Expression levels of ERVW-1, ERVFRD-1 and CGB after 2, 4 and 6 days of differentiation compared to day 0 levels assayed by RT-qPCR and normalized to GAPDH. Relative mRNA expression is shown as mean ± SEM (n = 3).
(B) Representative images of ChiPS4 fixed at day 0, day 4 and day 6 of differentiation and stained with nuclei marker Hoechst (blue), E-cadherin (green) and β-hCG (red).
(C) Zoomed pictures of E-cadherin and β-hCG signals in Day 6 cell population (corresponding to areas marked by a white square in B) are shown on the right to highlight local loss of E-cadherin matching with β-hCG expression areas. Scale bar, 100 μm. (Figure adapted from Lermant et al., iScience 20231).
Quantification and statistical analysis
-
1.
Barrier integrity can be quantified by measuring the fluorescence signal in the perfusion channel (Fluocells) and the adjacent ECM channel (Fluogel) of a chip. Open-access software such as ImageJ can be used for that step.
-
2.Determine the Fluogel / Fluocells ratio for each time point, corresponding to the relative leakage property of the barrier.
-
a.In case of a leak-tight barrier, this ratio will be low and remain constant over time.
-
b.In case of a leaky barrier, this ratio will increase over time eventually approaching 1.
-
a.
Limitations
Although the expression of various placental transporters in the model including GLUT1, FATP6, ZnT2, ZnT4 and CFTR has been validated by RNA-seq analysis,1 direct functional assays monitoring the uptake of reference compounds will need to be performed to confirm the model reliability for placental transport studies.
There are previous reports of trophoblast generated by hiPSC differentiation.7,8,9 The BAP differentiation protocol applied here is known to favor STB over extra-villous trophoblast (EVT) differentiation when performed under 20% oxygen conditions.5,10,3,7 The dominance of a STB phenotype in this model was further supported by a combination of transcriptomics and morphological criteria.1 Yet, it is likely that the obtained cell population is a mix of several trophoblast subtypes including a small proportion of EVT as revealed by the moderate expression of the EVT-specific marker HLA-G detected in the model.1 It is worth noting that the placenta in vivo will also have a mixture of trophoblast subtypes which are geographically arranged. Single-cell analyses would be required to determine the precise proportions of trophoblast subtypes in the placenta on a chip model. Depending on the expected outcomes, applying differentiation protocol variations such as the two-step protocols developed recently could be a way to achieve increased control over STB and EVT lineage commitment and develop more specific models of early placental processes.11,12
Finally, this protocol allows the generation of a minimalistic model of the placental barrier, including exclusively trophoblasts. Integrating other cell types that are part of the placental villi, such as endothelial cells or fibroblasts, will be useful to replicate the full placental barrier complexity. This can be easily achieved by seeding other cell types in the opposite channel, as demonstrated by Rabussier et al. who successfully co-cultured trophoblast cell lines and primary endothelial cells to replicate placental barrier functions and disease phenotypes.2
Troubleshooting
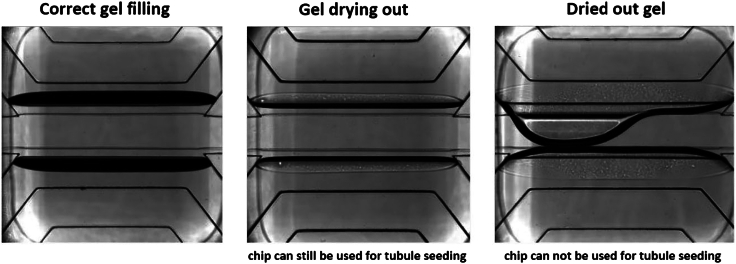
Problem 1
The gel loading is incomplete, or the gel is overflowing to the adjacent channel.
Potential solution
Correct gel loading can be checked by phase-contrast microscopy (Figure 6). In case of incomplete gel loading or overflow.
-
•
Make sure you place your pipette tip right above the top of the hole, perpendicular to the plate as contact between the droplet and the hole is essential for correct gel loading (Figure 6).
-
•
Make sure the pipet tip is not fully blocking the inlet hole (Figure 6).
-
•
If the gel loading is incomplete, increase the loading volume (i.e., to 2.3 μL).
-
•
In case of overflow, reduce the loading volume (i.e., to 1.7 μL).
Figure 6.
Gel loading in the OrganoPlate
Top panel shows pipette tip positioning for correct gel loading in the OrganoPlate. Bottom panel shows and overview of correct gel filling, incomplete gel filling, and overflow in the OrganoPlate.
Problem 2
The ECM gel dried out during loading and polymerization process.
Potential solution
This can happen when the loading process takes longer than expected, leading to the first chips starting to dry while the last are being loaded (Figure 7). To avoid that.
-
•
Make sure the loading process goes smoothly.
-
•
If the loading process is extended, regularly check the loaded gels under the microscope and quickly add HBSS to the gel inlet if starting to dry.
Figure 7.
ECM gel drying out due to prolonged gel loading or polymerization
Problem 3
Incomplete ECM gel polymerization.
Potential solution
The polymerization of certain ECM gels, e.g., collagen-I, is pH-dependent and acidic coating solutions may impair the integrity of the ECM gel in the gel channel. For this reason, make sure the NaHCO3 solution is at pH 9.3 before use and use pH neutral coatings in the perfusion channel.
Problem 4
Presence of air bubbles in the channels.
Potential solution
If air bubbles are introduced into the plate or channels this can impede cell growth, imaging and barrier formation assays. Problems can occur when pipetting viscous solution or leaving the plates to dry out.
-
•
Ensure correct pipetting of solutions.
-
•
First pipet into all inlets, then all outlets in the plate to avoid air trapped into the microfluidic lanes.
-
•
Avoid pipetting air into the OrganoPlate.
-
•
Use reverse pipetting mode at slow speeds for viscous liquids.
-
•
Ensure solutions do not dry out or that channels are left dry (omitting to be filled). The presence of liquid in the channel can be checked by eye: looking at the bottom of the plate, a liquid filled channel will appear black otherwise an empty (dry) channel will appear gray (Figure 6). A Phase contrast microscope can also be used to ascertain if a channel has been missed.
-
•
Ensure the OrganoPlate is under consistent rocking using the MIMETAS plate rocker.
Problem 5
Inadequate barrier formation.
Potential solution
Incomplete trophoblast differentiation or cell loss can compromise the barrier structural integrity. This will invalidate the use of the system to assess effects of various compounds or factors on placental barrier integrity.
-
•
Make sure the coating solution and cell suspension are dispatched evenly within the plate.
-
•
First check that the obtained barrier is completely leak-tight after 4 days of differentiation by performing the barrier integrity on one chip before initiating barrier integrity assays.
-
•
If the barrier is still leaky after 4 days, keep culturing cells in the differentiation medium for another 24 h to allow extra-time for barrier to completely form and check again.
Problem 6
Loss of focus during a barrier integrity assay imaging.
Potential solution
Image-based focusing algorithms can struggle with large fronts of dye. Turn off image-based auto-focusing and pre-define a single focal point (also known as Z-location). Laser-based focusing systems are unaffected by the presence of dye inside the channel.
Problem 7
Insufficient RNA yield.
Potential solution
-
•
Increase the number of pooled replicates.
-
•
Use TRIzol(R) RNA extraction method instead of RNeasy. Following the manufacturer’s protocol and using glycogen as a carrier generally results in higher yields. If using TRIzol, remove the lysate as soon as the culture is lysed and do not leave TRIzol in the OrganoPlate for longer than 5 min. Discard the OrganoPlate after usage of TRIzol.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Colin E. Murdoch (c.z.murdoch@dundee.ac.uk).
Technical contact
Further information and requests for technical details should be directed to and will be fulfilled by the technical contact, Agathe Lermant (a.lermant@dundee.ac.uk).
Materials availability
This study did not generate new unique reagents. Information on reagents used in this study is available in the key resources table.
Data and code availability
RNA-seq data set (fastq files) highlighted in this protocol and reported in Lermant et al. 2023 have been deposited with Zenodo community iPLACENTA https://zenodo.org/search?page=1&size=20&q=iplacenta under accession number https://doi.org/10.5281/zenodo.7510757.
Data has been deposited at https://doi.org/10.17632/wrxsr5drvy.1.
Original microscopy data can be accessed at OMERO (https://www.openmicroscopy.org/omero/) on request.
Acknowledgments
This work has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie agreement no. 765274, iPLACENTA. A.L. and G.R. are/were ESRs funded by iPLACENTA. C.E.M. is the coordinator of iPLACENTA (765274), and this work was further supported by Tenovus Scotland (T18-23) and FP7-Marie Sklodowska-Curie action no. 626633. Thanks to both Dr. James Cantley (School of Medicine, University of Dundee, United Kingdom) for the use of Incucyte live-cell imaging system and Prof. Albena Dinkova-Kostova, whose BBSRC grant BB/T017546/ funded the STELLARIS confocal microscope described in this protocol. We would like to acknowledge Dr. Iain Porter from University of Dundee Imaging Facility for acquiring confocal images of the placenta-on-a-chip.
Author contributions
A.L.: conceptualization, methodology, investigation, writing – first draft, and writing – review and editing. G.R.: methodology and writing – review and editing. H.L.L.: methodology and writing – review and editing. L.D.: methodology and writing – review and editing. C.E.M.: conceptualization, methodology, investigation, writing – review and editing, writing – first draft, project administration, supervision, and funding acquisition.
Declaration of interests
G.R. and H.L.L. are or were employees of MIMETAS, which is marketing the OrganoPlate. OrganoPlate is a registered trademark of MIMETAS. Copyright lies with MIMETAS for Figures 1C, 6, and 7.
References
- 1.Lermant A., Rabussier G., Lanz H.L., Davidson L., Porter I.M., Murdoch C.E. Development of a human iPSC-derived placental barrier-on-chip model. iScience. 2023;26 doi: 10.1016/j.isci.2023.107240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabussier G., Bünter I., Bouwhuis J., Soragni C., van Zijp T., Ng C.P., Domansky K., de Windt L.J., Vulto P., Murdoch C.E., et al. Healthy and diseased placental barrier on-a-chip models suitable for standardized studies. Acta Biomater. 2023;164:363–376. doi: 10.1016/j.actbio.2023.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan M.A., Yang Y., Jain A., Lyons A.S., Yang P., Brahmasani S.R., Dai A., Tian Y., Ellersieck M.R., Tuteja G., et al. Early onset preeclampsia in a model for human placental trophoblast. Proc Natl Acad Sci. 2019;116:4336–4345. doi: 10.1073/pnas.1816150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., Crandall L.J., Daigh C.A., Conard K.R., Piekarczyk M.S., et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 5.Amita M., Adachi K., Alexenko A.P., Sinha S., Schust D.J., Schulz L.C., Roberts R.M., Ezashi T. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soragni C., Vergroesen T., Hettema N., Rabussier G., Lanz H.L., Trietsch S.J., de Windt L.J., Ng C.P. Quantify permeability using on-a-chip models in high-throughput applications. STAR Protoc. 2023;4 doi: 10.1016/j.xpro.2023.102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garipcan A., Ozcimen B., Suder I., Ulker V., Onder T.T., Ozoren N. NLRP7 Plays A Functional Role in Regulating BMP4 Signaling During Differentiation of Patient-Derived Trophoblasts. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-020-02884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahern D.T., Bansal P., Armillei M.K., Faustino I.V., Kondaveeti Y., Glatt-Deeley H.R., Banda E.C., Pinter S.F. Monosomy X in isogenic human iPSC-derived trophoblast model impacts expression 1 modules preserved in human placenta. Proc Natl Acad Sci. 2022;119 doi: 10.1073/pnas.2211073119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Adachi K., Sheridan M.A., Alexenko A.P., Schust D.J., Schulz L.C., Ezashi T., Roberts R.M. Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure. Proc Natl Acad Sci. 2015;112:E2337–E2346. doi: 10.1073/pnas.1504778112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y., Zhou X., Huang W., Long P., Xiao L., Zhang T., Zhong M., Pan G., Ma Y., Yu Y. Generation of trophoblast-like cells from the amnion in vitro: A novel cellular model for trophoblast development. Placenta. 2017;51:28–37. doi: 10.1016/j.placenta.2017.01.121. [DOI] [PubMed] [Google Scholar]
- 11.Horii M., Li Y., Wakeland A.K., Pizzo D.P., Nelson K.K., Sabatini K., Laurent L.C., Liu Y., Parast M.M. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl. Acad. Sci. USA. 2016;113:E3882–E3891. doi: 10.1073/pnas.1604747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castel G., Meistermann D., Bretin B., Firmin J., Blin J., Loubersac S., Bruneau A., Chevolleau S., Kilens S., Chariau C., et al. Induction of Human Trophoblast Stem Cells from Somatic Cells and Pluripotent Stem Cells. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-seq data set (fastq files) highlighted in this protocol and reported in Lermant et al. 2023 have been deposited with Zenodo community iPLACENTA https://zenodo.org/search?page=1&size=20&q=iplacenta under accession number https://doi.org/10.5281/zenodo.7510757.
Data has been deposited at https://doi.org/10.17632/wrxsr5drvy.1.
Original microscopy data can be accessed at OMERO (https://www.openmicroscopy.org/omero/) on request.